Abstract
A new rhodamine-based Schiff base (L) has been synthesized and characterized by physicochemical and spectroscopic tools. This organic molecule selectively reacts with \(\hbox {Cu}^{2+}\) ions with a remarkably significant optical change, which supports the development of a chemosensor for \(\hbox {Cu}^{2+}\) ions as low as nanomolar level in aqueous medium. On the basis of the experimental work, the ‘turn-on’ colorimetric/fluorimetric spectroscopic change is due to \(\hbox {Cu}^{2+}\) ion-assisted hydrolysis followed by spirolactam ring opening of the probe (L) in 20 mM HEPES buffer [pH 7.4; water/acetonitrile (9:1 v/v)]. The competitive ions do not affect the selectivity and specificity of the probe (L) in the detection of \(\hbox {Cu}^{2+}\) ions. The cell imaging study using fluorescence microscope showed that this non-cytotoxic probe is useful to detect the distribution of \(\hbox {Cu}^{2+}\) ions in AGS cells lines.
Graphical abstract
A new turn-on non-cytotoxic rhodamine hydrazone derivative (L) senses \(\hbox {Cu}^{2+}\) ion in nano molar region selectively in 20 mM HEPES buffer [pH 7.4; water/acetonitrile (9:1 v/v)] and is an efficient biomarker to detect \(\hbox {Cu}^{2+}\) ions in living cells.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A chemosensor is such a compound that exhibits a very distinguishable and a significant change in electrical, electronic, magnetic, or optical signal when it binds to a specific guest counterpart like cations, anions or molecules, etc.[1] As the third most abundant transition metal ion, \(\hbox {Cu}^{2+}\) (after \(\hbox {Fe}^{2+}\) and \(\hbox {Zn}^{2+})\) plays a pivotal role in a variety of fundamental physiological processes in organisms ranging from bacteria to mammals involving cellular energy generation, oxygen transport and activation, and signal transduction.[2,3,4] In many metalloenzymes, including superoxide dismutase, cytochrome c oxidase, and tyrosinase copper ion plays a crucial role as a catalytic cofactor.[5] Due to its widespread use, \(\hbox {Cu}^{2+}\) ion is also a significant metal pollutant in the environment.[6] Though \(\hbox {Cu}^{2+}\) plays important role in many biochemical processes, alteration in the cellular homeostasis of copper ions was reported to be connected with some serious neurodegenerative diseases like Alzheimer’s disease,[7] Indian childhood cirrhosis (ICC),[8] Indian prion disease,[9] and Menkes and Wilson diseases.[10, 11] A high amount of copper for a short period of time can also lead to gastrointestinal disturbance, liver or kidney damage.[12] Free copper ions in a live cell catalyze the formation of reactive oxygen species (ROS) that can damage lipids, nucleic acids, and proteins. As a result, the maximum permissible level of copper in drinking water has been set at 1.3 ppm (\(\sim 20\,\upmu \hbox {M}\)) by the U.S. Environmental Protection Agency (EPA).[13]
Though, numerous methods for the detection of trace level of copper in the samples are available which include electrochemistry,[14] atomic absorption spectrometry,[15] inductively coupled plasma mass spectroscopy (ICPMS),[16] inductively coupled plasma atomic emission spectrometry (ICP-AES) [17] and voltammetry.[18] But these techniques are usually complicated, time-consuming and costly too.
Of the many different kinds of optical sensors, fluorescent chemosensors have several advantages over the other methods due to their intrinsic sensitivity, upfront application to fiber optical-based detection, and real-time monitoring with fast response time.[19, 20] Due to its intrinsic paramagnetic properties, \(\hbox {Cu}^{2+}\) ion has the propensity to quench the fluorescence of fluorescent metal chelators conferring a non-fluorescent state.[21, 22] In addition, most of the copper-selective sensors suffer from the interfering effect of cations such as \(\hbox {Ni}^{2+}\), \(\hbox {Ag}^{+}\), \(\hbox {Hg}^{2+}\) ions, etc.[23,24,25,26] Therefore, the development of a highly sensitive, selective turn-on fluorogenic probe working under a physiological condition with a fast response is highly desirable.
Rhodamine derivatives are non-fluorescent and colorless, whereas ring-opening of the corresponding spirolactam gives rise to strong fluorescence emission and a pink color. Several photosensing processes viz., photo-induced electron transfer (PET),[27] photo-induced charge transfer (PCT),[28] fluorescence resonance energy transfer (FRET),[29] intermolecular charge transfer (ICT),[30] chelation enhanced fluorescence (CHEF),[31] etc., are well known. Some hydroxynapthylidine derivatives and calixarene functionalised nanoparticles have been reported as \(\hbox {Cu}^{2+}\) ions sensor.[32, 33] Rhodamine and its derivatives have been widely used to design fluorescent chemosensors due to their good photostability, high extinction coefficient, high fluorescence quantum yield and broad fluorescence in the visible region of electromagnetic spectrum. Several rhodamine-based chemosensors for recognition of various metal ions are reported.[34,35,36,37,38,39] With these considerations, herein, we report synthesis of a salicylaldehyde appended rhodamine hydrazone derivative (L) for selective determination of \(\hbox {Cu}^{2+ }\)ions at physiological condition without interfering. Experimental findings suggest \(\hbox {Cu}^{2+}\) ions assisted spirolactam ring opening of (L) and the subsequent hydrolysis is responsible for fluorescence enhancement leading to off-on sensing.
2 Experimental
2.1 Materials and general methods
2.1.1 Materials
High-purity HEPES buffer and rhodamine B dye were purchased from Himedia Laboratories, India. 3,5-Di-tert-butyl-2-hydroxybenzaldehyde was purchased from Sigma Aldrich and different inorganic salts were purchased from Emplura, Merck. Analytical grade different solvents were purchased from commercial sources and they were used in this work without further purification (if not mentioned). Here, Milli-Q 18 \(\Omega \) water was used throughout the experiments. UV-Vis and IR spectral data were collected using a Shimadzu (model UV-1800) spectrophotometer and a Prestige-21 SHIMADZU FTIR spectrometer, respectively. A Bruker Avance DPX 500 MHz spectrometer \({}^{1}\hbox {H}\) NMR spectra were recorded in \(\hbox {DMSO-d}_{6}\). pH value of solution was adjusted using either 50 mM HCl or NaOH solution and the pH values were measured by using a Systronics digital pH meter (model 335). A Thermochem Exactive plus mass spectrometer was used to get the electronspray ionization mass spectra. Steady state fluorescence emission and excitation spectra were obtained with the help of a Hitachi-7000 spectrofluorimeter.
2.1.2 General methods
The fluorescence properties of the probe L were measured in 10% \(\hbox {CH}_{3}\hbox {CN}\) 20 mM HEPES buffer medium (pH 7.4) at \(25\,{^{\circ }}\hbox {C}\). To investigate the effect of different pH on the emission intensity of L, pH study was performed in 20 mM HEPES buffer solution by maintaining the pH using 50 mM HCl or 50 mM NaOH solution. Cell imaging study was carried out at biological pH \(\sim 7.4\) using 20 mM HEPES buffer solution. To check the selectivity of this probe L towards different metal ions, stock solutions (\(\sim 10^{-2}\,\hbox {M}\)) for L was prepared using different salts: such as, the chloride salts of \(\hbox {Ni}^{2+}\), \(\hbox {Co}^{2+}\), \(\hbox {Cu}^{2+}\), \(\hbox {Au}^{3+}\), \(\hbox {Hg}^{2+}\), \(\hbox {Ca}^{2+}\), \(\hbox {Mg}^{2+}\) and \(\hbox {Fe}^{3+ }\)ions; nitrate salts of \(\hbox {Na}^{+}\), \(\hbox {K}^{+}\), \(\hbox {Cr}^{3+}\) and \(\hbox {Ag}^{+ }\)ions; acetate salts of \(\hbox {Mn}^{2+}\) and \(\hbox {Zn}^{2+ }\)ions; and ferrous sulphate in 10% \(\hbox {CH}_{3}\hbox {CN}\) 20 mM HEPES buffer (pH 7.4) solvent. During this selectivity measurement, the concentration of these metal ions was taken fifty times greater than that of the probe (L). For fluorimetric titration study, solution of copper(II) chloride dihydrate in 10% \(\hbox {CH}_{3}\hbox {CN}\) 20 mM HEPES buffer (pH 7.4) was used. During this study, probe concentration was \(20.0\,\upmu \hbox {M}\) and gradually \(\hbox {Cu}^{2+}\) solution were added by varying \(\hbox {Cu}^{2+}\) concentration from 0 to \(60.0\,\upmu \hbox {M}\) and the corresponding spectra were recorded.
The path length of the cells used for absorption and emission studies was 1 cm. For UV-Visible and fluorescence titrations, a stock solution of L was prepared in 10% \(\hbox {CH}_{3}\hbox {CN}\) 20 mM HEPES buffer (pH 7.4) at room temperature. Working solutions of L and \(\hbox {Cu}^{2+}\) ions were prepared from their respective stock solutions. Fluorescence measurements were performed using a \(5\,\hbox {nm} \times 5\,\hbox {nm}\) slit setting for both excitation and emission spectra. All the fluorescence and absorbance spectra were taken after 20 min of mixing the \(\hbox {Cu}^{2+}\) ions and L. A series of solutions containing L and \(\hbox {CuCl}_{2}.2\hbox {H}_{2}\hbox {O}\) were prepared such that the total concentration of L (20 mM) remained constant in all the sets. The organic molecule (L) shows a very weak emission at 572 nm in 10% \(\hbox {CH}_{3}\hbox {CN}\) 20 mM HEPES buffer (pH 7.4) at \(25\,{^{\circ }}\hbox {C}\) when excited at 550 nm.
2.2 Synthesis of the probe (L)
The probe L was synthesised by a two step reaction (Scheme 1). At first, the rhodamine B-hydrazide was prepared following a literature method.[40] In brief, 85% hydrazine hydrate (4 mL) was added to a solution of rhodamine B (1.0 g, 2.1 mmol) in ethanol (40 mL). The solution was refluxed for 6 h. Then, the reaction mixture was evaporated under reduced pressure to give an orange coloured oil, which was then recrystallized from methanol-water to afford rhodamine B-hydrazide as a light-orange crystal (77%).
In the second step, 3,5-Di-tert-butyl-2-hydroxybenzaldehyde (234.33 mg, 1.0 mmol) dissolved in ethanol was added to the ethanolic solution of rhodamine-B hydrazide (456.25 mg, 1 mmol) with stirring. The resulting mixture was refluxed for 6 h. It was then evaporated to a small volume and cooled, from which white colored precipitate was filtered. The pure product was isolated from acetonitrile/methanol (3:1) mixed solvents on slow evaporation.
\(\hbox {C}_{43}\hbox {H}_{52}\hbox {N}_{4}\hbox {O}_{3}\): M.p.: \({>}250\,{^{\circ }}\hbox {C}\). HRMS in methanol: \([\hbox {M} + \hbox {H}]^{+}\), m/z, 673.3369 (100%) (calcd: m/z, 673.4118), where M = molecular weight of L (Figure S1), \({}^{1}\hbox {H}\) NMR (400 MHz, DMSO-d6): 11.18 (s, 1H); 8.85 (s, 1H); 7.94 (d, 1H); 7.61–7.66 (m, 2H); 6.956 (s.1 H); 6.36–6.45 (m.7H); 2.079 (s, 4H); 1.049–1.248 (m, 33H) (Figure S2), FT-IR (KBr, \(\hbox {cm}^{-1})\): \(\upnu _{C-H }\)(aromatic), 2960.63; \(\upnu _{C=O}\), 1691.05; \(\upnu _{CH=N}\), 1614.39; \(\upnu _{C=C}\), 1514.51; \(\upnu _{C-N}\), 1302.23; \(\upnu _{C-O-C}\), 1118.64; \(\upnu _{C-H}\)(bending), 788.15 (Figure S3). Yield: 78%.
2.3 Preparation of cell and in vitro cellular imaging with L
Maintenance of the AGS cell lines used in this study was done in Dulbecco’s modified eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 2 mM glutamax, \(100\,\upmu \hbox {g.mL}^{-1}\) of penicillin and \(100\,\upmu \hbox {g.mL}^{-1}\) of streptomycin at \(37\,{^{\circ }}\hbox {C}\) in a humidified incubator having 5% \(\hbox {CO}_{2}\). The cultures were grown as monolayer and accepted once in 4–5 days by trypsinizing with 0.25% Trypsin-EDTA. AGS cells (4 x \(10^{4}\,\hbox {cells/mm}^{2}\)), plated on cover slips, were incubated with L (2, 5 and \(10\,\upmu \hbox {M}\), 1% DMSO) for 30 min. After washing with 50 mM phosphate buffer of pH 7.4 containing 150 mM NaCl (PBS), required volumes of copper(II) chloride solution in DMSO were added such that the final concentration of copper(II) chloride adjusted to \(10.0\,\upmu \hbox {M}\) and \(20.0\,\upmu \hbox {M}\) (DMSO will be 1%) and incubated for 30 min. The cells were fixed with 4% paraformaldehyde for 10 min at room temperature (RT). After washing with PBS, cover slips were mounted in 90% glycerol solution containing Mowiol (as an anti-fade reagent) and sealed. Images were acquired using Apotome fluorescence microscope (Carl Zeiss, Germany) using an oil immersion lens at 63X magnification. The images were analyzed using the AxioVision Rel 4.8.2 (Carl Zeiss, Germany) software.[41]
2.4 Cell cytotoxicity assay
To verify the usefulness of this probe in the biological system, the cytotoxicity of L was checked through MTT [3-(4,5-dimethyl-thiazol-2-yl)-2,S-diphenyl tetrazolium bromide] assay experiment [42] To the respective probe (5, 10, 25, 50, and \(100\,\upmu \hbox {M}\)) solutions, \(10\,\upmu \hbox {L}\) of MTT solution (10 mg/mL PBS) was added to each well of a 96-well culture plate and incubated continuously at \(37\,{^{\circ }}\hbox {C}\) for 8 h. All the mediums were replaced with \(100\,\upmu \hbox {L}\) of acidic isopropanol in the wells. The intracellular formazan crystals (blue-violet) formed were solubilized with 0.04 N acidic isopropanol and the absorbance of the solution was measured at 550 nm with a microplate reader. Absorbance values were recorded as the mean ± S.D. of three independent experiments.
3 Results and Discussion
3.1 Synthesis and characterization
The rhodamine B-hydrazide derivative probe (L) was prepared from the reaction 3,5-Di-tert-butyl-2-hydroxybenzaldehyde (TBHB) and rhodamine B-hydrazide in ethanol (Scheme 1). The crude white precipitate obtained from the reaction mixture was purified through crystallisation from the solution of L in acetonitrile/methanol (3:1) mixed solvents on slow evaporation over a few days. The crystallised product was characterized by physicochemical and spectroscopic tools (Figures S1–S3, in Supplementary Information).
3.2 Absorption and fluorescence spectroscopic studies of L
The UV-Visible spectra of L recorded in 10% \(\hbox {CH}_{3}\hbox {CN}\) 20 mM HEPES buffer (pH 7.4) at \(25\,{^{\circ }}\hbox {C}\) shows an absorption maximum at 314 nm, which may be attributed to the intramolecular \(\uppi \)–\(\uppi \)* charge transfer transition.
On the stepwise addition of \(\hbox {Cu}^{2+}\) ions (0–\(60\,\upmu \hbox {M}\)) to the solution of L in 10% \(\hbox {CH}_{3}\hbox {CN}\) 20 mM HEPES buffer (pH 7.4), the absorption intensity at 314 nm increased gradually and a new peak at 560 nm was generated by ring opening with a visual color change from colorless to pink (Figure 1). In the presence of an excess of the biologically relevant transition metal ions (\(\hbox {Cr}^{3+}\), \(\hbox {Mn}^{2+}\), \(\hbox {Fe}^{3+}\), \(\hbox {Co}^{2+}\), \(\hbox {Ni}^{2+}\), \(\hbox {Zn}^{2+}\), \(\hbox {Cd}^{2+})\), alkali metal ions (\(\hbox {Na}^{+}\), \(\hbox {K}^{+})\), alkaline earth metal ions (\(\hbox {Ca}^{2+}\), \(\hbox {Mg}^{2+}\), \(\hbox {Al}^{3+})\) and other heavy metal ions (\(\hbox {Hg}^{2+}\), \(\hbox {Pb}^{2+}\)), no new band was produced as they were unable to go through the ring opening step.
Emission spectra of L (\(20\,\upmu \hbox {M}\)) in presence of \(\hbox {Cu}^{2+}\) ions (0–\(60\,\upmu \hbox {M}\)) (\(\lambda _{{\mathrm{em}}} = 572\, \hbox {nm}\), at \(\lambda _{{\mathrm{ex}}} = 550\,\hbox {nm}\)). Inset: Naked eye fluorescence color change: (A) L only, and (B) L in presence of \(\hbox {Cu}^{2+}\) ions in 10% \(\hbox {CH}_{3}\hbox {CN}\) 20 mM HEPES buffer (pH 7.4) at \(25\,{^{\circ }}\hbox {C}\).
The emission spectrum of L excited at 550 nm shows a fluorescence maximum at 572 nm in 10% \(\hbox {CH}_{3}\hbox {CN}\) 20 mM HEPES buffer (pH 7.4) at \(25\,{^{\circ }}\hbox {C}\). When various concentrations of \(\hbox {Cu}^{2+}\) ions (0–\(60\,\upmu \hbox {M}\)) were added fluorescence intensity increases with an increase in the concentration of \(\hbox {Cu}^{2+}\) ions (Figure 2).
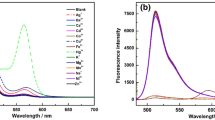
L showed an almost 68-fold increase in its fluorescence intensity with the addition of only 3.0 equivalents of \(\hbox {Cu}^{2+}\) ions. This consequential strong fluorescence emission is due to the ring-opening of the spirolactam system of rhodamine-B (Scheme 2). In addition, this visual and fluorescence color change due to \(\hbox {Cu}^{2+}\) ions were not perturbed by the presence of an excess of the biologically relevance transition (\(\hbox {Cr}^{3+}\), \(\hbox {Mn}^{2+}\), \(\hbox {Fe}^{3+}\), \(\hbox {Co}^{2+}\), \(\hbox {Ni}^{2+}\), \(\hbox {Zn}^{2+}\), \(\hbox {Cd}^{2+})\), alkali, alkaline earth metal ions (\(\hbox {Na}^{+}\), \(\hbox {K}^{+}\), \(\hbox {Ca}^{2+}\), \(\hbox {Mg}^{2+}\), \(\hbox {Al}^{3+})\) and other heavy metal ions (\(\hbox {Hg}^{2+}\), \(\hbox {Pb}^{2+})\) (Figure S4 in SI) and also these ions did not offer such visual and fluorescent color change property through ring opening. It reveals that the probe L has an excellent selectivity and specificity towards \(\hbox {Cu}^{2+}\) ions over the other cations.
3.3 pH study
The effect of pH on the emission characteristics of L has been examined. Different sets of an equimolar mixture of L and \(\hbox {Cu}^{2+}\) ions were adjusted to different pH (pH 4.0–11.0) and their emission intensities were measured (viz., Figure 3). This study clearly showed the maximum emission intensity of the [\(\hbox {Cu}^{2+}\) + L] system at pH 7.4, which is in support of estimation of \(\hbox {Cu}^{2+}\) ions in any matrix at physiological pH.
3.4 Mechanism
In the presence of \(\hbox {Cu}^{2+}\) ions, L undergoes hydrolysis leading to open ring rhodamine unit, evidenced by the mass spectra and IR spectra (Figures S5 and S6 in Supplementary Information) of the products obtained from the final mixture of the probe and the added 3.0 equivalents of \(\hbox {Cu}^{2+}\) ions. As a result of hydrolysis of the probe followed by the ring opening, the increase of the emission intensity was observed with increasing addition of \(\hbox {Cu}^{2+}\) ions (Figure 2). It may be due to more and more hydrolysis of L in this condition followed by the formation of copper(II) complex of the fragmented part, irreversibly (Figure S5 on SI). However, no colorimetric change or no fluorescence enhancement of L occurred in the presence of other ions but interestingly, changes in color/fluorescence was observed only in the presence of \(\hbox {Cu}^{2+}\) ions in similar condition. Based on the MS spectral analysis, it has also been observed that the selective \(\hbox {Cu}^{2+}\) ions-assisted hydrolysis has occurred and it is different from the previous reports.[43,44,45] The emission and absorption spectra of L in the presence of \(\hbox {Cu}^{2+}\) ions was measured for a time period of 30 min and it was found that after \({\sim }20\,\hbox {min}\) the emission and absorbance reach a saturation level (Figure 4 and Figure S7 in SI). From the above discussions, the plausible mechanistic pathway of selective sensing \(\hbox {Cu}^{2+}\) ions by the probe L is presented in Scheme 2.
3.5 Analytical figure of merit
To investigate the selectivity, representative ions such as \(\hbox {Na}^{+}\), \(\hbox {K}^{+}\), \(\hbox {Ca}^{2+}\), \(\hbox {Mg}^{2+}\), \(\hbox {Cr}^{3+}\), \(\hbox {Mn}^{2+}\), \(\hbox {Fe}^{2+}\), \(\hbox {Fe}^{3+}\), \(\hbox {Co}^{2+}\), \(\hbox {Ni}^{2+}\), \(\hbox {Cu}^{2+}\), \(\hbox {Zn}^{2+}\), \(\hbox {Cd}^{2+}\), \(\hbox {Hg}^{2+}\), \(\hbox {Pb}^{2+}\), \(\hbox {Ba}^{2+}\), \(\hbox {Sr}^{2+}\), \(\hbox {Ag}^{+}\) and \(\hbox {Au}^{3+}\) ions were added to a solution of L, keeping the other experimental conditions unchanged. Only in the case of addition of \(\hbox {Cu}^{2+}\) ions a visible color change (colorless to pink) occurred along with an enhancement of fluorescence intensity. No significant change in the fluorescence intensity was observed with the addition of 10 equivalents (excess) of the above metal ions. Changes in the fluorescence spectra of L (\(20\,\upmu \hbox {M}\)) upon the addition of metal ions are also checked (Figure 5). This selectivity of \(\hbox {Cu}^{2+}\) ion over all other ions is due to the selective hydrolysis of L. From the linear response curve (Figure S8 in Supplementary Information), it is revealed that the probe exhibits a linearity with concentration of \(\hbox {Cu}^{2+}\) ions up to \(\sim 46\,\upmu \hbox {M}\).
Fluorescence intensity of L in presence of different metal ions (at \(\lambda _{{\mathrm{em}}} = 572\,\hbox {nm}\)). (A) probe (L), (B) \(\hbox {Co}^{2+}\), (C) \(\hbox {Mn}^{2+}\), (D) \(\hbox {Al}^{3+}\), (E) \(\hbox {Fe}^{2+}\), (F) \(\hbox {Mg}^{2+}\), (G) \(\hbox {Hg}^{2+}\), (H) \(\hbox {K}^{+}\), (I) \(\hbox {Pb}^{2+}\), (J) \(\hbox {Na}^{+}\), (K) \(\hbox {Fe}^{3+}\), (L) \(\hbox {Cd}^{2+}\), (M) \(\hbox {Cr}^{3+}\), (N) \(\hbox {Ba}^{2+}\), (O) \(\hbox {Ni}^{2+}\), (P) \(\hbox {Zn}^{2+}\), (Q) \(\hbox {Ca}^{2+}\), (R) \(\hbox {Cu}^{2+}\), (S) \(\hbox {Ag}^{+}\), and (T) \(\hbox {Au}^{3+}\).
Fluorescence and bright field images of AGS cells after incubation with L (\(20\,\upmu \hbox {M}\)) followed by addition of \(\hbox {Cu}^{2+}\) ions, \((1, 1')\) \(0\,\upmu \hbox {M}\), \((2, 2')\) \(10\,\upmu \hbox {M}\) and \((3, 3')\) \(20\,\upmu \hbox {M}\), respectively. All the samples were excited at 550 nm by using a [63X] objective.
The detection limit (LOD) was calculated from the calibration curve based on the fluorescence enhancement at 572 nm (Figure 6) by magnifying the lower concentration region of \(\hbox {Cu}^{2+}\) ions, using the equation \(3\sigma /\hbox {S}\), where S is the slope of the line, and \(\sigma \) is the standard deviation of seven replicate measurements of the zero level.[46] LOD was found to be 30.75 nM. This indicates the efficiency of this probe towards the detection of \(\hbox {Cu}^{2+}\) ions in the nanomolar range.
3.6 Application
To investigate the utility of this probe (L) in biological systems, it was applied to AGS cell line. Here, L and \(\hbox {Cu}^{2+}\) were allowed to be taken up consecutively by the cells and the images of the cells were captured by fluorescence microscopy by excitation at \(\sim 550\,\hbox {nm}\) (Figure 7). Additionally, in vitro study showed that the probe L is non-cytotoxic towards the cells up to 8.0 h (Figure S9 in SI). These results indicate that the probe has a huge potentiality for both in vitro and in vivo applications as a \(\hbox {Cu}^{2+}\) sensor as well as for live cell imaging.
4 Conclusions
A new fluorescence turn-on rhodamine-based Schiff base (L) has been synthesized and characterized by physicochemical and spectroscopic methods. The spectrophotometric color formation (colorless to reddish pink) and fluorimetric emission due to the selective \(\hbox {Cu}^{2+}\) ion assisted hydrolysis, followed by spirolactam ring opening of the L in 10% \(\hbox {CH}_{3}\hbox {CN}\) 20 mM HEPES buffer (pH 7.4) at room temperature helps to detect \(\hbox {Cu}^{2+}\) ions as low as 30 nM in physiological condition. Interestingly, the presence of several competitive ions did not affect this color change. This non-toxic probe is also useful to identify the distribution of \(\hbox {Cu}^{2+}\) ions in living cells.
5 Supporting Information (SI)
Supplementary Information associated with this article is available at www.ias.ac.in/chemsci.
References
Jung H S, Kwon P S, Lee J W, Kim J-II, Hong C S, Kim J W, Yan S, Lee J Y, Lee J H, Joo T and Kim J S 2009 Coumarin-derived \(\text{ Cu }^{2+}\)-selective fluorescence sensor: Synthesis, mechanisms, and applications in living cells J. Am. Chem. Soc. 131 2008
Linder M C and Azam M H 1996 Copper biochemistry and molecular biology Am. J. Clin. Nutr. 63 797S
Uauy R, Olivares M and Gonzalez M 1998 Essentiality of copper in humans Am. J. Clin. Nutr. 67 952S
Gaggelli E, Kozlowski H, Valensin D and Valensin G 2006 Copper homeostasis and neurodegenerative disorders (Alzheimer’s, Prion, and Parkinson’s diseases and amyotrophic lateral sclerosis) Chem. Rev. 106 1995
Zhou Y, Wang F, Kim Y, Kim S-J and Yoon J 2009 \(\text{ Cu }^{2+}\)-selective ratiometric and “Off-On” sensor based on the rhodamine derivative bearing pyrene group Org. Lett. 11 4442
Royzen M, Dai Z and Canary J W 2005 Ratiometric displacement approach to Cu(II) sensing by fluorescence J. Am. Chem. Soc. 127 1612
Barnham K J, Masters C L and Bush A I 2004 Neurodegenerative diseases and oxidative stress Nat. Rev. Drug Discovery 3 205
Hahn S H, Tanner M S, Danke D M and Gahl W A 1995 Normal metallothionein synthesis in fibroblasts obtained from children with indian childhood cirrhosis or copper-associated childhood cirrhosis Biochem. Mol. Med. 54 142
Brown D R 2001 Copper and prion disease Brain Res. Bull. 55 165
Waggoner D J, Bartnikas T B and Gitlin J D 1999 The role of copper in neurodegenerative disease Neurobiol. Dis. 6 221
Vulpe C, Levinson B, Whitney S, Packman S and Gitschier J 1993 Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase Nat. Genet. 3 7
Sarkar S, Roy S, Sikdar A, Saha R N and Panja S S 2013 A pyrene-based simple but highly selective fluorescence sensor for \(\text{ Cu }^{2+}\) ions via a static excimer mechanism Analyst 138 7119
EPA US (1991) Maximum contaminant level goals and national primary drinking water regulations for lead and copper; final rule, Federal Register 56, 26460
Yang W, Jaramillo D, Gooding J J, Hibbert D B, Zhang R, Willett G D and Fisher K J 2001 Sub-ppt detection limits for copper ions with Gly-Gly-His modified electrodes Chem. Commun. 37 1982
Becker J S, Matusch A, Depboylu C, Dobrowolska J and Zoriy M V 2007 Quantitative imaging of selenium, copper, and zinc in thin sections of biological tissues (Slugs-Genus Arion) measured by laser ablation inductively coupled plasma mass spectrometry Anal. Chem. 79 6074
Becker J S, Zoriy M, Matusch A, Wu B, Salber D, Palm C and Becker J S 2010 Bioimaging of metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) Mass Spectrom. Rev. 29 156
Ensafi A A, Khayamian T, Benvidi A and Mirmomtaz E 2006 Simultaneous determination of copper, lead and cadmium by cathodic adsorptive stripping voltammetry using artificial neural network Anal. Chim. Acta 561 225
Zheng Y, Huo Q, Kele P, Andreopoulos F M, Pham S M and Leblanc R M 2001 A new fluorescent chemosensor for copper ions based on tripeptide glycyl-histidyl-lysine (GHK) Org. Lett. 3 3277
Lai C Y, Trewyn B G, Jeftinija D M, Jeftinija K, Xu S, Jeftinija S and Lin V S Y 2003 A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules J. Am. Chem. Soc. 125 4451
Shao N, Zhang Y, Cheung S, Yang R, Chan W, Mo T, Li K and Liu F 2005 Copper ion-selective fluorescent sensor based on the inner filter effect using a spiropyran derivative Anal. Chem. 77 7294
Lim M H, Wong B A, Pitcock W H, Mokshagundam D, Baik M-H and Lippard S J 2006 Direct nitric oxide detection in aqueous solution by copper(II) fluorescein complexes J. Am. Chem. Soc. 128 14364
Mondal B, Lohar S, Pal S, Maji A and Chattopadhyay P 2015 A new chemosensor selective for \(\text{ Cu }^{2+}\) ions through fluorescence quenching approach applicable to real samples J. Indian Chem. Soc. 92 1867
Mohammad R G, Tahereh P, Leila H B, Shohre R, Mohammad Y, Maryam R K, Abolghasem M, Hossein A and Mojtaba S 2001 Highly selective and sensitive copper(II) membrane coated graphite electrode based on a recently synthesized Schiff’s base Anal. Chim. Acta 440 81
Klein G, Kaufmann D, Schürch S and Reymond J-L 2001 A fluorescent metal sensor based on macrocyclic chelation Chem. Commun. 37 561
Gatta’s-Asfura K M and Leblanc R M 2003 Peptide-coated CdS quantum dots for the optical detection of copper(II) and silver(I) Chem. Commun. 39 2684
Rurack K, Kollmannsberger M, Genger U R and Daub J 2000 A selective and sensitive fluoroionophore for \(\text{ Hg }^{{{\rm II}}}\), \(\text{ Ag }^{{{\rm I}}}\), and \(\text{ Cu }^{{{\rm II}}}\) with virtually decoupled fluorophore and receptor units J. Am. Chem. Soc. 122 968
He G, Zhao X, Zhang X, Fan H, Wu S, Li H, He C and Duan C 2010 A turn-on PET fluorescence sensor for imaging \(\text{ Cu }^{2+}\) in living cells New J. Chem. 34 1055
Zhao H, Wang Y, Liu Z and Dai B 2014 Specific Cu(II) detection using a novel tricarbazolyl-tristriazolotriazine based on photoinduced charge transfer RSC Adv. 4 13161
Pal S, Sen B, Mukherjee M, Dhara K, Zangrando E, Mandal S K, Khuda-Bukhsh A R and Chattopadhyay P 2014 Effect of substituents on FRET in rhodamine based chemosensors selective for \(\text{ Hg }^{2+}\) ions Analyst 139 1628
Xu Z, Xiao Y, Qian X, Cui J and Cui D 2005 Ratiometric and selective fluorescent sensor for \(\text{ Cu }^{{{\rm II}}}\) based on internal charge transfer (ICT) Org. Lett. 7 889
Sen B, Mukherjee M, Banerjee S, Pal S and Chattopadhyay P 2015 A rhodamine-based ‘turn-on’ \(\text{ Al }^{3+}\) ion-selective reporter and the resultant complex as a secondary sensor for \(\text{ F }^{-}\) ion are applicable to living cell staining Dalton Trans. 44 8708
Dessingiou J, Khedkar J K and Rao C P 2014 Chemosensing ability of hydroxynaphthylidene derivatives of hydrazine towards \(\text{ Cu }^{2+}\): Experimental and computational studies J. Chem. Sci. 126 1135
Gunupuru R, Maity D, Bhadu G R, Chakraborty A, Srivastava D N and Paul P 2014 Colorimetric detection of \(\text{ Cu }^{2+}\) and \(\text{ Pb }^{2+}\) ions using calix[4]arene functionalized gold nanoparticles J. Chem. Sci. 126 627
Reddy U G, Ali F, Taye N, Chattopadhyay S and Das A 2015 A new turn on \(Pd^{2+}\)-specific fluorescence probe and its use as an imaging reagent for cellular uptake in Hct116 cells Chem. Commun. 51 3649
Ali F, Saha S, Maity A, Taye N, Si M K, Suresh E, Ganguly B, Chattopadhyay S and Das A 2015 Specific Reagent for Cr(III): Imaging cellular uptake of Cr(III) in Hct116 cells and theoretical rationalization J. Phys. Chem. B 119 13018
Mahato P, Saha S, Das P, Agarwalla H and Das A 2014 An overview of the recent developments on \(\text{ Hg }^{2+}\) recognition RSC Adv. 4 36140
Praveen L, Saha S, Jewrajka S K and Das A 2013 Self-assembly of modified rhodamine-6G with tri-block copolymer: unusual vesicle formation, pH sensing and dye release properties J. Mater. Chem. B 1 1150
Mahato P, Saha S, Suresh E, Liddo R D, Parnigotto P P, Conconi M T, Kesharwani M K, Ganguly B and Das A 2012 Ratiometric detection of \(\text{ Cr }^{3+}\) and \(\text{ Hg }^{2+}\) by a naphthalimide-rhodamine based fluorescent probe Inorg. Chem. 51 1769
Suresh M, Ghosh A and Das A 2008 A simple chemosensor for \(\text{ Hg }^{2+}\) and \(\text{ Cu }^{2+}\) that works as a molecular keypad lock Chem. Commun. 3906
Dujols V, Ford F and Czarnik A W 1997 A long-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water J. Am. Chem. Soc. 119 7386
McClintock J L and Ceresa B P 2010 Transforming growth factor-\(\alpha \) enhances corneal epithelial cell migration by promoting EGFR recycling Invest. Opthalmol. Vis. Sci. 51 3455
Banerjee S, Dixit A, Shridharan R N, Karande A A and Chakravarty A R 2014 Endoplasmic reticulum targeted chemotherapeutics: the remarkable photo-cytotoxicity of an oxovanadium(IV) vitamin-B6 complex in visible light Chem. Commun. 50 5590
Wang S, Wang Z, Yin Y, Luo J and Kong L 2017 Coumarin-naphthol conjugated Schiff base as a “turn-on” fluorescent probe for \(\text{ Cu }^{2+}\) via selective hydrolysis of imine and its application in live cell imaging J. Photochem. Photobiol. A Chem. 333 213
Sahana S, Mishra G, Sivakumar S and Bharadwaj P K 2017 Highly sensitive and selective “turn-on” chemodosimeter based on \(\text{ Cu }^{2+}\) - promoted hydrolysis for nanomolar detection of \(\text{ Cu }^{2+}\) and its application in confocal cell imaging J. Photochem. Photobiol. A Chem. 334 47
Wang L, Yan J, Qin W, Liu W and Wang R 2012 A new rhodamine-based single molecule multianalyte (\(\text{ Cu }^{2+}\), \(\text{ Hg }^{2+}\)) sensor and its application in the biological system Dyes Pigm. 92 1083
Pal S, Lohar S, Mukherjee M, Chattopadhyay P and Dhara K 2016 A fluorescent probe for the selective detection of creatinine in aqueous buffer applicable to human blood serum Chem. Commun. 52 13706
Acknowledgements
The financial assistance from Department of Science and Technology, Govt. of West Bengal (DST, GoWB, vide Project No. 698 (Sanc.)/ST/P/S & T/15-G/2015) is gratefully acknowledged. S. Lohar is thankful to UGC, New Delhi, India for a fellowship. The authors are indebted to Dr. Abhishek Mukherjee, Indian Institute of Chemical Biology, Kolkata for cell imaging and cell viability study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maji, A., Lohar, S., Pal, S. et al. A new rhodamine based ‘turn-on’ \(\hbox {Cu}^{2+}\) ion selective chemosensor in aqueous system applicable in bioimaging. J Chem Sci 129, 1423–1430 (2017). https://doi.org/10.1007/s12039-017-1349-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-017-1349-4