Abstract
Multiple shoot induction at an average of (15.25 ± 2.128 and 16.16 ± 3.851 shoots/explant) was obtained from Andrographis paniculata cotyledonary node explants on Murashige and Skoog’s (MS) medium supplemented with 2.0 mg/L 6-Benzylaminopurine (BAP) and Zeatin, respectively after 8 weeks of culture. Further proliferation of cultures in MS + BAP medium at a maximum of 150 ± 18.40 multiple shoots was obtained from single cotyledonary node explants after three subculture (84 days) in vitro passage. Elicitation as yield enhancement strategy, a protocol was developed using multiple shoots to treat the cultures with jasmonic acid (JA) and salicylic acid (SA). Various concentrations of JA (1, 5, 10, 25, 50, 75 and 100 µM) and SA (10, 20, 50 and 100 µM) were used for elicitation. Treatment of JA at 1.0 µM concentration resulted in 1.322 % dry weight (DW) with 2.6-fold increase in andrographolide production after fifth week compared to 0.508 % DW in untreated control. However, JA treatment at 25 and 50 µM promoted 3.3, 3.0-fold enhancement in andrographolide production (1.624 and 1.481 % DW), respectively; after eighth week compared to control. Treatment of 10, 20 and 50 µM SA resulted in 3.0, 3.4 and 3.1-fold andrographolide content (1.479, 1.654 and 1.483 % DW), increase after eighth week, respectively; compared to control (0.478 % DW). This is the first report on elicitation of A. paniculata multiple shoot cultures using signal molecules (JA and SA). The present findings may be helpful for in vitro manipulation and enhanced production of andrographolides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Andrographis paniculata (Wall. ex Nees) of family Acanthaceae is most popular for its various medicinal applications. It is one of the 28 species of Andrographis genus distributed in tropical Asia. This plant is widely used in India, China, Srilanka, Taiwan and other southeast Asian countries for the treatment of respiratory tract infection, common cold, fever, diarrhea, and liver disorders (Negi et al. 2008; Sareer et al. 2014; Wang et al. 2014). This herb is commonly known as Kalmegh which has a bitter taste due to the presence of bioactive diterpene lactones (Kumar et al. 2012). This herb produces many pharmaceutically important molecules such as diterpenoids, flavonoids and polyphenols (Chao and Lin 2010). The primary medicinal constituents of A. paniculata are andrographolides and related diterpene lactones which are found abundantly in leaf compared to other plant parts (Parasher et al. 2011).
A. paniculata has diverse pharmacological benefits such as antiviral (Niranjan et al. 2010), anti-malarial (Mishra et al. 2011), anti-helmintic (Singh et al. 2009), anti-oxidant (Lin et al. 2009), anti-bacterial (Burm et al. 2010) and for anticancer activity (Subramanian et al. 2012; Parveen et al. 2014). The active ingredients of A. paniculata has shown its interference with the viability of HIV-I virus. Further, a phase I trial of andographolide in HIV positive patients and normal volunteers was also undertaken (Calabrese and Berman 2000). Andrographolide, neoandrographolide, and 14-deoxy-11,12-didehydroandrographolide, have been studied for their anti-allergic, anti-inflammatory and cardiovascular effects (Yoopan et al. 2007; Wang et al. 2014). Currently, there is an increasing demand for plant-based medicines, in primary healthcare worldwide (De et al. 2012).
It is well established that the precursors of diterpene lactone andrographolides i.e. isopentenyl diphosphate and its isomer Dimethylallyl diphosphate are synthesized independently in the plastids through MEP/DXP (2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose-5-phosphate) pathway and in the cytosol through Mevalonic acid pathway (Seetha et al. 2005; Srivastava and Akhila 2010). Productivity of secondary metabolites in vitro can be augmented using different in vitro manipulation strategies and elicitation in particular may play an important role by inducing changes in biosynthetic pathways of the target cell (Leonard et al. 2009; Shilpa et al. 2010). Jasmonates (JAs) are a family of cyclopentanone compounds which are involved in plant stress responses and development (Wasternack and Hause 2013; Wasternack 2014). JA and SA are signal molecules involved in the activation of plant defense responses both in biotic and abiotic stresses and elicit wide range of secondary metabolites in plants (Pieterse et al. 2012; Baenas et al. 2014). It is an established fact that, signaling molecule treated plant cell cultures produce enhanced amount of secondary metabolites. Selection of appropriate elicitor is also important for enhanced secondary metabolite production at commercial scale (Ramakrishna and Ravishankar 2011; Murthy et al. 2014). JA and SA are the most successful chemical elicitors used in plant cell, tissue and organ cultures for production of bioactive compounds (Namdeo 2007; Matsuura et al. 2014; Murthy et al. 2014).
Production of andrographolides using in vitro culture systems will be of immense interest because in field cultivated A. paniculata, variation exists in growth and diterpene lactone content (Prathunturarug et al. 2007). Medicinal plants have been cultivated with laboratory-generated species based on chemical composition for commercial purpose (Pietrosiuk et al. 2007). Plant regeneration studies in A. paniculata are few (Purkayastha et al. 2008; Roy et al. 2009; Bansi and Rout 2013). A few reports on andrographolide production in A. paniculata using callus, cell suspension and adventitious root cultures are available (Praveen et al. 2009; Sharma and Jha 2012, 2013a). Recently, multiple shoot culture system has been exploited for elicitation studies and secondary metabolite production (Sivanandhan et al. 2013; Cheruvathur and Thomas 2014). Further, keeping in view the involvement of plastid in MEP/DXP pathway, exploitation of multiple shoot culture system finds its relevance for andrographolide production in vitro. Literature review showed that there was a limited study on elicitation in A. paniculata and related yield enhancement strategy. The present communication reports multiple shoot induction and enhanced andrographolide production in jasmonic/salicylic acid elicited multiple shoot cultures of A. paniculata.
Materials and methods
Germination of seeds
Seeds of A. paniculata were collected from the Regional Centre of CSIR-Central Institute of Medicinal and Aromatic Plants, Hyderabad, India. Seeds were washed using mild detergent (Teepol) solution followed by washing (seeds in mini cheese cloth bag) under running tap water for 15–20 min. Seeds were surface sterilized using 0.1 % (w/v) mercuric chloride solution for 2–3 min and washed 4–5 times with sterile double-distilled water (SDDW). The surface-sterilized seeds were inoculated on to semi-solid MS (Murashige and Skoog 1962) basal medium containing 30 g/L sucrose. Seeds were subsequently, incubated under controlled environmental conditions at 25 ± 1 °C in dark for germination.
Induction and maintenance of multiple shoots
Multiple shoot cultures of A. paniculata were induced using cotyledonary node (seedling devoid of root part) explants on full strength MS medium supplemented with 30 g/L sucrose and 1.0, 2.0, 3.0, 4.0 mg/L 6-benzylaminopurine (BAP); 1.0, 2.0, 3.0 and 4.0 mg/L kinetin (Kn) and 1.0, 2.0, 3.0 and 4.0 mg/L zeatin (Zt). The pH of the medium was adjusted to 5.8 ± 0.2 before addition of 0.9 % agar. The media was autoclaved at 121 °C for 15 min. The cultures were maintained in light provided by cool white fluorescent tube lights at 25 ± 1 °C and sub-cultured once at 4-week regular intervals. A minimum of twelve replicates of explants and different cytokinin concentrations was taken for each treatment. A total of thirty-six cotyledonary node explants were used for each treatment involving different cytokinins. The experiments were repeated thrice and data of respective experiments were recorded. Statistical analysis of data using mean, standard deviation and standard error was calculated.
Elicitation by signal compounds
Jasmonic acid (JA) and salicylic acid (SA) elicitors were evaluated for their influence on andrographolide accumulation in multiple shoot cultures of A. paniculata. Stock solutions of JA and SA, was prepared by dissolving 250 mg of each signal compound in 0.5 mL of dimethyl sulfoxide (DMSO) and absolute ethanol, respectively. Final stock solution of 25 mg/mL of JA and SA was prepared by adding SDDW and filter sterilized using 0.22 µM bacterial filtration unit (Millipore, Ireland). Filter sterilized JA (1, 5, 10, 25, 50, 75 and 100 µM) and SA (10, 20, 50 and 100 µM) was added aseptically to the melted multiple shoot culture growth agar medium using micropipettes (Thermo Scientifics Ltd. India). The 0.02 g of inoculums (axillary shoots) as explants (size ca. 2.0 cm) was inoculated onto multiple shoot culture media containing different concentrations of filter-sterilized JA and SA. Following elicitor treatment, multiple shoot cultures were incubated in culture room conditions in light. Further, subsequent to elicitor treatment multiple shoot cultures were harvested every week starting from first to eight weeks for the study of growth and andrographolide production.
Growth study for multiple shoot cultures
The fresh weight (FW) of control (untreated) and treated multiple shoot cultures were taken after removing the shoots from the semisolid medium. The fresh shoots were air dried at room temperature for 3 weeks, and dry weight (DW) was determined. The FW and DW data of multiple shoots were taken selecting six shoot bunches as replicates from each flasks extending over 8 weeks.
Ultrasonic extraction and quantitative analysis of andrographolide
Dry powdered material of untreated control and signal compound treated multiple shoot culture samples weighing 50 mg DW, was placed in a vial containing 5 mL of high- performance liquid chromatography (HPLC)-grade methanol and incubated for 1 h. The resulting extract was ultra-sonicated using an ultrasonic cleaning bath (Spectra Lab., Model UCB 30, India). After sonication for 30 min, the extract was initially filtered using Whatman filter paper No. 41. Further, the solution recovered was subjected to final filtration through 0.45 μM membrane (Millex HV, Millipore, Ireland) before its injection into HPLC system. The andrographolide fractions were analyzed using HPLC (Waters, USA). Waters Spherisorb C18 column (250 mm × 4.6 mm, 5 µM) was employed. Separation of compounds was carried out by isocratic elution with HPLC-grade methanol as mobile phase at 1 mL min−1 flow rate with 0.02 mL injection volume. The column was maintained at 25 °C. The elution was monitored at 230 nm. The software used was Empower pro and detector was photodiode array detector (PDA) with a retention time of 2.6 ± 0.3 min. Commercially available authentic standard andrographolide (98 % purity) was procured from Sigma-Aldrich, USA for analysis. All results were averaged over two consecutive experiments. Andrographolide content was expressed as % DW of control and elicited multiple shoot culture samples.
Results and discussion
Effect of cytokinins on induction of multiple shoot cultures
Cotyledonary node explants were found suitable for induction of multiple shoots in A. paniculata. The effect of various concentrations of BAP (1.0, 2.0, 3.0 and 4.0 mg/L), Kn (1.0, 2.0, 3.0 and 4.0 mg/L) and Zt (1.0, 2.0, 3.0 and 4.0 mg/L) in MS medium on multiple shoot induction showed variable responses after 4 and 8 weeks. Multiple shoot induction in terms of number of shoots/explant in A. paniculata gave almost similar results in BAP 2.0 mg/L (3.333 ± 0.465), Kn 2.0 mg/L (3.583 ± 0.711) and Zt 2.0 mg/L (3.916 ± 0.792) after 4 weeks (Fig. 1). The average number of shoots after 4 weeks in 1 mg/L BAP, Kn and Zt were 2.75 ± 3.09, 2.5 ± 0.39 and 2.56 ± 0.228, respectively. Lowest number of shoots in 3 mg/L BAP, Kn and Zt were 2.16 ± 0.29, 2 ± 0.34 and 2 ± 0.30, respectively. A decline in the number of shoots with 4 mg/L BAP, Kn and Zt were 1.58 ± 0.26, 1.41 ± 0.22 and 1.66 ± 0.30, respectively (Fig. 1a).
Further, subculture of multiple shoots for a duration of eight week on 2.0 mg/L BAP, Kn and Zt media generated increased number of shoots to 15.25 ± 2.128, 12.75 ± 3.035 and 16.166 ± 3.851, respectively (Fig. 1b). Minimum number of shoots 12.41 ± 2.44, 9.83 ± 2.65 and 11.33 ± 3.15 after 8 weeks in 1 mg/L BAP, Kn and Zt, respectively. The number of shoots up to 10.58 ± 2.61, 9.66 ± 2.44 and 10.58 ± 2.35 in 3 mg/L BAP, Kn and Zt were obtained, respectively. A decline in number of shoots 9 ± 1.83, 8.5 ± 2.58 and 8.41 ± 2.27 was observed with 4 mg/L BAP, Kn and Zt, respectively. In general, there was a decline in the number of shoots/explant on media with other concentrations of BAP (1.0, 3.0 and 4.0 mg/L), Kn (1.0, 3.0 and 4.0 mg/L) and Zt (1.0, 3.0 and 4.0 mg/L). BAP and Zt (2.0 mg/L) was found suitable for the growth of shoots up to eighth week.
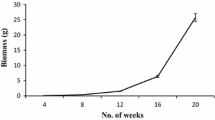
Primary shoots obtained from initially cultured cotyledonary node explants were repeatedly sub-cultured on MS medium supplemented with 2.0 mg/L BAP to generate large number of multiple shoots. BAP 2.0 mg/L was found to be most effective plant growth regulator for multiple shoot induction. A total of in an average 150 ± 18.40 shoots can be obtained from single cotyledonary node explant after 12 week (84 days) of culture when 8 week old individual shoots were sub-cultured for further proliferation (Figs. 2, 3a–d). Each single shoot was able to generate minimum of ten shoots in average. However, in a similar earlier report with A. paniculata 60–70 shoots was obtained after 90 days of in vitro passage (Purkayastha et al. 2008). In both the aforesaid findings cytokinin BAP was found superior in its response for multiple shoot induction. However, in a different study with A. paniculata we had observed, the Zt was found better than both Kn, BAP and thiodiazuron. Although, in this study higher number with an average of 62 shoots was obtained from stem base explants after 4 week of culture. But, maintenance of Zt promoted multiple shoots beyond 4 weeks was not suitable (Roy et al. 2009). However, in the present study this bottleneck was overcome by the induction and maintenance of multiple shoots in BAP containing medium. In fact, the multiple shoot cultures can be maintained in MS + BAP 2.0 mg/L medium beyond 2 years with similar growth pattern.
Influence of signal compounds (JA, SA) on growth and andrographolide production
In the present work, the effect of elicitation on growth and total andrographolide production in A. paniculata multiple shoot cultures were evaluated from first to 8 weeks. JA showed positive effect on growth and biomass production. However, on the other hand SA significantly reduced the biomass production in most of the treatments irrespective of all the concentrations used when compared to control shoot cultures. The effects of both the elicitors at varying concentrations on total andrographolide accumulation in multiple shoot cultures of A. paniculata showed changeable responses.
A comprehensive study on the effect of signal compounds such as JA and SA on fresh and dry weight of A. paniculata multiple shoot cultures revealed variable responses (Figs. 4a, b, 5a, b). FW of JA treated multiple shoot cultures were increased after first (all the concentrations), third (1 and 5 µM), fourth (all the concentrations except 100 µM), sixth (all the concentrations except 10, 50 and 100 µM), seventh (1 and 5 µM) and eighth (1 and 5 µM) week when compared to untreated control cultures. A decline in growth of shoots were observed from second to eighth week with 100 µM JA treatment compared to untreated control. However, in an earlier report all the concentrations (50-200 µM) of MeJA decreased the growth of Bacopa monnieri shoots (Sharma et al. 2013b). The growth study starting from first to eighth week revealed that highest biomass production of shoots (0.546 ± 0.097 g FW) was obtained with 5 µM treated multiple shoots after fourth week of culture compared to other concentrations of JA and untreated control (0.221 ± 0.040 g FW) shoot cultures (Fig. 4a). Best growth of control shoots (0.359 ± 0.139 g FW) was observed after fifth week. Seventh week JA (1, 5 µM) treated shoot cultures showed more FW when compared to control cultures. However, the treatment with these two concentrations (1, 5 µM), a meager increase in growth was observed after eighth week compared to control. There was an increase in growth of shoots at 25 and 75 µM JA dosage after sixth week compared to1 and 5 µM JA. Increased treatment of JA (50, 100 µM) decreased the growth drastically even lower than untreated control shoot cultures after sixth week. Similar type of response was also reported in shoot cultures of Hypericum hirsutum and Hypericum maculatum (Coste et al. 2011). In the present study, there was no significant difference in biomass between 1 µM JA treated and control shoots after fifth week but at 25 µM concentrations there was inhibitory effect on growth of shoots when compared to control shoot cultures (Fig. 4a). Elicitors are signal compounds, which induce or enhance the biosynthesis of metabolites by activating pathways in response to exogenous stresses (Kuzmaa et al. 2009). Jasmonic acid and its derivatives are stress signaling molecules involved in plant defense and regulate various metabolic pathways along with other plant hormones (Avanci et al. 2010; Ballare 2011; Pirbalouti et al. 2014). These molecules are responsible for enhanced production of different secondary metabolites and act in plant chemical defense mechanism (Frankfater et al. 2009; Murthy et al. 2014). In the past, a number of elicitors were used for increased production of plant secondary metabolites. JA and its derivatives were proven very effective in enhancing secondary metabolite production in different plant cell cultures (De Geyter et al. 2012).
It is well known that SA is an endogenous signaling molecule involved in plant defense and growth responses (Hayat et al. 2010; Boatwright and Pajerowska-Mukhtar 2013). In general, the findings in the present study indicated that SA was not a good promoter for growth rather generated less biomass compared to untreated control. SA concentrations, such as 10, 20 and 50 µM showed optimum growth, but; still lesser than control and further reduction in growth at 100 µM salicylic acid (Fig. 4b). Thus, increased concentration of SA suppressed the growth and production of andrographolides in shoot cultures of A. paniculata. Similar observations on reduced biomass production in Digitalis purpurea shoots with increasing concentrations of SA has also been reported (Patil et al. 2013). SA (10, 20, 50 and 100 µM) was very effective after fifth, sixth, seventh and eighth week. SA treatment resulted in adverse influence on overall growth of multiple shoot cultures. SA significantly reduced the biomass production irrespective of all concentrations used when compared to control shoots except with 10 µM SA after second week. The second week 10 µM SA treated shoots gave higher biomass (0.216 ± 0.055 g FW) compared to untreated control. Nevertheless, compared to control a marginal increase in growth was observed after eighth week when multiple shoots were treated with both 10 and 50 µM SA.
Compared to other concentrations of JA and control shoot cultures, DW was high after third and fourth week when 1 and 5 µM JA treatment was given. However, maximum DW (0.088 ± 0.011 g DW) of shoots was obtained with 5 μM JA compared to control (0.035 ± 0.004 g DW) after fourth week of culture. Higher DW was obtained after sixth week with 1, 25 and 75 µM JA action. On the other hand, shoot DW was more after seventh week following 1 and 5 µM JA application. Very low growth of shoot cultures was observed with 1, 5 and 10 µM JA on eighth week when compared to control shoots cultures (Fig. 5a). SA treated shoot cultures at different concentrations and culture duration revealed differential growth responses. Maximum shoot biomass (0.035 ± 0.007 g DW) was more with 10 µM SA after second week and 10 and 50 µM after eighth week when compared to control shoot cultures (Fig. 5b). Previous reports on the effect of SA on biomass production gave similar credence to the results observed in the present study. High concentration of SA (100 µM) resulted in inhibition of biomass from first to eight weeks and similar observations were also observed with multiple shoot cultures of Ruta graveolens (Diwan and Malpathak 2010) and Withania somnifera (Sivanandhan et al. 2013).
The influence of signal molecules JA and SA on andrographolide production in A. paniculata multiple shoot cultures revealed irregular responses. HPLC analysis of standard andrographolide, untreated control and randomly selected elicited multiple shoot cultures was carried out (Fig. 6a–c). Addition of 75 µM JA resulted in increased andrographolide production (0.813 % DW) when compared with untreated control cultures (0.548 % DW) after first week. Cultures treated with 50 µM JA and the analysis after second week resulted in comparable amount of andrographolide production (0.793 % DW) compared to 0.582 % DW in control cultures. Earlier, a treatment of 50 µM MeJA (derivative of JA) promoted enhanced production of bacoside A in multiple shoot cultures of B. monnieri (Sharma et al. 2013b). Further, a marginal decline in andrographolide content (0.776 % DW) was observed with 5 µM JA treatment after third week with control (0.590 % DW). Addition of JA at concentrations 1 and 25 µM and analysis of shoot cultures at the end of fifth week was effective and andrographolide content significantly enhanced up to 1.322 % (2.6-fold) and 1.253 % (2.4-fold), respectively; compared to control cultures (0.508 % DW). An increase in andrographolide content (1.012 % DW) was observed with 1 µM JA dosage after seventh week compared to control (0.891 % DW). However, a significantly sharp increase in andrographolide content of 1.624 (3.3-fold) and 1.481 % DW (3.0-fold) was obtained after eighth week with 25 and 50 µM JA concentrations, respectively (Fig. 7a). In an earlier finding it was found that a, longer exposure of Salvia officinalis shoots to high concentrations of MeJA treatment resulted in lower diterpenoid content, although the content were significantly higher compared to controls (Izabela and Halina 2009). In an earlier report threefold increase in bacoside content was obtained with an elicitor treatment of 1.0 mg/L JA (Sharma et al. 2015). A general trend of increase in andrographolide content was observed after eighth week with all treatments ranging from 1, 5, 10, 75 and 100 µM JA when compared to control. All the concentrations of elicitor (MeJA) facilitated higher bacoside A content, however; 50 µM MeJA treatment resulted in highest (twofold) production (Largia et al. 2015). JA at 1, 25, and 50 µM resulted in increased production of andrographolide. Higher concentrations of JA (75 and 100 µM) resulted in reduced andrographolide production. Comparable results on higher concentrations of JA (500 µM) was observed with decreased hypericin content in H. hirsutum and H. maculatum (Coste et al. 2011).
Addition of 10, 20 and 100 µM SA resulted in increased andrographolide content of 1.259 % DW, 1.323 % DW and 1.243 % DW, respectively; after fifth week of culture when compared to 0.804 % DW content following 50 µM treatment. Treatment of 20 and 50 µM SA was effective after sixth week old cultures in increasing the andrographolide content of 1.551 % and 1.494 %, respectively; than control (0.877 % DW). Further, a minor increase in andrographolide content of 1.169 % and 1.132 % DW was observed in 10 and 100 µM treated cultures, respectively after six week of culture. Previously, 50 µM SA resulted in enhanced digitoxin and bacoside production in D. purpurea and B. monnieri shoots; respectively (Patil et al. 2013; Sharma et al. 2015). The presence of higher levels of salicylic acid (100 µM) decreased the andrographolide production when compared to other concentrations. This observation is similar to previous reports on secondary metabolites production in multiple shoot cultures of H. hirsutum and H. maculatum (Coste et al. 2011). Addition of 10, 20, 50 and 100 µM SA resulted in enhanced andrographolide content of 1.481, 1.442, 1.467 and 1.352 % DW, respectively; compared to control (0.891 % DW) after seventh week. Treatment of shoots with 10, 20, 50 and 100 µM SA also resulted in improved andrographolide production of 1.479 % (3.0-fold), 1.654 % (3.4-fold), 1.483 % (3.1-fold) and 1.275 % DW(2.6-fold), respectively; contrast to control (0.478 % DW) after eighth week (Fig. 7b). Further, elicitation with 50 µM SA produced about threefold higher content of bacoside A; respectively, when compared to untreated control shoots (Largia et al. 2015). In the present study similar response was observed following 50 µM SA elicitation. After eighth week low concentration of SA (20 µM) enhanced the production of andrographolide and it was the highest compared to other concentrations of SA. However, higher concentration of 100 µM SA decreased the andrographolide content. SA at higher concentrations (200 and 250 µM) decreased withanolides production, although their accumulation was significantly higher when compared to untreated control (Sivanandhan et al. 2013). Decrease in bacoside content with increased concentration of SA (100 and 200 µM) was observed in B. monnieri shoot cultures (Sharma et al. 2015).
There are several reports on the influence of many signal compounds including JA, SA and other chemical elicitors on in vitro culture systems (callus, cell suspension, adventitious roots, hairy roots and multiple shoot cultures etc.) for enhanced secondary metabolites production (Karuppusamy 2009; Ramakrishna and Ravishankar 2011). A number of other reports include valtrate production in Valeriana amurensis adventitious root cultures (Cui et al. 2012), glucosinolates production in Sinapis alba hairy root cultures (Kastell et al. 2013) and enhancement of vindoline and vinblastine production in suspension-cultured cells of Catharanthus roseus by artemisinic acid elicitation (Liu et al. 2014). Besides the use of signal molecules as elicitor heavy metals can also influence improved production of total andrographolides in cell suspension culture of A. paniculata (Gandi et al. 2012).
Conclusion
These results suggest that, andrographolide accumulation in multiple shoots of A. paniculata was influenced by both the elicitors (JA, SA) bringing about enhancement and variation. Out of the two elicitors, JA could result in increase of biomass at certain concentrations but SA showed inhibitory effect irrespective of different concentrations used. However, both the elicitors were found to be very effective in eliciting increased andrographolide production. Our results showed that the application of signal molecules allowed optimal production of andrographolide in multiple shoot cultures of A. paniculata. Synthesis of secondary metabolites requires the plant tissue to perceive and react to various environmental signals in an interactive manner. The exogenous presence of elicitors (signal molecules), helps to alter the cellular dynamics and regulate the cellular environ (Kohli et al. 2013; Murthy et al. 2014). Outcome of the present study may be exploited for further enhancement in andrographolide production through biotechnological interventions in the diterpene lactone biosynthetic pathway using molecular approaches.
Abbreviations
- BAP:
-
6-Benzylaminopurine
- DW:
-
Dry weight
- FW:
-
Fresh weight
- HPLC:
-
High performance liquid chromatography
- JA:
-
Jasmonic acid
- Kn:
-
Kinetin
- MS:
-
Murashige and Skoog (1962)
- SA:
-
Salicylic acid
- Zt:
-
Zeatin
References
Avanci NC, Luche DD, Goldman GH, Goldman MH (2010) Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Genet Mol Res 16:484–505
Baenas N, García-Viguera C, Moreno DA (2014) Elicitation: a tool for enriching the bioactive composition of foods. Molecules 19:13541–13563Ballare CL (2011) Jasmonate-induced defenses: a tale of intelligence, collaborators and rascals. Trends Plant Sci 16:249–257
Ballare CL (2011) Jasmonate-induced defenses: a tale of intelligence, collaborators and rascals. Trends Plant Sci 16:249–257
Bansi TS, Rout GR (2013) Plant regeneration protocol of Andrographis paniculata (Burm. f.) -an important medicinal plant. Afr J Biotechnol 12:5738–5742
Boatwright JL, Pajerowska-Mukhtar K (2013) Salicylic acid: an old hormone up to new tricks. Mol Plant Pathol 14:623–634
Burm F, Kumar OA, Naidu LM, Rao KG (2010) In vitro antibacterial activity in the extracts of Andrographis paniculata. Int J Pharm Tech Res 2:1383–1385
Calabrese C, Berman Babish JG (2000) A phase I trial of andographolide in HIV positive patients and normal volunters. Phytother Res 14:333–338
Chao WW, Lin BF (2010) Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin Med 17:1–15
Cheruvathur MK, Thomas TD (2014) High frequency multiple shoot induction from nodal segments and rhinacanthin production in the medicinal shrub Rhinacanthus nasutus (L.) Kurz. Plant Growth Regul 74:47–54
Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell, Tissue Organ Cult 106:279–288
Cui L, Wang ZY, Zhou XH (2012) Optimization of elicitors and precursors to enhance valtrate production in adventitious roots of Valeriana amurensis Smir. ex Kom. Plant Cell, Tissue Organ Cult 108:411–420
De Geyter N, Gholami A, Goormachtig S, Goossens A (2012) Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci 17:349–359
De S, Dey YN, Sarkar P, Gaidhani S (2012) An overview of angiogenesis and renal cell carcinoma. Int J Nutr Pharmacol Neurol Dis 2:3–7
Diwan R, Malpathak N (2010) Bioprocess optimization of furanocoumarin elicitation by medium renewal and re-elicitation: a perfusion-based approach. Appl Biochem Biotech 163:756–764
Frankfater CR, Dowd MK, Triplett BA (2009) Effect of elicitors on the production of gossypol and methylated gossypol in cotton hairy roots. Plant Cell, Tissue Organ Cult 98:341–349
Gandi S, Rao K, Chodisetti B, Giri A (2012) Elicitation of andrographolide in the suspension cultures of Andrographis paniculata. Appl Biochem Biotechnol 168:1729–1738
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68:14–25
Izabela G, Halina W (2009) The effect of methyl jasmonate on production of antioxidant compounds in shoot cultures of Salvia officinalis L. Herb Pol 55:238–243
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plant Res 3:1222–1239
Kastell A, Smetanska I, Ulrichs C, Cai Z, Mewis I (2013) Effects of phytohormones and jasmonic acid on glucosinolate content in hairy root cultures of Sinapis alba and Brassica rapa. Appl Biochem Biotechnol 169:624–635
Kohli A, Sreenivasulu N, Lakshmanan P, Kumar PP (2013) The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Rep 32:945–957
Kumar A, Dora J, Singh A, Tripathi R (2012) A review on king of bitter (Kalmegh). Int J Res Pharm Chem 2:116–124
Kuzmaa L, Bruchajzer E, Wysokinska H (2009) Methyl jasmonate effect on diterpenoid accumulation in Salvia sclarea hairy root culture in shake flasks and sprinkle bioreactor. Enz Microb Technol 44:406–410
Largia MJV, Pothiraj G, Shilpha J, Ramesh M (2015) Methyl jasmonate and salicylic acid synergism enhances bacoside A content in shoot cultures of Bacopa monnieri (L.) Plant Cell Tissue Organ Cult (Online First)
Leonard E, Runguphan W, Connor Prather KJ (2009) Opportunities in metabolic engineering to facilitate scalable alkaloid production. Nat Chem Biol 5:292–300
Lin FL, Wu SJ, Lee SC, Ng LT (2009) Antioxidant, antioedema and analgesic activities of Andrographis paniculata extracts and their active constituent andrographolide. Phyto Res 23:958–964
Liu J, Zhu J, Tang L, Wen W, Lv S, Yu R (2014) Enhancement of vindoline and vinblastine production in suspension-cultured cells of Catharanthus roseus by artemisinic acid elicitation. World J Microbiol Biotechnol 30:175–180
Matsuura HN, Rau MR, Fett-Neto AG (2014) Oxidative stress and production of bioactive monoterpene indole alkaloids: biotechnological implications. Biotechnol Lett 36:191–200
Mishra K, Dash AP, Dey N (2011) Andrographolide: a novel antimalarial diterpene lactone compound from Andrographis paniculata and its interaction with curcumin and artesunate. J Trop Med 2011 Article ID 579518:1–6
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell, Tissue Organ Cult 118:1–16
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites: a review. Phcog Rev 1:69–79
Negi AS, Kumar JK, Luqman S, Shanker K, Gupta MM, Kbanuja SP (2008) Recent advances in plant hepatoprotectives: a chemical and biological profile of some important leads. Med Res Rev 28:746–772
Niranjan A, Tewari SK, Lehri A (2010) Biological activities of Kalmegh (Andrographis paniculata Nees) and its active principles-a review. Ind J Nat Prod Resour 1:125–135
Parasher R, Upadhyay A, Khan NA, Dwivedi SK (2011) Biochemical estimation and quantitative determination of medicinally important andrographolide in Andrographis paniculata at different growth stages. J Environ Agric Food Chem 10:2479–2486
Parveen R, Ahmad FJ, Iqbal Z, Singh M, Kamal YT, Ahmad S (2014) Simultaneous estimation of anti-cancer terpenoids in pharmaceutical nanoformulation by RP-HPLC and HPTLC. Acta Chromatogr 26:391–400
Patil JG, Ahire ML, Nitnaware KM, Panda S, Bhatt VP, Kishor PB, Nikam TD (2013) In vitro propagation and production of cardiotonic glycosides in shoot cultures of Digitalis purpurea L. by elicitation and precursor feeding. Appl Microbiol Biotechnol 97:2379–2393
Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:489–521
Pietrosiuk A, Furmanowa M, Lata B (2007) Catharanthus roseus micropropagation and in vitro techniques. Phytochem Rev 6:459–473
Pirbalouti AG, Sajjadi SE, Parang K (2014) A review (research and patents) on jasmonic acid and its derivatives. Arch Pharm Chem Life Sci 347:229–239
Prathunturarug S, Schaffner W, Berger BK, Pank F (2007) Variation in growth and diterpene lactones among field-cultivated Andrographis paniculata. J Nat Med 61:159–163
Praveen N, Manohar SH, Naik PM, Nayeem A, Jeong JH, Murthy HN (2009) Production of andrographolide from adventitious root cultures of Andrographis paniculata. Curr Sci 96:694–697
Purkayastha J, Sugla T, Paul A, Solleti S, Sahoo L (2008) Rapid in vitro multiplication and plant regeneration from nodal explants of Andrographis paniculata : a valuable medicinal plant. In Vitro Cell Dev Biol Plant 44:442–447
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731
Roy S, Giri A, Bhubaneswari P, Narasu ML, Giri CC (2009) High frequency plant regeneration via direct organogenesis in Andrographis paniculata: an important medicinal plant. Med Aromat Plant Sci Biotechnol 3:94–96
Sareer O, Ahmad S, Umar S (2014) Andrographis paniculata: a critical appraisal of extraction, isolation and quantification of andrographolide and other active constituents. Nat Prod Res 9:1–21
Seetha K, Banerjee NS, Omkumar RV, Purushothama MG (2005) Cloning and characterization of partial promoter of HMGCoA reductase from Andrographis paniculata (Burm.f.) Wall.ex Nees - a tropical medicinal plant. J Plant Biochem Biotechnol 14:41–45
Sharma SN, Jha Z (2012) Production of andrographolide from callus and cell suspension culture of Andrographis paniculata. J Cell Tissue Res 12:3423–3429
Sharma P, Yadav S, Srivastava A, Shrivastava N (2013a) Methyl jasmonate mediates upregulation of bacoside a production in shoot cultures of Bacopa monnieri. Biotechnol Lett 35:1121–1125
Sharma SN, Jha Z, Sinha RK (2013b) Establishment of in vitro adventitious root cultures and analysis of andrographolide in Andrographis paniculata. Nat Prod Commun 8:1045–1047
Sharma M, Ahuja A, Gupta R, Mallubhotla S (2015) Enhanced bacoside production in shoot cultures of Bacopa monnieri under the influence of abiotic elicitors. Nat Prod Res 29:745–749
Shilpa K, Varun K, Lakshmi BS (2010) An alternate method of natural drug production: eliciting secondary metabolite production using plant cell culture. J Plant Sci 5:222–247
Singh S, Mehta A, John J, Mehta P (2009) Anthelmintic potential of Andrographis paniculata, Cajanus cajan and Silybum marianum. Pharmacogn J 1:71–73
Sivanandhan G, Rajesh M, Arun M, Jeyaraj M, Kapil Dev G, Arjunan A, Manickavasagam M, Muthuselvam M, Selvaraj N, Ganapathi A (2013) Effect of culture conditions, cytokinins, methyl jasmonate and salicylic acid on the biomass accumulation and production of withanolides in multiple shoot culture of Withania somnifera (L.) Dunal using liquid culture. Acta Physiol Plant 35:715–728
Srivastava N, Akhila A (2010) Biosynthesis of andrographolide in Andrographis paniculata. Phytochem 71:1298–1304
Subramanian R, Asmawi MZ, Sadiku A (2012) A bitter plant with a sweet future? A comprehensive review of an oriental medicinal plant: Andrographis paniculata. Phytochem Rev 11:39–75
Wang J, Yang W, Wang G, Tang P, Sai Y (2014) Determination of six components of Andrographis paniculata extract and one major metabolite of andrographolide in rat plasma by liquid chromatography–tandem mass spectrometry. J Chromatogr B 951–952:78–88
Wasternack C (2014) Action of jasmonates in plant stress responses and development—applied aspects. Biotechnol Adv 32:31–39
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058
Yoopan N, Thisoda P, Rangkadilok N, Sahasitiwat S, Pholphana N, Ruchirawat S, Satayavivad J (2007) Cardiovascular effects of 14-deoxy-11,12-didehydroandrographolide and Andrographis paniculata extracts. Planta Med 73:503–511
Acknowledgments
Authors would like to thank sponsors University Grants Commission (UGC), New Delhi OU-DST-PURSE project Department of Science and Technology (DST), New Delhi for financial support. Mr. Mohd. Zaheer thanks UGC and DST (OU-DST-PURSE), New Delhi for the Research Fellowships.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaheer, M., Giri, C.C. Multiple shoot induction and jasmonic versus salicylic acid driven elicitation for enhanced andrographolide production in Andrographis paniculata . Plant Cell Tiss Organ Cult 122, 553–563 (2015). https://doi.org/10.1007/s11240-015-0787-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0787-2