Abstract
Two novel conjugated Schiff bases were prepared and characterized by IR, 1H NMR, and elementary analysis. Their fluorescence measurements indicated that both compounds have excellent performance on fluorescence emission, and the intensity of fluorescence emissions was enhanced sharply by adding Co2+. These compounds can be used as highly sensitive fluorescence turn-on sensors for Co2+ with the detection limit below 10−14 M.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
As an essential micronutrient, cobalt plays an important role in metabolic processes [1] of the human body. As a transition metal ion, cobalt supplementation benefits the formation of biological compounds such as Vitamin B12, which is widely responsible for the prevention of pernicious anemia [2]. The excess or lack of cobalt can cause a series of diseases [3]. Furthermore, in our daily life, cobalt is usually identified as the environmental pollutant. So, it is necessary to detect Co2+ for human health and environmental protection.
In recent years, many methods have been developed to trace the behaviors of Co2+ and to determine its concentration, which mainly refer to spectrophotometric [4, 5] and fluorophotometric [6] methods. Furthermore, chemists have made much progress in Co2+ detection [7–9]. Owing to the advantages in efficiency, sensitivity, and selectivity [10–12], the fluorophotometric method is widely used by many groups [13–15]. But the sensitivity [16] and selectivity [17] of the reported fluorescence sensors are not satisfactory. All these obstacles limit the development of Co2+ fluorescence probes. Therefore, it is important to search for new fluorophores of Co2+, especially those that can overcome the disadvantages.
As reported in the literature [18], the fluorescent chemosensors for transition metal ions are numerous [10, 19]. The sensors based on Schiff base are widely used. A novel probe has been developed to behave as a highly selective fluorescent pH sensor [20]. A sensitive turn-on fluorescent Zn2+ chemosensor based on Schiff base has been reported as applying to the detection of intracellular Zn2+ and could be used as a potential recyclable component in sensing materials [21]. Owing to a special fragment consisting of the C=N group with a lone electron pair, Schiff base can cooperate with many metal ions [19]. However, according to the selectivity researches of many groups [22–24], rare Schiff base can detect Co2+. Up to now, there have been few reports on fluorescence chemical sensors based on Schiff base for Co2+. In this work, two new conjugated Schiff bases based on carbazole were synthesized and used as fluorescent chemosensors for Co2+. Experiments revealed that both compounds have superior sensitivity and can be used as turn-on fluorescence sensors for detection of Co2+.
Scheme 1 exhibits the synthesis routes of both compounds; the detailed experiments are shown in the supporting information. The 4-carbazol-9-ylbenzaldehyde was synthesized according to the previously reported method [25].
The optical properties of compounds 1 and 2 have been investigated by UV–Vis absorption and F-4500 fluorescence spectrometer. The spectra are shown in Fig. 1 and the optical data are listed in Table 1. Both compounds exhibited long-wavelength absorption bands centered at 376 and 333 nm, respectively, which could be assigned to the n-π* transition associated with imine chromophore [26, 27]. The maximum emission wavelengths (λ em) of both compounds were 495 and 397 nm, respectively, when excited at their maximum absorption wavelength (λ abs). The fluorescent intensity of compound 1 was 222 a.u. when the excitation voltage was 900 V. However, the compound 2 was 246 a.u. with a lower excitation voltage (400 V). The compound 1 was poorly fluorescent, which can be explained by the isomerization of the C=N double bonds in the excited state [28]. The isomerization could break a molecular coplanar structure and, consequently, have a great effect on the conjugation of compound 1. However, the special structure of compound 2 provided a steric hindrance effect, which restricted the isomerization of the C=N double bonds. In addition, from Fig. 1, we also found that the emission spectrum of compound 1 was broad. This was attributed to the structure of compound 1, and several allowed transitions occurred when excited due to the poor conjugacy.
Table 2 depicts the emission data of compound 1 and compound 2 in solvents with various polarities. It is easily seen that the fluorescence intensity of two compounds decreased with increasing solvent polarity, which can be explained by the “twisted intra-molecular charge transfer” (TICT) mode [29]. According to the TICT model and Table 2, the larger the solvent polarity, the more obvious the solvation for the non-emissive TICT state, so the compounds gave low fluorescence intensity in higher polar solvent. On the other hand, with the increase of solvent polarity, fluorescence spectra of two compounds showed a remarkable red-shift. For example, λ em of compound 1 was located at 451 nm in THF and red-shifted to 458 and 495 nm in Chloroform and DMSO, respectively. This was because the fluorophore has a larger dipole moment in the excited state than in the ground state, as the enhanced dipole–dipolar interactions caused by the increasing polarity of solvent will lead to a more significant energy level decrease for the exited state.
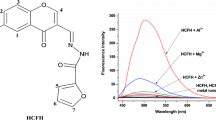
As shown in Fig. 2, the emission spectra for compounds were observed with Co2+ concentrations increasing from 1.0 × 10−14 to 1.0 × 10−8 M. The fluorescence emissions of compounds 1 and 2 could be enhanced 17-fold and 3.4-fold, respectively, when 1.0 × 10−8 M Co2+ was added to the solution. The improvement of the fluorescence originates from the strong chelation-enhanced fluorescence effect (CHEF) [30] between Schiff base and Co2+. Therefore, a large fluorescence enhancement was observed. This indicated that both compounds exhibited a high sensitivity to Co2+ in the solvent. The enhancement processes can be analyzed by the Stern–Volmer relationship,
where I 0 and I are the maximum fluorescence intensities in the absence and presence of Co2+, [Q] is the concentration of Co2+. By fitting the linear curve (Fig. 2), the Stern–Volmer fluorescence enhancement constant K sv of compound 1 and compound 2 were −0.00225 and −0.00436 L/mol, respectively. The results suggested that both exhibited a certain extent of sensitivity toward Co2+.
In addition, it is interesting that a blue shift occurs for compound 1 from 448 nm to 375 nm with the presence of Co2+; however, no shift occurs for compound 2. This indicated the Co2+ can significantly affect the excited state of compound 1, but have no effect on compound 2.
As a probe, the highly selective response to target ions is an essential requirement. The spectral changes were examined upon addition of various other metal ions. The experiments were carried out in DMSO with emission wavelengths at 495 nm for compound 1 and 397 nm for compound 2, respectively. As shown in Fig. 3, fluorescence emissions of the two compounds were significantly enhanced with Co2+. However, when the interfering ions, such as Cu2+, Cd2+, Zn2+, and Ni2+, were added individually to the solution of compounds, the fluorescence intensity of compound 1 has a relatively slight change, but the emission intensity of compound 2 was also dramatically enhanced. This indicated that the recognition to Co2+ for compound 1 was not significantly perturbed by other coexisting metal ions, and that compound 1 has a high selectivity toward Co2+. In contrast, compound 2 has a poor selectivity for Co2+.
In summary, two new conjugated Schiff base have been prepared. These compounds were easily obtained in high yield using low-cost commercially available materials. Significant emission enhancements in the wavelengths of the compounds were detected in the presence of Co2+. While compound 1 exhibited high selectivity for Co2+ over other metal ions with 17-fold fluorescence enhancement, when 1.0 × 10−8 M Co2+ ions were added, the selectivity of compound 2 was poor. Furthermore, the detection limit of the two compounds could reach below 10−14 M in solvents. This suggests that both compounds could serve as fluorescence probes for Co2+.
References
E.J. Underwood, Trace Elements in Human and Animal Nutrition, 4th edn. (Academic, New York, 1977)
C.D. Klaassen, Casarett and Doull’s Toxicology the Basic Science of Poisons. (McGraw-Hill, New York, 1999)
T.D. Luckey, B. Venugopal, A Metal Toxicity in Mammals, vol. 2 (Plenum, New York, 1979)
M.D.F.A. Ahmed, Y. Lingappa, Int. J. Curr. Pharm. Res. 3, 56–58 (2011)
V.S.A. Devi, V.K. Reddy, Int. J. Anal. Chem. doi: 10.1155/2012/981758
X.L. Wang, W.Y. Zheng, H.Y. Lin et al., Tetrahedron Lett. 50, 1536–1538 (2009)
D. Maity, T. Govindaraju, Inorg. Chem. 50, 11282–11284 (2011)
Y. Au-Yeung, E.J. New, C.J. Chang, Chem. Commun. 48, 5268–5270 (2012)
D. Maity, V. Kumar, T. Govindaraju, Org. Lett. 14, 6008–6011 (2012)
P. Li, L.B. Fang, H. Zhou, W. Zhang et al., Chem. Eur. J. 17, 10520–10523 (2011)
L. Zhou, P.Y. Cai, Y. Feng et al., Anal. Chim. Acta 735, 96–106 (2012)
W.H. Hsieh, C.F. Wan, D.J. Liao et al., Tetrahedron Lett. 53, 5848–5851 (2012)
M. Shamsipur, M. Sadeghi, K. Alizadeh et al., Anal. Chim. Acta 630, 57–66 (2008)
C.Y. Li, X.B. Zhang, Z. Jin et al., Anal. Chim. Acta 580, 143–148 (2006)
J.H. Ye, L.J. Duan, L.L. Jin, Adv. Mater. Res. 554–556, 2045–2048 (2012)
H.Y. Luo, X.B. Zhang, C.L. He et al., Spectrochim. Acta Part A 70, 337–342 (2008)
M. Montalti, L. Prodi, N. Zaccheroni, J. Mater. Chem. 15, 2810–2814 (2005)
C. Bargossi, M.C. Fiorini, M. Montalti et al., Coord. Chem. Rev. 208, 17–32 (2000)
A. Pui, Polyhedron 30, 2127–2131 (2011)
Y. Zhou, Z.X. Li, S.Q. Zang et al., Org. Lett. 14, 1214–1217 (2012)
U.C. Saha, K. Dhara, B. Chattopadhyay et al., Org. Lett. 13, 4510–4513 (2011)
S. Khatua, S.H. Choi, J. Lee, Inorg. Chem. 48, 1799–1801 (2009)
N. Xie, Y. Chen, Chin. J. Chem. 24, 1800–1803 (2006)
J.B. Li, Q.H. Hu, X.L. Yu et al., J. Fluoresc. 21, 2005–2013 (2011)
J.Y. Wu, Y.L. Pan, X.J. Zhang et al., Inorg. Chem. Acta. 360, 2083–2091 (2007)
H.H. Xu, X. Tao, Y.Q. Li et al., Spectrochim. Acta Part A 91, 375 (2012)
H.P. Deng, B.S. Zhu, L. Song et al., Polym. Chem. 3, 421–428 (2012)
J.S. Wu, W.M. Liu, X.Q. Zhuang et al., Org. Lett. 9, 33–36 (2007)
N. Sarkar, K. Das, D.N. Nath, K. Bhattacharyya, Langmuir 10, 326–329 (1994)
L. Wang, W. Qin, X. Tang et al., Phys. Chem. A. 115, 1609–1616 (2011)
Acknowledgments
This work is supported by Shandong Provincial Natural Science Foundation, China (ZR2011BM011) and A Project of Shandong Province Higher Educational Science and Technology Program (J12LD07).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bu, J., Duan, H., Wang, X. et al. New turn-on fluorescence sensors for Co2+ based on conjugated carbazole Schiff base. Res Chem Intermed 41, 2767–2774 (2015). https://doi.org/10.1007/s11164-013-1385-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1385-8