Abstract
Cecropia peltata is popularly known as “guarumbo” in Mexico and is used in traditional medicine for treatment of diabetes mellitus. C. peltata plants were cultivated in a hydroponic system under controlled conditions. Gradients of light (20, 30 and 100 μmol m−2 s−1) and nitrate concentrations (13, 2 and 0.2 mM) were applied to estimate their effect on biomass allocation and accumulation of bioactive (chlorogenic acid and isoorientin) phenolic compounds over a 28-day period. According to carbon nutrient balance (CNB) hypothesis predictions, biomass accumulation in foliage was stimulated by the highest irradiance (100 μmol m−2 s−1); similarly, at highest irradiance in combination with lowest nitrate concentration (0.2 mM), root growth was stimulated (root-to-shoot ratio increased twofold with respect to the control). In these conditions, total phenolics (TP) and chlorogenic acid (CGA) contents were higher in aerial parts than in roots, with a 3.8-fold increase in TP and a 7.7-fold increase in CGA in foliage with respect to the control plants. Isoorientin was accumulated at very low levels. Antioxidant activity and total phenolic content showed a strong positive correlation. Phenylalanine ammonia-lyase activity (PAL) in aerial parts exhibited significant changes (>twofold) by highest irradiance. C. peltata plants allocate biomass and/or phenolic compounds to compensate the oxidative damage (increase in MDA levels) due to changes in light and nitrate restriction. The results are the basis for the establishment of a system of C. peltata culture in view of the potential use of C. peltata in therapeutic preparations for the treatment of diabetes mellitus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants are an important source of secondary metabolites with pharmacological properties and have been used throughout history to prevent and cure diseases in humans (Raskin et al. 2002; Cai et al. 2004). However, in the widespread consumption of some medicinal species from natural growing areas or field cultivation, essential topics regarding quality control, safety, efficacy and environmental conservation have not been fully considered (Mosaleeyanon et al. 2005; Street and Van Staden 2008). Within this context, the establishment of controlled crops is a potential strategy for an adequate supply of authentic plant raw material, with homogenous morphologic characteristics and chemical constituents that are likely to be commercially exploited (Raskin et al. 2002; Mosaleeyanon et al. 2005).
It is known that raw material quality in medicinal plant cultivation is determined by dry matter yield as well as by the content of bioactive compounds (target compounds, e.g., phenolics); both parameters are afforded mainly by the environment in which the plants grow, development status and seasonal collection (Gray et al. 2003). Biomass is partitioned among plant organs to optimize the uptake of light, carbon dioxide, nutrients and water to maximize plant growth rate (Ryser and Eek 2000). Furthermore, it has been reported that to improve the obtainability of a limited resource, the plant component responsible for its achievement (roots for minerals, shoots for light) grows relatively more than under unrestricted conditions (López-Bucio et al. 2003; Grechi et al. 2007).
Biomass allocation is commonly referred to as the distribution of carbon (C) among plant organs; however, the term is also applied to functional activities within organs, such as distribution of defensive compounds (Coviella et al. 2002). The carbon nutrient balance (CNB) hypothesis predicts that the pattern of allocation to defensive compounds depends on the relative availability of carbon and nutrients, as well as on their relationship with the plant growth rate. Under conditions of high photosynthesis rate or reduction of available nitrogen (N), production of C-based defense compounds will be favored, whereas under the opposite conditions, N-based defense compound synthesis will be carried out (Coviella et al. 2002; Fritz et al. 2006; Grechi et al. 2007). According to these predictions, it has been demonstrated that the production of phenolics can be stimulated by low nitrate levels (Armstrong et al. 1970; Fritz et al. 2006; Palumbo et al. 2007; Le Bot et al. 2009), high light (Hemm et al. 2004; Jaakola et al. 2004; Mosaleeyanon et al. 2005) and other biotic and abiotic stresses, e.g., pathogen attack, mechanic wounding, ultraviolet (UV) radiation, low temperatures and nutrient deficit (Bernards and Ellis 1991; Kováčik and Bačkor 2007; Clé et al. 2008; Jansen et al. 2008). Phenylalanine ammonia-lyase enzyme (PAL) catalyzes the conversion of phenylalanine into trans-cinnamic acid; therefore, this enzyme causes the flux of primary into secondary metabolites in the phenolics pathway. PAL activity and other phenylpropanoid enzymes are mainly regulated via de novo synthesis. Hemm et al. (2004) showed that PAL genes are highly expressed in light-grown Arabidopsis roots, and Fritz et al. (2006) reported that PAL gene expression was induced by nitrate deficiency in Nicotiana tabacum plants.
Cecropia peltata (Loefl) and C. obtusifolia trees (Cecropiaceae), popularly known as “guarumbo” in Mexico, are used in traditional medicine for the treatment of diabetes mellitus (Argueta et al. 1994; Aguilar et al. 1998), and clinical studies have validated their effectiveness against this disease (Herrera-Arellano et al. 2004; Revilla-Monsalve et al. 2007). Guarumbo leaves contain phenolic compounds such as chlorogenic acid (CGA) and isoorientin (ISO) (Andrade-Cetto and Wiedenfeld 2001; Nicasio et al. 2005; Nicasio-Torres et al. 2009); both compounds possess hypoglycemic, hypolipidemic and antioxidant properties (Ko et al. 1998; Rodríguez de Sotillo and Hadley 2002). Additionally, it has been reported that CGA stimulates glucose uptake in both insulin-sensitive and -resistant adipocytes (Alonso-Castro et al. 2008). Some Mexican industries have been currently employing guarumbo leaves for the production of drugs (Químicos y Vegetales Rowi, S.A. de C.V., Zapopan, Jalisco; Ciencia y Salud: Fórmulas Herbolarias, México, D.F.), and guarumbo leaves have been recently exported to Europe for the same purpose (Gutiérrez-Domínguez and Betancourt-Aguilar 2008).
The present need for a quality-controlled supply of C. peltata raw material for further clinical investigation and potential development of a phytopharmaceutical requires standardized and controlled production systems. C. peltata and C. obtusifolia multiplication was reported through in vitro plantlet apical buds, which preserved the ability to produce both bioactive compounds (Nicasio-Torres et al. 2009). Nonetheless, to our knowledge, studies have not been reported on guarumbo plants improving the bioactive compound contents by abiotic stress induction. Considering that biomass yield and phenolic contents in guarumbo are important parameters in the quality of this medicinal species, the aim of this study was to explore the effect of light and nitrate supply on biomass allocation, as well as bioactive CGA, ISO and total soluble phenolic compounds contents in a controlled hydroponic culture of C. peltata plants.
Materials and methods
Plant material
Mature fruits were obtained from a Cecropia peltata tree previously collected in Cunduacán, Tabasco State, and acclimated in Xochitepec, Morelos State, Mexico. This specimen was identified (code number 14123) by Abigail Aguilar, Director of the Mexican Institute of Social Security’s (IMSSM) medical plants herbarium of Mexico (Nicasio-Torres et al. 2009). Seeds were surface sterilized by successive immersion in 70% ethanol for 5 min, which was followed by immersion in a commercial bleach solution (Cloralex®:water, 1:5 v/v) for 10 min. Disinfected seeds were washed three times with sterile distilled water. Subsequently, the seeds were transferred aseptically to glass jars containing 30 ml of solid half-strength Murashige and Skoog (1962) medium, supplemented with 1.5% (w/v) sucrose and 0.3% (w/v) phytagel, and adjusted to pH 5.7 prior to autoclaving (121°C for 18 min). Seeds in glass jars were incubated at 26 ± 2°C during a light:dark (16:8-h) photoperiod under 30 μmol m−2 s−1 of irradiance supplied by cool-white fluorescent lamp.
Hydroponic culture
Eight-week-old seedlings (5 cm in height) were acclimatized to ex vitro conditions as described by Osuna et al. (2008). For a short time, plantlets were hydroponically grown in plastic containers (covered with transparent plastic bags to prevents desiccation) with 60 ml of slightly modified Hoagland solution (Hoagland and Arnon 1950): 13 mM NO3 −, 6 mM K+, 4 mM Ca2+, 2 mM SO4 2−, 2 mM Mg2+, 1 mM H2PO4 −, 46.2 μM H3BO3, 17.9 μM Fe2+-K2EDTA, 9.1 μM Mn2+, 0.76 μM Zn2+, 0.31 μM Cu2+ and 0.10 μM MoO4 2− to pH 5.8. The nutrient solution was renewed every 7 days and relative humidity was reduced gradually until the plastic bags were removed from the plants. After 4 weeks, plants were transplanted to 300-ml potting foam cups filled with mineral perlite and were watered with nutrient solution (50 ml) every other day during 4 weeks. Conditions in the growth chamber were 25°C and light:dark (16:8-h) photoperiod under 30 μmol m−2 s−1 irradiance supplied by cool-white fluorescent lamp. Sixteen-week-old plants were employed as experimental units.
Experimental design
Nine treatments (Table 1) were applied by means of a combination of two factors: irradiance (20, 30 and 100 μmol m−2 s−1) and nitrate concentration in the nutrient solution (13, 2, and 0.2 mM). To ensure adequate supply of K+ and Ca2+, the salts withdrawn from the nutrient solution were replaced by equivalent moles of K2SO4 and CaCl2. Plants were watered with 50 ml of nutritional solution every other day for a period of 28 days. All experiments were carried out in the growth room. Control conditions were 30 μmol m−2 s−1 of irradiance and 13 mM of nitrate concentration. Cool-white fluorescent lamps were utilized to modify the amount of light reaching the plants. At the beginning of the experiment (d0), three plants were harvested as referents of initial values from the parameters to be evaluated. The remainder samples were collected at day 28 (d28). A second experimental plant batch was achieved under 30 and 100 μmol m−2 s−1 of irradiances in combination with 13 and 0.2 mM nitrate concentrations for chlorophyll, hydrogen peroxide and lipid peroxidation (MDA) evaluations in fresh vegetal material.
Chemical analyses from fresh material
Chlorophyll
The concentration of total chlorophyll was determined using the method of Lichtenthaler (1987) in the acetone (80%) extract acquired from fully expanded third young leaf. The absorbance of cleared extract was measured at 663.2 and 648.8 nm.
Hydrogen peroxide and lipid peroxidation
The endogenous H2O2 level was determined as H2O2–titanium complex produced as a result of the reaction of tissue-H2O2 with titanium tetrachloride by the method of Brennan and Frenkel (1977). Lipid peroxidation in leaf tissue was determined in terms of malondialdehyde (MDA) content by thiobarbituric acid (TBA) reaction as described by Heath and Packer (1968). MDA equivalent was calculated from the difference in absorbance at 532 and 600 nm utilizing extinction coefficient of 155 mM−1cm−1.
Harvesting and growth parameters
Plants were carefully separated into aerial parts and roots, and were dried at room temperature under darkness conditions. Fresh mass (FM) and dry mass (DM) of each sample were registered, and root-to-shoot ratio (RSR) was determined as the relation of root/aerial part (g g−1) of DM.
Chemical analyses from dry material
Obtaining extract
Finely ground dry material (0.2 g) was separately extracted three times by maceration at room temperature with methanol (1:100, w/v) during 24 h, followed by filtration through filter paper (Whatman No. 1). Extracts were pooled and concentrated to dryness under reduced pressure and stored in glass containers (Nicasio et al. 2005; Nicasio-Torres et al. 2009).
Total soluble phenolic compounds (TP)
The concentration of TP was determined by the Folin-Ciocalteu colorimetric method (Cai et al. 2004; Silva et al. 2007). Extracts were re-dissolved in methanol (1 mg ml−1) and oxidized with Folin-Ciocalteu reagent (10% v/v); after 10 min, the reaction was neutralized with saturated sodium carbonate solution (8%, w/v). After incubation for 90 min at room temperature (23°C), absorbance was measured at 760 nm using a Jenway 6305 spectrophotometer. Quantification was performed on the basis of the CGA standard curve. Results were expressed as milligram of TP equivalents (eq) to CGA per gram DM, as well as milligram of TP equivalents (eq) to CGA per plant.
Antioxidant activity (AA)
AA was carried out by the improved ABTS·+ method described by Re et al. (1999). The ABTS·+ radical cation was generated by reacting 7 mM ABTS and 2.45 mM potassium persulfate after incubation at room temperature (23°C) under darkness conditions for 16 h. The ABTS·+ solution was diluted with water to an absorbance of 0.700 ± 0.050 at 734 nm (Jenway 6305 spectrophotometer). Extracts were re-dissolved in methanol (1.58 mg ml−1), and 20 μl was mixed thoroughly with 1 ml of ABTS·+ solution. The reactive mixture was allowed to stand at room temperature for 5 min and absorbance was immediately recorded at 734 nm. CGA standard solutions in methanol (0–200 μg ml−1) were prepared and assayed under the same conditions. Results were expressed in terms of CGA equivalent antioxidant activity per gram of DM.
HPLC analysis
HPLC analyses for CGA (Sigma Chemical Co., St Louis, MO, USA) and ISO (Indofine Chemical Co., Inc., USA) were conducted on a Waters (Separation Module 2695) HPLC with a system controller (Millennium System Manager Software) and under conditions previously established by Nicasio-Torres et al. (2009). Samples (60-μl) were eluted onto a Spherisorb ODS-2 column (250 mm × 4.6 mm, 5 μm) at a 1 ml min−1 flow rate with a binary gradient of mobile phases as follows: (A) H2O–(H3PO4 0.5%), and (B) CH3CN–CH3OH (1:1) within 32 min. Peak purity of the tested sample was determined by comparing its UV spectra with the authentic standard. Calibration curves were constructed with standard solutions of 20, 40, 80 and 100 μg ml−1 of CGA and ISO in methanol by monitoring absorbance at 300 nm employing a Waters 2996 photodiode array detector. Standard curve linear regressions based on peak area of standards utilizing a zero-intercept model showed R 2 = 0.999.
Phenylalanine ammonia-lyase (PAL) activity
PAL was extracted and its activity was determined, making some modifications to the method described by Kováčik and Bačkor (2007). Fresh samples (0.2 g) were mixed in the tissue homogenizer during 50 s with 3 ml of cold sodium borate buffer 0.1 M (pH 8.8) containing 20 mM of β-mercaptoethanol. The mixture was maintained for 30 min in an ice bath (4°C), then centrifuged at 12,500 rpm at 4°C during 20 min, and this final extract was employed for the enzymatic assay. The reaction mixture with 2.1 ml of sodium borate buffer 0.1 M (pH 8.8) and 0.5 ml of extract was pre-incubated at 40°C for 5 min; subsequently, the reaction was initiated by the addition of 0.3 ml of l-phenylalanine 100 mM. After 1 h of incubation at 40°C, the reaction was stopped by the addition of 0.1 ml of HCl 6 N. Simultaneously, the blank for every sample was carried out with 2.4 ml of sodium borate 0.1 M (pH 8.8), 0.5 ml of the extract and 0.1 ml of HCl 6 N. Change of absorbance was evaluated after 1 h at 277 nm. Enzymatic activity was reported as U g−1 of fresh mass (FM), where U = difference of absorbance after 1 h at 277 nm h−1.
Statistical analysis
Data were analyzed by two-way analysis of factorial variance (ANOVA); p values of ≤0.05 were considered to be important. Significant differences among treatment means were calculated by Tukey test. After linear regression of the data of TP versus AA, the square of the Pearson correlation coefficient (R 2) was reported along with its p value; correlations with p values of ≤0.01 were considered as significant (SAS, 9.1; SAS Institute, Inc.).
Results
Growth parameters
During plant development, we observed that at 30 and 100 μmol m−2 s−1 of irradiance, the plants’ foliage presented leaves with chlorosis (yellowing) and a reddish tonality in the vascular tissue area with a nitrate deficiency (Fig. 1). Similarly, we observed that plants developed with greater irradiance presented more abundant secondary roots after 28 days of culture.
With regard to growth under control conditions (30 μmol m−2 s−1 irradiance and 13 mM nitrate), dry biomasses of aerial parts and roots (Fig. 2) increased nearly threefold with respect to initial biomasses (d0) at the end of the experiment.
Combined effects of irradiance and nitrate supply on dry mass accumulation in aerial parts and roots of Cecropia peltata plants sampled prior to (d0) and at the end of the experiment (d28). Vertical bars represent the standard error (SE) of mean (n = 3). An asterisk indicates a significant difference against the control (C) according to the Tukey multiple range test
In aerial parts, after 28 days, significant differences of dry mass (DM) were not found among low-irradiance treatments (20 and 30 μmol m−2 s−1); however, in treatments with higher irradiance (100 μmol m−2 s−1), DM increased significantly (F = 87.68; p < 0.0001; Tukey0.05 = 0.22), up to 1.7-fold more than that of the control. Under this higher-irradiance condition, we observed a non-significant decrease in DM accumulation on reducing the nitrate concentration (13, 2, and 0.2 mM).
At the beginning of the experiment, chlorophyll concentration was significantly higher than that obtained at the final time in all treatments (Table 2). H2O2 concentration did not show significant differences among all treatments; however, MDA content was significantly increased 2.1- and 2.5-fold with respect to the control in the treatments with 0.2 mM of nitrate at 30 and 100 μmol m−2 s−1 of irradiance, respectively.
In roots, statistical analysis indicated that biomass accumulation was generated due to the interaction of greater irradiance and lower concentration of nitrate (F 3×3 = 5.92; p = 0.0032). In the treatment with highest irradiance, in an effect contrary to the tendency observed in aerial parts, DM increased on diminishing the concentration of nitrate.
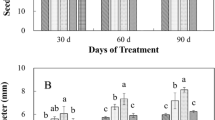
By means of the root-to-shoot ratio (RSR) parameter (Fig. 3), we analyzed the difference in growth between aerial parts and roots. In the control, at 28 days we obtained a relation similar to that of the beginning (d0). Likewise, in low-irradiance treatments (20 and 30 μmol m−2 s−1), we found no significant differences in RSRs at 28 days with respect to the control; in contrast, in 100 μmol m−2 s−1 treatments, the relation of RSR increased significantly (F = 111.76; p < 0.0001; Tukey0.05 = 0.02), up to nearly 2.0-fold, which was favored on diminishing the concentration of nitrate (F = 29.37; p < 0.0001; Tukey0.05 = 0.02).
Combined effects of irradiance and nitrate supply on root-to-shoot ratio (RSR) (g g−1DM) of Cecropia peltata plants sampled prior to (d0) and at the end of the experiment (d28). Vertical bars represent the standard error (SE) of mean (n = 3). An asterisk indicates a significant difference against the control (C) according to the Tukey multiple range test
Chemical analysis and PAL activity
The accumulation of total soluble phenolics (TP) was determined both in aerial parts and in roots (Tables 3, 4). On initiation (d0), the concentration of aerial parts was 1.8 times greater than that of the roots, a relation that increased 2.3-fold at the end of the study (d28) under control conditions (30 μmol m−2 s−1 irradiance and 13 mM nitrate). The abundance of CGA in aerial parts is noteworthy; at the beginning (d0), a content presented that was twofold greater than that of the roots, and at 28 days this relation increased from six to eightfold in the different treatments. On the other hand, as a general tendency, PAL activity was greater in roots than in aerial parts.
As an important tendency in aerial parts, we observed that TP, AA and CGA contents increase as irradiance increases, and the concentration of nitrate decreases (Table 3), an effect contrary to that of biomass allocation (Fig. 2). Significant increments in the TP, AA and CGA parameters (Table 5) were obtained under the condition of greater irradiance (100 μmol m−2 s−1) and lower concentration of nitrate (0.2 mM), whereas PAL activity increased significantly only with greater irradiance.
Regarding roots, none of the factors evaluated affected PAL activity (Tables 4, 5). Significant increases in TP and AA parameters were obtained under the condition of greater irradiance (100 μmol m−2 s−1) and lower nitrate concentration (0.2 mM) because of the interaction of both factors for TP, and independently to the same extent by the irradiance as by the nitrate concentration in the case of AA. CGA content was favored only by the effect of greater irradiance (Table 5).
Through linear regression analysis between TP and AA contents obtained in the different treatments (Table 1; Fig. 4), we found a significant positive correlation in the aerial parts (R 2 = 0.9; p < 0.001; n = 10), as well as in the roots (R 2 = 0.8; p < 0.001; n = 10). In the comparison of the straight-line equation of aerial parts (y = 0.9893x + 0.253) and roots (y = 1.2753x + 0.7647), the greater value of the slope for roots is highlighted.
Relationship between antioxidant activity (AA) and total soluble phenolics (TP) of Cecropia peltata plant aerial part extracts grown with a range of irradiance and nitrate concentrations (Table 1). The ‘best-fit’ line shown was generated by linear regression of the data (n = 10). Vertical and horizontal bars represent the standard error (SE) of means (n = 3)
A commercially useful expression of the results of FT and CGA contents is to consider total biomass per plant. In Figs. 5 and 6, total yields are represented under the different study conditions. We observe that accumulation of phenolics is greater in aerial parts (6.5-fold) than in roots (Fig. 5). TP levels in aerial parts increase significantly under greatest irradiance (F = 33.99; p < 0.0001; Tukey0.05 = 4.97) and least nitrate concentration (F = 5.34; p = 0.0151; Tukey0.05 = 4.97), that is, 3.8-fold in aerial parts and 7.6-fold in roots with respect to the control. In the roots, we found an interaction between greatest irradiance and least nitrate concentration (F 3×3 = 9.76; p = 0.0002; Tukey0.05 = 0.55).
Combined effects of irradiance and nitrate supply on total soluble phenolics (TP) in Cecropia peltata plant aerial parts and roots sampled prior to (d0) and at the end of the experiment (d28). Vertical bars represent the standard error (SE) of mean (n = 3). An asterisk indicates a significant difference against control (C) according to the Tukey multiple range test
Combined effects of irradiance and nitrate supply on chlorogenic acid content (CGA) in Cecropia peltata plant aerial parts and roots sampled prior to (d0) and at the end of the experiment (d28). Vertical bars represent the standard error (SE) of mean (n = 3). An asterisk indicates a significant difference against the control (C) according to the Tukey multiple range test
With respect to CGA (Fig. 6), its contents per plant is the result of the interaction of both factors (irradiance and nitrate concentration), both in the aerial parts (F 3×3 = 4.8; p = 0.0082; Tukey = 0.305) and in the roots (F 3×3 = 5.35; p = 0.0051; Tukey0.05 = 0.019). The greatest increments were also obtained with the highest irradiance and least nitrate concentration, that is, 7.7-fold in aerial parts and 10.4-fold in roots with respect to the control. In addition, CGA content in the aerial part was 19.2-fold greater than that in the root. Notwithstanding this, under the condition of highest irradiance, CGA accumulation was greater under nitrate-deficiency conditions (0.2 mM). It is noteworthy that being under the same conditions also allowed obtaining greatest biomass in the plant (Fig. 2).
In Fig. 7, the chromatographic profiles of aerial-part extracts obtained under the greatest-irradiance condition are present, in which we observe a general tendency in the increase of CGA signal (retention time = 11.0–11.9 min; λ max = 326.7 nm) due to the effect of nitrate-concentration diminution, while the ISO flavonoid (retention time = 16.0–16.5 min; λ max = 350.6 nm) presented low levels that could not be quantified under the conditions of the system employed. Additionally, in aerial parts, we found two signals similar to isoorientin, which, because of its retention time (17.7 and 18.6 min) and maximal absorption in UV (λ max = 337.4 and 339.8 nm), might correspond to certain structurally related compounds.
a HPLC profiles of methanolic extracts of aerial parts from plant development with 100 μmol m−2 s−1 irradiance and three different levels of nitrate; chlorogenic acid contents (CGA) correspond to the signal of chlorogenic acid peak (rt = 11.0–11.9 min; λ max = 350.6 nm). b Structural representation of chlorogenic acid
Discussion
C. peltata is an arboreal species in its adult state and the gathering of its leaves in different status of development, in addition to environmental factors, can signify important variation in its chemical composition and have repercussions on its pharmacological efficacy (Alonso-Castro et al. 2008). The culture of “guarumbo” under controlled conditions is a viable alternative for obtaining reliable, safe and effective plant material, in addition to being a useful system for increasing the yields of quality parameters that favor the commercialization of this medicinal species. In the present study, by means of the manipulation of the light and nitrate supply, we modified the growth and phenolic compounds accumulation of C. peltata plants.
In control plants (30 μmol m−2 s−1 of irradiance and 13 mM of nitrate), accumulation of total biomass increased due to the growth effect during the 28 days of the study (Fig. 2). Regarding the remainder of the treatments, variation in light and nitrate supply exerted an impact on yield and distribution of biomass. Plants at least levels of irradiance (20 and 30 μmol m−2 s−1), despite their not having a nutrient limitation (13 mM of nitrate), developed low biomass levels in foliage. It has been mentioned for different species that during growth, photosynthetic capacity is dependent on the intensity of the light. Plants developed under low irradiance would contain a lower amount of their photosynthetic components and, consequently, a lower CO2 fixation rate (Horton 2000; Mosaleeyanon et al. 2005; Poorter et al. 2006). Contrariwise, under the same nitrate condition (13 mM) and with the increase of irradiance up to 100 μmol m−2 s−1, plants increased their biomass significantly, a response that coincided with other reports in the literature (Ryser and Eek 2000; Mosaleeyanon et al. 2005; Grechi et al. 2007). With the irradiance increase, CO2 fixation increases, thus a greater content of photosynthates in leaves is available for new tissue translocation and growth (Horton 2000). One visual characteristic of plants under this treatment of high irradiance and high nitrogen concentration was the tendency of the foliage to lose turgidity, which may be related to a greater stomatic opening, which in turn permits greater transpiration in leaves.
Hydrogen peroxide concentration did not show significant changes in all treatments, probably due to an increase of antioxidant systems such as peroxidase enzyme (POD), as was observed by Konieczny et al. (2008) in zygotic embryos from Heliantus annus.
Under greatest-irradiance conditions, no significant diminution of foliar biomass caused by reduction in nitrate supply (2 and 0.2 mM) could be related with the photoinhibition process in plants. The overexcited chlorophylls incite the formation of oxygen singlet (ROS) in the thylakoid membranes, causing damage in photosystem components, especially in phostosystem II, an effect known as photoinhibition. In addition, it is well known that ROS-induced membrane lipid peroxidation is a reference for cellular damage based on oxidative stress (Khan and Panda 2008). MDA is formed by decomposition of polyunsaturated fatty acids and used as an indicator of oxidative stress (Tewari et al. 2006). The significant increase in MDA levels associated with lipid peroxidation are linked with oxidative stress in C. peltata plant development under conditions of nitrate deficiency (0.2 mM), mainly under 100 μmol m−2 s−1 of irradiance.
It has been reported that there is partial closing of the stomas under nitrate-deficiency conditions (Cabrera 2002); thus, the capacity is limited for utilizing high levels of light, and the leaves absorb more luminous energy than that required for the assimilation of available CO2. In these treatments with nitrate deficiency, we observed greater turgidity in foliage, which suggests a smaller stomatic opening. Consequently, diminution of intracellular CO2 reduces photosynthesis and subsequently plant growth. Another important characteristic comprised symptoms of chlorosis in leaves (Table 2; Fig. 1) due to the diminution of chlorophyll related with nitrogen deficiency in the tissues, as reported previously in Hypericum perforatum plants (Briskin et al. 2000). On the other hand, in rice plants grown in a high-luminosity environment, Horton (2000) refers to the decrease in the chlorophyll content as a photoprotective response to the excess of light.
Nitrate-deficiency conditions (0.2 mM) also favored biomass allocation toward the roots (greater RSR value up to nearly 2.0-fold). Grechi et al. (2007) reported that changes in the RSR of Vitis vinifera plants are controlled by equilibrium aspects in the C:N internal levels. López-Bucio et al. (2003), in Arabidopsis plants, refer to the same allocation effect toward roots and postulate that nutrients such as nitrate possess signaling functions with a profound impact on radicular-system architecture. Also, it was reported that nitrate not only exerts an effect on the roots, but also functions as a signal for the metabolism and development of the entire plant (Takei et al. 2002; Urbanczyk-Wochniak and Fernie 2005; Miller et al. 2007).
The results of this study are consistent with the functional-equilibrium hypothesis (FEH) (Marcelis and Heuvelink 2007), in which biomass allocation was modeled on the whole plant, and in which it was established that plants, on finding themselves in an environment limited by an essential resource (e.g., nitrogen), respond with an increase in allocation toward the structure responsible for acquisition of the element that is restricted (Wilson 1988; Grechi et al. 2007). With these results, the plasticity of C. peltata is corroborated to distribute its resources under limited nutrient conditions in an efficient manner.
Allocation patterns toward compounds of defense (phenolics) found in this study are congruent with the predictions of the CNB hypothesis: When the production of C is greater than that required for growth, the excess of C is allocated to the formation of secondary metabolites (Bryant et al. 1983; Coviella et al. 2002; Mosaleeyanon et al. 2005; Le Bot et al. 2009). The results of the production of secondary metabolites (TP) comply with the CNB hypothesis. In aerial parts, in highest-irradiance treatments (100 μmol m−2 s−1 irradiance), TP content (Table 3) increases as nitrate concentration decreases (0.2 mM), a condition under which nitrogen limitation is perceived. Observation of the reddish tonality in vascular tissue zones of leaves (Fig. 1) might be related with the accumulation of phenolic compounds (e.g., anthocyanins). Under these conditions, the significant increase of TP can be interpreted as: photosynthesis surpasses the growth demand, and, on there being an excess of carbohydrates that accumulate in plants with nitrate deficiency, the C assimilated is available for biosynthesis of compounds that do not require N (e.g., phenolics). Phenolic accumulation due to light increase or nitrate deficiency has been reported in different studies (Briskin and Gawienowski 2001; Coviella et al. 2002; Mosaleeyanon et al. 2005; Fritz et al. 2006; Poorter et al. 2006; Grechi et al. 2007; Palumbo et al. 2007; Le Bot et al. 2009). Under high-irradiance and nitrate-deficiency conditions, the assimilated carbon is directed toward the following two key components: (a) the biomass increase in roots, as previously mentioned, and (b) toward the increase of levels of carbon-rich secondary metabolites, such as phenolics.
In the experimental model of this study, the combination of high-irradiance and nitrate-deficiency factors could be stress factors in C. peltata plants through the formation of reactive oxygen species (ROS), which are strongly oxidating molecules (Laloi et al. 2006). It has been established that accumulation of ROS causes disequilibrium in intracellular redox balance, known as oxidative stress (Tewari et al. 2006; Khan and Panda 2008). One of the cellular mechanisms for avoiding tissue damage is the production of antioxidant compounds for ROS uptake (Dixon and Paiva 1995; Grace and Logan 2000; Winkel-Shirley 2002; Cai et al. 2004). According to the results, under highest-irradiance and greatest nitrate-deficiency conditions (100 μmol m−2 s−1 irradiance and 0.2 mM nitrate), aerial parts presented the highest concentrations of TP (up to 6.5-fold) in comparison with the content in roots, which suggests that the oxidative-stress level in aerial parts is greater because these are in direct contact with light, while the roots are not, thus requiring lower levels of antioxidant response. Correlation analyses suggest that phenolic compounds contribute significantly to the antioxidant response of the extracts evaluated, and that in aerial parts (R 2 = 0.9; p < 0.001; n = 10) there is a greater association between TP content and AA (R 2 = 0.8; p < 0.001; n = 10). Likewise, comparison of the slopes of aerial parts (0.9893) and roots (1.2753) suggests that compounds with greater antioxidant activity are found in root extracts.
As mentioned previously, CGA content (Fig. 6) increased importantly (7.7-fold in aerial parts and 10.4-fold in roots with respect to the control) under greatest-irradiance and least nitrate-concentration conditions (100 μmol m−2 s−1 irradiance and 0.2 mM nitrate). CGA is a low-molecular weight phenylpropanoid that possesses an important antioxidant function in the cells of photosynthetic tissues (Sakihama et al. 2002; Heo et al. 2007), counterattacking the damaging effects of the ROS (Clé et al. 2008). As an antioxidant, CGA (Fig. 7b) possesses three important characteristics for its structure–function relationship: (a) the catechol group; (b) the double link conjugated in the propanoid-residue chain and (c) the sterification of the cafeic-acid terminal carboxyl group with a quinic-acid–OH group for the formation of CGA (Grace and Logan 2000). Additionally, these authors have proposed that CGA could perform another important function in a dissipation mechanism of photochemical energy. An increase has been reported in other species of the concentration of CGA of up to fourfold in Nicotiana tabaccum plants because of the diminution of nitrate from 12 to 0.2 mM (Fritz et al. 2006), and due to the excess of light in Solanum lycopersicum from 200 to 900 μmol m−2 s−1 (Urbanczyk-Wochniak and Fernie 2005).
With respect to ISO, the culture conditions employed did not stimulate its accumulation, which coincides with other studies that refer to ISO as an inducible phytoalexin in response to biotic stress (McNally et al. 2003), although other factors, such as developmental state or exposure to sunlight (UV), might be required to stimulate its biosynthesis and accumulation in C. peltata plants (Clé et al. 2008; Nicasio-Torres et al. 2009).
The C. peltata-plant extracts obtained in this study presented a bioactive compound chemical profile (a greater content of CGA than ISO), similar to extracts evaluated in clinical and laboratory pharmacological assays in which its antidiabetic properties were corroborated (Herrera-Arellano et al. 2004; Revilla-Monsalve et al. 2007). In addition, the activity of the extract is not solely due to these compounds, but also to the complex mixture of the different metabolites, although the latter have not been chemically characterized (Nicasio-Torres et al. 2009).
PAL activity was observed to be increased only in aerial parts by the effect of greatest irradiance; however, its activity in aerial parts as well as in roots does not correlate with the increments presented in phenolic levels on diminishing nitrate content. In this respect, it is known that PAL is the key enzyme in phenolic-compound biosynthesis, and it has been reported that it is activated and its expression induced in response to different stress conditions, among these, an increase in light (Hemm et al. 2004; Jaakola et al. 2004) and nitrate deficiency (Fritz et al. 2006). Different studies mention that PAL-activity increases during the first days of stress, and once the required compounds have been synthesized, the enzyme’s activity decreases (Bernards and Ellis 1991; Kováčik and Bačkor 2007). When C. peltata plants were submitted to different treatments, PAL activity could have increased to a maximum in days prior to the analysis, with which phenolics were synthesized and later diminished to levels observed at the end of the treatments (d28).
Conclusion
In this study, we demonstrated that C. peltata plants distribute the carbon assimilated in photosynthesis as a response to the availability of light and nitrate, whether due to the structural formation of the different organs (biomass) and/or to the accumulation of secondary metabolites with functions of protection and employment of nutritional resources. Under the condition of high irradiance, growth of foliage and roots was favored; in addition to these latter components, nitrate limitation interacted with light to increase their biomass. Similarly, in the treatment with higher irradiance and low nitrate concentration, we obtained greater TP (3.8-fold) and CGA (7.7-fold) contents in plant foliage with respect to the control: TP and AA content correlated positively, while PAL activity increased mainly in the aerial parts due to the effect of greater irradiance, and there were no important changes in PAL activity in the roots. Allocation differences of phenolic compounds in the distinct supply levels of light and nitrate in C. peltata plants are congruent with the predictions of the CNB hypothesis.
The culture of C. peltata under controlled conditions does not require plant development until adult age, because it has been reported that the metabolites of interest are present in the early stages of their development (Nicasio-Torres et al. 2009). The results of this study are the basis for the establishment of a system of culture of C. peltata by means of nitrate limitation under high-irradiance conditions for supplying high-quality prime matter, in view of the potential use of C. peltata in therapeutic preparations for the treatment of diabetes mellitus.
Abbreviations
- AA:
-
Antioxidant activity
- CGA:
-
Chlorogenic acid
- CNB:
-
Carbon nutrient balance
- DM:
-
Dry mass
- FM:
-
Fresh mass
- ISO:
-
Isoorientin
- MDA:
-
Malondialdehyde
- PAL:
-
Phenylalanine ammonia-lyase
- ROS:
-
Reactive oxygen species
- RSR:
-
Root-to-shoot ratio
- TBA:
-
Thiobarbituric acid
- TP:
-
Total soluble phenolics
References
Aguilar A, Camacho JR, Chino S, Jácquez P, López ME (1998) Plantas medicinales del herbario del IMSS, su distribución por enfermedades. Instituto Mexicano del Seguro Social y Grupo Roche Syntex de México, México, p 40
Alonso-Castro AJ, Miranda-Torres AC, González-Chávez MM, Salazar-Olivo LA (2008) Cecropia obtusifolia Bertol and its active compound, chlorogenic acid, stimulate 2-NBDglucose uptake in both insulin-sensitive and insulin-resistant 3T3 adipocytes. J Ethnopharmacol 120:458–464
Andrade-Cetto A, Wiedenfeld H (2001) Hypoglycemic effect of Cecropia obtusifolia on streptozotocin diabetic rats. J Ethnopharmacol 78:145–149
Argueta A, Cano L, Asselein L, Rodarte ME (1994) Atlas de las Plantas de la medicina tradicional mexicana. Instituto Nacional Indigenista (INI) II, México, pp 706–707
Armstrong GM, Rice LMR, Wender SH (1970) The effect of nitrogen deficiency on the concentration of caffeoylquinic acids and scopolin in tobacco. Phytochemistry 9:945–948
Bernards MA, Ellis BE (1991) Phenylalanine ammonia-lyase from tomato cell cultures inoculated with Verticillium albo-atrum. Plant Physiol 97:1494–1500
Brennan T, Frenkel C (1977) Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol 59:411–416
Briskin DP, Gawienowski MC (2001) Differential effects of light and nitrogen on production of hypericins and leaf glands in Hypericum perforatum. Plant Physiol Biochem 39:1075–1081
Briskin DP, Leroy A, Gawienowski M (2000) Influence on the production of hypericins by St John’s wort. Plant Physiol Biochem 38:413–420
Bryant JP, Chapin FS III, Klein DR (1983) Carbon nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357–368
Cabrera HM (2002) Ecophysiological responses of plants in ecosystems with Mediterranean-like climate and high mountain environments. Rev Chil His Nat 75:625–637
Cai Y, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74:2157–2184
Clé C, Hill LM, Niggeweg R, Martin CR, Guisez Y (2008) Modulation of chlorogenic acid biosynthesis in Solanum lycopersicum: consequences for phenolic accumulation and UV-tolerance. Phytochemistry 69:2149–2156
Coviella CE, Stipanovic RD, Trumble JT (2002) Plant allocation to defensive compounds: interactions between elevated CO2 and nitrogen in transgenic cotton plants. J Exp Bot 53(367):323–331
Dixon RA, Paiva NL (1995) Stress induced phenylpropanoids metabolism. Plant Cell 7:1085–1097
Fritz C, Palacios N, Fiel R, Stitt M (2006) Regulation of secondary metabolism by the carbon–nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoids metabolism. Plant J 46:553–548
Grace SC, Logan BA (2000) Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos Trans R Soc Lond B Biol Sci 355:1499–1510
Gray DE, Pallardy SG, Garret HE, Rottinghauss GE (2003) Effect of acute drought stress and time of harvest on phytochemistry and dry weight of St. John’s wort leaves and flowers. Planta Med 69:1024–1030
Grechi I, Vivin Ph, Hilbert G, Milin S, Robert T, Gaudillère JP (2007) Effect of light and nitrogen supply on internal C:N balance and control of root-to-shoot biomass allocation in grapevine. Environ Exp Bot 59:139–149
Gutiérrez-Domínguez MA, Betancourt-Aguilar Y (2008) El mercado de plantas medicinales en México: situación actual y perspectivas de desarrollo. http://www.geocities.com/redmexicana/colombia.doc (accessed July 2009)
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetic and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hemm MR, Rider SD, Ogas J, Murry DJ, Chapple C (2004) Light induces phenylpropanoids metabolism in Arabidopsis roots. Plant J 38:765–778
Heo HJ, Jun Kim Y, Chung D, Kim D (2007) Antioxidant capacities of individual and combined phenolics in a model system. Food Chem 104:87–92
Herrera-Arellano A, Aguilar-Santamaría L, García-Hernández B, Nicasio-Torres P, Tortoriello J (2004) Clinical trial of Cecropia obtusifolia and Marrubium vulgare leaf extracts on blood glucose and serum lipids in type 2 diabetics. Phytomedicine 11:561–566
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular 347. University of California Agricultural Experiment Station, Berkeley, pp 1–32
Horton P (2000) Prospects for crop improvement through the genetic manipulation of photosynthesis: morphological and biochemical aspects of light capture. J Exp Bot 51:475–485
Jaakola L, Määttä-Riihinen K, Kärenlampi S, Hohtola A (2004) Activation of flavonoid biosynthesis by solar radiation in bilberry (Vaccinium myrtillus L.) leaves. Planta 218:721–728
Jansen MAK, Hectors K, O’Brien NM, Guisez Y, Potters G (2008) Plant stress and human health: do human consumers benefit from UV-B acclimated crops? Plant Sci 175:449–458
Khan MH, Panda SK (2008) Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol Plant 30:81–89
Ko FN, Chu CC, Lin CN, Chang CC, Teng CM (1998) Isoorientin-6”-O-glucoside, a water-soluble antioxidant isolated from Gentiana arisanensis. BBA 1389:8190. (Abstract)
Konieczny R, Libik M, Tuleja M, Niewiadomska E, Miszalski Z (2008) Oxidative events during in vitro regeneration of sunflower. Acta Physiol Plant 30:71–79
Kováčik J, Bačkor M (2007) Phenylalanine ammonia-lyase and phenolic compounds in Chamomile tolerance to cadmium and copper excess. Water Air Soil Pollut 185:185–193
Laloi C, Przybyla D, Apel K (2006) A genetic approach towards elucidating the biological activity of different reactive oxygen species in Arabidopsis thaliana. J Exp Bot 57(8):1719–1724
Le Bot J, Bénard C, Robin C, Bourgaud F, Adamowicz S (2009) The ‘trade-off’ between synthesis of primary and secondary compounds in young tomato leaves is altered by nitrate nutrition: experimental evidence and model consistency. J Exp Bot 60(15):4301–4314
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6:280–287
Marcelis LFM, Heuvelink E (2007) Concepts of modeling carbon allocation among plant organs. In: Vos J, Marcelis LFM, Visser PHB, Struik PC, Evers JB (eds) Functional–structural plant modelling in crop production, 1st edn. Springer, Berlin, pp 103–111
McNally DJ, Wurms KV, Labbé C, Quideau S, Bélanger RR (2003) Complex C-glicosyl flavonoid phytoalexins from Cucumis sativus. J Nat Prod 66:1280–1283
Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM (2007) Nitrate transport and signaling. J Exp Bot 58(9):2297–2306
Mosaleeyanon K, Zobayed SMA, Afreen F, Kozai T (2005) Relationships between net photosynthetic rate and secondary metabolite contents in St. John’s wort. Plant Sci 169:523–531
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nicasio P, Aguilar S, Aranda E, Ortíz S, González M (2005) Hypoglycemic effect and chlorogenic acid content in two Cecropia species. Phytother Res 19:661–664
Nicasio-Torres P, Erazo-Gómez JC, Cruz-Sosa F (2009) In vitro propagation of two antidiabetic species known as guarumbo: Cecropia obtusifolia and Cecropia peltata. Acta Physiol Plant 31(5):905–914
Osuna L, Mora A, Ventura E, Jiménez E, Bazaldúa C, Jiménez A (2008) Micropropagation of Aristolochia elegans (Mast.). J Crop Sci Biotech 10(3):141–146
Palumbo MJ, Putz FE, Talcott ST (2007) Nitrogen and gender effects on the secondary metabolism of yaupon, a caffeine-containing North American holly. Oecologia 151:1–9
Poorter H, Pepin S, Rijkers T, Jong Y, Evans JR, Körner C (2006) Construction costs, chemical composition and payback time of high- and low-irradiance leaves. J Exp Bot 57(2):355–371
Raskin I, Ribnicky DM, Komamytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N, O’Neal JM, Comwell T, Pastor I, Friendler B (2002) Plants and human health in the twenty-first century. Trends Biotechnol 20:522–531
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9/10):1231–1237
Revilla-Monsalve MC, Andrade-Cetto A, Palomino-Garibay MA, Wiedenfeld H, Islas-Andrade S (2007) Hypoglycemic effect of Cecropia obtusifolia Bertol aqueous extract on type 2 diabetic patients. J Ethnopharmacol 111:636–640
Rodríguez de Sotillo DV, Hadley M (2002) Chlorogenic acid modifies plasma and liver concentrations of cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J Nutr Biochem 13:717–726
Ryser P, Eek L (2000) Consequences of phenotypic plasticity vs. interspecific differences in leaf and root traits for acquisition of aboveground and belowground resources. Am J Bot 87(3):402–411
Sakihama Y, Cohen MF, Grace SC, Yamashi H (2002) Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology 177:67–80
Silva EM, Souza JNS, Rogez H, Rees JF, Larondelle Y (2007) Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem 101:1012–1018
Street RA, Van Staden SJ (2008) South African traditional medicinal plant trade- challenges in regulating quality, safety and efficacy. J Ethnopharmacol 119:705–710
Takei K, Takahashi T, Sugiyama T, Yamaya T, Sakakibara H (2002) Multiple routes communicating nitrogen availability from roots to shoots: a signal transduction pathway mediated by cytokinin. J Exp Bot 53(370):971–977
Tewari RK, Kumar P, Sharma PN (2006) Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Sci Hortic 108:7–14
Urbanczyk-Wochniak E, Fernie AR (2005) Metabolic profiling reveals altered nitrogen nutrient regimes have diverse effects on the metabolism of hydroponically-grown tomato (Solanum lycopersicum) plants. J Exp Bot 56(410):309–321
Wilson JB (1988) A review of evidence on the control of shoot:root ratio in relation to models. Ann Bot 61:433–449
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223
Acknowledgments
This research was partially supported by a scholarship from the Consejo Nacional de Ciencia y Tecnología-México (CONACyT-México) (CVU 42514) and from the Instituto Mexicano del Seguro Social (IMSS) (99183755).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Z.-L. Zhang.
Rights and permissions
About this article
Cite this article
Mora Izquierdo, A., Nicasio Torres, M.d.P., Sepúlveda Jiménez, G. et al. Changes in biomass allocation and phenolic compounds accumulation due to the effect of light and nitrate supply in Cecropia peltata plants. Acta Physiol Plant 33, 2135–2147 (2011). https://doi.org/10.1007/s11738-011-0753-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0753-5