Abstract
Coumestrol (CMS), one of the soybean isoflavonoids which contains several benefits for maintaining skin function including antiageing properties. In this study, we evaluated various explant sources and plant genotypes to determine competent soybean adventitious root materials for the mass production of CMS, and investigated their skin care efficacies to be used as a novel cosmetic ingredient. Adventitious roots were directly induced from in vitro seedling derived from the mature seeds, extracts were prepared and refluxed for enzymatic deglycosylation. In vitro cell cytotoxicity was evaluated using normal human dermal fibroblast and murine B16 melanoma cells after treatment with increasing concentrations of methanol soybean adventitious roots extracts for 72 h. Finally, in vitro cell assays on HDF cells were performed to evaluate the effect of the soybean adventitious roots extracts in collagen production. The root induction frequency and biomass productivity were significantly affected by plant genotypes, explant sources, the type of auxin used and its concentration. The total CMS production (per 1 L medium) after 4 weeks of culture in a bulb-type bubble bioreactor (3 L capacity) was the highest in the adventitious roots induced from the radicles of Glycine max, ‘Sinhwakong’. Different strengths of Murashige and Skoog (MS) medium were tested to develop culture protocols and the highest total CMS production (per 1 L medium) was observed at 1/2 MS. The content of coumestrin, the glycoside form of CMS, was higher than that of CMS in the roots cultured in 1/2 MS medium for 4 weeks in a bioreactor. The final content of CMS in the ethanol extract after enzymatic deglycosylation was 81.3-fold higher than non-enzymatic deglycosylation. Almost all the coumestrin in the roots were converted to CMS. Further, the enriched CMS root did not exhibit any cell cytotoxicity in normal human dermal fibroblast (HDF) and murine B16 melanoma cells (B16) for 72 h. In addition, in vitro collagen production assay on HDF cells showed that the enriched CMS root increased the collagen production compared to the coumestrol, daidzein, and non-enzyme-treated sample. Thus, enriched CMS root could be potential ingredient for the cosmetic applications
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the range of applications for high-value plant-based bioactive compounds used as active materials in pharmaceuticals, health foods, and cosmetic products has increased. Phytoestrogens are one such type of bioactive compound in high demand in industry and are usually extracted from the seeds of leguminous plants, which contain three main compounds in four different chemical forms: the aglycones, daidzein, genistein, and glycitein. The glucosides daidzin, genistin, and glycitin; their malonyl glucoside and acetyl glucoside forms (Wang and Murphy 1994). Coumestrol (CMS), a well-known potent phytoestrogen, is a trace isoflavonoid present in Glycine max (commonly known as soybean) belonging to the coumestan family, derived from the precursor daidzein. In natural conditions, the CMS level in plants is increased, and it acts as phytoalexin under stress conditions, such as drought, germination, fungal infection, or exposure to chemical compounds to protect plant survival (Simons et al. 2011, 2012). A recent study confirmed that CMS exerts an anti-cancer, anti-obesity, and neuroprotective effects without the induction of significant side effects (Castro et al. 2012; Lee et al. 2013; Taxvig et al. 2013) and is a novel dietary compound for the prevention and improvement of UVB-associated skin ageing (Park et al. 2015). However, the amount of CMS in natural plants is very low and markedly affected by harvest time and environmental conditions (Tripathi et al. 2016); thus, these factors limit the use of CMS in industrial applications.
The plant tissue culture technology has enabled the controlled production of target biomass and bioactive compounds in large quantity as well as the development of a pilot-scale production system using bioreactors (Gaid et al. 2016). Adventitious roots—derived from plant tissues in a culture medium—are one of the most attractive materials for the production of biomass with bulk of valuable phytochemicals within a limited time and space which does not involve genetic modification (Saeed et al. 2017). In vitro soybean cultures have been produced using cells (Federici et al. 2003; Zacharius and Kalan 1990), somatic embryos (Lazzeri et al. 1987) and hairy roots (Theboral et al. 2014). These cultures were further used for the production of macro compounds such as daidzein, genistein, glycitein, and their glycosides. However, there are no reports pertaining to the production of highly valuable CMS through soybean adventitious roots.
In recent years, there has been a growing interest in developing novel plant-based materials to be used in the cosmetic industry, soybean adventitious roots exhibit a suitable phytochemical profile for such purpose. Compounds such as CMS, daidzein, genistein, glycitein, and their glycosides are widely used in the cosmetic industry. To assess the effects of the soybean adventitious roots extracts on cell viability, and its influence in collagen production, murine melanoma B16 and human dermal fibroblast skin cells were used as in vitro models.
In the present study, we identified the most competent soybean adventitious roots for the mass production of CMS through the assessment of different explant sources and plant genotypes, namely elite cultivars and semi-wild soybeans. In addition, we developed a tissue culture protocol to enhance the biomass using deglycosidase derived from Aspergillus aculeatus. Finally, the potential as novel plant-based cosmetic material of extracts derived from the soybean adventitious roots was evaluated in skin cells.
Materials and methods
Adventitious root induction and continuous bioreactor culture
Adventitious roots were directly induced from in vitro seedling derived from the mature seeds. Each sterilized seed of the two genotypes, ‘Sinhwakong’ (SHK; an elite cultivar) and ‘Napjakong’ (NJK; a semi-wild soybean), was placed on full strength of Murashige and Skoog medium (MS medium; Duchefa, Haarlem, The Netherlands) (Murashige & Skoog 1962) supplemented with 30 g L−1 sucrose and 2.3 g L−1 Gelrite™ (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for germination. After each seedling was grown to ~ 10 cm, the cotyledons, hypocotyls, and radicles were excised from the seedlings and transferred to full strength of MS medium supplemented with different concentration of indole-3-butyric acid (IBA; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and 1-naphthaleneacetic acid (NAA; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) to induce adventitious roots. The induced adventitious roots were propagated in the full strength of MS medium containing 4 mg L−1 IBA without Gelrite™ in 3-L bulb-type bubble bioreactors. The bioreactor culture was initiated by inoculation with fresh adventitious roots at a density of 4.0 g L−1 and the aeration volume in the bioreactors was adjusted to 0.1 vvm (air volume/culture volume per min) using air flow meters (RMA series; Dwyer Instruments Inc., Michigan, USA). The adventitious roots were maintained by sub-culturing in fresh liquid medium every 3 weeks in the dark at 22 ± 1 °C.
Determination of optimal MS medium salt strength
To determine the optimal MS medium salt strength for the promotion of root growth and CMS accumulation, the adventitious roots were inoculated in 3-L bulb-type bubble bioreactors supplemented with different strengths of 2 L of MS medium (0.25, 0.5, 1.0, and 2.0 X), 4 mg L−1 IBA, and 30 g L−1 sucrose and inoculum density of 4 g L−1. The other culture conditions were the same as described above.
Determination of the root weight
After culture for 4 weeks in the bioreactor, the roots were separated from the liquid culture medium and their fresh weight was measured after blotting away the surface water. The fresh roots were dried at 60 °C for 24 h and the dry weight was recorded.
Preparation of root extract
The ground dry roots were extracted in 30 volumes of 80% (w/v) ethanol at 20–25 °C (room temperature) for 24 h with agitation. The resulting extract was filtered through a 0.2 µm membrane filter (Advantec 110 mm; Toyo Roshi Kaisha Ltd., Tokyo, Japan) and the solvent was evaporated to produce a concentrated powder. The CMS contents of the obtained powders were determined and their skin care efficacies were evaluated.
Determination of extraction yield
The ethanol extracts were transferred to a tared round bottom flask (W1) and evaporated under vacuum in a rotary evaporator to a constant weight (W2). The value of the extract yield was calculated from the following formula: extract yield = (W2 − W1)/initial dry weight used for extraction.
Enzymatic deglycosylation of the ethanol extracts
The ethanol extracted root powders were refluxed in 50 volumes of 2% (w/v) Pectinex ultra SP-L™ (Novozymes, Copenhagen, Denmark) at 45 °C with agitation. After 48 h, the cooled reaction solution was centrifuged and lyophilized to yield a concentrated powder. The CMS contents of the powders were determined and the skin care efficacies were evaluated.
Determination of CMS contents and total production
The content of CMS was determined using a high-performance liquid chromatography (HPLC) system (Waters e2695 separation module; Waters Chromatography, Milfold, USA) equipped with a UV detector (Waters 2998 photodiode array detector; Waters Chromatography, Milfold, USA) and a Mightysil RP-18 GP 250–4.6 column (5 µm, 4.6 × 250 mm; Kanto Chemicals Company Inc., Tokyo, Japan). The mobile phase consisted of (A) water plus 0.1% acetic acid (w/v) and (B) acetonitrile plus 0.1% (w/v) acetic acid. The A and B ratio at 0, 65, 70, 77, and 80 min was set to 95:5, 62:38, 10:90, 95:5, and 95:5, respectively. The flow rate of the mobile phase was 1.0 mL min−1 and the injection volume for both samples and standards was 10 µL. The CMS content was detected at 342 nm and the standards were obtained from Indofine Chemical Company Inc. (Hillsboro, USA). The measurements of CMS were integrated by comparison with the external standard calibration curve. The value of the total production was calculated from the following formula: total production = mean of dry weight (g L−1) × mean of CMS content (mg g−1 DW).
Cell culture
B16 skin melanoma and Human dermal fibroblast (HDF) cell lines were obtained from the Korean Cell Line Bank (Seoul, Korea). Arbutin was obtained from Abcam (Cambridge, UK.), α-MSH was purchased from Sigma (St. Louis, MO). Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS) and Penicillin–streptomycin solution were purchased from GenDEPOT (Barker, USA), soluble 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Life technologies (Eugene, USA) and Human Collagen Type X (COL10) ELISA kit was purchased from Blue Gene (Shanghai, China). Cell lines were cultured in DMEM and supplemented with 10% of FBS and 1% penicillin–streptomycin at 37 °C in a humidified 95% air and 5% CO2 atmosphere as described previously (Saeed et al. 2017).
Cell viability assay
Effect of enriched CMS Root on cell viability was measured by MTT assay method (Saeed et al. 2017). Coumestrol (Sigma-Aldrich, St. Louis, MO, USA), daidzein and non-enzyme treated root sample were used as assay control. Cells were seeded at a density of 1 × 105 in 96 well plate and cultured for 24 h. At 95% confluency, cells were treated with various concentratios of enriched CMS Root, coumestrol, daidzein or non-enzyme treated root sample for 72 h. After the incubation period, 10 μL of MTT assay solutions (5 mg mL−1 in PBS) was added to each well and further incubated at 37 °C for 3 h; then finally, 100 μL dimethyl sulfoxide was added to dissolve the formazan crystals. The absorbance was measured at 570 nm with an ELISA reader.
Effect on collagen-I production
HDF cells were used for collagen-I estimation, the cells (1 × 104 cells mL−1) were seeded into 40-mm Petri plates and incubated for 24 h at 37 °C. The cells were treated with enriched CMS Root, coumestrol, daidzein or non-enzyme treated root sample and incubated for 72 h at 37 °C. ELISA, respectively. After incubation period, the supernatant was collected and collagen-1, was estimated using commercially available ELISA kit.
Statistical analysis
All data are presented as mean ± standard error. All experiments were independently performed for three times. The mean values of the treatment groups were compared with untreated groups using Student’s t test. Statistical significance was assigned at *p < 0.05, **p < 0.01, ***p < 0.001***.
Results
Adventitious root induction
The adventitious roots were induced from various combinations of three explant sources and two genotypes of G. max through the inoculation of each explant on full strength of MS medium supplement with different concentrations of NAA and IBA (0–8.0 mg L−1); the initial adventitious root formation was visible after incubation for 5 days. Significant differences in root induction frequency were observed depending on the explant sources, and auxin types and concentrations. The hypocotyls and radicles of both soybean genotypes, SHK and NJK, were successfully induced after 2 weeks of inoculation (Table 1). However, the final root induction frequency was higher when the radicles were used as the original induction materials instead of the other explant sources. When the cotyledons were inoculated on the MS medium supplemented with NAA and IBA, the root induction frequency was extremely low for the selection of an adventitious root line to sub-cultures, and swelling and callus formation were visible rather than root induction. In terms of the root morphology, different characteristics were observed after NAA and IBA treatments (Fig. 1). On IBA-containing medium, the adventitious roots emerged directly from each explant without callus formation, and developed into fine, thin, and long roots with optimal morphological characteristics for continuous sub-culture in a bioreactor (data not shown). In contrast, the media supplemented with NAA, the developed adventitious roots were thick and short. Suitable root morphologies, with high root induction frequencies of both soybean genotypes, were observed in growth medium that contained 4 mg L−1 IBA after 2 weeks of inoculation. These results indicated that 4 mg L−1 IBA was the optimal auxin composition for adventitious root induction from the hypocotyls and radicles of SHK and NJK soybean genotypes, as evidenced by the high root induction frequency and the optimal morphological characteristics for continuous culture.
Determination of potential soybean cultivars for adventitious roots production
The four adventitious root lines induced from each hypocotyl and radicle of SHK and NJK were cultured on full strength MS medium containing 4 mg L−1 IBA to determine the competent soybean adventitious root line for mass CMS production. The content of CMS in the roots (per g dry weight) was not significantly affected by the explant source or genotypes; the CMS content in the four different root lines were similar after 4 weeks of bioreactor culture (Table 2). However, the yields and the total CMS production (per 1 L medium) in the two root lines originated from SHK were higher than those that originated from NJK. In particular, the total CMS production (per 1 L medium) in the roots induced from the radicles of SHK was the highest, almost fourfold greater than that from the radicles of NJK. Therefore, the roots induced from the radicles of SHK were the most competent soybean adventitious root materials for the mass production of CMS, according to the final CMS productivity.
Determination of optimal MS medium salt strength
To develop a protocol for the culture of selected soybean adventitious roots for the large-scale production of biomass and CMS, different salt strengths of MS medium were tested. Biomass productivity was greatly influenced by the total salt strength of the medium and this phenomenon also affected the final total CMS production (per 1 L medium) after 4 weeks of culture in a bioreactor (Table 3). A large variability in dry weight (between 1.1 and 5.3 g L−1) was observed within four treatments after 4 weeks of bioreactor culture and the highest fresh and dry weight, 60.4 g L−1 and 5.3 g L−1, respectively, was achieved with 0.5 MS. The content of CMS in the roots (per g dry weight) at low salt strengths (0.25 and 0.5 MS) was higher than that at high salt strengths (1.0 and 2.0 MS); the highest total CMS production (per 1 L medium) was observed at 0.5 MS and was 15.5-fold higher than that at 2.0 MS. Medium salt strength also affected the morphological characteristics of the adventitious roots. The roots cultured at 2.0 MS were shorter, thicker, and less numerous compared with those cultured at other treatments, whereas the roots cultured at 0.25 MS were too thin for continuous subculture (Fig. 2). Therefore, 0.5 MS was determined as a suitable medium salt strength for both root growth and CMS accumulation in the roots; thus, the optimization of bioreactor culture conditions will benefit the large-scale production of CMS derived from soybean adventitious roots for commercialization.
Enzymatic deglycosylation to CMS
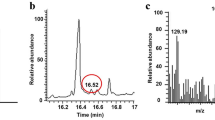
The final content of CMS in the ethanol extract was greatly increased after enzymatic deglycosylation by treatment with a pectinase containing β-glucosidase derived from Aspergillus aculeatus, which can remove glucoses from the glucosides to produce to the aglycone moieties (Table 4). The CMS content in the ethanol extract after enzymatic conversion was 81.3-fold higher than that of before enzymatic deglycosylation. This high conversion rate indicated that almost all the coumestrin in the roots were converted to CMS. Moreover, the conversion of coumestrin to coumesterol was also confirmed by the HPLC analysis, which suggested a clear indication in the rise of peak in coumeserolin the enzyme-treated samples compared to the non-enzyme treated (Fig. 3).
In vitro cytotoxicity
To evaluate the cytotoxic effect of enriched CMS Root on HDF and B16 cells, we carried out the MTT assay for 3 days. The cell viability is higher than 95% even at the highest concentration of 100 µg mL−1 for both cell lines. We compare these result to the MTT result of coumestrol, daidzein or root control sample, which shows that coumestrol alone induce cell death at concentration higher than 5 µg mL−1 for both HDF and B16 cells (Figs. 4 and 5). However, the result of the MTT assay for HDF and B16 is similar to those treated with daidzein or non-enzyme treated root sample when we compared to those treated with the enriched CMS Root sample.
Cell viability of HDF cells after treatment with a Daidzein b Coumestrol c non-enzyme treated root sample and denriched CMS root after 24 h. Cells (1 × 105 cells/well) were incubated with various concentrations (5–100 μg mL−1). Cell viability was determined by 3-(4,5-dimethyl-thiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Note that samples a, c, and d did not show any toxicity effect up to 100 μg mL−1. Results are expressed as a percentage of sample-treated control and presented as mean ± SD of three separate experiments. *p < 0.05, **p < 0.01, ***p < 0.001*** versus control by Student’s t test
Cell viability of B16 cells after treatment with a Daidzein, b Coumestrol, c non-enzyme treated root sample, and d enriched CMS root after 24 h. Cells (1 × 105 cells/well) were incubated with various concentrations (5–100 μg mL−1). Cell viability was determined by 3-(4,5-dimethyl-thiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Note that samples a, c, and d did not show any toxicity effect up to 100 μg mL−1. Results are expressed as a percentage of sample-treated control and presented as mean ± SD of three separate experiments. *p < 0.05, **p < 0.01, ***p < 0.001 *** versus control by Student’s t test
Effect on collagen-I production
To evaluate the effect of enriched CMS Root sample on collagen-I production in fibroblast cells, ELISA was performed. HDF treated with enriched CMS Root sample showed an increase in collagen-I production from 2.7 ng mL−1 to 3.9 ng mL−1 at 1 μg and 5 μg mL−1, respectively (Fig. 6).
Comparative study of the effect on collagen-I production in HDF cells treated with Daidzein, Coumestrol, non-enzyme treated root sample (1 µg mL−1 and 5 µg mL−1) or enriched CMS root (1 µg mL−1 and 5 µg mL−1) collagen-I in cell supernatants were estimated by ELISA and results ng mL−1 of collagen-I. Results are expressed as a percentage of sample-treated control and presented as mean ± SD of three separate experiments. *p < 0.05, **p < 0.01, ***p < 0.001 *** versus control by Student’s t test
Discussion
Adventitious root induction
Auxins are the most commonly employed plant growth regulators for adventitious root induction and the application of a single auxin or combined auxins is considered to exert a major influence on the initial root induction frequency and root morphology (Saeed et al. 2017). Bellamine et al. (1998) reported that auxin exerts the primary role in root formation through its involvement in successive and interdependent phases and that root formation was completely inhibited when anti-auxins were supplied in the medium. Therefore, there have been numerous reports on the optimization of auxin types and concentrations with different explant sources, such as the root, leaf, stem, and petiole, for adventitious root induction in many industrially important plant species (Saeed et al. 2017; Khan et al. 2015). Silja and Satheeshkumar (2015) had reported that Plumbago rosea adventitious root induction was observed in all media supplemented with various combinations of IAA and IBA. Khan et al. (2017) also confirmed that Fagonia indica adventitious root induction was observed in all media supplemented with four single auxins. Our results also verified the positive effects of auxin for the initial adventitious root induction of G. max, even if the induction frequency and morphology were dependent on various parameters. Plant cells have totipotency, which is the ability to differentiate into other cell types. This ability can result in different morphological characteristics based on the medium compositions and can allow the creation of new explant types that fit the researcher’s purposes from in vitro or natural materials, from organs to undifferentiated cells, or from calluses to organs. The characteristics of in vitro explants are stability under controlled in vitro conditions, which allows the cultured explants to survive, proliferate, and accumulate phytochemicals for a long time (Federici et al. 2003). Owing to these characteristics, in vitro explants, such as adventitious roots, cells, calluses, and embryos, can be considered plant stem cells and therefore provide alternative raw materials for the mass production of biomass and plant-based valuable compounds for industrial applications.
Determination of competent soybean adventitious roots
A large variability in biomass productivity, morphological characteristics, and phytochemical compositions was observed in different root lines derived from the same species (Jayakodi et al. 2014) and in different explant types induced from the same mother plants (Charchoglyan et al. 2007). Federici et al. (2003) had reported the isoflavone contents of the 40 different cell lines induced from four soybean cultivars. A 500-fold variability was observed in the cell lines, between 0.10 mg g−1 dry weight and 46.3 mg g−1 dry weight of isoflavones. Therefore, the researchers emphasized the importance of cell line selection for the successful production of the target bioactive compounds. Lee et al. (2017) had determined the primary metabolite profile and gene expression pattern using five adventitious root lines derived from five ginseng cultivars with different morphological characteristics. Their results showed that the trait of the mother plants was expressed directly in their adventitious roots. SHK is an elite cultivar soybean that has been bred to increase biomass productivity and to accumulate health-beneficial compounds. In contrast, NJK is a semi-wild soybean that shows a low growth rate in natural conditions. Therefore, the four soybean adventitious root lines used in this experiment showed different phenomena, the root lines induced from SHK showed higher total CMS production (per 1 L medium) than those from NJK, because of the high biomass productivity influenced from the traits of their mother plants. Therefore, the adventitious root lines induced from the radicles of SHK soybean were selected as a competent material for CMS production, as evidenced by the total high CMS productivity and the optimal morphological characteristics for continuous bioreactor culture.
Determination of optimal MS medium salt strength
During the culture period, in vitro explants consume water and minerals from the culture medium for their survival, growth, and development; the absorption capacity of each explant is affected by the water potential in the medium (Amirouche et al. 1985). Full strength MS medium, which is used widely in in vitro cultures, contains high levels of nitrogen, potassium, and certain micronutrients (Lee and Paek 2012) reported that nitrogen sources in the MS medium, such as NH4+ and NO3−, are key macroelements that influence biomass accumulation. They confirmed that these residual nitrogen sources were almost zero in the 0.25 MS medium at the end of the culture period, which was presumably one of the primary reasons for the strong suppression of root growth (Lee and Paek 2012). Therefore, the root growth of the soybean adventitious roots cultured at 0.25 MS, the lowest salt strength, was inhibited because of the lack of essential minerals for their anabolism. In contrast, the root growth of the roots cultured at 2.0 MS, the highest salt strength, was inhibited because of the suppression of the absorption of water and minerals from the culture medium because of high osmotic stress.
A low nitrogen content in the medium, within the limits tolerated by the plant, often benefits secondary metabolite accumulation (Knobloch and Berlin 1983; Phillips and Henshaw 1977). In soybean adventitious roots, nitrogen starvation had a positive effect on the accumulation of CMS in the roots (per g dry weight) and this undernourishment phenomenon probably contributed to the stimulation of plant defense mechanisms; the CMS content at low salt strengths (0.25 and 0.5 MS) was almost threefold higher than that at the higher salt strengths (1.0 and 2.0 MS). Different media composition affects not only the accumulation of secondary metabolites in the roots, but also the morphological characteristics. Silja and Satheeshkumar (2015) reported that the 2 × salt media induced callus formation and became thicker, darker in colour, and inhibited the proliferation of Plumbago rosea adventitious roots compared with that grown in media with lower salt strengths. In soybean adventitious roots, the roots cultured at 2.0 MS medium were not observed to have callus formation, but were observed to swell and inhibit root elongation when compared with that at optimal salt strength. Earlier reports emphasized that it was necessary to optimize the salt strength of the medium to achieve maximum biomass and target compound production in the adventitious root cultures of P. rosea (Silja et al. 2015), Periploca sepium (Zhang et al. 2011), and Gynura procumbens (Saiman et al. 2012). The optimization of environmental conditions will benefit the large-scale production of CMS derivatives from soybean adventitious roots.
Enzymatic deglycosylation to CMS
The content of coumestrin, the glycoside form of CMS, was higher than that of CMS in the soybean adventitious roots cultured at 0.5 MS medium after 4 weeks of bioreactor culture. Previous studies also confirmed that the major isoflavone forms in the soybean callus strains were glycosides and malonyl conjugates (> 95% of the total) (Federici et al. 2003; Kim et al. 2012. However, the aglycones showed significantly higher estrogenic activity than the glycosides. In recent years, many studies have focussed on the conversion from the macro compounds to the more active minor compounds via methods, such as heating, acid treatment, alkali treatment, and enzymatic conversion. Microbial or enzymatic approaches are considered more attractive methods because of the mild reaction conditions required and environmental compatibility (Chang et al. 2014). Yuk et al. (2016) reported that they isolated some strains of β-glucosidase-producing microorganisms, which showed a high activity for the conversion of major ginsenosides into rare ginsenosides. Based on their results, the content of the rare ginsenosides was significantly higher than that after microbial transformation in the ginseng adventitious roots. They emphasized that these findings may not only solve the problem of the low productivity of metabolites in adventitious root cultures, but may also result in the development of a valuable new method for the manufacture of trace compounds in nature.
Although the content of CMS in soybean adventitious roots was low, the content of the glycoside form in the roots was high. Aspergillus species are known to be a useful source of β-glucosidase, and the enzymatic deglycosylation of soybean adventitious roots using a β-glucosidase to convert the glycosides into CMS was simple and had a high conversion rate. These enzymatic approaches increased the final productivity of CMS and increased the value of the soybean adventitious roots.
Investigation of skin care efficacies
The development of biocompatible and effective compound is important for the treatment of particular diseases. CMS has been shown to possess diverse skin pharmacological effects, including anti-cancer, antioxidant, antiageing, and anti-melanogenic effects (Zafar et al. 2017; Montero et al. 2019; Park et al. 2015). However, it important to assess the potential cytotoxic effect of new compounds. In the present study we investigated the safety and beneficial effect on skin cell of the mass production batch of soybean advantage root contain higher concentrations of bioactive CMS. For the study we selected HDF and B16 skin cell lines and treated them with different concentration of soybean adventitious root extract containing higher concentrations of bioactive CMS using MTT assay method. Control wells were incubated with fresh culture media, and experiments were repeated in triplicates. The groups treated with daidzein, non-enzyme treated root sample and enriched CMS root did not exhibit any significant cytotoxic effecton HDF and B16. Soybean adventitious root with enriched CMS has shown to be safe to HDF and B16 skin cells. (Figs. 4 and 5). However the cell viability of HDF and B16 was more sensitive to commercial coumestrol as it decreased with the increase in concentrations.
For all the groups treated with daidzein, non-enzyme treated root sample and enriched CMS root there were no visible changes in the cell morphology between treated cell and control groups after 72 h.
CMS has been reported to possess antioxidant, collagen synthesis facilitation and antiageing effect. (Zafar et al. 2017; Montero et al. 2019). Among all the biological effects of CMS on skin, collagen synthesis are of remarkable importance. Collagen represents the main component of the extracellular matrix of dermal connective tissue. Our study shows that non-enzyme treated root sample and enriched CMS significantly increase the collagen-1 production (Fig. 6). Hense, the current results suggested that Soybean advantages root can be used as a potential ingredient in cosmetic formulations.
Conclusion
In an attempt to improve the CMS productivity, we induced adventitious roots from the radicles of an elite soybean cultivar (‘Sinhwakong’) and investigated the optimal MS medium salt strength. The CMS content from the ethanolic extracts of the root culture was enriched after deglycosylation of the extract. Considering the phytochemical profile and pharmacological efficacies of soybean adventitious root in skin, we have successfully exploited adventitious root extract enriched with CMS and found it was non-toxic to HDF and B16 cell lines. Thus, the present results suggested that the soybean adventitious roots extract act as potential antioxidant and protective skin care agents in cosmetics products.
References
Amirouche L, Stuchbury T, Matthews S (1985) Comparisons of cultivar performance on different nutrient media in a routine method for potato micropropagation. Potato Res 28:469–478
Bellamine J, Penei C, Greppin H, Gaspar T (1998) Confirmation of the role of auxin and calcium in the late phases of adventitious root formation. J Plant Growth Reg 26:191–194
Castro CC, Pagnussat AS, Orlandi L, Worm P, Moura N, Etgen AM, Netto CA (2012) Coumestrol has neuroprotective effects before and after global cerebral ischemia in female rats. Brain Res 1474:82–90
Chang KH, Jo MN, Kim KT, Paik HD (2014) Evaluation of glucosidases of Aspergillus niger strain comparing with other glucosidases in transformation of ginsenoside Rb1 to ginsenosides Rg3. J Ginseng Res 38:47–51
Charchoglyan A, Abrahamyan A, Fujii I, Boubakir Z, Gulder TAM, Kutchan TM, Vardapetyan H, Bringmann G, Ebizuk Y, Beerhues L (2007) Differential accumulation of hyperforin and secohyperforin in Hypericum perforatum tissue cultures. Phytochemistry 68:2670–2677
Federici E, Touche´ A, Choquart S, Avanti O, Fay L, Offord E, Courtois D (2003) High isoflavone content and estrogenic activity of 25-year-old Glycine max tissue cultures. Phytochemistry 64:717–724
Gaid M, Haas P, Beuerle T, Scholl S, Beerhues L (2016) Hyperforin production in Hypericum perforatum root cultures. J Biotech 222:47–55
Jayakodi M, Lee SC, Park HS, Jang WJ, Lee YS, Choi BS, Nah GJ, Kim DS, Natesan S, Sun C, Yang TJ (2014) Transcriptome profiling and comparative analysis of Panax ginseng adventitious roots. J Ginseng Res 38:278–288
Khan MA, Abbasi BH, Shah NA, Yücesan B, Ali H (2015) Analysis of metabolic variations throughout growth and development of adventitious roots in Silybum marianum L. (Milk thistle) a medicinal plant. Plant Cell Tiss Org Cult 123:501–510
Khan T, Abbasi BH, Khan MA, Azeem M (2017) Production of biomass and useful compounds through elicitation in adventitious root cultures of Fagonia indica. Ind Crops Prod 108:451–457
Kim EH, Ro HM, Kim SL, Kim HS, Chung IM (2012) Analysis of isoflavone, phenolic, soya sapogenol, and tocopherol compounds in soybean (Glycine max (L.) Merrill) germplasms of different seed weights and origins. J Agric Food Chem 60:6045–6055
Knobloch KH, Berlin J (1983) Influence of phosphate on the formation of theindole alkaloid and phenolic compounds in cell suspension cultures of Catharanthus roseus L. comparison of enzyme activities and product accumulation. Plant Cell Tiss Org Cult 2:333–340
Lazzeri PA, Hildebrand DF, Collins GB (1987) Soybean somatic embryogenesis: effects of hormones and culture manipulations. Plant Cell Tiss Org Cult 10:197–208
Lee EJ, Paek KY (2012) Enhanced productivity of biomass and bioactive compounds through bioreactor cultures of Eleutherococcus koreanum Nakai adventitious roots affected by medium salt strength. Ind Crops Prod 36:460–465
Lee YH, Yuk HJ, Park KH, Bae YS (2013) Coumestrol induces senescence through protein kinase CKII inhibition-mediated reactive oxygen species production in human breast cancer and colon cancer cells. Food Chem 141:381–388
Lee YS, Park HS, Lee DK, Jayakodi M, Kim NH, Lee SC, Kundu A, Lee DY, Kim YC, In JG, Kwon SW, Yang TS (2017) Comparative analysis of the transcriptomes and primary metabolite profiles of adventitious roots of five Panax ginseng cultivars. J Ginseng Res 41:60–68
Montero G, Arriagada F, Günther G, Bollo S, Mura F, Berríos E, Morales J (2019) Phytoestrogen coumestrol: antioxidant capacity and its loading in albumin nanoparticles. Int J Pharm 1(562):86–95
Murashige T, Skoog F (1962) A revise medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Park CM, Joung MS, Paek KY, Choi JW (2010) Inhibitory effect of Jewel orchid (Anoectochilus formosanus) plantlet extract against melanogenesis and lipid droplet accumulation. J Soc Cosmetics Kor 36:145–150
Park GE, Baek SH, Kim JE, Lim TG, Lee CC, Yang H, Kang YG, Park JS, Augustin M, Mrosek M, Lee CY, Dong ZG, Huber R, Lee KW (2015) Flt3 is a target of coumestrol in protecting against UVB-induced skin photoaging. Biochem Pharmacol 93:473–483
Phillips R, Henshaw GG (1977) The regulation of synthesis of phenolics in stationary phase cell cultures of Acer pseudoplatanus L. J Exp Bot 28:785–794
Saeed S, Ali H, Khan T, Kayani W, Khan MA (2017) Impacts of methyl jasmonate and phenyl acetic acid on biomass accumulation and antioxidant potential in adventitious roots of Ajuga bracteosa Wall ex Benth., a high valued endangered medicinal plant. Physiol Mol Biol Plants 23:229–237
Saiman MZ, Mustafa NR, Schulte AE, Verpoorte R, Choi YH (2012) Induction, characterization, and NMR-base metabolic profiling of adventitious root cultures from leaf explants of Gynura procumbens. Plant Cell Tiss Org Cult 109:465–475
Silja PK, Satheeshkumar K (2015) Establishment of adventitious root cultures from leaf explants of Plumbago rosea and enhanced plumbagin production through elicitation. Ind Crops Prod 76:479–486
Simons R, Vincken JP, Bohin MC, Kuijpers TF, Verbruggen MA, Gruppen H (2011) Identification of prenylated pterocarpans and other isoflavonoids in Rhizopus spp. elicited soya bean seedlings by electrospray ionisation mass spectrometry. Rapid Commun Mass Spectrom 25:55–65
Simons R, Gruppen H, Bovee TF, Verbruggen MA, Vincken JP (2012) Prenylated isoflavonoids from plants as selective estrogen receptor modulators (phytoSERMs). Food Funct 3:810–827
Song X, Wu H, Piao X, Yin Z, Yin C (2017) Microbial transformation of ginsenosides extracted from Panax ginseng adventitious roots in an airlift bioreactor. Electronic J Biotechnol 26:20–26
Taxvig C, Specht IO, Boberg J, Vinggaard AM, Nellemann C (2013) Dietary relevant mixtures of phytoestrogens inhibit adipocyte differentiation in vitro. Food Chem Toxicol 55:265–271
Theboral J, Sivanandhan G, Subramanyam K, Arun M, Selvaraj N, Manickavasagam M, Ganapathi A (2014) Enhanced production of isoflavones by elicitation in hairy root cultures of Soybean. Plant Cell Tiss Org Cult 117:477–481
Tripathi P, Rabara RC, Reese RN, Miller MA, Rohila JS, Subramanian S, Shen QJ, Morandi D, Bücking H, Shulaev V, Rushton PJ (2016) A toolbox of genes, proteins, metabolites and promoters for improving drought tolerance in soybean includes the metabolite coumestrol and stomatal development genes. BMC Genomics 17:1–22
Wang H, Murphy PA (1994) Isoflavone composition of American and Japanese soybeans in Iowa: effects of variety, crop year, and location. J Agric Food Chem 42:1674–1677
Yuk HJ, Song YH, Curtis-Long MJ, Kim DW, Woo SG, Lee YB, Uddin Z, Kim CY, Park KH (2016) Ethylene induced a high accumulation of dietary isoflavones and expression of isoflavonoid biosynthetic genes in Soybean (Glycine max) Leaves. J Agric Food Chem 64:7315–7324
Zacharius RM, Kalan EB (1990) Isoflavonoid changes in soybean cell suspensions when challenged with intact bacteria or fungal elicitors. J Plant Physiol 135:732–736
Zafar A, Singh S, Naseem I (2017) Cytotoxic activity of soy phytoestrogen coumestrol against human breast cancer MCF-7 cells: Insights into the molecular mechanism. Food Chem Toxicol 99:149–161
Zhang J, Gao WY, Wang J, Li XL (2011) Effects of explants types and media salt strength on growth and secondary metabolites accumulation in adventitious roots of Periploca sepium Bunge. Acta Physiol Plant 33:2447–2452
Acknowledgement
This work (Grant no: C0267437) was supported by business for Academic-Industrial cooperative establishments funded by Korea Small and Medium Business Administration in 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest. The authors are sole responsible.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, E.J., Jiménez, Z., Seo, KH. et al. Mass production of coumestrol from soybean (Glycine max) adventitious roots through bioreactor: effect on collagen production. Plant Biotechnol Rep 14, 99–110 (2020). https://doi.org/10.1007/s11816-019-00589-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-019-00589-2