Abstract
Fungal endophytes are the subject of intense research due to their plant growth-promoting properties and production of plant growth regulators (PGRs). This study evaluated the potential synergistic interaction between indole-3-acetic acid (IAA) and crude fungal extract (CFE) derived from Rhizopus oryzae (coriander roots isolate), on 24 growth attributes in spinach. Coriander roots were investigated for fungal endophytes, the isolate was assessed for IAA production, and the CFE was examined for plant growth-promoting properties. Groups of spinach plants were aerially sprayed with CFE or IAA separately and concurrently every week for 42 days in a research greenhouse. In total, 15/24 vegetative and 9/24 physiochemical plant traits were monitored for improvements. Morphological identification of Rhizopus oryzae was confirmed through 18S sequencing and was registered for 35.2 mg L−1 of IAA in culture. Among the three CFE treatments (CFE-100, CFE-250, CFE-500), only CFE-500 exhibited bioactivities and promoted 15/24 growth attributes. Similarly, IAA alone improved 19/24, while concurrent treatment with CFE and IAA interactively affected 23/24 plant features. Briefly, 18/24 synergistic, 3/24 additive, and 3/24 antagonistic interactions were registered between CFE and IAA. Taken together, CFE exhibited growth-promoting properties when used individually but synergistically improved 18/24 plant traits when applied concurrently with IAA. It is obvious that the isolate is a potential biofertilizer and industrial organism and may be trial inoculated to variety of plant roots for enhancing agricultural productivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sustainable agricultural productivity is desirable to meet the food demand of increasing global population. Currently, huge quantities of organic and inorganic fertilizers are used by the farmers to enhance crop growth and yield. However, long-term application of synthetic fertilizers influences the soil negatively and ultimately contributes to the soil pollution. Therefore, agriculturists are now turning to adapt eco-friendly biological fertilizers (Suebrasri et al. 2020). Certain fungi establish a mutually beneficial association with plants without causing disease symptoms and are called endophytes. This is an extreme adaptation where the fungus resides inside the plant tissues, protected from environmental stresses like mycoparasites, rival microbes, direct sunlight, UV radiation, extreme temperatures, and desiccation. In this safe ecological niche, the symbiont receives minerals and organic nutrients (e.g., carbohydrates, proteins, vitamins, organic acids) and in return prepare host-specific active metabolites that assist the host in its physiological functions (Baron and Rigobelo 2022; Roberts 2022). The endophytes enable the host to resist biotic (birds, insects, and mammals) and abiotic (drought, salt, and heat) stresses (Hussain et al. 2020a; Turbat et al. 2020). On average, every plant harbors more than one endophyte, and therefore over one million fungal strains may be associated with 300,000 higher plants (Fouda et al. 2015).
Auxins, abscisic acid, gibberellins, cytokinins, and ethylene are important plant hormones regulating almost every aspect of plant growth and development. Production of plant hormones is the most important attribute associated with fungal endophytes (Jahn et al. 2021; Hori et al. 2021). Auxin or indole-acetic-acid (IAA) is considered the principal plant hormone involved in plant growth and development. It is a multifunctional PGR, controlling cell division, elongation, differentiation, phototropic and geotropic responses, flower formation, fruit ripening, senescence, and apical dominance (Pradhan and Tayung 2019). Auxin is a signaling molecule and affects the expression of many genes that are involved in the physiological process of plant growth and development. Therefore, IAA producer endophytes have received much attention recently (Zhang et al. 2022; Jahn et al. 2021).
Medicinal plants offer a huge repository of fungal endophytes and need to be fully explored for unique strains with biotechnological features. Coriander (Coriandrum sativum Linn) is a well-known medicinal, spice, and flavoring crop, possessing medicinal and nutritional values. It belongs to the Apiaceae family and is widely used for treating various health issues (Aftab et al. 2020). Coriander was selected for this study because of efficient growth under a variety of environments and stressed conditions (Khodadadi et al. 2017). Based on current literature, there was no study on the fungal endophytes associated with coriander roots and their potential role in plant growth promotion. The growth-promoting properties of the fungal isolate were assessed using Spinach (Spinacia oleracea L.) plant.
The effects of growth-promoting endophytes and synthetic plant hormones on plant growth and development have gained considerable attention and are under intense research recently (Turbat et al. 2020; Rabiei et al. 2020; Hussain et al. 2020a). The idea of improved plant growth and development through combination of two or more synthetic plant hormones is well established in plant sciences (El-Karamany et al. 2019). However, reports on the combined effect of synthetic plant hormones and extract derived from growth-promoting fungal endophytes are limited. Compared to fungus inoculum, CFE application has the advantage to be prepared on large scale industrially, by culturing the most efficient endophytic strain for extraction. Interaction between CFE and synthetic PGRs may produce synergetic, additive, or antagonist effects on plant growth and development. The current study was aimed (1) to characterize fungal endophytes isolated from coriander roots for IAA production and (2) to assess the growth-promoting properties of CFE derived from the isolate singly and (3) potential synergistic effect of CFE by applying concurrently with IAA in spinach. Similarly, the level of growth-promoting activities in CFE will be used to (4) hypothesize industrial potential of the strain.
2 Materials and Methods
2.1 Plant Collection and Sampling Area
Plant collection was made from 5 different points at Peshawar (34° 1′ 33.3012″ N and 71° 33′ 36.4860″ E) KPK (Khyber Pakhtunkhwa), Pakistan, in the months of July and August 2019. Ten young, healthy, and fresh whole plants from each point were collected in sterile plastic bags and transferred to the Biotechnology Laboratory at Sarhad University Peshawar, Pakistan. Plant samples were stored at 4°C until further procedures, the next day. Randomly, 1 plant from each point was autoclaved to be used as negative control.
2.2 Surface Sterilization
Plant samples were prepared for inoculation as described by Turbat et al. (2020). Clay particles were removed by washing the plant thoroughly with running tap water and then rinsing thrice with sterilized dH2O. Roots were separated from the whole plant with a sterilized blade and cut into 1-cm-long sections for inoculation. Five root sections from each area were prepared which collectively resulted in 25 inoculations. Each section was surface sterilized by immersing in 70% ethanol for 1 min, followed by immersion in 2% sodium hypochlorite for 5 min and soaked again in 70% ethanol for ½ min. Sterilized root sections were rinsed thrice with autoclaved dH2O and wrapped in sterilized filter paper for 6 h. To check the success of sterilization, water from the last washing step was collected in a disinfected beaker and streaked on potato dextrose agar (PDA) plate for fungal growth.
2.3 Isolation of Endophytic Fungi
Root sections were carefully placed on Sabouraud Dextrose Agar plates (supplemented with 50 µg ml−1 of penicillin-G) with sterilized forceps and incubated at 27°C for 7 days. The growth was checked daily until the fungal colonies appeared on the plates. Percent frequency of colonization was determined by using Eq. 1 (Mehmood et al. 2018).
Fungal colonies having similar shape and color were sub-cultured on PDA slants. The re-culturing was repeated multiple times until the pure culture was obtained. Preliminary identification of the isolate was performed through microscopic study.
2.4 Molecular Characterized
Phenol/chloroform/iso-amyl alcohol (PCI) method described by Gul et al. (2017) and Katoch et al. (2017) was used to extract genomic DNA. Fungal isolate was identified by sequencing the ITS region of 18S DNA. The target gene was amplified using the primers NS1 5′ (GTA GTC ATA TGC TTG TCT C) 3′ and NS8 5′ (TCC GCA GGT TCA CCT ACG GA) 3′. Polymerase chain reaction was performed in a 50 μl reaction mixture containing 27.5 μl master mix, 13.5 μl deionized water, 1 μl of each primer, and 7 μl of genomic DNA. For amplification, thermal cycler (T100 Bio-Rad, USA) was initially set at 94°C for 3 min to activate Taq polymerase. This was followed by 35 cycles of 94°C (1 min for denaturation), followed by 55°C (30-s primer annealing), 72°C (1 min for extension), and final step of 72°C for 10 min. Services of MS Macrogen Korea were obtained for sequencing and purification of amplified DNA (size 1757 nucleotides). Sequencing errors were determined and cleaned with Finch TV (Version 1.4.0), while alignment was performed with Codon Code Aligner (trial version V. 8.0.2). To find out the identity values, the sequence was analyzed and compared with the sequences present in the GenBank database using BLAST tools from the NCBI website. Phylogenetic tree was constructed using MEGAX32 software and by applying neighbor-joining method with a confidence level of 1000 repeat bootstrap value. The sequence was deposited into the NCBI GeneBank under the Accession No MW331683.
2.5 IAA Quantification
IAA produced in the culture was qualitatively detected (Suebrasri et al. 2020) and then quantified using spectrophotometric methods of Suebrasri et al. (2020) with minor modifications. The quantification was made using standard curve technique. Ten dilutions (5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 µg ml−1) of IAA were prepared from stock solution in ethanol. Five milliliters from culture was centrifuged at 8000 rpm for 5 min at RT. One milliliter from each supernatant and standard IAA solution was mixed with 2 ml of Salkowski’s reagent and kept in dark for 120 min. Optical density was read at 530 nm and the standard curve used for IAA quantification. The cultures were filtered through sterilized filter paper (Whatman No. 1), and the culture filtrate (CF) was subjected to HPLC with a UV detector set at 280 nm and column size C18 (5 μm; 25 × 0.46 cm).
2.6 Extract Preparation and Greenhouse Experiment
The isolate was cultured in Erlenmeyer flasks (250 ml) containing potato dextrose broth (150 ml), by inoculating a 5-mm plug, taken from PDA culture. The culture was allowed to grow at 27°C for 7 days. The mycelia were crushed with glass spheres and separated through vacuum filtration, with 0.45-μm filter paper. The filtrate was then extracted with 50 ml of ethyl-acetate (1:1 ratio) using a separating funnel. The extract was subjected to reduced pressure using rotary vacuum evaporator at 40°C and 20 mbar, until concentrated to semisolid crude form (Suebrasri et al. 2020; Turbat et al. 2020). Plant growth-promoting activity of CFE alone and synergistic effect in combination with IAA were assessed in an experimental greenhouse using spinach plant. The focused plant characters included whole plant lengths (WPLs), shoot lengths (SLs), root lengths (RLs), number of leaves/plant (NOL/P), leaf blade lengths (LBLs), leaf blade widths (LBWs), flag leaf area by manual method (FLAM), flag leaf area by camera scanner (FLACS), total soluble protein (TSP), total soluble sugar (TSS), fresh shoot weight (FSW), dry shoot weight (DSW), shoot water contents (SWC), fresh root weight (FRW), dry root weight (DRW), root water contents (RWCs), root/shoot ratio (R/S), chlorophyll a (Chl-a), chlorophyll b (Chl-b), total chlorophyll (Chl-t), total carotenoids (TCar), β-carotene (βCar), and xanthophyll (Xan).
Flat-leafed spinach seeds were kindly obtained from PCSIR Laboratory Peshawar, Pakistan. The experiment was carried out in randomized complete block design, comprised of 6 treatments weekly for 4 weeks as CFE-100, CFE-250, and CFE-500 (received 100, 250, and 500 mg L−1 of CFE, respectively); IAA-25 (received 25 mg L−1 of IAA); CFE-250+IAA-25 (received combination of 250 mg L−1 CFE and 25 mg L−1 IAA); and N-control (received sterilized dH2O only). A total of 120 healthy seeds were selected and sterilized as described above (Section 2.2.). The disinfected seeds were placed evenly on water soaked sterilized filter paper in Petri plates (10 seeds in each) and allowed to grow for 7 days at 25°C in the dark (Gong et al. 2021). Equal sized 6 germinated seeds were transferred into 500-ml plastic pots, packed uniformly with 500 g of soil. Cultivated layer of soil (0–20 cm) from a clean pristine site was used for pot packing. To prepare a homogenized soil sample, unnecessary pebbles were removed and sieved with 2-mm sieve. To prevent unwanted weed growth and destroy phytopathogenic spores, the soil was sterilized by autoclaving at 121° C for 15 min. Textural class of pot soil was checked and was found loamy with 35% silt, 28% sand, and 37% clay. Important soil features were assessed and found as cation exchange capacity (mEq) = 41.5, electrical conductivity = 1080, organic matter = 3.2, organic carbon = 1.6, and pH = 6.7.
After the seedlings successfully established roots (3 days), the density was reduced to 3 healthy plantlets per pot and shifted to greenhouse. Cultivated plantlets were allowed to grow at 8–20°C, 60–80% humidity, and 14-h illumination (8000 lux) period and watered with deionized water, as described by Gong et al. (2021). Thereafter, plantlets in each pot were aerially sprayed with 100, 250, and 500 mg L−1 (prepared in dH2O) of CFE and 25 mg L−1 IAA separately and 25 mg L−1 IAA combined with 250 mg L−1 CFE. Depending on the age and size of the plantlet, 5–10 ml (approximately) of each solution was used on the plantlet in the pot, using a hand pump. Negative control received autoclaved dH2O. During the experimental period, all groups of plants were irrigated twice a day with a fixed amount (30 ml) of autoclaved tap water. On the 42nd day of experiment, the plants were harvested and assessed for vegetative and physiochemical features. Cumulative time duration from seed selection to grownup plants was 52 days in total.
Both treated and control groups were carefully uprooted from the pot. Soil particles were removed by rinsing the whole plant slowly with tap water. To minimize root damage, a delicate paint brush was used to clean roots. All plants were blotted dry by mildly pressing between the paper towels for ½ min. Keeping record of each group, lengths of whole plant, roots, and shoots were measured with a ruler. Roots and shoots (before the individual leaves separated) were then separated, and fresh weights of whole plant and separated parts were measured with a digital balance. Three leaves from each group were randomly selected for chlorophylls, carotenoids, soluble sugar, and soluble protein investigations. The remaining leaves and roots were dried for 48 h at 75°C in a hot air oven and analyzed for dry weights. Root to shoot ratio was determined simply by dividing root dry weight by shoot dry weight. Total water content in the whole plant, shoots, and roots was calculated with Eq. 2 (Bueno and Cordovilla 2021).
where WCPP is water content in plant part, FW fresh weight, and DW dry weight.
The number of leaves per plant in each group was counted. Flag leaf area was calculated manually, as well as with Easy Leaf Area Software using scanner camera (Cam Scan) in cell phone (Samsung, J7 prime) following the stepwise instructions provided by the App makers.
2.7 Estimation of Chlorophyll a and b and Total Chlorophyll
Rabiei et al. (2020) were followed to assess chlorophyll a (Chl-a), chlorophyll b (Chl-b), and total chlorophyll (Chl-T) in spinach leaves treated with CFE singly and in combination of CFE with IAA. One gram of healthy fresh leaf was crushed finely with mortar and pestle and extracted with 10 ml of acetone and water mixture (4:1) by shaking for 2 min. After homogenization, the samples were centrifuged (5000 rpm) for 10 min at 20°C. Supernatants were subject to spectrophotometry (Hitachi U-2001 Japan) at 652 and 665.2 nm. The levels of Chl-a, Chl-b, and Chl-T were calculated with Eqs. 3, 4, and 5, respectively (Arnon formulas).
2.8 Estimation of Carotenes and Carotenoids
Β-carotene (βCar), xanthophyll (Xan), and total carotenoids (TCar) were extracted with an extraction mixture containing 4 parts acetone and 1 part water (as described for chlorophyll pigments in Section 2.11). For β-carotene and total carotenoids, the absorption was read at 453, 470, 505, 645, and 663 nm and calculated using Eqs. 6 and 7 (Branisa et al. 2014).
Xanthophyll was estimated by subtracting beta-carotene from total carotenoids.
2.9 Total Soluble Protein (TSP) and Total Soluble Sugar (TSS) Contents
Fresh whole plant (500 mg) was crushed in liquid nitrogen and homogenized in 10 ml of potassium-phosphate-buffer (pH 7.5). After centrifugation (2000 rpm) for 15 min at 4°C, the supernatant was mixed with Bradford reagent (500 ml) and allowed to react for 25 min at room temperature (25±2). The absorbance was read at 595 nm, and protein level was measured (μg g−1), by using standard curve, prepared with bovine serum albumin (Aslam et al. 2016). To determine soluble sugar, 500 mg of fresh whole plant was crushed in 5 ml of 80% ethanol at 80°C in a water bath. After centrifugation (5000 rpm) for 15 min, the supernatant was separated, and the residue was re-extracted with 5 ml of 80% ethanol. The two extractions were mixed and diluted to 50 ml. One milliliter from each sample was mixed with 5 ml of anthrone-sulfuric-acid reagent and measured at 627 nm after cooling to room temperature (Qi et al., (2020).
2.10 Statistical Analysis
Microsoft Excel sheet and GraphPad Prism (version 5.03) were used for statistical analysis. Colonization frequency, total water content, Chl-a, Chl-b, Chl-T, βCar, TCar, and Xan were calculated with Microsoft Excel sheet using Eqs. 1, 2, 3, 4, 5, 6, and 7, respectively. Synergistic effects were calculated by comparing the sum of changes (%) due to application of 250 mg L−1 CFE and 25 mg L−1 IAA separately, with the changes (%) occurring due to 250 mg and IAA collectively. For descriptive analysis, two-way ANOVA was applied. The data for vegetative and physiochemical characters was measured in triplicate.
3 Results
3.1 Colonization and Identification
We observed 13 identical colonies on different culture plates. The colonization frequency was 52%. Based on macroscopic (colony) and microscopic (mycelial) features, a single isolate was identified from root system of coriander. The identity of fungal endophyte was confirmed from 18S gene sequencing, where the strain showed 99.5% homology (Fig. 1) to the sequence of Rhizopus oryzae (Accession No gi: 106364874), downloaded from NCBI database. The isolate belonged to Genus Rhizopus, class Zygomycete, phylum Zygomycota, and Kingdom Eukaryota.
3.2 IAA Detection and Quantification
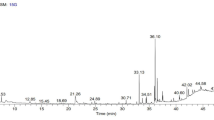
Formation of characteristic pink color with addition of Salkowski’s reagents confirmed the presence of auxin in culture supernatant. Quantitative assessment revealed 35.2 mg L−1 of IAA in Potato Dextrose Broth-based culture filtrate. The R square value of the standard curve was 0.9843. Production of IAA was further confirmed with HPLC-MS/MS analyses using the Multiple Reaction Monitoring (MRM) model.
3.3 Green House Experiment
Figures 2 and 3 present the average effects of each treatment on growth, Table 1 on biomass, Fig. 4 on biochemical parameters, and Table 2 on the physiological features of spinach. Weekly application of CFE or IAA separately and in combination (CFE-250+IAA-25) for 42 days in a research greenhouse influenced 14/15 vegetative (Fig. 2a, b, c, and d; Fig. 3a, b, c, and d; and Table 1) and 9/9 physiochemical (Fig. 4a and b and Table 2) features in spinach.
Effects of various treatments on sizes of whole plant (a), shoot (b), root (c), and number of leaves per plant (d). *** indicate significance at p < 0.001, ** indicate significance at p < 0.01; ns, not significant; CFE, crude fungal extract; IAA, indole-3-acetic acid; CFE-100, received 100 mg L−1 of extract; CFE-250, received 250 mg L−1 of extract; CFE-500, received 500 mg L−1 of extract; IAA-25, received 25 mg L−1 of IAA; CFE-250+IAA-25, received 250 mg L−1 of extract; and 25 mg L−1 of IAA, N-control, received sterilized dH2O
Effects of various treatments on leaf lengths (a), widths (b), and flag leaf area (FLA) by manual method (c) and by Easy Leaf Area Scanner software (d). *** indicate significance at p < 0.001, * indicate significance at p < 0.05; ns, not significant; CFE, crude fungal extract; IAA, indole-3-acetic acid; CFE-100, received 100 mg L−1 of extract; CFE-250, received 250 mg L−1 of extract; CFE-500, received 500 mg L−1 of extract; IAA-25, received 25 mg L−1 of IAA; CFE-250+IAA-25, received 250 mg L−1 of extract; and 25 mg L−1 of IAA, N-control, received sterilized dH2O
Effects of various treatments on total soluble protein (a) and total soluble sugar (b) in spinach. *** indicate significance at p < 0.001; ns, not significant; CFE, crude fungal extract; IAA, indole-3-acetic acid; CFE-100, received 100 mg L−1 of extract; CFE-250, received 250 mg L−1 of extract; CFE-500, received 500 mg L−1 of extract; IAA-25, received 25 mg L−1 of IAA; CFE-250+IAA-25, received 250 mg L−1 of extract; and 25 mg L−1 of IAA, N-control, received sterilized dH2O
Among the three CFE treatments (CFE-100, CFE-250, CFE-500), only CFE-500 showed significant bioactivity and improved 15/24 (8/15 vegetative and 7/9 physiochemical) plant attributes during the course of study. Four vegetative (FSW, DSW, FRW, and DRW) and 4 physiochemical traits (Chl-a, Chl-b, Chl-T, and Xan) were improved at p < 0.05, 2 physiological (Chl-a/b and TCar) at p > 0.01 while six (WPL, SLs, LBLs, FLAM, FLACS, and TSPs) at p < 0.001. Compared to N-control, the maximum increase (109.4%) was noted in Chl-b.
Similarly, the P-control (25 mg L−1 of IAA) improved 19/24 plant features when aerially sprayed for 42 days. Statistics of our data show that 7/15 vegetative and 5/9 physiochemical plant traits improved at p < 0.05, 3 vegetative and 3 physiochemical at p < 0.01, and 1 physiochemical plant character at p < 0.001.
The highest improvements were registered in plants treated concurrently with CFE and IAA compared to either component alone. Out of 24 plant traits, combined application (CFE-250+IAA-25) promoted 23 characters in spinach. Moreover, co-application of extract and IAA promoted 2/15 vegetative and 1/9 physiochemical at p < 0.05, 3 vegetative and 3 physiochemical at p < 0.01, and 9/15 vegetative and 5/9 physiochemical plant trait at p < 0.001.
3.4 Interaction of CFE with IAA
Interactive effects due to combined application of CFE and IAA were calculated using percent improvement in each plant character (Table 3). Compared to the effects of IAA (41.03%) and CPE (15.21%) on WPLs separately, the growth improved synergistically to 77.61% when the plant was treated concurrently with CPE and IAA. Similar synergisms were also registered for SLs, RLs, LBLs, LBWs, FLAM, FLACS, FSW, DSW, FRW, TSP, TSS, Chl-b, TCar, Chl-t, βCar, and Xan. On average, 17/24 characters were synergistically improved, 4 showed additive (SWC, DRW, RWC, Chl-a,) properties, while 3 were recorded for antagonistic (NOL/P, R/S, Chl-a/b) effects. Maximum synergism was recorded for TSP and TSS by showing 208% and 321% increases, compared to expected additive effects.
4 Discussion
Medicinal plants offer a huge repository of endophytes that may produce novel metabolites of pharmaceutical, industrial, and agricultural importance. Production of PGRs is a unique feature and notable example of bioactive metabolites associated with fungal endophytes (Mehmood et al. 2018). We isolated an important filamentous fungus R. oryzae from the roots of coriander which is well documented for the production of enzymes, lipids, ethanol, pigments, polyphosphates, chitin/chitosan, and acidic metabolites (Dzurendova et al. 2021; Yin et al. 2020; Lopez-Fernandez et al. 2020; Benabda et al. 2019). To our knowledge, this is the first report about the isolation of R. oryzae from the roots of coriander. Previously, R. oryzae was also reported from the roots of Artemisia nilagirica (Myrchiang et al. 2014), Crocus sativus (Chamkhi et al. 2018), Helianthus annuus, and Glycine max (Hussain et al. 2020b).
Microbial endophytes help their host plant by protecting against environmental stresses (biotic and abiotic) and promote growth by producing a variety of metabolites. Of the many plant hormones, fungal IAA is considered the most important plant hormone involved in promoting plant growth and development (Pradhan and Tayung 2019). In this regard, a large number of fungal strains have been investigated for IAA production recently (Jahn et al. 2021; Suebrasri et al. 2020; Pradhan and Tayung 2019; Mehmood et al. 2018). The current isolate (R. oryzae) was evaluated and confirmed for remarkable quantities of extracellular IAA (35.2 mg L−1) on 7th day of inoculation. In similar investigations, Turbat et al. (2020) reported 15 fungal isolates belonging to the genera Alternaria, Didymella, Fusarium, and Xylogone for IAA production. Fouda et al. (2015) recorded IAA production by Penicillium chrysogenum, Alternaria alternata, and an unidentified fungus they call sterile hyphae from Asclepias sinaica. In another study, Jagannath et al. (2019) isolated Cymbidium aloifolium, from an epiphytic orchid, and characterized it for 10.7±1.97 mg L−1 of IAA.
Interaction among biological molecules is an established phenomenon in nature (Limo et al. 2018). The outcome of molecular interaction may be synergistic, antagonistic, or additive (Huang and Falco 2021). Synergism is the desired property when observed among therapeutic or agronomic molecules. Researchers in health sciences employ combinatorial therapies (drug-drug, herb-drug, and herb-herb) to achieve better curative effects, dosage compliance, and extended spectrum of action (Moukette et al. 2017; Yuan et al. 2017). In a similar way, agronomists combine various plant growth-promoting entities to synergize agricultural productivity and quality of food. The major combinations used in agronomic researches include phytohormones-phytohormones (Zhang et al. 2022; Hussain et al. 2020a; El-Karamany et al. 2019), various pesticides (Han et al. 2020), phytohormones with plant-extract (Aslam et al. 2016), pesticide-CFE (Patel et al. 2020), phytohormone-CFE (Turbat et al. 2020), microorganism-microorganism (Xie et al. 2021; Hori et al. 2021), microorganism with plant-extract (Suriani et al. 2021), and hydrolysate from several rhizobacteria (Redondo-Gomez et al. 2022). For the current research, a new idea was proposed to assess the combined performance of phytohormones (IAA) with CFE (from auxin producer endophyte) on plant growth and development.
In plant-endophyte association, PGRs are directly secreted into the plant tissues where growth signals are initiated for biological processes. Published data show that cultured endophytes secret extracellular PGRs that are accumulated in the growth media and can be extracted with suitable solvents. Therefore, three gradient concentrations of ethyl acetate extract (CFE-100, CFE-250, and CFE-500) were aerially sprayed, and the effects were recorded on 24 plant features. Only 500 mg L−1 of extract displayed bioactivities and produced statistically significant effects on spinach growth. Externally applied hormones are generally absorbed through diffusion across the cuticle and plasma membrane and can easily pass through stomata. The overall impact of CFE-500 was slightly weaker than the effects produced with 25 mg L−1 of IAA. This clearly indicated that the CFE-500 solution had PGRs with potency almost equal to 25 mg L−1 of IAA. It was further noted that CFE-500 improved 8/15 vegetative and 7/9 physiochemical plant characters all at p < 0.05. On the other hand, 100 and 250 mg L−1 of CFE exhibited weak bioactivities, indicating that CFE-100 and CFE-250 solutions had PGRs levels below the threshold concentrations required for triggering biological processes. Analysis of the overall results suggests that the improvement recorded in spinach growth with CFE-500 was due to extracellular IAA and other PGRs produced by endophytic fungus in the culture (Jahn et al. 2021; Suebrasri et al. 2020). The results obtained in this study are in agreement with the findings of Aslam et al. (2016), who observed that Moringa oleifera leaf extract (MOLE) improved Chl-a, Chl-b, Chl-T, and carotenoid contents in spinach plants. Similarly, Jagannath et al. (2019) treated Macrotyloma uniflorum and Vigna unguiculata seeds with culture filtrate from Colletotrichum gloeosporioides and observed significant improvement in seed germination, root lengths, and shoot lengths.
Plant growth-promoting properties of auxins, abscisic acid, gibberellins, cytokinins, and ethylene are well established and are the most used plant hormones in agriculture. Depending on the plant part and the type of receptors, plant hormones control a wide range of plant features involved in overall plant yield. By acting on specific receptor, each plant hormone initiates a specific metabolic pathway for plant growth and development. However, IAA is regarded as the major PGR control and involved in multiple plant growth traits (Pradhan and Tayung 2019). In the current investigation, the positive control group of spinach plants was weekly treated with 25 mg L−1 of IAA for 42 days. The results revealed that treatment with IAA promoted 9/15 vegetative and 9/9 physiochemical traits in spinach plants. Similarly, Hanaa and Safaa (2019) assessed 7 vegetative and 1 physiological traits of wheat plant and registered significant promotion of different plant features after treatments with IAA at different stages of growth. Increases in leaf number, sizes and surface area, fresh and dry weights of plant, photosynthetic pigments, protein, and carbohydrate contents have also been observed by other researchers after foliar application of IAA (Hussain et al. 2020a, Aslam et al. 2016; Suebrasri et al. 2020; Turbat et al. 2020; El-Karamany et al. 2019).
It is well established that combined application of two or more plant hormones improves the overall efficiency of plant growth and development. In this regard, plant growth-promoting properties of various plant hormones like auxin and gibberellic acid (GA3) have been studied extensively. Pradhan and Tayung (2019) reported that GA3 and IAA collectively regulate plant growth and development by triggering cell division, elongation, tissue differentiation, phototropic and geotropic responses, lateral root initiation, flower formation, embryogenesis, fruit ripening, senescence, and apical dominance. Recently, Hussain et al. (2020b) assessed the combined effect of IAA and GA3 in 4 cultivars of pea and recorded significant improvement in 14 growth characters of the plant. In similar study, El-Karamany et al. (2019) noticed that combined application of IAA and GA3 synergistically promoted pods weight/plant, pods No/plant, seeds No/pod, straw yield, plant height, plant biomass, seed biomass, N in seeds (%), protein in seeds (%), and total carbohydrates.
In spite of extensive research on interaction between different plant growth regulators, reports on combined effects of synthetic plant hormones and CFE obtained from growth-promoting fungal endophytes are limited. The ability of CFE to improve growth-promoting properties of synthetic plant hormones might be a new approach that may help to increase agricultural productivity in the future. To assess synergistic effects, 250 mg L−1 of CFE and 25 mg L−1 IAA were collectively applied through aerial spray for the same period of time. Although 250 mg L−1 of CFE was weak bio-stimulant separately, however, concurrent application of CFE-250 with IAA-25 produced synergistic effects on 10/15 vegetative and 7/9 physiochemical plant traits focused in this research. Based on the recorded data, all the three types of interactions (additive, synergistic, and antagonistic) were observed for concurrent application of CFE and IAA. The overall plant growth pattern due to combined treatment (CFE-250+IAA-25) indicated the production of multiple growth-promoting entities by the strain that may have collectively magnified growth effects of IAA. Compared to a mixture of synthetic PGRs, interaction among the crude extract (both fungal and plant) and phytohormones is more complex and difficult to elucidate scientifically (Huang and Falco 2021). However, many bioactives in crude extract and several target proteins initiate a network of reactions, leading to amplification or suppression of metabolic processes. Receptor-mediated interaction between bioactive components and phytohormones may induce or inhibit targeted metabolic enzymes and transporter protein (Zhang et al. 2022; Huang and Falco 2021; Yuan et al. 2017). Fungi are amazing factories of bioactive metabolites. The culture filtrate from fungal isolate generally contains several metabolites, many of which may show bioactivity. Of the 22,500 bioactive metabolites so far obtained from microorganisms, approximately 9000 were originally screened from fungi (Sanchez and Demain 2017). Depending on the types of bioactive metabolites in the culture filtrate and their interaction with targeted protein, the CFE may change the track of plant growth and development. The current synergetic, additive, or antagonistic effects on the growth pattern in spinach may be the results of interaction between IAA and the multiple bioactive components produced by the strain.
Generally, high levels of bioactivities in crude extract can indicate industrial potential of an organism (Dzurendova et al. 2021). The data generated in this research revealed that CFE-250 alongside IAA-25 produced synergistic effects in 18/24 plant growth traits compared to the overall effect of each component. Extract induced synergistic increase in bioactivities indicated the production of novel PGRs in the culture. To effectively use these beneficial PGRs in agriculture, the strain can be a candidate for commercial production of extract in the future.
5 Conclusion
This study revealed that Rhizopus oryzae was the only fungal endophyte isolated from coriander roots where it was benefiting the host by supplying auxin. The strain was confirmed for in vitro production of indole-acetic-acid (IAA), and the extract obtained from culture filtrate exhibited growth-promoting properties in spinach. Concurrent application of crude fungal extract influenced the growth-promoting properties of IAA, which indicated interaction between the bioactive components of extract and synthetic plant hormones. All the 3 types of interactions were registered; however, synergism was the predominant feature recorded for several plant traits, compared to additive or antagonistic effects in this study. High-level synergistic benefits registered for fungal extract suggested the possible industrial role of the strain. The synergistic growth effects noted for fungal extract were not simple to be postulated and need to be analyzed using multifaceted approaches in field application.
Data Availability
All data generated or analysed during this study are included in this published article.
References
Aftab A, Haider MA, Ali Q, Malik A (2020) Genetic evaluation for morphological traits of Coriandrum sativum grown under salt stress. Biol Clin Sci Res J 52:1–7. https://doi.org/10.54112/bcsrj.v2021i1.52
Aslam M, Sultana B, Anwar F, Munir H (2016) Foliar spray of selected plant growth regulators affected the biochemical and antioxidant attributes of spinach in a field experiment. Turk J Agric For 40:136–145. https://doi.org/10.3906/tar-1412-56
Baron NC, Rigobelo EC (2022) Endophytic fungi: a tool for plant growth promotion and sustainable agriculture. Mycology 13:39–55. https://doi.org/10.1080/21501203.2021.1945699
Benabda O, M’hir S, Kasmi M, Mnif W, Hamdi M (2019) Optimization of protease and amylase production by Rhizopus oryzae cultivated on bread waste using solid-state fermentation. J Chem ID 3738181. https://doi.org/10.1155/2019/3738181
Branisa J, Jenisova Z, Porubska M, Jomova K, Valko M (2014) Spectrophotometric determination of chlorophylls and carotenoids. an effect of sonication and sample processing. J Microbiol Biotechnol Food Sci 3:61–64
Bueno M, Cordovilla MdP (2021) Plant growth regulators application enhance tolerance to salinity and benefit the halophyte Plantago coronopus in saline agriculture. Plants 10:1872. https://doi.org/10.3390/plants10091872
Chamkhi I, Sbabou L, Aurag J (2018) Endophytic fungi isolated from Crocus sativus L. (Saffron) as a source of bioactive secondary metabolites. Pharmacogn Mag 10:1143–1148. https://doi.org/10.5530/pj.2018.6.195
Dzurendova S, Losada CB, Dupuy-Galet BX, Fjaer K, Shapaval V (2021) Mucoromycota fungi as powerful cell factories for modern biorefinery. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-021-11720-1
El-Karamany MF, Sadak MS, Bakry BA (2019) Synergistic effect of indole acetic acid and gibberellic acid on mung bean grown under sandy soil conditions. J Applied Sci 19:718–724. https://doi.org/10.3923/jas.2019.718.724
Fouda AH, Hassan SE, Eid AM, Ewais EE (2015) Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.). Ann Agric Sci 60:95–104. https://doi.org/10.1016/j.aoas.2015.04.001
Gong Q, Li Z, Wang L et al (2021) Gibberellic acid application on biomass, oxidative stress response, and photosynthesis in spinach (Spinacia oleracea L.) seedlings under copper stress. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-13745-5
Gul F, Hussain A, Jan G, Hamayun M (2017) Genomic DNA extraction for molecular identification of endophytic fungi: an easy and efficient protocol. Biosci Biotechnol Res Asia 14:667–671. https://doi.org/10.13005/bbra/2492
Han H Jr, GJP, Guo H, Yu Q, Powles SB, (2020) Mechanistic basis for synergism of 2,4-D amine and metribuzin in Avena sterilis. J Pestic Sci 45:216–222. https://doi.org/10.1584/2Fjpestics.D20-028
Hanaa H, Safaa A (2019) Foliar application of IAA at different growth stages and their influenced on growth and productivity of bread Wheat (Triticum aestivum L.). IOP Conf Series: J Phys. https://ui.adsabs.harvard.edu/link_gateway/2019JPhCS1294i2029H/https://doi.org/10.1088/1742-6596/1294/9/092029
Hori Y, Fujita H, Hiruma K, Narisawa K, Toju H (2021) Synergistic and offset effects of fungal species combinations on plant performance. Front Microbiol 12:713180. https://doi.org/10.3389/fmicb.2021.713180
Huang Z, Falco KA (2021) Synergy assessment for plant growth by independent joint action theory. HortScience 56:623–626. https://doi.org/10.21273/HORTSCI15731-21
Hussain A, Mehmood A, Qadir M et al (2020) Thermal stress alleviating potential of endophytic fungus Rhizopus oryzae inoculated to sunflower (Helianthus annuus L.) and soybean (Glycine max L.). Pak J Bot 52:1–10. https://doi.org/10.30848/PJB2020-5(10)
Hussain K, Anwer S, Nawaz K et al (2020) Effect of foliar applications of IAA and GA3 on growth, yield and quality of pea (Pisum sativum L.). Pak J Bot 52:447–460. https://doi.org/10.30848/PJB2020-2(32)
Jagannath S, Konappa NM, Alurappa R, Chowdappa S (2019) Production, characterization of indole acetic acid and its bioactive potential from endophytic fungi of Cymbidium aloifolium L. J Biol Act Prod Nat 9:387–409. https://doi.org/10.1080/22311866.2019.1688684
Jahn L, Hofmann U, Ludwig-Muller J (2021) Indole-3-acetic acid is synthesized by the endophyte Cyanodermella asteris via a tryptophan-dependent and -independent way and mediates the interaction with a non-host plant. Int J Mol Sci 22:2651. https://doi.org/10.3390/ijms22052651
Katoch M, Phull S, Vaid S, Singh S (2017) Diversity, phylogeny, anticancer and antimicrobial potential of fungal endophytes associated with Monarda citriodora L. BMC Microbiol 17:44-56. https://doi.org/10.1186%2Fs12866-017-0961-2
Khodadadi M, Dehghani H, Javaran MJ (2017) Quantitative genetic analysis reveals potential to genetically improve fruit yield and drought resistance simultaneously in coriander. Front Plant Sci 8:568. https://doi.org/10.3389/fpls.2017.00568
Limo MJ, Sola-Rabada A, Boix E et al (2018) Interactions between metal oxides and biomolecules: from fundamental understanding to applications. Chem Rev 118:11118–11193. https://doi.org/10.1021/acs.chemrev.7b00660
Lopez-Fernandez J, Benaiges MD, Valero F (2020) Rhizopus oryzae Lipase, a promising industrial enzyme: biochemical characteristics, production and biocatalytic applications. Catalysts 10:1277. https://doi.org/10.3390/catal10111277
Mehmood A, Hussain A, Irshad M et al (2018) In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis 2018:1–12. https://doi.org/10.1007/s13199-018-0583-y
Moukette BM, Moor VJA, Nya CPB et al (2017) Antioxidant and synergistic antidiabetic activities of a three-plant preparation used in Cameroon folk medicine. Int Sch Res Notices ID 9501675. https://doi.org/10.1155/2017/9501675
Myrchiang P, Dkhar MS, Devi HR (2014) Studies on endophytic fungi associated with medicinally important aromatic plant Artemisia nilagirica (C.B. Clarke) Pamp. and their antagonistic activity against Phytophthora infestans. J Adv Lab Res Biol 5:112-119. Retrieved from https://e-journal.sospublication.co.in/index.php/jalrb/article/view/202
Patel JS, Selvaraj V, Gunupuru LR, Rathor PK, Prithiviraj B (2020) Combined application of Ascophyllum nodosum extract and chitosan synergistically activates host-defense of peas against powdery mildew. BMC Plant Biol 20:113–23. https://doi.org/10.1186/s12870-020-2287-8
Pradhan J, Tayung K (2019) Seeds endophytic fungi of some plant species and their potential for producing indole acetic acid (IAA). Res J Pharm Biol Chem Sci 5:504–514. https://doi.org/10.26479/2019.0503.42
Qi J, Liu W, Jiao T, Hamblin A (2020) Variation in morphological and physiological characteristics of wild Elymus nutans ecotypes from different altitudes in the Northeastern Tibetan Plateau. J Sens 2020: Article ID 2869030. https://doi.org/10.1155/2020/2869030
Rabiei Z, Hosseini SJ, Pirdashti H, Hazrati S (2020) Physiological and biochemical traits in coriander affected by plant growth-promoting rhizobacteria under salt stress. Heliyon 6:05321. https://doi.org/10.1016/j.heliyon.2020.e05321
Redondo-Gomez S, Garcia-Lopez JV, Mesa-Marin J et al (2022) Synergistic effect of plant-growth-promoting rhizobacteria improves strawberry growth and flowering with soil salinization and increased atmospheric CO2 levels and temperature conditions. Agronomy 12:2082. https://doi.org/10.3390/agronomy12092082
Roberts EL. (2022). Plant growth promoting fungal and bacterial endophytes of tall fescue: a review. Grass Research 2:2. https://doi.org/10.48130/GR-2022-0002
Sanchez S, Demain AL (2017) Bioactive products from fungi. J Food Bioact 11:59–87. https://doi.org/10.1007/2F978-3-319-51639-4_3
Suebrasri T, Harada H, Jogloy S, Ekprasert J, Boonlue S (2020) Auxin-producing fungal endophytes promote growth of sunchoke. Rhizosphere 16:100271. https://doi.org/10.1016/j.rhisph.2020.100271
Suriani NL, Suprapta DN, Indrayani AW et al (2021) The synergistic action of three piper plant extracts and biofertilizer for growth promotion and biocontrol of blast disease in red rice. Sustainability 13:10412. https://doi.org/10.3390/su131810412
Turbat A, Rakk D, Vigneshwari A et al (2020) Characterization of the plant growth-promoting activities of endophytic fungi isolated from Sophora flavescens. Microorganisms 8:1–15. https://doi.org/10.3390/2Fmicroorganisms8050683
Xie L, Bi Y, Ma S, Shang J, Hu Q, Christie P (2021) Combined inoculation with dark septate endophytes and arbuscular mycorrhizal fungi: synergistic or competitive growth effects on maize? BMC Plant Biol 21:498–509. https://doi.org/10.1186/s12870-021-03267-0
Yin L, Luo X, Zhang Y, Zheng W, Yin F, Fu Y (2020) Comparative proteomic analysis of Rhizopus oryzae hyphae displaying filamentous and pellet morphology. 3 Biotech 10:469. https://doi.org/10.1007/s13205-020-02458-0
Yuan H, Ma Q, Cui H et al (2017) How can synergism of traditional medicines benefit from network pharmacology? Molecules 22:1135. https://doi.org/10.3390/2Fmolecules22071135
Zhang M, Gao C, Xu L et al (2022) Melatonin and indole-3-acetic acid synergistically regulate plant growth and stress resistance. Cells 11:3250. https://doi.org/10.3390/cells11203250
Acknowledgements
The laboratory facilities provided by the Sarhad University of Science and Technology Peshawar, Pakistan, are highly acknowledged.
Author information
Authors and Affiliations
Contributions
Conceptualization, validation, writing—original draft: Mohammad Parvez. Investigation, methodology, data analysis: Murad Khan, Hira Sajid, and Gul e-Rana. Review and editing: Farrukh Hussain
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parvez, M., e-Rana, G., Hussain, F. et al. Concurrent Application of Indole Acetic Acid and Crude Fungal Extract from Rhizopus oryzae Synergistically Improved Vegetative and Physiochemical Attributes in Spinach. J Soil Sci Plant Nutr 23, 2287–2298 (2023). https://doi.org/10.1007/s42729-023-01179-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01179-6