Abstract
Two novel and potential Schiff base sensor compounds were synthesized by the one pot condensation method starting from 2-hydroxy-1-naphthaldehyde. Both the Schiff bases were characterized by IR, UV,1H-NMR, 13C-NMR and mass spectral studies. The cation sensing expertise of the Schiff bases was evaluated by naked eye, UV–Vis and fluorescence spectroscopic methods in aqueous methanolic medium. The cobalt(II), nickel(II) and copper(II) ions were selectively detected by both the receptors. The detection limits for the ions were found to be in the order of 10−6 M to 10−7 M. Stability constants of the complexes formed by the receptors with the three metal ions were determined by the Benesi–Hildebrand plot method. Stoichiometry of the complexes were ascertained as 1:1 (M:R) by Job’s plot method. Mode of interactions were corroborated by 1H-NMR and IR titrations. The structure of the complexes formed during the recognition process was authenticated by the ESI–mass spectroscopy method. The metal ion sensing property of the receptor is applied in bio imaging studies of human Hela cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synthetic small molecules that induce marked changes in the fluorescence properties which form the basis for determination of analytes may be called fluorescent probes that on account of the strong ability in amelioration of analytical sensitivity and in usage in invivo imaging studies have been extensively investigated and widely used in many fields; of these, fluorescent probes for transition metal ions such as Cu(II) and Co(II) gain importance because of the biological relevance. As a matter of fact high-sensitive probes are required for practical applications in the light of the fact that these metal ions are present in bio systems only at low concentrations.
Generally, Schiff bases possess notable biological activity and are used for complex formation. In recent years research studies on the sensing properties of many Schiff bases are reported. But Schiff base chemosensors recognizing the three main group metals are rare. In the present study we have described the metal detecting sensibility of two Schiff base receptors namely N,N′-Bis(2-hydroxynaphthylidene)-4-methoxyphenylmethanediamine and N,N′-Bis(2-hydroxynaphthylidene)-4-chlorophenylmethanediamine [1] by the colorimetric method, UV–Vis method and fluorescence spectroscopic methods [2]. In day to day life, the use of metals has become an indispensable one. Nevertheless, the use proves to be counter productive should it exceed the limit. In the case of copper which is sine–qua-non for enzyme activities, when the limit of its presence exceeds a certain amount it leads to Wilson’s disease which causes disorders of organs like liver, brown rings in cornea of eyes and ataxia in children [3, 4]. Cobalt, the main component of vitamin B12, performs a crucial role in many biological functions like the synthesis of DNA and also activates several metalloenzymes [5, 6]. However, if it exceeds the limit, it can affect haemoglobin content and sometimes leads to polycythaemia. Excess nickel causes skin diseases. It also interferes in the activities of enzymes like isocitrate dehydrogenase [7]. In the present study, we developed Schiff base based multi-ion metal sensors. The sensing activity was checked with absorption and emission spectra.
Experimental methods
Synthesis of schiff bases
N,N ′-Bis(2-hydroxynaphthylidene)-4-methoxyphenylmethanediamine[BHNMPMD]
Mixture of 2-hydroxy-1-naphthaldehyde (2 mmol),4-chlorobenzaldehyde or 4-methoxybenzaldehyde (1 mmol) (receptor 1/receptor 2) and ammonium acetate(2 mmol) were stirred well in the presence of triethylamine for about 10 min, Yellow colour liquid obtained was dissolved in ethanol and kept overnight [8]. The yellow colour solid formed was separated and washed with diethyl ether and recrystallized from ethanol. M.pt. 178 °C and 228 °C. Yield 98%.
Characterisation of receptor 2
IR (KBr, cm−1): ν(O–H) 3331; ν(C=N) 1621; ν(C–O) 1230; ν(C–H) 752; 1HNMR (300 MHz, DMSO, TMS, ppm): δ 10.71(s, 2H; –OH); δ 9.88(s, 2H; –CH=N); δ 6.71– δ 8.20(m, 16H; Ar–H); δ 5.10(s, CH); δ 3.35(s, OCH3);13CNMR (500 MHz, DMSO, TMS ppm): δ 150.80 (s, C–OH); δ 131.54(s, –CH=N); δ 113.50– δ 131.09(m, Ar–C); δ 75.24(s, CH); 40.10(s, OCH3); UV/Vis (DMSO, nm): λmax 269, 280 and 291 (π–π* transitions of aromatic ring); 321, 335 and 416 (π–π* transition within the –C=N–); MS (EI) m/z[M]+ calcd for C29H21O2N2OCH3: 459.5450, found: 459.5452. Elemental analysis calcd. for C29H21O2N2OCH3: C 78.41%, H 5.26%, N 6.10%, O 10.23%; found: C 79.25%, H 0.99%, N 4.36%, O 15.4%.
Elemental analysis for receptor 1
Elemental analysis calcd. for C29H21O2N2Cl: C 74.91%, H 4.55%, N 6.02%, O and Cl 14.51%; found: C 79.75%, H 4.88%, N 4.51%, O and Cl 10.86%.
Results and discussion
To explore the metal ion detecting ability, the receptors 1 and 2 were taken in the concentration of 1 × 10−4 M in methanol medium. The concentration of metal ions was maintained at 3 × 10−3 M in aqueous medium.
UV–Visible spectroscopic studies
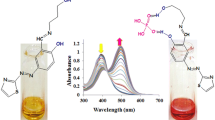
The UV–visible spectrum of receptor 1 exhibited three bands while that of receptor 2 showed four bands prominently. The receptors were made to interact with one equivalent of different metal ions such as (Na(I), Mg(II), Ca(II), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Sr(II), Cd(II), Ba(II), Hg(II), Pb(II) and Al(III)). Co(II), Ni(II) and Cu(II) introduced changes in the absorption pattern of both the receptors. These changes are due to the formation of coordination complexes between the receptors and the respective metal ions. The complex formation is possible because of the presence of four donor sites, namely two azomethine nitrogens and two naphtholic oxygens present in the receptors. The complex formation is facilitated by the chelate ring formation. Notable colour changes were also observed with the naked eye during these interactions [9,10,11] indicating the occurrence of d–d transitions in the metals initiated by the receptors resulting in the formation of coordination complexes between the receptor and the metal ions. Receptor 1 was changed from pale yellow to leaf green, dark green and purple, whereas receptor 2, which is also yellow in colour, converted into ink blue, bluish violet and deep purple in the presence of cobalt, nickel and copper metal ions and are depicted in Figs. 1 and 2 (Scheme 1).
The detection of all the three metal ions resulted in the emergence of a new band in the region of 585–600 nm in the absorption spectrum of both the receptors (Figs. 3, 4). In the presence of receptor, the d-orbitals in the metals will undergo splitting losing the degeneracy. As a result d–d transitions will take place in the metal ion. This is the reason for the formation of a new band in the region of 590 nm which is also responsible for the observation of bright colours. In the case of detection of all the three metal ions the new bands observed are of high intensity. These additional high intense bands may be attributed to the transfer of electrons from the orbital primarily located on the donor moiety of the Schiff base receptor to an orbital located on the metal ions. More specifically, the transition occurs between the HOMO of the donor and the LUMO of the acceptor in the formed complexes and those transitions are charge transfer transitions.
In the case of cobalt the new band at 598 nm may be due to the 4T1g(F) → 4T2g, 4T1g(F) → 4A2g(F) and 4T1g (F) → 4A2g(P) transitions [12]. The Ni(II) shows the new band at 595 nm, which may be assigned to the transition 1A1g → 1A2g [13]. Similarly, in copper 2Eg–2T2g transition [14] is responsible for the appearance of a new band at 585 nm and the appearance of bright colour in the presence of receptors. In addition the tracing of Ni(II) was accomplished with the appearance of another new band at 370 nm in both the receptors, and there was a concomitant disappearance of bands at 457 nm and 277 nm in receptor 1 and receptor 2 respectively. The trapping of Cu(II) resulted in a blue shift to an extent of 27 nm in the band at 438 nm of receptor 1 and a red shift of 34 nm in the band at 332 nm of receptor 2. The lone pair on imine nitrogen is involved in conjugation with the phenyl rings in the ligand which are donated to the copper metal for coordination. Because of the unavailability of the lone pair for conjugation, a blue shift has been observed.
These interactions indicate the formation of complexes between the Schiff base receptors and the metal ions and are further ratified by UV–visible titrations involving sequential addition of metal ions to solutions of receptors from 0.1 equivalents to 1 equivalent and are depicted in Figs. 5 and 6. During these additions, intensity of the new peaks at 594–595 nm and other blue shifted and red shifted peaks increased gradually. The formation of isobestic points further confirms the complex formation [15]. No other metal ions initiated changes in the absorption behaviour of both the receptors. The association constant [16] of each receptor metal ion complex was calculated using Benesi–Hildebrand plots and is listed in Table 1. It is clear that copper-receptor 1 and cobalt-receptor 2 complexes are stronger than other complexes.
a UV–Visible titrations of the receptor 1 with Co(II) ion; inset: change of absorbance on addition of Co(II)ion at 595 nm; b UV–Visible titrations of the receptor 1 with Ni(II) ion; inset: changes of absorbance on addition of Ni(II)ion at 595 nm; c UV–Visible titrations of the receptor 1 with Cu(II) ion; inset: changes of absorbance on addition of Cu(II)ion at 584 nm
a UV–Visible titrations of the receptor 2 with Co(II) ion; inset: change of absorbance on addition of Co(II)ion at 595 nm; b UV–Visible titrations of the receptor 2 with Ni(II) ion; inset: changes of absorbance on addition of Ni(II)ion at 595 nm; c UV–Visible titrations of the receptor 2 with Cu(II) ion; inset: changes of absorbance on addition of Cu(II)ion at 585 nm
Fluorescence spectroscopic studies
We have performed fluorescence spectroscopic studies to explore whether the synthesized Schiff base receptor can find application as fluorescent probes for the detection of Co(II), Ni(II) and Cu(II). Receptor 2 was more fluorescent when compared to receptor 1. This is due to the presence of chromophore –OCH3. The fluorescence spectrum of receptor 1 showed an emission band at 504 nm and that of receptor 2 at 302 nm. The addition of 1 equivalent of Co(II), Ni(II) and Cu(II) (3 × 10−3 M) to a 1 × 10−4 M solution of receptor 1 resulted in quenching. Similar was the case with the receptor 2. The predominant quenching was caused by Co(II), Ni(II) and Cu(II). The quenching process can be explained by means of PET effect that is the electron transfer occurring between the excited fluorophore and the donor species of the receptor. In the present case the energy of HOMO of the donor is higher than the HOMO of the fluorophore. When the receptor is irradiated with light it will absorb a photon and an electron is promoted from the HOMO to the LUMO. Consequently a hole will be created in the HOMO which will be filled by an electron from the donor part of the receptor preventing the decay of the excited state leading to quenching of fluorescence [17, 18]. Other metal ions that caused no spectacular changes in the emission pattern are shown in Figs. 7 and 8. The above results were further checked and confirmed by titrations between the receptors and metal ions involving stepwise addition of the latter to the former and are sketched in Figs. 9 and 10.
a Fluoresence titrations spectrum of receptor 1 with Co(II) ion; inset: change of fluoresence emission on addition of Co(II) ion at 501 nm; b fluorescence titration spectrum of receptor 1 with Ni(II) ion; inset: change of fluorescence emission on addition of Ni(II) ion at 499 nm; c fluorescence titration spectrum of receptor 1 with Cu(II) ion; inset: change of fluorescence emission on addition of Cu(II) ion at 509 nm
a Fluoresence titrations spectrum of receptor 2 with Co(II) ion; inset: change of fluoresence emission on addition of Co(II) ion at 301 nm; b fluorescence titration spectrum of receptor 2 with Ni(II) ion; inset: change of fluorescence emission on addition of Ni(II) ion at 301 nm; c fluorescence titration spectrum of receptor 2 with Cu(II) ion; inset: change of fluorescence emission on addition of Cu(II) ion at 302 nm
Selectivity and reversibility
The selective interaction of receptor 1 and receptor 2 with Co(II), Ni(II) and Cu(II) were evidenced by the competitive titration experiments (Figs. 11, 12). The Receptor 1 made to interact with 1 equivalents of the above three metal ions. In the presence of 1 equivalent of each of other metal ions, small hindrance was caused by Fe3+, Al3+and Pb2+for the detection of cobalt and by Fe for the detection of Ni2+. No hindrance was produced for copper detection. With receptor 2, the cobalt ion detection was intervened by Al3+and Pb2+, whereas nickel and copper ion sensing processes were not altered by any metal [19, 20]. The sensing behaviour of both the receptors were found to be reversible. This was ascertained by the reproduction of receptor florescence spectrum from the cation trapped spectrum of the receptors (Figs. 13, 14) [21, 22].
a Fluorescence intensity of the receptor 1 with 1 equiv. of Co(II) and 1 equiv. of other metal ions stated; b fluorescence intensity of the receptor 1 with 1 equiv. of Ni(II) and 1 equiv. of other metal ions stated; c fluorescence intensity of the receptor 1 with 1 equiv. of Cu(II) and 1 equiv. of other metal ions stated
a Fluorescence intensity of the receptor 2 with 1 equiv. of Co(II) and 1 equiv. of other metal ions stated; b fluorescence intensity of the receptor 2 with 1 equiv. of Ni(II) and 1 equiv. of other metal ions stated; c fluorescence intensity of the receptor 2 with 1 equiv. of Cu(II) and 1 equiv. of other metal ions stated
Means of interaction
To validate the mechanism of interaction of the receptors with metal ions 1H NMR, IR and mass titrations were performed. The coordination between the receptors and the metal ions were ascertained to occur through –OH group of two naphthyl rings in a deprotonated fashion and two imine nitrogen atoms.
1H NMR titrations
1H NMR titrations were performed in aqueous DMSO-d6 medium (Fig. 15). The addition of one equivalent of metal ion to the receptor 2 results in the disappearance of signal due to two naphtholic protons of the receptor. The signal due to imine proton experienced a downfield shift because azomethine nitrogen donate its lone pair of electrons to the metal, thereby experiencing a deshielding effect. Thus indicating the coordination of imine nitrogen to the metal ion. Hence it can be concluded that the metals are detected by the receptor through the formation of coordination compound via the binding sites of two imine nitrogen and two naphtholic groups in a deprotonated fashion.
IR titrations
IR titrations were performed in methanol medium (Figs. 16, 17). In receptor 1 and receptor 2, the band due to C=N appeared at 1658 and 1659 cm−1 respectively. With the addition of copper ion, the bands were shifted to 1660 and 1661 cm−1 respectively [23]. The bands due to –OH group present around 3440 cm−1 were also shifted to an extent of 3 cm−1. The emanation of bands ranging from 403 to 425 cm−1 correspond to a metal nitrogen bond, further confirming the coordination through the nitrogen of a C=N group [24]. The emergence of new bands ranging from 591–618 cm−1 corresponding to M–O bond are due to the coordination of a receptor through its phenolic oxygens [25].
Mass titrations
The pattern of complexes formed during the interaction between the receptors and the metal ions was confirmed by mass spectral studies. The mass spectra of receptor 1 with copper showed the molecular ion peak at 590.9703 (calcd. 590.5651) indicating the coordination of two molecules of methanol in addition to the receptor. In the case of Co(II) two molecules of the solvent DMSO were involved in coordination along with a receptor (1) molecule leading to hexa coordinated Co(II) complex. This is evidenced by the presence of a M-2 peak observed at 676.0345 (calcd. 678.1381).
Stoichiometry and detection limit
Stoichiometry of the complexes formed between receptors and the respective metal ions was determined as 1:1 by Job’s plot method [26, 27] and are shown in Figure S5–S10. The limit of detection of the two receptors for Co(II), Ni(II) and Cu(II) are given in Table 1 and are falling in the range from 10−6 to 10−7 M.
Fluorescence activity towards hela cell lines
The 5 × 105 cells/ml of Hela cells were seeded into the cover slip containing a six well tissue culture plate and incubated at 37 °C in a 5% Co2 incubator for 24 h. After incubation, 102 μg/ml of CuCl2 was added and incubated for 30 min at 37 °C in a 5% Co2 incubator. Finally, 132 μg/ml of BHNCPMD was directly added to the coverslip culture and evaluated immediately by a confocal microscope using a fluorescent filter (Ex. 318 nm and Em. 354 nm). Low fluorescence emission was observed on the cells treated with BHNCPMD ligand with CuCl2 stimulation, indicating that the fluorescence is well quenched after the addition of CuCl2 (Fig. 18) [10, 15].
Conclusion
A new Schiff base, namely N,N′-Bis(2-hydroxynaphthylidene)-4-methoxyphenylmethanediamine synthesised and characterized by IR, UV, 1H NMR and mass spectral studies and another Schiff base N,N′-Bis(2-hydroxynaphthylidene)-4-chlorophenylmethanediamine reported as a ligand for complex preparation by the authors have been exposed to sensor study. The execution of the study of cation recognizing properties revealed their selectivity, sensitivity reversibility and good detection limit. The fluorescence property of receptor 1 is applied in cell imaging studies. To conclude, the two receptors synthesized by one pot synthesis may act as highly affordable sensor materials.
References
S. Santhi, S. Amala, Chem. Sel. 3, 7378 (2018)
S.M. Basheer, A.C. Willis, A. Sreekanth, J. Lumin. 183, 266 (2017)
Y.W. Choi, G.J. Park, Y.J. Na, H.Y. Jo, S.A. Lee, G.R. You, C. Kim, Sens. Actuators B 194, 343 (2014)
S.M. Basheer, M. Muralisankar, T.V. Anjana, K.N. Aneesrahman, A. Sreekanth, Spectrochim. Acta Part A 182, 95 (2017)
S.M. Basheer, J. Haribabu, N.S.P. Bhuvanesh, R. Karvembu, A. Sreekanth, J. Mol. Struct. 1145, 347 (2017)
S.M. Basheer, S.L.A. Kumar, M.S. Kumar, A. Sreekanth, Mater. Sci. Eng. C 72, 667 (2017)
J. Bu, H. Duan, X. Wang, T. Xu, X. Meng, D. Qin, Res Chem. Intermed 41, 2767 (2015)
H. Naeimi, K. Rabiei, F. Salimi, Bull. Korean Chem. Soc. 29, 2445 (2008)
T. Ghosh, B. G. Maiya, A. Samanta, Dalton Trans. 795 (2006)
X. Tang, J. Han, Y. Wang, L. Ni, X. Bao, L. Wang, W. Zhang, Spectrochim. Acta Part A 173, 721 (2017)
A. Maji, S. Thlohar, S. Pal, P. Chattopadhyay, J. Chem. Sci. 129, 1423 (2017)
G.G. Mohamed, N.A. Ibrahim, H.A.E. Attia, Spectrochim. Acta 72, 610 (2009)
N. Malviya, P. Mandal, M. Das, R. Ganguly, S. Mukhopadhyay, J. Coord. Chem. 70, 261 (2017)
N. Raman, S. Ravichandran, C. Thangaraja, J. Chem. Sci. 116(4), 215 (2004)
A.K. Mandal, M. Suresh, E. Suresh, Sensors Actuators B 145, 32 (2010)
Z. Aydin, M. Keles, Turk. J. Chem. 41, 89 (2017)
L. Fan, T.R. Li, B.D. Wang, Z.Y. Yang, C.J. Liu, Spectrochim. Acta Part A 118, 760 (2014)
Y. Chen, M. Ying, Y. Chen, M. Hu, Y. Lin, D. Chen, X. Li, M. Zhang, X. Yun, J. Zhou, E. He, S. Skog, Int. J. Clin. Oncol. 15, 359 (2010)
M. Zhang, W. Lu, J. Zhou, G. Du, L. Jiang, J. Ling, Z. Shen, Tetrahedron 70, 1011 (2014)
L. Zang, D. Wei, S. Wang, S. Jiang, Tetrahedron 68, 636 (2012)
S.P. Wu, Z.M. Huang, S.R. Liu, P.K. Chung, J Fluoresc. 22, 253 (2012)
M.X. Liu, T.B. Wei, Q. Lin, Y.M. Zhang, Spectrochim. Acta Part A. 79, 1837 (2011)
S. Santhi, S. Amala, R. Suganya, IJEAS 4(10), 9 (2017)
S. Jyothi, G.R. Rao, K. Shashank, K. Sridhar, A. Reddy, P. Someshwar, S.J. Swamy, Indian J. Chem. 53A, 535 (2014)
S. Santhi, S.M. Basheer, S. Amala, J. Chem. Sci. 130, 153 (2018)
S. Kim, J.Y. Noh, K.Y. Kim, J.H. Kim, H.K. Kang, S.W. Nam, S.H. Kim, S. Park, C. Kim, J. Kim, Inorg. Chem. 51, 3597 (2012)
W. Wang, W.J. Yuan, Q.L. Liu, Y.N. Lei, S. Qi, Y. Gao, Heterocycl. Commun. 20(5), 289 (2014)
Acknowledgements
The authors are thankful to the Director, SAIF, IIT Madras, Chennai for providing analytical support. The authors wish to express their thanks to the Secretary, Principal, Vice-Principal and faculty members of Department of Chemistry, Seethalakshmi Ramaswami College, Tiruchirappalli, Tamil Nadu for providing laboratory facilities and support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santhi, S., Amala, S., Renganathan, R. et al. Colorimetric and fluorescent sensors for the detection of Co(II), Ni(II) and Cu(II) in aqueous methanol solution. Res Chem Intermed 45, 4813–4828 (2019). https://doi.org/10.1007/s11164-019-03862-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03862-9