Abstract
The aerial tissues of Tilia americana var. mexicana produce compounds with anxiolytic activity, such as quercetin 3-O-β-d-glucoside and tiliroside, in addition to ones with anti-inflammatory properties, such as scopoletin. These three compounds were initially identified in callus cultures of apical buds. In the present study, suspension cultures from leaf explant callus were established; the accumulation of scopoletin and quercetin 3-O-β-d-glucoside in these cultures were found to be cell-growth-associated using cell growth and active compound-production kinetics assays. The effects of varying the nitrate and copper concentrations in Murashige and Skoog (MS, 27.4 mM total nitrates and 0.01 µM copper) medium on the growth of a suspension culture of T. americana cells and on the production of active compounds were tested by means of central composite design (CCD) generally used in the response surface methodology (RSM). Cell growth, measured as maximal biomass, improved when the total nitrate concentration decreased in the MS medium to 13.7 mM (p < 0.01) regardless of the copper concentration. As a phytoalexin, scopoletin accumulated rapidly in plants after pathogen infection, in the suspension cultures scopoletin yield was stimulated by increased copper concentration to 1.2 μM (p < 0.01). According to the C:N hypothesis, the carbon excess generated by nitrates reduced to 8.03 mM (p < 0.01) stimulated the production of quercetin 3-O-β-d-glucoside. Cell suspension of T. americana represents a potential biotechnological alternative for industrial exploitation in a stirred-tank bioreactor using a two-phase process: (1) the first step will be to grow the cell suspension, (2) the second stage will consist in handle the suspension culture towards the production of anxiolytic compounds or towards the production of anti-inflammatory compounds. As well as to evaluate another elicitors to stimulate tiliroside production in the T. americana suspension cultures.
Key message
Tilia americana cells grown in a two-phases suspension culture system produce more scopoletin and quercetin 3-O-β-d-glucoside when exposed to increased concentrations of copper and decreased concentrations of total nitrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tilia americana var. mexicana (Schlecht.) Hardin is the only species from the family Tiliaceae distributed in North America and four different varieties of this tree exist in Mexico (Hardin 1990). T. americana var. mexicana grows from Chihuahua and Coahuila to Guerrero and Oaxaca in low mountain forests, warm climates, and sub-warm climates (Martínez 1987; Hardin 1990; Pavón and Rico-Gray 2000). In traditional Mexican medicine, T. americana is mainly utilized for its tranquilizing and sedative effects, as well as under conditions associated with inflammation and pain (Martínez and Matuda 1979; Aguilar et al. 1994; Argueta et al. 1994; Viola et al. 1994; Monroy-Ortiz and Castillo-España 2007). The methanolic extracts from inflorescences and bracts of T. americana have demonstrated the presence of glycosylated flavonoids derived from quercetin and kaempferol (Fig. 1), their properties to treat conditions such as anxiety and depression were validated in murine models (Aguirre-Hernández et al. 2007; Herrera-Ruiz et al. 2008; Aguirre-Hernández et al. 2010; Noguerón-Merino et al. 2015). Additionally, a synergic effect was observed upon combining the flavonoids quercetin, rutin, and isoquercitrin (quercetin 3-O-β-d-glucoside) on the inhibition of the GABAergic system, such as GABA/BDZ and 5HT1A serotonin, to achieve sedative and anxiolytic effects (Aguirre-Hernández et al. 2016). Moreover, the neuroprotective properties of the hexane and water extracts of inflorescences of T. americana were corroborated in mice and guinea pigs models of intestinal ischemia in situ and cerebral ischemia (Angeles-López et al. 2013, 2015).
The collection of bracts and flowers from T. americana trees in their natural habitat has been restricted since 2001 by the Mexican Ministry of the Environment and Natural Resources (SEMARNAT 2010), as it is considered to be at risk of extinction. Consequently, identifying any means of preserving the T. americana medicinal tree is mandatory. There are few reports related to the micropropagation of T. americana from axillary buds derived from seedlings (Zurita-Valencia et al. 2014) and through the rooting of cuttings by application of Radix® rooter powder 10,000 ppm of indole-3-butyric acid (IBA) (Muñoz-Flores et al. 2011; Flores-Sánchez et al. 2019).
Calluses cultures from apical and axillary buds from T. americana cuttings were developed in MS medium supplemented with 0.005 mg L−1 of thidiazuron (TDZ) in combination with 0.1 mg L−1 of IBA. The methanolic extracts of leaves and calluses showed anti-inflammatory activity in a tetradecanoylphorbol-13-acetate (TPA)-induced ear edema model, with a median effective dose (ED50) of 0.38 mg per ear for the leaf extract and 1.73 mg per ear for the callus extract. Quercetin 3-O-β-d-glucoside and tiliroside anxiolytic compounds and the anti-inflammatory compound scopoletin were identified in the methanol extracts (Fig. 1). Cell suspension cultures of T. americana represents a biotechnological alternative with the potential for bioactive compound production and industrial exploitation. The aim of this study was to culture T. americana cells in suspension and to evaluate the production of anxiolytic compounds already identified from this tree, as well as of scopoletin, derived from the leaves and calluses (Flores-Sánchez et al. 2019).
The numerous interactions between the components in Murashige and Skoog (MS) medium (Murashige and Skoog 1962) and their effects on cellular metabolism and the production of bioactive compounds have been documented extensively (Rozita et al. 2005; Fritz et al. 2006; Maksymiec 2007; Lea et al. 2007; Zhou and Zhong 2009; Mora-Izquierdo et al. 2011; Nicasio-Torres et al. 2012, 2016). In this study, scopoletin and quercetin 3-O-β-d-glucoside production was characterized in T. americana cells grown in suspension in MS medium; after this phase, modifications to the (1) total nitrates and (2) copper contents in the MS medium were evaluated as strategies to promote cell growth and as abiotic stimulation to improve the production of scopoletin and quercetin 3-O-β-d-glucoside from the cultures. The outcome was analyzed by means of the central composite design (CCD), the most widely used in the response surface methodology (RSM), an efficient statistical experimental approach used in the optimization process. The CCD consists of a factorial design (FD) with points to the center plus adding a star design (SD) used to model the curvature with respect to each factor (Van Ryswyk and Van Hecke 1991; Palasota and Deming 1992; Gilmour 2006; Bruns et al. 2006; Bezerra et al. 2008; Hanchinal et al. 2008; Pérez-Hernández et al. 2019).
Materials and methods
Calluses
Tilia americana var. mexicana calluses were previously developed from leaf explants of branches (Fig. 2a, b) collected in Mexicapan, Mexico State, Mexico in October and November 2015. This species was authenticated by Abigail Aguilar, M.Sc., Head of the Herbarium at the Instituto Mexicano del Seguro Social in Mexico City [IMSSM] and vouchers were stored for reference under #IMSSM-5099.
Leaves from Tilia americana var. mexicana cuttings (a) used as explants (b) and cultivated in Murashige and Skoog (MS) medium for calluses generation (c) with 2.0 mg L−1 of 2,4-dichlorophenoxiacetic acid (2,4-D) mixed with kinetin (Kin) at 0.5 mg L−1, and d cells in suspension started with these calluses and stained by means of the Evans blue
Callus grown in sterile MS medium with 2,4-dichlorophenoxyacetic acid (2,4-D) at 0.5, 1.0 or 2.0 mg L‒1 mixed with kinetin (Kin) at 0.5 mg L‒1, supplied with 30.0 g L−1 of sucrose and adjusted to pH 5.7; 3.0 g L−1 of PhytaGel (Sigma-Aldrich, México) as a gelling and 1.0 g L−1 of Polyvinylpolypyrrolidone as an antioxidant. Calluses were incubated at 26 ± 2 °C, with a 16 h:8 h (light:dark) photoperiod under 50 μM m−2 s−1 warm, white-fluorescent light intensity, and 60% relative humidity. Calluses were transferred into new medium every 5 weeks.
Cell suspension cultures
After 15 months of callus development (Fig. 2c), T. americana cell suspension in batch cultures were started with a 6% inoculum of fresh friable callus in 80 mL of liquid MS medium supplemented with 2,4-D (2.0 mg L‒1), Kin (0.5 mg L‒1), and sucrose (30.0 g L‒1), adjusted to pH 5.7, and sterilized. Flasks of cell suspensions were placed in an orbital shaker (New Brunswick Scientific Co., New Brunswick, NJ, USA) at 110 rpm and incubated under the same conditions employed for the callus cultures. Successfully established T. americana cell suspensions were transferred into new medium under sterile conditions every 2 weeks using the same inoculum. Cell viability was determined considering membrane integrity by means of the Evans blue dye (0.25%, w/v) exclusion test (Orozco-Sanchez et al. 2011).
Growth kinetics of cell suspension culture
The batch cell suspension cultures of T. americana were developed over a period of 28 days, during which nine flasks were analyzed at the beginning of the study (day 0) and every following Monday, Wednesday, and Friday until the end of the experimental period. Each flask was vacuum-filtered in a Buchner funnel (Whatman filter paper No. 1, 5.5-cm diameter); the retained biomasses were washed with distilled water and then dried in an oven (Thelco 160 DM) at 65 °C for 48 h.
Cell growth
The growth curve of T. americana was constructed by registration of the mean of the cell biomass in dry weight (DW, g L−1) throughout the culture period. The DW of the maximal cell biomass and the time required for its growth were recorded. The growth index (GI) was calculated considering the maximal biomass obtained at the end of the logarithmic growth period, minus the inoculum, and divided by the inoculum. Maximal growth rate (μmax) was calculated by generating linear regression equations of a semi-log calculation of the biomasses from the logarithmic growth phase versus time (graph not shown). Doubling time (Dt) was determined from the equation Dt = ln 2/µmax and biomass produced according to the sucrose content (30.0 g L−1) were determined based on the theoretical value (Y = 0.5 g of biomass/g of sucrose) reported for plants (Katoh and Yoshida 2009).
Extraction of cells in suspension
Dry biomasses (200 mg) from six flasks taken at the beginning (0 days) and six flasks after 7, 14, 21, and 28 days of batch cell suspension cultures were extracted at room temperature at a 1:20 (w/v) proportion with methanol three times (24 h for each procedure). The extracts obtained for each sample were filtered through filter paper, pooled, concentrated to dryness (Pérez-Hernández et al. 2014; Nicasio-Torres et al. 2016; Flores-Sánchez et al. 2019), and dissolved in high-purity methanol (Merck, Mexico, Mexico) for chromatographic analysis. Production kinetics were obtained by quantifying the scopoletin and quercetin 3-O-β-d-glucoside levels in these methanolic extracts.
HPLC conditions for quercetin 3-O-β-d-glucoside and scopoletin analyses
HPLC analyses were performed using a Waters system (2695 Separation Module) coupled to a diode array detector (2996) with a 190–600-nm detection range and operated through the Manager Millennium software system (Empower 1; Waters Corp., Boston, MA, USA). Separation of the compounds from the biomass extracts for quercetin 3-O-β-d-glucoside and scopoletin quantification was performed in a Spherisorb® RP-18 column (250 × 4.6 mm, 5 µm; Waters); a constant temperature of 25 °C was maintained during all analyses. Samples (20 μL) were eluted at a 1.0 mL min−1 flow rate with (A) high-purity H2O with CF3COOH to 0.5% v/v (TFA, Sigma-Aldrich, Mexico, Mexico) and (B) high-purity CH3CN gradient (Merck) mobile phases and were detected by absorbance at λ = 343 nm for scopoletin and λ = 355 nm for quercetin 3-O-β-d-glucoside (Pérez-Hernández et al. 2014; Nicasio-Torres et al. 2016; Flores-Sánchez et al. 2019). Analyses of scopoletin (99%, Sigma-Aldrich) and quercetin 3-O-β-d-glucoside (90%, Sigma-Aldrich) were performed by comparing their retention times (9.54 min and 11.2 min, respectively) and the absorbance spectra, and quantification by method of external standard with calibration curves (Flores-Sánchez et al. 2019).
Effect of nitrate and copper on cell growth and scopoletin and quercetin 3-O- β-d-glucoside production
Experimental design
Total nitrates (27.4 mM, 11.5 mM KNO3, and 15.9 mM NH4NO3) and copper (0.1 μM CuSO4) contents in the complete MS medium were used as independent variables (X1 and X2, 0 and 0, central points) in a 2K FD, in which these factors were coded at two levels (+ 1, − 1). The codification of the experimental design is shown in Table 1.
The cell growth (maximal biomass DW, Y1), productions (µg L−1) and/or yields (μg per g of dry biomass) of scopoletin (Y2) and quercetin 3-O-β-d-glucoside (Y3) were compared using an analysis of variance (ANOVA), which generated the following linear mathematical model:
where: Y = predicted responses according to the model (Y1 = maximal biomass, productions and/or yields of Y2 = scopoletin, and Y3 = quercetin-3-O-β-d-glucoside); β0 = intercept term; β1 = coefficient indicative of the linear effect of total nitrates on the response; β2 = coefficient indicative of the linear effect of copper on the response; β1,2 = coefficient of the interaction effect of total nitrates and copper on the response.
With this model, it was possible to detect the levels of total nitrate and copper that would define the optimal concentrations for the growth (Y1) and for the yields of scopoletin (Y2) and quercetin 3-O-β-d-glucoside (Y3) at day 14 of culture, period established for maximal cell growth (Van Ryswyk and Van Hecke 1991; Palasota and Deming 1992). A central composite design (CCD) for the two independent variables was performed to five codified levels (− 1.41, − 1, 0, 1, and 1.41).
Analytical determinations
Cell growth
At the end of the culture period (14 days), six flasks for each experimental condition were withdrawn. The cell suspension from each flask was vacuum-filtered in a Buchner funnel (Whatman Paper No. 1, 5.5 cm diameter) and the retained biomass was washed with distilled water. Subsequently, the biomass was dried in an oven at 65 °C for 48 h, the cell-biomass DW determined (g L−1), and the mean cellular-biomass growth rate under each nutrient condition recorded (Y1).
Quercetin 3-O-β-d-glucoside and scopoletin yields
Biomasses (200 mg) from each flask were extracted with methanol for the HPLC analyses of scopoletin (Y2) and quercetin 3-O-β-d-glucoside (Y3). Reported yields represent the mean of 6 independently grown flasks from each experimental condition.
Cell growth (biomass DW, Y1) and yields (μg per g of dry biomass) of scopoletin (Y2) and of quercetin 3-O-β-d-glucoside (Y3) of CCD were analyzed with an ANOVA assay, the results of which can be expressed according to the following quadratic equation:
β0 = the intercept term; β1 = linear effect of total nitrate on response; β2 = linear effect of copper on response; β1,1 = quadratic effect of total nitrate on response; β2,2 = quadratic effect of copper on response; β1,2 = coefficient of the effect of the interaction between variables.
Using the above model, the response surface was plotted for each response variable.
To know whether the production of both compounds was favored by abiotic stimulation, the yields of scopoletin and quercetin 3-O-β-d-glucoside productions were compared with an ANOVA and a Tukey’s post-test (ρ ≤ 0.05).
Results and discussion
Cell suspension growth
All the calluses generated from leaf explants of T. americana var. mexicana in MS medium with 2,4-d at 0.5, 1.0 or 2.0 mg L‒1 mixed with kinetin (Kin) at 0.5 mg L‒1 were friable and presented a dark brown color, and in the subsequent changes to fresh medium the coloration changed to a light brown color (Fig. 2c). The T. americana cell suspension started with this callus and cultivated in batch in full MS medium (27.4 mM of total nitrates and 0.1 µM copper) was constituted of small cell aggregates light brown in color (Fig. 2d). In the logarithmic growth phase, the culture appeared brown in color, possibly due to the production of phenolic compounds (Bourgaud et al. 2001; Matkowski 2008). When the culture reached its stationary phase, the light-brown color was recovered and retained until the end of the culture (day 28).
The kinetics of cell suspensions demonstrated a sigmoid growth pattern (Fig. 3): the lag phase lasted 2 days; thereafter, the logarithmic growth phase began and lasted 12 days, obtaining maximal biomass (17.86 g DW L−1) at day 14 followed by the stationary phase; subsequently, the cellular biomass gradually decayed until the end of the culture period. The growth parameters of batched cells grown in suspension, for example, GI = 4.81 ± 0.88, Dt = 6.603 ± 0.78 days, µ = 0.107 ± 0.011 days−1, and the cellular biomass produced with respect to the carbon source (sucrose) Y = 0.637 ± 0.067 g DW biomass/g sucrose, can be found within the values already reported for another woody species, such as Taxus globosa (Tapia et al. 2013), Bursera linanoe (Pavón-Reyes et al. 2016), Prosopis laevigata (Trejo-Espino et al. 2011), and Pinus pinaster (Azevedo et al. 2008).
The largest biomass in the complete MS medium was obtained at day 14 (Fig. 3), then, the cells in suspension cultivated in MS medium with different concentration of total nitrates and copper of 2K FD (Table 1) were stopped and analyzed at this point. The effect of both nutrients and their interaction were significant for the growth of cellular suspension (p < 0.01). The negative effect of total nitrates and the positive effect of copper indicate that, independently or in combination, the levels of total nitrates should be decreased and copper should be increased to optimize the growth of the cell suspension according to the following equation \(Y_{1} = 18.98 - 2.79X_{1} + 0.49X_{2} - 0.59X_{1} X_{2}\).
With this model, it was possible to determine the concentrations of total nitrates and copper that would define the optimal concentrations for the growth of cells in suspension (Y1) to complete a CCD with five (− 1.41, − 1, 0, 1, and 1.41) codified levels (Table 3). Coefficients of CCD model indicated ratify that total nitrate concentration should decrease and copper concentration increase independently or interaction (p < 0.01) in order to favor the growth of cells in suspension according to the following equation \(Y_{1} = 18.05 - 1.8X_{1} + 0.63X_{2} - 0.6X_{1} X_{2} + 1.06 X_{1}^{2} - 0.98 X_{2}^{2}\). The highest maximal biomasses were obtained with 13.7 mM of total nitrates in combination with 1 µM of copper (Table 3, Fig. 4a).
Morphologically, the T. americana cells cultured in suspension grew as very small multicellular aggregates that were light brown in color (Fig. 5e). The cells assumed similar characteristics when cultivated without copper (Fig. 5c); however, they were dark brown in color when the concentration of total nitrates was increased (Fig. 5b), light yellow when the copper concentration was increased (Fig. 5d), and gray-green in color when the total nitrate concentration was reduced (Fig. 5a). Based on the C:N hypothesis, the color change and increase in the maximum biomass, that occurs in cells grown in MS medium with reduced total nitrate content (Fig. 5a), could be due to the synthesis of compounds with many carbon, such as phenolic, by increasing the formation of cell agglomerates probably by lignification of the cell wall (Fritz et al. 2006). The change in cellular characteristics caused by the increased copper content (Fig. 5d) could be due to interactions between this metal and the hydroxyl groups of lignin and cellulose in the cell wall, which would create conditions that are unfavorable for cell growth (Chen et al. 2004).
Scopoletin and quercetin 3-O-β-d-glucoside production
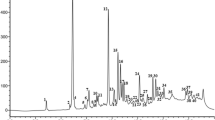
Methanolic extracts from calluses developed with 1.0 and 2.0 mg L−1 of 2,4-d mixed with 0.5 mg L−1 of kin and cells in suspension biomass shown a similar TLC and HPLC profile. The presence of scopoletin and quercetin 3-O-β-d-glucoside in the methanolic extracts of the dry biomasses from cells in suspensions was confirmed by comparing the retention time, the absorption spectrum, and by co-elusion with the standards (Fig. 6). The production of both compounds was growth-associated (Fig. 3) and the highest concentrations were obtained during the maintenance phase. The maximal production of both compounds occurred concurrently with maximal biomass (14 days). The content of quercetin 3-O-β-d-glucoside (669.15 µg L−1) was greater than that determined for scopoletin (394.78 µg L−1).
Given that scopoletin and quercetin 3-O-β-d-glucoside production was growth-associated, the effect of total nitrates and copper concentration was evaluated at day 14 of culture in the 2K FD experiments. According to ANOVA, scopoletin (p < 0.01) and quercetin 3-O-β-d-glucoside production (p < 0.01) was maximized by the combined effect of both factors. To optimize scopoletin production, similar to cell growth, nitrate concentrations must be reduced and copper increased independently or in combination according to the equation \(Y_{2} = 163.11 - 63.66X_{1} + 21.68X_{2} - 13.29X_{1} X_{2}\); while for quercetin 3-O-β-d-glucoside production, nitrate and copper concentrations must decrease independently or in combination according to the equation \(Y_{3} = 491.84 - 129.60X_{1} - 49.31X_{2} - 38.81X_{1} X_{2}\) (Table 1). To evaluate the effect of both elicitors on scopoletin and 3-O-β-d-glucoside production, the yields (µg g−1) of each compound from the 2K FD experiments were compared (Table 1). The β values from the ANOVA (ρ < 0.01) and the equation \(Y_{2} = 9.31 - 1.62X_{1} + 0.66X_{2} + 0.35X_{1} X_{2}\), scopoletin yields could be optimized by an independent effect of total-nitrate-concentration reduction and a copper-concentration increase (Table 2). When quercetin 3-O-β-d-glucoside yields were compared, ANOVA showed a significant effect (ρ < 0.01) by the reduction of both nutrients according to the equation \(Y_{3} = 26.28 - 1.10X_{1} - 1.24X_{2} - 0.17X_{1} X_{2}\) (Table 1).
To know whether the production of both compounds was favored by abiotic stimulation, the yields of scopoletin and quercetin 3-O-β-d-glucoside productions were compared with an ANOVA and a Tukey’s post-test (ρ ≤ 0.05). The highest scopoletin and quercetin 3-O-β-d-glucoside yields were obtained with 27.4 mM and 13.7 mM of total nitrate (Table 2); with 13.7 mM of total nitrate concentration the growth of cells in suspension was improved (Table 1). The copper concentrations evaluated exhibited no significant effect (ρ > 0.05) for these responses.
With the aim to improve scopoletin and quercetin 3-O-β-d-glucoside yields a CCD (+1.414, 0, − 1.414) was completed for intracellular analyses at 14 day. The CCD model coefficients indicated that total nitrate concentration should decrease and/or copper concentration increase independently (p < 0.01) in order to favor scopoletin production (Table 3; Fig. 4b). Instead of favoring quercetin 3-O-β-d-glucoside production, independently, the total nitrate and copper concentrations should be reduced (Table 3; Fig. 4c). Maximal level of scopoletin (threefold increase) was obtained with 27.4 mM of nitrate and 1.2 μM of copper. For quercetin 3-O-β-d-glucoside (threefold increase), the best condition was 8.03 mM of total nitrate and 0.1 μM of copper (Table 3). The copper content and total nitrate concentrations that favors the scopoletin and quercetin 3-O-β-d-glucoside production affect the cell growth of the T. americana cell suspension; the condition that best supported its growth was 13.7 mM of total nitrate and 1 μM of copper (Table 3; Fig. 4a).
There are other reports on the production of secondary metabolites in cell suspension cultures of plants stimulated by modification of the nitrogen source or with copper utilized as an abiotic elicitor, but there are few reports in which both nutrients were modified at the same time. One example of the modification of both factors and evaluated by CCD was showed in cell suspension cultures of Sphaeralcea angustifolia. Scopoletin production by cell suspension cultures of T. americana is greater than that produced by the cultures of S. angustifolia in flasks grown in complete MS medium, and reduced medium in nitrates (2.74 mM). Similar to the results described here, the reduction of the total nitrate content stimulated its production. Copper concentrations of 2.35 µM with 2.42 mM of total nitrates were reported to enable the highest levels of coumarins (4.0 mg L−1) and sphaeralcic acid (6.1 mg L−1) production in suspensions cultures of S. angustifolia; interestingly, this condition did not affect cell growth (Pérez-Hernández et al. 2014, 2019; Nicasio-Torres et al. 2016). In Angelica archangelica suspension cultures, altering the copper content (5-50 μM) stimulated with a dose-dependent effect on scopoletin production (Siatka et al. 2017). These results are similar to those obtained with the copper increase at 1.2 μM, unrelated to the nitrate concentration, for scopoletin production in the cell-suspension T. americana cultures.
The production of scopoletin and other coumarins has also been demonstrated using in vitro cultures of different plant species, such as Ammi majus (Staniszewska et al. 2003) with the use of elicitors (e.g., 1,2,3-benzothiadiazole-7-carbothioic acid, S-methyl ester (BION®), and Enterobacter sakazaki lysate). Scopoletin was identified in the callus culture derived from hypocotyls of A. majus seedlings; the most effective elicitor was the E. sakazaki lysate. Moreover, Siatka and Reichling (2000) reported a 12-fold increase in the accumulation of scopoletin in cell suspension cultures of Archangelica officinalis treated with Fusarium oxysporum compared with the control (0.2 mg mL−1).
There was an increase in the accumulation of quercetin 3-O-β-d-glucoside when the T. americana cell suspension was cultivated in MS medium with 8.03 mM of total nitrates; increasing the copper concentration diminished the production of this flavonoid. Korsangruang et al. (2010), using the cell suspensions of Pueraria candollei var. candollei and Pueraria candollei var. mirifica, reported that a five-fold increase in the concentration of the nutrient in the culture medium (0.5 to 2.5 μM of copper) led to a slight accumulation of total isoflavonoids in the stationary phase of both cultures, possibly induced by copper. A similar effect was observed on increasing gingenosides’ production in Panax ginseng roots by increasing the copper content from 5 to 25 μM in a bioreactor culture (Ali et al. 2006).
There are, to our knowledge, few reports of quercetin 3-O-β-d-glucoside production using cell suspension cultures; the existing reports are mainly oriented toward the bioconversion of quercetin in vitro cultures of Vitis sp., Ipomea batata, and Crocus sativum to quercetin glucosides among them quercetin 3-O-β-d-glucoside. Glycosyltransferase enzyme regulates the conversion of quercetin to glycosyl esters and stored them in vacuoles (Kodama et al. 1990; Kokubo et al. 1991). Several glycosylated flavonoids derived from quercetin and kaempferol were identified in leaves and inflorescence T. americana (Aguirre-Hernández et al. 2007; Herrera-Ruiz et al. 2008; Aguirre-Hernández et al. 2010; Noguerón-Merino et al. 2015).
These T. americana suspension cultures did not produce tiliroside anxiolytic compound. Tiliroside production was recently reported in T. americana calluses established from apical buds cultivated in MS medium with 0.005 mg L−1 of TDZ in combination with 0.1 mg L−1 of IBA. Conversely, scopoletin and quercetin-3-O-β-d-glucoside contents are higher in the suspensions cultures to those detected in apical bud calluses (Flores-Sánchez et al. 2019). The difference in the chemical profile could be due to the growth regulators employed for callus generation, as well as to the origin of the explant.
Conclusions
Tilia americana cells grown in suspension cultures produce the anxiolytic quercetin 3-O-β-d-glucoside and the anti-inflammatory scopoletin. By modifying the nutrient conditions of the MS culture medium using a mathematical model of CCD, it was possible to stimulate cellular growth by reducing the amount of total nitrates and increasing the copper content. Scopoletin production was mainly associated with the increase of copper concentration, indicating that this metabolite is inducible as phytoalexin. Future studies should include a broader range (particularly higher) of copper concentration. The CCD-based analysis indicated that the production of quercetin 3-β-d-glucoside was stimulated by reducing the total nitrate content; according to the C:N hypothesis, the carbon excess generated under this condition could be used for quercetin 3-β-d-glucoside production. It will be necessary to cultivate T. americana cell suspensions in the best condition to support the growth and transiently modify the nutritional conditions to temporarily increase scopoletin or quercetin 3-O-β-d-glucoside production. As well as to evaluate another abiotic or biotic elicitors to stimulate tiliroside production in the T. americana suspension cultures.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- CCD:
-
Central composite design
- Dt:
-
Duplication time
- DW:
-
Dried weight
- FD:
-
Factorial design
- GI:
-
Growth index
- IBA:
-
Indol-3-butyric acid
- Kin:
-
Kinetin
- µ:
-
Maximal growth rate
- MS:
-
Murashige and Skoog
- rf:
-
Reference front
- RSM:
-
Response surface methodology
- SD:
-
Star design
- TDZ:
-
Thidiazuron
- TPA:
-
12-O-Tetradecanoylphorbol-13-acetate
References
Aguilar A, Camacho JR, Chino S, Jacquez P, López ME (1994) Herbario medicinal del Instituto Mexicano del Seguro Social. Información Etnobotánica. Instituto Mexicano del Seguro Social, Mexico City, p 218
Aguirre-Hernández E, Martínez A, González-Trujano M, Moreno J, Vibrans H, Soto-Hernández M (2007) Pharmacological evaluation of the anxiolytic and sedative effects of Tilia americana L. var. mexicana in mice. J Ethnopharmacol 109:140–145. https://doi.org/10.1016/j.jep.2006.07.017
Aguirre-Hernández E, González-Trujano M, Martínez A, Moreno J, Kite G, Terrazas T, Soto-Hernández M (2010) HPLC/MS analysis and anxiolytic-like effect of quercetin and kaempferol flavonoids from Tilia americana L. var. mexicana. J Ethnopharmacol 127:91–97. https://doi.org/10.1016/j.jep.2009.09.044
Aguirre-Hernández E, González-Trujano ME, Terrazas T, Herrera-Santoyo T, Guevara-Fefer P (2016) Anxiolytic and sedative-like effects of flavonoids from Tilia americana var. mexicana: GABAergic and serotonergic participation. Salud Mental 39:37–46. https://doi.org/10.17711/SM.0185-3325.2015.066
Ali MB, Hahn EJ, Paek KY (2006) Copper-induced changes in the growth, oxidative metabolism, and saponin production in suspension culture roots of Panax ginseng in bioreactors. Plant Cell Rep 25(10):1122–1132. https://doi.org/10.1007/s00299-006-0174-x
Angeles-López GE, González-Trujano ME, Déciga-Campos M, Ventura-Martínez R (2013) Neuroprotective evaluation of Tilia americana and Annona diversifolia in the neuronal damage induced by intestinal ischemia. Neurochem Res 38:1632–1640. https://doi.org/10.1007/s11064-013-1065-5
Angeles-López GE, González-Trujano ME, Gómez C, Chánez-Cárdenas ME, Ventura-Martínez R (2015) Neuroprotective effects of Tilia americana var. mexicana on damage induced by cerebral ischaemia in mice. Nat Prod Res 30:2115–2119. https://doi.org/10.1080/14786419.2015.1110701
Argueta A, Cano L, Rodarte M (1994) Atlas de las plantas de la medicina tradicional mexicana. Tomo III. Instituto Nacional Indigenista, Mexico City, p 1337
Azevedo H, Dias A, Tavares RM (2008) Establishment and characterization of Pinus pinaster suspension cell cultures. Plant Cell Tis Org Cult 93(1):115–121. https://doi.org/10.1007/s11240-008-9349-1
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76(5):965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161(5):839–851. https://doi.org/10.1016/s0168-9452(01)00490-3
Bruns RE, Scarminio IS, de Barros Neto B (2006) Statistical design-chemometrics, vol 25. Elsevier, Amsterdam
Chen CT, Chen TH, Lo KF, Chiu CY (2004) Effects of proline on copper transport in rice seedlings under excess copper stress. Plant Sci 166(1):103–111. https://doi.org/10.1016/j.plantsci.2003.08.015
Flores-Sánchez K, Cruz-Sosa F, Zamilpa-Alvarez A, Nicasio-Torres P (2019) Active compounds and anti-inflammatory activity of the methanolic extracts of the leaves and callus from Tilia americana var. mexicana propagated plants. Plant Cell Tiss Organ Cult 137(1):55. https://doi.org/10.1007/s11240-018-01550-x
Fritz C, Palacios N, Fiel R, Stitt M (2006) Regulation of secondary metabolism by the carbon nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J 46(4):533–548. https://doi.org/10.1111/j.1365-313X.2006.02715.x
Gilmour SG (2006) Response surface designs for experiments in bioprocessing. Biometrics 62:323–331. https://doi.org/10.1111/j.1541-0420.2005.00444.x
Hanchinal VM, Survase SA, Sawant SK, Annapure US (2008) Response surface methodology in media optimization for production of β-carotene from Daucus carota. Plant Cell Tiss Org Cult 93(2):123–132. https://doi.org/10.1007/s11240-008-9350-8
Hardin JW (1990) Variation patterns and recognition of varieties of Tilia americana s.l. Syst Bot 15:33–48. https://doi.org/10.2307/2419014
Herrera-Ruiz M, Román-Ramos R, Zamilpa A, Tortoriello J, Jiménez-Ferrer E (2008) Flavonoids from Tilia americana with anxiolytic activity in plus-maze test. J Ethnopharmacol 118:312–317. https://doi.org/10.1016/j.jep.2008.04.019
Katoh S, Yoshida F (2009) Cell kinetics in biochemical engineering: a textbook for engineers, chemists and biologists. Wiley, Weinheim, pp 47–54
Kodama T, Ishida H, Kokubo T, Yamakawa T, Noguchi H (1990) Glucosylation of quercetin by a cell suspension culture of Vitis sp. Agric Biol Chem 54(12):3283–3288. https://doi.org/10.1080/00021369.1990.10870473
Kokubo T, Ambe Y, Nakamura M, Yamakawa T, Noguchi H, Kodama T (1991) Quercetin 3-O-β-D-glucopyranoside and Isorhamnetin 3-O-β-D-glucopyranoside formation from quercetin by cell cultures of Ipomoea batatas and Crocus sativum. Agric Biol Chem 55(2):613–614. https://doi.org/10.1080/00021369.1991.10870595
Korsangruang S, Soonthornchareonnon N, Chintapakorn Y, Saralamp P, Prathanturarug S (2010) Effects of abiotic and biotic elicitors on growth and isoflavonoid accumulation in Pueraria candollei var. candollei and P. candollei var. mirifica cell suspension cultures. Plant Cell Tiss Org Cult 103(3):333–342. https://doi.org/10.1007/s11240-010-9785-6
Lea US, Slimestad R, Smedvig P, Lillo C (2007) Nitrogen deficiency enhances expression of specific MYB and bHLH transcription factors and accumulation of end products in the flavonoid pathway. Planta 225:1245–1253. https://doi.org/10.1007/s00425-006-0414-x
Maksymiec W (2007) Signaling responses in plants to heavy metal stress. Acta Physiol Plant 29:177–187. https://doi.org/10.1007/s11738-007-0036-3
Martínez SM (1987) Plantas autóctonas y productos volcánicos de las inmediaciones de Morelia. Universidad Michoacana de San Nicolás de Hidalgo, Morelia
Martínez M, Matuda E (1979) Flora del Estado de México. Tomo III. Ed. Biblioteca enciclopédica del Estado de México, Mexico City, p 495
Matkowski A (2008) Plant in vitro culture for the production of antioxidants—a review. Biotechnol Adv 26(6):548–560. https://doi.org/10.1016/j.biotechadv.2008.07.001
Monroy-Ortiz C, Castillo-España P (2007) Plantas medicinales utilizadas en el estado de Morelos. Universidad Autónoma del Estado de Morelos, Morelos, pp 251–253, 319
Mora-Izquierdo A, Nicasio-Torres P, Sepúlveda-Jiménez G, Cruz-Sosa F (2011) Changes in biomass allocation and phenolic compounds accumulation due to the effect of light and nitrate supply in Cecropia peltata plants. Acta Physiol Plant 33(6):2135–2147. https://doi.org/10.1007/s11738-011-0753-5
Muñoz-Flores HJ, Orozco-Gutiérrez G, García-Magaña J, Coria-Ávalos VM, Salgado-Garciclia R, Santiago-Santiago MR (2011) Épocas de colecta y tratamiento para enraizamiento de estacas de cirimo Tilia mexicana Schlecht (Tiliaceae). Rev Mex Cienc For 2(3):13–23
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nicasio-Torres P, Meckes-Fischer M, Aguilar-Santamaría L, Garduño-Ramírez ML, Chávez-Ávila VM, Cruz-Sosa F (2012) Production of chlorogenic acid and isoorientin hypoglycemic compounds in Cecropia obtusifolia calli and in cell suspension cultures with nitrate deficiency. Acta Physiol Plant 34(1):307–316. https://doi.org/10.1007/s11738-011-0830-9
Nicasio-Torres P, Pérez-Hernández J, González-Cortázar M, Meckes-Fischer M, Tortoriello J, Cruz-Sosa F (2016) Production of potential anti-inflammatory compounds in cell suspension cultures of Sphaeralcea angustifolia (Cav.) G. Don. Acta Physiol Plant 38(8):209. https://doi.org/10.1007/s11738-016-2211-x
Noguerón-Merino MC, Jiménez-Ferrer E, Román-Ramos R, Zamilpa A, Tortoriello J, Herrera-Ruiz M (2015) Interactions of a standardized flavonoid fraction from Tilia americana with serotoninergic drugs in elevated plus maze. J Ethnopharmacol 164:319–327. https://doi.org/10.1016/j.jep.2015.01.029
Orozco-Sanchez F, Sepúlveda-Jiménez G, Trejo-Tapia G, Zamilpa A, Rodríguez-Monroy M (2011) Oxygen limitations to grow Azadirachta indica cell culture in shake flasks. Rev Mex Ing Quím 10:343–352
Palasota JA, Deming SN (1992) Central composite experimental designs: applied to chemical systems. J Chem Educ 69(7):560. https://doi.org/10.1021/ed069p560
Pavón NP, Rico-Gray V (2000) An endangered and potentially economic tree of Mexico: Tilia mexicana (Tiliaceae). Econ Bot 54:113–114
Pavón-Reyes L, Evangelista-Lozano S, Sepúlveda-Jiménez G, Chávez-Ávila V, Rodríguez-Monroy M (2016) Cell culture of Bursera linanoe in a stirred tank bioreactor for production of linalool and linalyl acetate. Nat Prod Commun 12(3):319–322
Pérez-Hernández J, González-Cortázar M, Marquina S, Herrera-Ruiz M, Meckes-Fischer M, Tortoriello J, Cruz-Sosa F, Nicasio-Torres P (2014) Sphaeralcic acid and Tomentin, anti-inflammatory compounds produced in cell suspension cultures of Sphaeralcea angustifolia. Planta Med 80(02/03):209–214. https://doi.org/10.1055/s-0033-1360302
Pérez-Hernández J, Martínez-Trujillo A, Nicasio-Torres P (2019) Optimization of active compounds production by interaction between nitrate and copper in Sphaeralcea angustifolia cell suspension using response surface methodology. Plant Cell Tiss Organ Cult 136(2):407–413. https://doi.org/10.1007/s11240-018-1516-4
Rozita O, Abdullah MA, Hasan MA, Marziah M, Siti MMK (2005) Optimization and elucidation of interactions between ammonium, nitrate and phosphate in Centella asiatica cell culture using response surface methodology. Biotechnol Bioprocess Eng 10:192–197. https://doi.org/10.3844/ajassp.2004.215.219
Secretaria de Medio Ambiente y Recursos Naturales (SEMARNAT) (2010) Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesdo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario oficial de la federación, 30 Diciembre de 2010. México, D.F. 77 pp. http://dof.gob.mx/normasOficiales/4254/semarnat/semarnat.htm
Siatka T, Reichling J (2000) Stimulation of scopoletin accumulation in Archangelica officinalis Hoffm. cell suspension cultures by fungal elicitors. Herba Polonica 46(1):12–17
Siatka T, Chlebek J, Host’alkova A (2017) Copper (II) sulfate stimulates scopoletin production in cell suspension cultures of Angelica archangelica. Nat Prod Commun 125(11):1779–1780
Staniszewska I, Królicka A, Maliński E, Łojkowska E, Szafranek J (2003) Elicitation of secondary metabolites in in vitro cultures of Ammi majus L. Enzyme Microb Technol 33(5):565–568. https://doi.org/10.1016/S0141-0229(03)00180-7
Tapia N, Zamilpa A, Bonfil M, Ventura E, Cruz-Vega D, Del Villar A, Cruz-Sosa F, Osuna L (2013) Effect of the culture medium and biotic stimulation on taxane production in Taxus globosa Schltdl. in vitro cultures. Acta Physiol Plant 35:3447–3455. https://doi.org/10.1007/s11738-013-1380-0
Trejo-Espino JL, Rodríguez-Monroy M, Vernon-Carter EJ, Cruz-Sosa F (2011) Establishment and characterization of Prosopis laevigata (Humb. & Bonpl. ex Willd) MC Johnst. cell suspension culture: a biotechnology approach for mesquite gum production. Acta Physiol Plant 33(5):1687–1695. https://doi.org/10.1007/s11738-010-0705-5
Van Ryswyk H, Van Hecke GR (1991) Attaining optimal conditions: an advanced undergraduate experiment that introduces experimental design and optimization. J Chem Educ 68(10):878. https://doi.org/10.1021/ed068p878
Viola H, Wolfman C, de Stein ML, Wasowski C, Peña C, Medina JH, Paladín AC (1994) Isolation of pharmacologically active Benzodiazepine receptor ligands from Tilia tomentosa (Tiliaceae). J Ethnopharmacol 44:47–53. https://doi.org/10.1016/0378-8741(94)90098-1
Zhou X, Zhong JJ (2009) Effect of initial ammonium concentration on taxoid production and biosynthesis genes expression profile in suspension cultures of Taxus chinensis cells. Eng Life Sci 9(3):261–266. https://doi.org/10.1002/elsc.200800109
Zurita-Valencia W, Gómez-Cruz JE, Atrián-Mendoza E, Hernández-García A, Granados-García ME, García-Magaña JJ, Salgado-Garciglia R, Sánchez-Vargas NM (2014) Establecimiento de un método eficiente de germinación in vitro y micropropagación del cirimo (Tilia mexicana Schlecht.) (Tiliaceae). Polibotanica 38:129–144
Acknowledgements
This work was supported by Basic Grant 302000 from the Consejo Nacional de Ciencia y Tecnología, México (CONACyT-México) for the doctoral studies of Daniel Cisneros-Torres at the Biotechnology Doctoral Program of UAM-Iztapalapa and by Complementary Grant 99187269 from the Instituto Mexicano del Seguro Social (IMSS).
Author information
Authors and Affiliations
Contributions
As a Ph.D. student, Daniel Cisneros-Torres participated in all of the experimental work under the advice of the co-authors, in the collection, analysis, interpretation of the data, and the writing of the manuscript. Francisco Cruz-Sosa supervised the establishment of the factorial design experiments, provided scopoletin and quercetin 3-O-β-d-glucoside standards, and he was Daniel Cisneros-Torres’ thesis co-advisor. Manasés González-Cortazar participated in the extraction and in the establishment of analytical methods for compound quantification. Aurora Martínez-Trujillo participated in the concept and design of the factorial design and supported Daniel Cisneros-Torres in the statistical analyses. Pilar Nicasio-Torres supervised the establishment of the cell-suspension cultures and growth and production kinetics, extractions of biomasses; she was also Daniel Cisneros-Torres’s thesis co-advisor, in addition to participating in the writing of the manuscript and approving the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Ali R. Alan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cisneros-Torres, D., Cruz-Sosa, F., González-Cortazar, M. et al. Enhancing the production of scopoletin and quercetin 3-O-β-d-glucoside from cell suspension cultures of Tilia americana var. mexicana by modulating the copper and nitrate concentrations. Plant Cell Tiss Organ Cult 139, 305–316 (2019). https://doi.org/10.1007/s11240-019-01683-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01683-7