Abstract
A novel sensor based on acetylferrocene-containing Schiff base (ASB) was synthesized by reaction of α-chloroacetylferrocene and N-(salicylidene)-l-valinmethylester. The structure of the compound was characterized by using elemental analysis and Fourier-transform infrared (FT-IR), 1H nuclear magnetic resonance (NMR), and 13C NMR spectroscopy. Its metal-cation-sensing properties were investigated spectrofluorometrically. ASB served as selective chemosensor for Zn2+ and Cd2+ towards alkali, alkaline-earth, and various heavy-metal ions. It showed significant fluorescence enhancement for Zn2+ and Cd2+ ions, stemming from C=N isomerization and chelation-enhanced fluorescence. The binding modes of the complexes were determined to have 1:1 complexation stoichiometry, and the binding constants were calculated as (6.93 ± 0.25) × 106 M−1 for ASB·Zn 2+ and (7.49 ± 0.18) × 105 M−1 for ASB·Cd 2+ using the nonlinear curve-fitting method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Development of fluorogenic sensors for transition-metal ions is especially attractive due to their excellent sensitivity, simplicity, and rapid response [1–5]. Among transition-metal ions, discrimination of Zn2+ and Cd2+ has attracted considerable attention from researchers because they occupy the same group of the Periodic Table. Zn2+ is an essential cofactor in biological processes, being the second most plentiful transition-metal ion in the human body, unlike Cd2+, which plays a different role as a toxic metal ion in biochemical processes [6–10]. However, to date, only a few literature reports are available on applications of fluorescence sensors for zinc and cadmium discrimination [11, 12]. Many researchers have been attracted to develop single chemosensors to distinguish metal ions [9, 13]. They focus on development of simple probes with high reliability, sensitivity, and selectivity, and easy synthesis [14–16].
Several Schiff base fluorescent probes have been developed, because Schiff bases are known to be good ligands due to their selective chelating properties for metal ions [17–20]. C=N isomerization in Schiff bases generally results in weak fluorescence emission due to the attached chromophore, but high-intensity emission can be obtained after this isomerization is stopped by bonding to metal ions [21]. In addition, ferrocene has excellent ability to store electrons, which not only strengthens the coordination between the sensor and metal ion, but also lowers the detection limit of metal ion [22]. Although numerous ferrocene-based sensors for transition-metal ions have been studied [23–26], there are few reports on efficient cation sensing based on simply and easily synthesized Schiff base ferrocene-based sensors [27].
In this work, to explore the selective sensing properties of ferrocenyl Schiff base towards transition-metal ions, a novel acetylferrocene-containing Schiff base (ASB) was synthesized. This compound was characterized by elemental analysis and FT-IR, 1H NMR, and 13C NMR spectroscopy. Its metal-ion-sensing properties were also evaluated fluorometrically. The selective sensor properties of compound ASB for different metal ions (Na+, Hg2+, Ca2+, Cu2+, Ni2+, Cd2+, Pb2+, Zn2+, Sr2+, Mg2+, Li+, Na+, K+, Rb+, Ag+, Cs+, and Cr3+) were investigated in detail. The results show that compound ASB exhibits fluorescent sensor properties for Zn2+ and Cd2+ in CH3CN.
Experimental
Materials and methods
l-Valine methylester hydrochloride, salicylaldehyde, ferrocene, chloroacetyl chloride, magnesium sulfate (Mg2SO4), and aluminum chloride (AlCl3) were obtained from Sigma-Aldrich and used as received. Petroleum ether, dichloromethane (CH2Cl2), triethylamine, and acetonitrile (CH3CN) were purchased from Merck. All heavy-metal salts were used as perchlorate compounds (MClO4, M = Na+, Hg2+, Ca2+, Cu2+, Ni2+, Cd2+, Pb2+, Zn2+, Sr2+, Mg2+, Li+, Na+, K+, Rb+, Ag+, Cs+, and Cr3+) and dried over P2O5 prior to use. Melting points were determined on a Büchi B-540 apparatus in sealed capillary and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a Varian 400-MHz spectrometer in CDCl3 and dimethyl sulfoxide (DMSO)-d6. FT-IR spectra of solid samples were recorded on a PerkinElmer Spectrum 100 FT-IR spectrometer (Universal/ATR sampling accessory). Elemental analyses were performed on a Thermo Flash 2000 Scientific model analyzer. Fluorescence spectra were measured using a PTI QuantaMaster 400 Fluorometer spectrophotometer at room temperature.
Synthesis of α-chloroacetylferrocene (1)
α-Chloroacetylferrocene was prepared from ferrocene and chloroacetyl chloride in CH2Cl2 according to a previously published method [28]. Solution of ferrocene (9.3 g, 50 mmol) in CH2Cl2 (40 mL) was added to stirred solution of chloroacetyl chloride (4 mL, 50 mmol) and AlCl3 (7.0 g, 50 mmol) in CH2Cl2 (90 mL) at 0 °C under N2 atmosphere. After 5 h, the solution was extracted three times with water (180 mL), the organic layer was dried with Mg2SO4 and concentrated in vacuo, and the residue was subjected to silica gel chromatography (petroleum ether:dichloromethane = 1:10) to afford orange compound 1. Yield: 17 %, m.p.: 93 °C. 1H NMR (DMSO-d6, 400 MHz, δ, ppm): 4.85 (t, J = 1.84 Hz, 2H, Cp); 4.76 (s, 2H, CH2Cl); 4.64 (t, J = 1.84 Hz, 2H, Cp); 4.26 (s, 5H, Cp). IR (cm−1): 1675 (CO). Anal. calcd. for C12H11ClFeO: C, 54.85; H, 4.19. Found: C, 54.23; H, 4.82 %.
Synthesis of N-(salicylidene)-l-valinmethylester (2)
l-Valine methylester hydrochloride was condensed with salicylaldehyde by the following procedure: Compound 2 was prepared according to literature [29]. Triethylamine (3 mL, 20 mmol) was added to suspension of (l)-valine methylester hydrochloride (20 mmol) in 90 mL dichloromethane. Salicylaldehyde (20 mmol) was added slowly until the color of the reaction mixture turned yellow. The suspension was stirred at room temperature overnight. The solution was washed twice with water, and the organic layer was dried with Mg2SO4. The drying agent was filtered off and the filtrate concentrated in vacuo. The remaining yellow oil crystallized while standing at room temperature. Yield 2.71 g (57 %), m.p.: 35 °C. 1H NMR (CDCl3, 400 MHz, δ, ppm): 13.37 (s, 1H, OH); 8.57 (s, 1H, N=CH); 7.49–6.90 (m, 4H, Ar–H); 3.98 (d, 1H, CH); 3.69 (s, 3H, CH3); 2.27 (m, 1H, CH); 0.91 (d, 3H, CH3); 0.89 (d, 3H, CH3). IR (cm−1): 2966, 2874, 2726, 1741, 1627, 1578. Anal. calcd. for C13H17O3N: C, 66.36; H, 7.28; N, 5.95. Found: C, 66.32; H, 7.35; N, 5.83 %.
Synthesis of acetylferrocene-containing Schiff base (ASB)
Compound 2 was condensed with α-chloroacetylferrocene (1) by the following procedure (Scheme 1): α-Chloroacetylferrocene (2 mmol) was added to a solution of compound 2 (2 mmol) and triethylamine (0.3 mL, 2 mmol) in 30 mL dichloromethane, and the reaction mixture was stirred at 0 °C under N2 atmosphere. After 7 h, the solution was extracted three times with water (60 mL), and the organic layer was dried with Mg2SO4 and concentrated in vacuo. The remaining dark-red oil crystallized while standing at room temperature. Yield 0.77 g (84 %), m.p.: 54 °C. 1H NMR (CDCl3, 400 MHz, δ, ppm): 8.30 (s, 1H, N=CH); 7.33–6.89 (m, 4H, Ar–H); 4.83 (t, 2H, Cp); 4.60 (t, 2H, Cp); 4.42 (s, 2H, CH2Cl); 4.25 (s, 5H, Cp); 3.73 (d, 1H, CH); 3.74 (s, 3H, CH3); 2.37 (m, 1H, CH); 0.97 (d, 3H, CH3); 0.95 (d, 3H, CH3). 13C NMR (CDCl3, 100 MHz, δ, ppm): 195.31, 171.56, 166.56, 161.08, 132.76, 131.69, 118.70, 117.13, 77.93, 75.89, 73.08, 70.18, 69.54, 52.16, 46.08, 31.84, 19.42, 18.18. IR (cm−1): 3029, 2951, 1738, 1681, 1627, 1580, 1169. Anal. calcd. for C25H27O4NFe: C, 65.03; H, 5.85; N, 3.03. Found: C, 65.51; H, 5.34; N, 3.35 %.
Fluorescence studies
To reveal its recognition properties, binding affinity, and sensitivity, the interactions of ASB with various metal ions (Hg2+, Ca2+, Cu2+, Ni2+, Cd2+, Pb2+, Zn2+, Sr2+, Mg2+, Li+, Na+, K+, Rb+, Ag+, Cs+, and Cr3+) were systematically investigated by recording the fluorescence response. Stock solutions were prepared by dissolving their hexahydrate perchlorate salts in CH3CN (5.0 × 10−2 M). The calculated amount of metal solution was added to ASB solution, and the final concentrations of the solution were adjusted to the desired value by adding extra CH3CN. The resulting solution was transferred in a quartz cuvette (3 mL; path length, 1 cm). Fluorescent measurements were recorded after 3 min.
Results and discussion
Structural characterization
The FT-IR spectrum of compound 1 exhibited characteristic absorption bands at 3089 and 1448 cm−1, indicating presence of aromatic C–H and C=C in cyclopentadienyl ring. The stretching frequency at 1675 cm−1 was assigned to C=O vibrations. The other absorptions at 2937 and 779 cm−1 were attributed to presence of C–H and C–Cl groups, respectively [28, 30].
FT-IR spectra of aldehydes and amines generally exhibit signals at 1645–1665 cm−1 and between 2964 and 3427 cm−1, attributed to C=O and NH2 stretching vibration. After reaction, a new band was observed at 1627 cm−1, attributed to azomethine (CH=N) group, in the FT-IR spectrum of compound 2. This finding/change showed that amino and aldehyde groups of the starting reactants converted to corresponding Schiff bases [31]. Other absorption bands were seen at 2966 cm−1 for (C–H)arom., 2874 cm−1 for (C–H)alph., and 1578 cm−1 for (C=C). Vibration of carbonyl group was observed at 1741 cm−1 in the amino acid methyl ester [32]. While the O–H stretching frequency of compound 2 was expected in the 3500–3800 cm−1 region, this signal was displaced to the 2726 cm−1 region due to the O–H···N intramolecular hydrogen bond (Scheme 2). According to this result, the compound is planar in structure, and hence compound 2 has sufficient intramolecular distance to form hydrogen bond [33].
After reaction of compound 1 with compound 2, the C–Cl (779 cm−1) band disappeared and a new band was observed at 1176 cm−1, attributed to etheric (C–O–C) group, in the FT-IR spectrum of ASB. The FT-IR spectra of compounds 2 and ASB are shown in Fig. 1.
The 1H NMR and 13C NMR spectra of compounds 1, 2, and ASB were recorded (solvent, DMSO-d6 and CDCl3). Detailed structural information was provided by 13C NMR spectra.
In the 1H NMR spectrum of compound 1, the triplet signals observed in the region 4.64–4.85 ppm and the singlet signal observed at 4.26 ppm were attributed to –CH2 and –CH protons in acetyl and ferrocenyl group, respectively. All protons were found in their expected region [28, 30].
In the 1H NMR spectrum of compound 2, the singlet signals at 8.57 and 13.37 ppm were attributed to azomethine and phenolic protons, respectively. Aromatic protons in benzene ring were also observed at 7.49–6.90 ppm as multiplet. All of the aliphatic, aromatic, and azomethine carbon (CH=N) shifts were confirmed in the expected regions [29]. Other peaks of 2, i.e., doublet signal at 3.98 ppm and multiplet signal at 2.27 ppm, and singlet signal at 3.69 ppm and two doublet signals at 0.91–0.89 ppm, were attributed to –CH and –CH3 protons, respectively (Fig. 2a) [29]. While obtaining ASB, phenolic OH band at 13.37 ppm disappeared (Fig. 2b).
The interpretation of the 13C NMR spectrum showed peaks for the various carbon atoms in ASB as follows: The azomethine (CH=N) group carbon showed peak at 161.08 ppm (Fig. 3). The aromatic carbons in benzene ring showed peak in the range of 132.76–117.13 ppm. The substituted methyl groups in the valine methyl ester exhibited peaks at 52.16, 31.84, and 18.18–19.42 ppm, attributed to O–CH3 (C10), –CH (C11), and –CH3 (C12, C13) carbons, respectively [29]. Carbon bonded with carbonyl group exhibited a peak at 171.56 ppm. The signals at 75.89, 73.08, 69.54, 70.18, 195.31, and 46.08 ppm were attributed to C1, C2, C3, C4, C5, and C6 carbons of acetylferrocenyl group, respectively [30].
Fluorescence study
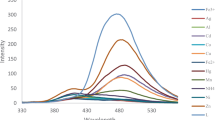
To determine the binding properties of metal ions to ASB, the fluorescent response of ASB for various metal ions including Hg2+, Ca2+, Cu2+, Ni2+, Cd2+, Pb2+, Zn2+, Sr2+, Mg2+, Li+, Na+, K+, Rb+, Ag+, Cs+, and Cr3+ was investigated. ASB showed high, substantial change in presence of Zn2+ and Cd2+ ions. As seen in Fig. 4, ASB in CH3CN showed very weak fluorescence emission with excitation at 378 nm. When 1 equiv of various metal ions such as Hg2+, Ca2+, Cu2+, Ni2+, Cd2+, Pb2+, Zn2+, Sr2+, Mg2+, Li+, Na+, K+, Rb+, Ag+, Cs+, and Cr3+ was added, solution of ASB exhibited no or small significant increase of fluorescence, except for Cd2+ and Zn2+. ASB showed selective fluorescence enhancement with Zn2+ at 452 nm, even though there was a relatively smaller fluorescence increase with Cd2+. The fluorescence enhancement of ASB in presence of Zn2+ and Cd2+ can be explained by considering the C=N isomerization. Because C=N isomerization in compounds with unbridged C=N structure is the predominant decay process of excited states, such compounds exhibit very poor fluorescence. It is known that compounds containing covalently bridged C=N structure show higher fluorescence intensity owing to restriction of C=N isomerization in the excited states [34]. Binding of Zn2+ and Cd2+ to ASB can inhibit C=N isomerization, resulting in a dramatic increase of fluorescence intensity [35]. Chelation of ASB with Zn2+ and Cd2+ can induce rigidity in the complexes, causing a large chelation-enhanced fluorescence (CHEF) effect with drastic enhancement of fluorescence [36]. The CHEF value was determined to be 13 for the case of ASB·Zn 2+ and 5 for the case of ASB·Cd 2+.Footnote 1 The fluorescence changes under UV lamp (at 365 nm) were also detected by naked eye (Fig. 4, inset). These results indicated that ASB can readily distinguish Zn2+ and Cd2+ from biologically and environmentally relevant metal ions by enhanced fluorescence [37].
The changes in the emission spectrum of ASB as a function of Zn2+ and Cd2+ concentration are shown in Fig. 5. When fluorescent titration was performed with Zn2+ or Cd2+, the emission intensity increased up to 10 equiv, then no change was observed. The fluorescence intensity was enhanced almost 12.73-fold upon addition of 10 equiv Zn2+ and 4.59-fold upon addition of 10 equiv Cd2+. The fluorescence intensity of ASB increased linearly with Zn2+ and Cd2+ up to 1:1 mol ratio, then remained constant with increasing amount of Zn2+ or Cd2+. These changes indicate that ASB and the metal ions undergo 1:1 complex formation (Fig. 5a, b, insets) considering the mole fraction method.
Considering the fluorescence changes and complexation ratio (1:1 stoichiometry) between ASB and the metal ions at lower equivalents, the nonlinear curve-fitting procedure was used to determine the binding constants (K a) by applying Eq. (1) [38].
where r is the added equivalents of cation, \(I_{\hbox{min} }^{0}\), I, and \(I_{\hbox{max} }^{0}\) are the fluorescence of free ligand, ligand plus r equivalents of cation, and ligand plus large excess cation solution, respectively, \(C_{\hbox{min} }^{0}\) is the concentration of free ligand, and K a is the binding constant. The nonlinear curve fits based on Eq. (1) obtained using Sigma Plot 10.0 are presented in Fig. 6. The binding constant K a for 1:1 stoichiometry of the complex between ASB and the metal ions was calculated as (1.38 ± 0.25) × 106 M−1 for ASB·Zn 2+ and (7.49 ± 0.18) × 105 M−1 for ASB·Cd 2+ from the curve fitting. These results show that the binding affinity between ASB and Zn2+ is stronger than that between ASB and Cd2+.
Nonlinear curve fitting of Eq. (1) for fluorescence change at 452 nm with respect to added amount of a Zn2+ in the range of 0.1–100 μM (correlation coefficient of the nonlinear curve fitting 0.9910) and b Cd2+ in the range of 0.5–500 μM (correlation coefficient of nonlinear curve fitting 0.9877)
Considering the results for the stoichiometric ratio and binding constants, structures for the ASB·Cd 2+ and ASB·Zn 2+ complexes are proposed in Scheme 3.
To calculate the detection limit, calibration curves were obtained from plots of fluorescence intensity versus added Zn2+ and Cd2+ concentration (Fig. 7). The detection limit for Zn2+ and Cd2+ was determined to be 0.68 and 0.94 µM, respectively, according to DL = 3.3σ/S.Footnote 2 These results clearly demonstrate that ASB was highly efficient in sensing Zn2+ or Cd2+. The reversibility of the sensor was also examined. The fluorescence intensity decreased almost to that of bare ASB when 10 μM ethylenediaminetetraacetic acid (EDTA) was added to solution of ASB·Zn 2+ and ASB·Cd 2+. These results show that compound ASB could be regenerated for repeated use as an optical sensor.
Conclusions
We describe herein preparation of a novel acetylferrocene-containing Schiff base (ASB) and characterization of its structure by using elemental and spectroscopic (FT-IR, 1H NMR, 13C NMR) methods. ASB displayed selective and sensitive fluorescence response to Zn2+ and Cd2+ in CH3CN. Addition of Zn2+ and Cd2+ showed drastic enhancement of emission intensity, meaning that ASB could be used as a dual sensor in CH3CN for these metal ions. In other words, ASB·Zn 2+ showed high fluorescence enhancement, even though ASB·Cd 2+ showed relatively smaller fluorescence increase. The binding modes of Zn2+ and Cd2+ to ASB were examined by nonlinear curve fitting, proving that ASB coordinates with the metal ions in a 1:1 complex. Simultaneously, chelation between the ligand and metal ion causes a large CHEF effect, which induces the increase of fluorescence intensity. In particular, ASB can detect Zn2+ selectively in CH3CN in presence of other metal ions, except Cd2+ ion. This sensitive and selective fluorescent sensor can provide guidance for the development of new chemosensors.
Notes
The CHEF is defined as I max/I 0, where I max corresponds to the maximum emission intensity of the receptor–metal complex, while I 0 is the maximum emission intensity of the free receptor. For recent and relevant examples of heavy- and transition-metal cation (HTM) chemosensors based on chelation-enhanced fluorescence (CHEF).
Guidance for industry Q2B validation of analytical procedures: methodology, Nov 1996.
References
M. Kaur, P. Kaur, V. Dhuna, S. Singh, K. Singh, Dalton Trans. 43, 5707–5712 (2014)
S. Maiti, Z. Aydin, Y. Zhang, M. Guo, Dalton Trans. 44, 8942–8949 (2015)
B. Sen, M. Mukherjee, S. Banerjee, S. Pal, P. Chattopadhyay, Dalton Trans. 44, 8708–8717 (2015)
S. Anbu, R. Ravishankaran, M.F.C.G. Da Silva, A.A. Karande, A.J.L. Pombeiro, Inorg. Chem. 53, 6655–6664 (2014)
V.K. Gupta, N. Mergu, L.K. Kumawat, A.K. Singh, Sens. Actuators B 207, 216–223 (2015)
J. Wang, W. Lin, W. Li, Chem. Eur. J. 18, 13629–13632 (2012)
S.K. Lee, M.G. Choi, J. Choi, S.K. Chang, Sens. Actuators B 207, 303–307 (2015)
Q. Zhao, R. Li, S. Xing, X. Liu, T. Hu, X. Bu, Inorg. Chem. 50, 10041–10046 (2011)
Z. Liu, C. Zhang, W. He, Z. Yang, X. Gao, Z. Guo, Chem. Commun. 46, 6138–6140 (2010)
V.K. Gupta, M.R. Ganjali, P. Norouzi, H. Khani, A. Nayak, S. Agarwal, Crit. Rev. Anal. Chem. 41, 282–313 (2011)
F. Xiao, J. Shen, J. Qu, S. Jing, D.R. Zhu, Inorg. Chem. Commun. 35, 69–71 (2013)
J.T. Hou, B.Y. Liu, K. Li, K.K. Yu, M.B. Wu, X.Q. Yu, Talanta 116, 434–440 (2013)
Y. Ma, F. Wang, S. Kambam, X. Chen, Sens. Actuators B 188, 1116–1122 (2013)
K. Dutta, R.C. Deka, D.K. Das, Spectrochim. Acta A 124, 124–129 (2014)
R. Borthakur, U. Thapa, M. Asthana, S. Mitra, K. Ismail, R.A. Lal, J. Photochem. Photobiol. A Chem. 301, 6–13 (2015)
B. Kashyap, K. Dutta, D.K. Das, P. Phukan, J. Fluoresc. 24, 975–981 (2014)
H. Ye, F. Ge, Y.M. Zhou, J.T. Liu, B.X. Zhao, Spectrochim. Acta A 112, 132–138 (2013)
J.H. Hu, J.B. Li, J. Qi, Y. Sun, Sens. Actuators B 208, 581–587 (2015)
R. Pandey, R.K. Gupta, M. Shahid, B. Maiti, A. Misra, D.S. Pandey, Inorg. Chem. 51, 298–311 (2011)
V.K. Gupta, A.K. Singh, L.K. Kumawat, Sens. Actuators B 195, 98–108 (2014)
L. Wang, H. Li, D. Cao, Sens. Actuators B 181, 749–755 (2013)
Y.S. Mi, Z. Cao, Y.T. Chen, Q.F. Xie, Y.Y. Xu, Y.F. Luo, J.J. Shic, J.N. Xiang, Analyst 138, 5274–5280 (2013)
C.K. Kumar, R. Trivedi, L. Giribabu, S. Niveditha, K. Bhanuprakash, B. Sridhar, J. Organomet. Chem. 780, 20–29 (2015)
P. Chinapang, V. Ruangpornvisuti, M. Sukwattanasinitt, P. Rashatasakhon, Dyes Pigments 112, 236–238 (2015)
J. Shen, T. Liu, Y. Li, W. Ji, S. Jing, D.R. Zhu, G.F. Guan, Inorg. Chem. Commun. 44, 6–9 (2014)
S.J. Ponniah, S.K. Barik, A. Thakur, R. Ganesamoorthi, S. Ghosh, Organometallics 33, 3096–3107 (2014)
V. Uahengo, B. Xiong, P. Zhao, Y. Zhang, P. Cai, K. Hu, G. Cheng, Sens. Actuators B 190, 937–945 (2014)
L. Zhu, D. Zhang, D. Qu, Q. Wang, X. Ma, H. Yian, Chem. Commun. 46, 2587–2589 (2010)
G. Warncke, U. Böhme, B. Günther, M. Kronstein, Polyhedron 47, 46–52 (2012)
O. Dogan, V. Senol, S. Zeytinci, H. Koyuncu, A. Bulut, J. Organomet. Chem. 690, 430–434 (2005)
M.L. Sundararajan, T. Jeyakumar, J. Anandakumaran, B.K. Selvan, Spectrochim. Acta A 131, 82–93 (2014)
J. Müller, G. Kehr, R. Fröhlich, G. Erker, Eur. J. Inorg. Chem. 2005, 2836–2841 (2005)
D. Tomczyk, L. Nowak, W. Bukowski, K. Bester, P. Urbaniak, G. Andrijewski, B. Olejniczak, Electrochim. Acta 121, 64–77 (2014)
J. Wu, W. Liu, J. Ge, H. Zhang, P. Wang, Chem. Soc. Rev. 40, 3483–3495 (2011)
Y.J. Lee, C. Lim, H. Suh, E.J. Song, C. Kim, Sens. Actuators B 201, 535–544 (2014)
L. Wang, W. Qin, X. Tang, W. Dou, W. Liu. J. Phys. Chem. A 115, 1609–1616 (2011)
X. Liu, N. Zhang, J. Zhou, T. Chang, C. Fangab, D. Shangguan, Analyst 138, 901–906 (2013)
H. Bingol, E. Kocabas, E. Zor, A. Coskun, Talanta 82, 1538–1542 (2010)
Acknowledgments
This work was supported by Scientific Research Projects (BAP 13201019) of Selcuk University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Findik, M., Ucar, A., Bingol, H. et al. Fluorogenic ferrocenyl Schiff base for Zn2+ and Cd2+ detection. Res Chem Intermed 43, 401–412 (2017). https://doi.org/10.1007/s11164-016-2630-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2630-8