Abstract
Background and aims
Crop diversity has been repeatedly shown to support multiple ecosystem functions, both directly and indirectly, driven by interspecific root-root interactions. Despite continuous advances in this field, some research gaps remain, and we need to pay more attention to the design and management of multi-species and multi-cultivar systems in the future.
Scope
We review advances in intercropping in enhanced ecosystem functioning in competition-based and facilitation-based intercropping systems via root-root interactions. We also consider recent achievements in yield stability and soil fertility. We address several perspectives to focus on towards more sustainable agriculture via intercropping or cultivar mixtures in the future.
Conclusions
In competition-based systems, scramble competition via root-root competition and contest competition involving allelochemicals offset yield advantages of target crop species. However, niche differentiation and selection of desirable crop combinations to minimize negative effects through secondary metabolites may also help to gain yield advantages in intercropping and cultivar mixtures. In facilitation-based systems, selecting genotypes of facilitated species with root traits that best match the facilitator may strengthen the facilitative interactions in resource enrichment and disease and pest control. We need more long-term research to explore the effects of belowground processes on soil fertility, ecosystem stability, adaptation, and mitigation of climate change to establish sustainable agroecosystems in the future. It is also urgent to develop new methods to link belowground processes to functioning in multi-species and multi-cultivar agroecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crop diversification, including temporal diversification (e.g., rotations, multiple cropping, and cover crops after harvest of the main crop) and spatial crop diversification (e.g., intercropping, species mixtures, cultivar mixtures, cover crops and agroforestry), have repeatedly been shown to enhance ecosystem functioning, such as enhanced yield and stability, increased resource-use efficiency, enhanced soil fertility, reduced crop disease, and minimized environmental costs (Li et al. 2021b; Renard and Tilman 2019; Tamburini et al. 2020; Yang et al. 2021a). Competition, complementarity, and facilitation play pivotal roles in shaping community structure and driving productivity; the net outcome is determined by their relative strengths (Callaway 2007; Jiao et al. 2021a). A large number of case studies and reviews indicate that enhanced ecosystem functioning in diverse agroecosystems is derived from temporal and spatial niche complementarity (Brooker et al. 2015; Homulle et al. 2021; Li et al. 2020b; Yang et al. 2021a; Yu et al. 2015, 2016).
Aboveground complementarity promotes light interception and use efficiency by differences in crop height and light requirement; the greater temporal complementary use of resources also enhances yield benefits (Yu et al. 2015; Zhang et al. 2008). Belowground complementarity increases water and nutrient acquisition by niche differentiation (e.g., hydraulic lift by deep-rooting species) and resource partitioning (e.g., crop species use different forms of a given nutrient) (Homulle et al. 2021; Li et al. 2021a). Interspecific facilitation is also a main driver enhancing ecosystem functioning via resource enrichment, disease and pest protection, and toxin alleviation in multi-cropping systems (Brooker et al. 2021; Li et al. 2014, 2021a; Yang et al. 2021b).
Our evidence supports the complementarity theory for above- (Zhang et al. 2015b, 2017, 2021b) and belowground components (Li et al. 1999; Liu et al. 2015, 2020; Xia et al. 2013b). Numerous studies have emphasized the importance of belowground processes in positive biodiversity-ecosystem functioning (BEF) relationships in both natural ecosystems and agroecosystems (Brooker et al. 2015, 2021; Homulle et al. 2021; Li et al. 2014; Wright et al. 2017b; Yu et al. 2021). Therefore, this review mainly summarizes and synthesizes the advances in belowground processes that drive yield benefits and sustainability of intercropping systems.
We have spent 30 years exploring belowground mechanisms underlying enhanced ecosystem functioning in several intercropping combinations. Some experimental methods are widely used to uncover belowground interactions in intercropping systems. More evidence and belowground mechanisms have been uncovered around the world which broadened the view and promoted advances in crop-diversification research. Therefore, below, we first review some widely-accepted methods to study root-root interactions, and then summarize recent advances in belowground interspecific competition and facilitation that determine the outcome of diverse agroecosystems. We also focus on the effect of intercropping on mechanisms underlying maintaining or improving soil fertility. Then, we review the yield stability and acclimation to climate change via belowground processes in agroecosystems with intercropping. Finally, we provide some perspectives for future crop diversification research from the point of view of root-root interactions.
Methods to explore root-root interactions in intercropping
Root sampling and measurement are critical for understanding belowground interactions between plants in a real soil environment or under field condtions. Researchers have gradually overcome the difficulty in measuring and quantifying root systems (Freschet et al. 2021).
Excavation of roots is a method that can directly show the architectural, morphological and chemical traits of the root system (Böhm 2012; Freschet et al. 2021). Trenches are dug in vertical and horizontal planes to study root distribution in intercropping and monoculture plots in a field experiment (Li et al. 2006). The depth extends to 100 to 130 cm in different periods, and the width is 100 cm to facilitate excavation. After digging, the working face or wall of the profile is smoothed by carefully removing the soil. A cleaned profile is used to observe the root distribution of different species. Monolith excavation such as a “shovelomics approach” (Böhm 2012) usually takes soil monoliths from the soil surface and collects the root systems. Standard shovels are used to excavate a soil cylinder of 40 cm diameter and a depth of 25 cm with the plant in the center. Root crown architecture is evaluated after removing the soil adhering to the root crown (Trachsel et al. 2011).
Soil core samplings with a soil auger take a cylindrical volume of soil, and the roots are then separated from the soil by washing. In different experiments, the auger diameter should be adjusted to the mean root diameter and root length density, ranging from 2 to 10 cm. Soil cores (5.5 cm diameter) are collected at 10 cm intervals to a maximum depth of 100 cm to determine the vertical root distribution in intercropping and monocropping plots in different growing stages (Liu et al. 2015; Xia et al. 2013b; Wu and Guo 2014) developed a conventional soil core sampling method with wide corer (50 cm diameter, 55 cm deep), sampling machinery, and analysis software, providing a novel technique for quantifying the root architecture of field-grown crops.
Rhizoboxes are usually designed for glasshouse experiments to study root-root interaction with high precision. Rhizoboxes and pots with different barrier types are designed to determine the interactions between roots. A solid barrier eliminates root interactions and solute movement, making it similar to a monoculture; a mesh barrier prevents root intermingling but permits the exchange of root exudates. No barrier allows full root-root interactions, i.e. true intercropping (Garland et al. 2017; Li et al. 1999). Similar experiments have also been constructed in the field to study the effect of root interactions on crop growth; a trench (1 m deep, 0.1 m wide, and 8 m long) is excavated between the two crop strips and the root barrier material (nylon mesh or a flexible plastic sheet) are buried in each trench before sowing (Li et al. 2007; Wang et al. 2018). However, the method causes severe soil disturbance in field experiments in the first year. It probably gives more reliable results in the second year.
Minirhizotrons provided non-destructive, repeated monitoring of fine roots over time with tubes (typically 2–5 cm in diameter and up to 2 m in length) installed in the soil in which small video cameras or scanners are inserted at regular intervals (Hendrick and Pregitzer 1996; Iversen et al. 2012). Combined with results from minirhizotrons in the field and glasshouse experiments, researchers estimated the P facilitation by cluster roots to neighbor plants with non-cluster roots (Teste et al. 2020). In a rhizobox experiment, Zhang et al. (2020a) used a minirhizotron camera and showed that maize (Zea mays L.) roots grow along with faba bean (Vicia faba L.) roots in maize/faba bean intercropping. Compared with minirhizotrons, root windows are transparent viewing planes installed in soil with much larger viewing areas (Smit et al. 2000), which may be more suitable in root-root interaction studies.
Tracer techniques are usually applied by adding tracers to the soil dissolved in water either by spraying an area around target plants or by injecting them into the soil, then harvesting the plant after a certain period and measuring the tracer concentrations (Xiao et al. 2004; Hoekstra et al. 2014). Tracers are usually included in two classes: radioisotopes and stable isotopes. Radioisotopes were popular a few decades ago, they were used to determine root growth, activity, and distribution by injecting tracers into the soil at different depths and lateral distances from the target plants. For example, Hauggaard-Nielsen et al. (2001) used radioactive phosphorus (32P) to assess the root depth and lateral root distribution in barley (Hordeum vulgare L.)/pea (Pisum sativum L.) intercropping. Stable isotopes are usually used in more recent studies, which focus on nutrient and water uptake. For example, fertilizer labeled with enriched 15N was used to study the nitrogen (N) uptake of different plants at different times or depths or of different chemical forms which can determine niche differentiation between neighboring plants (von Felten et al. 2009).
The 15N natural abundance method is often used in studies of N cycling in ecosystems. Based on differences in 15N abundance between legumes, non-legumes, and atmospheric N2, this method can assess the reliance upon N2 fixation for legumes and the transfer of N from the legume to neighboring non-legumes (Peoples et al. 2015). For example, Chapagain and Riseman (2014, 2015) used this method to quantify N2 fixation by faba bean and pea, and subsequent transfer to non-legume neighbors. Similarly, 13C natural abundance can be used to separate roots from C3 and C4 plants (Hobbie and Werner 2004).
The collection and measurement of root exudates can help uncover nutrient-mediated and signal-mediated root-root interactions in intercropping systems. Oburger and Jones (2018) compared the methods to sample root exudates in different substrates. Measuring root-released or rhizosphere soil phosphatases, protons and carboxylates, shows the differences in P mobilization among species and reveals interspecific P facilitation between efficient P-mobilizing species and inefficient P-mobilizing species in intercropping (e.g., Li et al. 2007). The measurement of hormones and secondary metabolites may further help detect potential signaling components that trigger root-root competition (e.g., Kong et al. 2018) and facilitation (e.g., Li et al. 2016a).
Adopting these wide-accepted methods improves the exploration of root-root interaction in intercropping, which helps uncover belowground competition and facilitation between crop species in both field and glasshouse experiments.
Competition
Competition for resources is one of the fundamental mechanisms determining the structure and development of plant communities. In diverse agroecosystems, intra- and interspecific competition among crop individuals is vital for the growth and yield of components in multi-cropping systems (Vandermeer 1992). Compared with shoot competition for light, root competition is generally greater in nutrient-poor environments. Interspecific root competition is generally more important when the neighbor is a strong competitor, like a Poaceae species (Kiaer et al. 2013; Ravenek et al. 2016). Due to the simultaneous use of two or more crop species/cultivars in the same field, competition, especially between root systems, is considered a key factor that impacts growth and yield in diverse agroecosystems compared with monocultures (Caballero et al. 1995; Nassab et al. 2011). For example, when maize is grown next to wheat (Triticum aestivum L.), shoot growth and horizontal root distribution of maize are inhibited by the presence of wheat (Fig. 1).
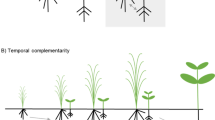
Field observations of competition-based and facilitation-based intercropping systems. (a) Above- and belowground performance of maize intercropped with wheat (at the left) and faba bean (at the right). Maize grown with wheat experiences strong competition. In contrast, the leaves of maize are greener when intercropped with faba bean. Photo credit: Long Li. (b) Compared with a monoculture, the lateral distribution of maize roots is suppressed in intercropping, while the roots of maize grow toward faba bean. Photo credit: Long Li. (c) Maize root distribution in monoculture, maize/wheat intercropping, and maize/faba bean intercropping. Maize roots cannot expand beneath the wheat area, while they grow in the faba bean area (Li et al. 2006)
Intra- and interspecific competition can be driven through resource depletion, i.e. scramble competition, and by root secondary metabolites, allelochemicals that inhibit the access of neighbors to resources, i.e. contest competition (Fig. 2, Schenk 2006). In scramble competition, root-root interactions are thought to mainly occur via water and nutrient competition whereby these resources acquired by an individual plant would no longer be available to a neighboring plant (Bechmann et al. 2014; Chen et al. 2012; Fransen et al. 2001; Guderle et al. 2018; Mommer et al. 2011). Competitive ability, earlier emergence, and the competition-recovery processes together determine the outcome of competition-based intercropping. The response of root plasticity in morphological and physiological to belowground resource use may be driven by competition for nutrients and water (Bechmann et al. 2014; Guderle et al. 2018).
Cereals show greater competitive ability than neighbors in belowground competition in barley/white lupin (Lupinus albus L.), wheat/white lupin, barley/vetch (Vicia sativa L.), wheat/vetch, wheat/maize intercropping systems, thus promoting shoot growth of cereals (Li et al. 2001a, b; Mariotti et al. 2009; Yin et al. 2020). The roots of intercropped wheat expand in the soil area under maize plants, exhibiting much greater root length density than in monoculture (Fig. 1c, Li et al. 2006; Ma et al. 2019a). The greater phenotypic plasticity of wheat roots attributes to the greater N uptake in intercropping (Liu et al. 2015). A field study further shows that rainfed condition promotes the horizontal distribution of intercropped wheat roots than supplementary irrigation treatment, strengthening the advantages of wheat (Ma et al. 2019a). In agroforestry systems, tree species exhibit a greater root competitive ability than crop species. Belowground competition in wheat/jujube (Zizyphus jujuba Mill.) and wheat/walnut (Juglans regia L.) agroecosystems results in variation of roots traits of both intercropped species (Wang et al. 2014a; Zhang et al. 2013, 2015a). The strength of interspecific competition is linked to the distance between different species. In addition, higher irrigation levels in apple (Malus pumila Mill.)/soybean (Glycine max (L.) Merr.) intercropping divert the fine roots of apple trees to the topsoil layer and intensify competition. The strong plasticity and the growth and distribution of roots increase water-use efficiency compared with monocultures (Fig. 2, Zheng et al. 2021).
Species that are sown earlier may explore the soil volume freely and occupy the dominant niche, while the later-sown species likely experience interspecific competition and inhibition during the co-growth stage (Homulle et al. 2021; Li et al. 2001b). After the early-maturing crop harvest, the late-maturing crops recover via root plasticity and use of extra nutrients under the area where the early-maturing crop was growing (Liu et al. 2015, 2020; Ma et al. 2020). Interspecific interactions lead to an increase for dominant species (e.g., wheat in wheat/maize intercropping), but a decrease for subordinate species (e.g., maize in wheat/maize intercropping) in terms of yield and nutrient acquisition during the co-growth stage (Li et al. 2001a). The yield benefit of wheat comes from the competitive advantages in the co-growth stage, and the yield and nutrient acquisition advantages of the subordinate species (e.g., maize) is derived from the recovery in the late growth stage after the dominant species has been harvested (Li et al. 2001b). A recovery effect for the late-maturing crop that follows the early-maturing crop has also been reported in wheat/soybean (Li et al. 2001a, b), maize/pigeon pea (Cajanus cajan L.) (Dalal 1974), maize/chickpea (Cicer arietinum L.) (Xia et al. 2013b), and maize/pea (Tan et al. 2020; Zhao et al. 2016) intercropping systems. Studies also showed that the growth trajectories in wheat/maize and barley /maize exhibit a temporal niche differentiation, which compensates the growth of intercropped maize after the earlier-maturing crop harvest (Zhang et al. 2015b, 2017). Intercropped crops with different growth periods lead to shifts from competition to complementarity via improved spatial-temporal niche differentiation.
In contest competition, allelopathy via root exudates affects belowground interactions in a species- and dose-specific manner and is common in both natural and agricultural ecosystems (Fig. 2, Bertin et al. 2003; Wang et al. 2021c). Previous studies have reviewed the main allelochemical compounds (Macias et al. 2019) and signaling molecules (Wang et al. 2021c) in root-root interactions. The main allelochemicals include phenolic compounds (e.g., simple phenolics, flavonoids, coumarins, quinones), terpenoids (e.g., monoterpenes, sesquiterpenes, diterpenes, triterpenes and steroids), and N-containing allelochemicals (e.g., alkaloids, benzoxazinoids) (Macias et al. 2019). The identification of signaling molecules is much more difficult than that of allelochemicals. Recent studies show that jasmonic acid, (-)-loliolide, ethylene, allantoin, canonical strigolactones and some noncanonical strigolactones are root-secreted signaling chemicals in root-root interactions (Lopez-Raez et al. 2017; Wang et al. 2021c). Allelochemicals have a negative effect on the receiving plants. For example, Kong et al. (2018) showed that when wheat is grown with 100 other plant species, wheat can detect neighbors and respond by increasing allelochemical release in a density-dependent manner. They further identified that (-)-loliolide and jasmonic acid are signaling molecules triggering allelochemical production in cereals (Kong et al. 2018; Li et al. 2020c).
A shift of the soil microbiome may mediate the effects of allelochemicals on target species (Fig. 2). A previous review indicates that microbiomes may enhance or alleviate allelopathic effects on the receiving plant species (Cipollini et al. 2012). For example, microbes may enhance the distribution of allelochemicals via common mycorrhizal networks which intensifies the negative effects; microorganisms may also degrade allelochemicals and enhance plant tolerance to allelochemicals in diverse plant communities (Cipollini et al. 2012). Another case study shows that cover crops may suppress weeds through allelochemicals, mainly at the early stage. The effects only last a limited time because soil microbes exhibit an antagonistic interaction with allelochemicals. Instead, microbiomes may suppress weeds in the later phase in agroecosystems (Luo et al. 2016a).
Plant species detect and recognize neighboring species and respond intraspecifically between kin and non-kin individuals via root exudates (Biedrzycki and Bais 2010; Biedrzycki et al. 2010; Dudley and File 2007; Murphy et al. 2017a). The first example of kin recognition was found in Cakile edentula, which exhibited different performances when grown with the same population than when growing with unrelated plants. When grown with a non-relative neighbor, C. edentula produced more root biomass and directed its root towards the stranger. However, the species tends to avoid belowground competition in the presence of a kin (Dudley and File 2007). Similar kin recognition patterns are also found in rice (Oryza sativa L.) (Yang et al. 2018) and soybean (Murphy et al. 2017b).
Yang et al. (2018) showed that an N-rich allantoin compound secreted by roots is important in kin recognition in rice. Xu et al. (2021) further grew allelopathic rice cultivars with kin pairs and non-kin pairs; they found that roots of target rice individuals grow more towards neighboring weeds than towards their relatives. The altered root replacement enhances interspecific competition with weeds and decreases the production of allelochemicals to inhibit weeds. This indicates that a desirable combination of relatedness allows plant cultivars to inhibit weeds more economically without compromising yield. Another study found that Pseudotsuga menziesii shares more carbon (C) to neighbor individuals through ectomycorrhizal networks in kin pairs than in non-kin pairs which may be mediated by signaling molecules (Pickles et al. 2017).
Therefore, when selecting crop combinations with a strong competitor, a sufficient recovery stage and great root trait plasticity are needed for the less competitive crop to overcompensate yield after the competitor is harvested (i.e. temporal niche differentiation). We also need to consider secondary metabolites-mediated contest competition when establishing intercropping systems. Notably, allelochemicals released by the target crop species is a double-edged sword, which may suppress the growth of neighboring crop species, while the desirable cultivar mixtures also inhibit the growth of weeds without comprising yield.
Mechanisms that drive interspecific competition and facilitation in intercropping. Interspecific competition may be driven by resource depletion of competition-dominated species via root traits (scramble competition). The phenotypic plasticity of root traits also plays an important role for competitive-dominated species to get advantages at co-growth stages and for subordinate species to recover after the harvest of dominant species. It is also mediated by the effects of secondary metabolites (e.g., allelochemicals) on the soil microbiome. Belowground facilitation might result from resource enrichment (e.g., N, P, micronutrients, and water). The root traits (e.g., root morphological traits and root exudate signaling molecules) that match facilitating and facilitated species may play important roles. The enrichment of beneficial microbiome, which attenuates negative plant-soil feedback, also contributes to interspecific facilitation involving disease, pathogen or pest protection
Facilitation
It is well-documented that enhanced performance in diverse plant communities is mainly derived from complementarity, which is usually considered as resource complementarity, niche partitioning and facilitation (Barry et al. 2019; Loreau and Hector 2001). A large body of case studies (e.g., Cardinale et al. 2007; Ceulemans et al. 2017; Giles et al. 2017; Tang et al. 2019; Zhang et al. 2014), theoretical research (e.g., Wang et al. 2021d), and reviews (e.g., Barry et al. 2019; Homulle et al. 2021; Li et al. 2020a, b) have illustrated the importance of resource complementarity and niche partitioning in the positive biodiversity-ecosystem functioning (BEF) relationship in natural systems and agroecosystems. For example, in a maize/faba bean intercropping system, the maize grows better than of maize grown next to wheat (Fig. 1a). We showed that the shallow roots of faba bean have a limited lateral root distribution and allow intercropped maize roots to extend under faba bean plants and explore a larger soil volume for nutrients (i.e. spatial complementarity, Fig. 1b-c, Li et al. 2006).
Facilitation occurs when the presence of one or more species increases the performance of neighboring species (Callaway 2007). In a facilitation-dominated crop combination, the first-harvested crop species (e.g., faba bean) obtains a yield advantage, and the neighboring crop species (e.g., maize) may have the same yield as the monocrop, or overyield in the co-growth stage, i.e. a win-win situation. Facilitative mechanisms that explain species-specific overyielding in diverse plant communities include direct abiotic facilitation (e.g., aboveground microclimate amelioration and belowground resource enrichment), and indirect biotic facilitation (e.g., microbe-mediated nutrient mobilization, pathogen and pest protection, and biotic feedback) (Fig. 2, Brooker et al. 2021; Li et al. 2014; Wright et al. 2017b; Yu et al. 2021).
Interspecific N facilitation through N2 fixation by legume is a widespread mechanism in diverse natural ecosystems and agroecosystems (Fig. 2). Legume/cereal intercropping has been widely practiced as a sustainable agricultural system, because legumes can increase ecosystem N input by symbiotic N2 fixation. A recent study showed that yield advantages in maize/soybean and maize/peanut (Arachis hypogaea L.) are related to a positive complementarity effect in the absence of N fertilization. This suggests facilitation of N acquisition by intercropped legumes (Zhang et al. 2021b). Nitrogen can be transferred from legumes to neighbors through root exudates, common mycorrhizal networks, or rhizodeposits (Li et al. 2009a; Thilakarathna et al. 2016; van der Heijden and Horton 2009; Wahbi et al. 2016; Zhang et al. 2020b). Notably, N facilitation acts in a reciprocal manner in intercropping systems, i.e. legumes not only enhance N uptake of neighboring cereals, but specific cereals also promote N2 fixation of legumes by root exudates (Li et al. 2016a).
Intercropping legumes with cereal crops enhances the biological N2 fixation of legumes (Fan et al. 2006; Xiao et al. 2004). This is because of the greater competitive ability of maize for soil mineral N which reduces nitrate accumulation (Li et al. 2005, 2011), and alleviates the inhibitory effect of N on legume nodulation and N2 fixation, enhancing N2 fixation in intercropped legumes (Li et al. 2009b). Wheat and barley exhibit a greater competitive ability for soil N than maize (Zhang et al. 2015b, 2017); however, a stimulatory effect on faba bean is not observed in wheat/faba bean and barley/faba bean intercropping systems (Fan et al. 2006). Thus, we surmise this relationship is species-specific. Combining field studies with glasshouse experiments, we found that root exudates of maize promote faba bean nodulation, whereas exudates of wheat and barley do not. Faba bean treated with root exudates of maize elicits a two-fold increase in exudation of flavonoids, and an increased expression of genes mediating nodulation and auxin (Li et al. 2016a). Genistein, a flavonoid released from maize roots, plays an important role as a signaling molecule in this facilitative root-root interaction.
Intercropping may also increase the abundance of some N2-fixing microbes, e.g., Rhizobium hainanense, Rhizobium leguminosarum, and Frankia (Chen et al. 2018). Wheat exudates probably enhance nodulation of neighboring faba bean by specific flavonoids such as flavonol, isoflavone, chalcone and hesperetin (Liu et al. 2017). Using 13 C-DNA-based stable isotope probing, Hu et al. (2021) further identified nine key bacterial genera (i.e. Agromyces, Arthrobacter, Bacillus, Gemmatimonas, Heliobacillus, Lysobacter, Natronocella, Paenibacillus and Sorangium) in the rhizosphere of the faba bean that are highly related to N2 fixation. These key bacteria may be affected by root exudates and contribute more than 50% of N2 fixation in intercropped faba bean (Hu et al. 2021). Therefore, there are three pathways for efficient use of N in cereal/legume intercropping via facilitation. One is facilitative effects on non-legume species via N transfer by legumes; the second is enhanced symbiotic N2 fixation of legumes which results from soil mineral N dilution due to competition for soil mineral N by cereals; the third is enhanced nodulation of legumes, driven by signaling molecules in root exudates from neighboring species and a shift of the soil microbiome.
Facilitation of P and micronutrient acquisition via root-root interactions and microbial processes are widely-accepted mechanisms driving overyielding in intercropping (Fig. 2, Dai et al. 2019; Dissanayaka et al. 2015; Khashi u Rahman et al. 2021; Li et al. 2014, 2016b; Tang et al. 2014, 2016; Xue et al. 2016; Zhang et al. 2016, 2020a). Efficient P-mobilizing legumes may mobilize either inorganic P, e.g., faba bean, or organic P, e.g., chickpea, by releasing large amounts of protons and carboxylates, or acid phosphatases, respectively (Li et al. 2003a, 2004, 2007, 2014; Zhou et al. 2009). The increased soil P availability facilitates the growth of neighboring species (Li et al. 2014).
Our field studies showed that grain yield and P uptake of intercropped faba bean and maize are greater than those of the corresponding crop species in monoculture (Li et al. 2003b; Zhang and Li 2003). In a meta-analysis, Tang et al. (2021b) showed that 90% of intercropping combinations exhibit P-uptake advantages. Interspecific P facilitation and P-use efficiency are greater when fertilizer input is reduced and may be associated with more N2 fixation of legumes (Tian et al. 2020; Xia et al. 2013a). We showed that the enhanced soil P availability by root exudates from legumes facilitates neighboring inefficient P-mobilizing species with root interactions in intercropping systems under supplies of both organic P and sparingly-soluble inorganic P (Li et al. 2003a, 2004, 2007, 2014). This facilitative interaction may not only save P fertilizer (Li et al. 2007), but also enhance apparent recovery of fertilizer P in intercropping in both infertile (Mei et al. 2012) as well as fertile soils (Xia et al. 2013a).
The strength of P facilitation also requires greater plasticity of root traits that allow the facilitated species to interact with the facilitator (Fig. 2, Yu et al. 2020a). Using different barrier types, we showed that species exhibiting a better match of root traits to a facilitator can obtain more benefits in a community (Yu et al. 2020a). In response to the facilitator, root trait plasticity is also affected by soil nutrient availability and neighbor identity (Abakumova et al. 2016; Schneider and Lynch 2020; Zhang et al. 2016, 2020a). In a tomato (Solanum lycopersicum L.) / onion (Allium cepa L.) intercropping system, root exudates of intercropped tomato shifted bacterial composition under different P-application sources. The increased abundance of P-solubilizing microbes is positively associated with plant-available soil P concentration, suggesting an interaction between root exudates and soil microbial composition to increase soil P availability in intercropping systems (Khashi u Rahman et al. 2021). Using a 41 K-labeling method, asymmetric interspecific facilitation via K exchange through common mycorrhizal networks in tomato/onion intercropping was shown (Gao et al. 2021). Based on our previous studies and recent advances in belowground root-root interactions, we proposed that belowground facilitation requires trait matching in natural ecosystems as well as agroecosystems (Yu et al. 2021).
A large body of research has indicated that maize enhanced iron (Fe) and zinc (Zn) uptake of legumes in intercropping (Zuo et al. 2000, 2003, 2004; Zuo and Zhang 2008, 2009). Field and glasshouse experiments showed that the phytosiderophores secreted by maize facilitate Fe acquisition of peanut in maize/peanut intercropping (Jiao et al. 2021b; Xiong et al. 2013). The dynamics in rhizosphere effects and molecular mechanisms in maize/peanut intercropping was summarized in a previous review (Dai et al. 2019). The shifts of microbial community composition and functioning may also directly or indirectly contribute to micronutrient facilitation (Dai et al. 2019), while belowground processes need further work to help better use intercropping to secure micronutrient supply in agroecosystems.
Beyond nutrient-uptake facilitation, diverse agroecosystems also promote water acquisition, protect plants against hazardous substances (e.g., metals and allelochemicals), diseases and pests via root-root interactions (direct abiotic facilitation) and plant-soil biota interactions (indirect biotic facilitation) (Fig. 2). Intercropping enhances water-use efficiency and saves water with mulching in arid regions (Mao et al. 2012). Hydraulic redistribution by roots and common mycorrhizal networks increases ecosystem functioning in agroforestry and intercropping systems (Dawson 1993; Pang et al. 2013; Singh et al. 2019), as has been covered in recent reviews (Bayala and Prieto 2019; Homulle et al. 2021). Specific species mixtures might also mitigate autotoxic effects and enhance nutrient availability through chemical facilitation and a shift of microbial composition (Xia et al. 2016, 2019). Intercropping upland rice with Solanum nigrum, a cadmium (Cd)-hyperaccumulating species, may mitigate Cd accumulation in rice. This is associated with shifts in rhizosphere processes in rice and S. nigrum, together with interactions between root exudates and AMF that block Cd uptake of rice and enhance Cd acquisition of S. nigrum (Yang et al. 2021b).
Cultivar mixtures and intercropping also reduce diseases and pests, increasing productivity via asymmetric facilitation of target species or cultivars and reducing the need for pesticides. The role of root exudates and microbiome in disease and pest inhibition in diverse plant communities includes negative density dependence, direct inhibition by root exudates, and indirect biotic facilitation by soil microbiome (Brooker et al. 2021; Mwakilili et al. 2021; Stomph et al. 2020; Zhang et al. 2019a, b; Zhu et al. 2000; Homulle et al. 2021; van Ruijven et al. 2020). Beyond the above mechanisms, root exudates of wheat may also induce positive microbial legacy effects, which suppress pathogens and increase microbes that are beneficial to intercropped faba bean in the long run (Wang et al. 2021a). Interactions among trophic levels may also contributes to plant health. For example, co-inoculation of AMF and earthworms may interactively enhance yield via increased nutrient availability (Zarea et al. 2009). Earthworms also inhibit infection by nematodes and increase plant resistance to herbivores by modulating plant chemistry and soil properties (Xiao et al. 2019). However, in a tomato/leek (Allium porrum L.) intercrop, the authors found that inoculation of AMF increases root-knot nematode infection, and earthworm and AMF co-inoculation does not facilitate plant growth (Detrey et al. 2022). Another study also showed that facilitative pairwise microbial interaction promotes pathogen invasion, while antagonistic interaction suppresses pathogen invasion in the tomato rhizosphere (Li et al. 2019). Therefore, specific microbiome and interkingdom combinations are needed to protect target species from pathogens and herbivore attacks in diverse agroecosystems.
This section reviews the mechanisms underlying belowground direct abiotic facilitation (e.g., resource enrichment) and indirect biotic facilitation (e.g., disease inhibition) in intercropping systems. Belowground facilitation may function in a universal manner through the amelioration of soil environments or in a species-specific fashion via the trait matching between facilitator and facilitated species (e.g., trait plasticity and root exudate signaling, Yu et al. 2021). Therefore, specific belowground processes between crop species should be considered to strengthen interspecific facilitation in future intercropping design.
Belowground processes and soil fertility in agroecosystems with intercropping
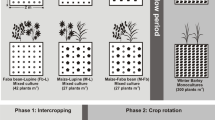
As reviewed above, intercropping enhances grain yield and nutrient uptake via belowground processes, while it also means multi-cropping removes more nutrients from the soil than monocultures. Therefore, it is crucial to understand whether soil fertility would increase, stay the same or decrease in the long run. Therefore, we established four long-term field experiments with substantial soil fertility gradients in Gansu province and Ningxia Autonomous Region, Northwest China, to examine the effects of intercropping on soil fertility and sustainability. Two experiments, conducted in fertile soils in Baiyun village and Wuwei City, Gansu province, were started in 2003 and 2009, respectively; the third experiment, on moderately fertile soils in Jingtan village, Baiyin City, Gansu province, was started in 2009; and the fourth experiment, in newly-reclaimed desert soils in Xingsheng village, Hongsibu County, Wuzhong City, Ningxia Autonomous Region, was also started in 2009 (Fig. 3). Here, we review the advances of the effects of diverse cropping systems on soil physical, chemical, and biological properties.
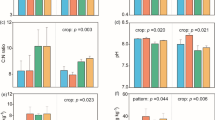
Soil physical properties are of fundamental importance in ensuring soil functions and ecosystem services (Rabot et al. 2018). As a key indicator of soil physical properties, soil aggregates significantly determine soil functions, such as protecting soil organic matter from microbial decomposition, affecting soil water and oxygen movement, as well as nutrient cycling and conditions for crop growth (Six et al. 2004; Tisdall and Oades 1982). Soil aggregation is affected by many factors, such as root traits, soil microorganisms and fauna, inorganic binding agents, and environmental variables (Six et al. 2004). Our long-term field experiments show that intercropping systems exhibit a greater macro-aggregate (> 2 mm) content than monocultures (Li et al. 2021b; Tian et al. 2019), which is in line with other experiments (Garland et al. 2017; Nyawade et al. 2019). This change could be associated with the changes of root traits, microorganisms, and soil environments in crop diversification (Fig. 4). First, root traits play a fundamental role in soil aggregation, including root biomass, root architecture, and root physiology. More root production in intercropping provides more binding agents for aggregate formation and stabilization (Chen et al. 2019b; Nyawade et al. 2019). More root exudates associated with root biomass act as binding agents of soil particles that contribute to soil aggregation (Peres et al. 2013). Species with finer roots enhance soil aggregation through entangling particles by roots and root hairs, e.g., Lolium perenne (grass), while species with thicker roots have a negative effect on soil aggregation, e.g., Trifolium repens (legume) (Gould et al. 2016). Second, changed soil microbiome and fauna in diverse systems affect aggregate formation. Tian et al. (2019) showed that intercropping enhances soil macro-aggregates by decreasing the relative abundance of soil Nitrospirae in poor-fertility soil, by increasing AMF biomass in medium-fertility soil, or by increasing the relative abundance of soil Sordariales in high-fertility soil. Fungal mycelium acts as a “sticky string bag” that entangles soil particles and cements particles together by producing extracellular polysaccharides. Mucilage exuded by bacteria and fungi also improves soil aggregation (Six et al. 2004). In contrast, increased earthworm biomass in diverse systems may destabilize soil aggregates due to increased mechanical breakdown (Peres et al. 2013). Finally, soil environmental variables induced by diversification, such as increased soil organic matter content and a decreased soil C: N ratio, indirectly affect aggregate formation. Soil organic matter is a crucial binding agent of soil aggregates, and a lower C: N ratio increases the formation of soil aggregates through enhanced microbial activity (Gamboa et al. 2020; Peres et al. 2013).
Belowground processes and mechanisms that may change soil fertility in intercropping. The greater root biomass likely drives belowground processes by increasing carbon (C) input in multi-species and multi-cultivar systems. The diverse root exudates may, directly and indirectly, affect soil C sequestration by specific soil microbiomes, macro-aggregate formation and physical protection, quality of root residual and decomposition rates. The biological N2 fixation by legumes also directly enhances nitrogen (N) sequestration or affects soil N storage through a shift of the soil microbiome. The changes of C and N sequestration via belowground processes in intercropping then may affect greenhouse gas emissions. However, the effects of root exudates on the soil microbiome, enzyme activity, deep soil C and N processes, or microbial effect on soil C and N sequestration, and the linkage between soil C and N storage and greenhouse gas emission needs further research (“?” and dashed lines)
Soil chemical properties, especially soil organic carbon (SOC) and total soil N, are essential for soil fertility and sustainability (Fig. 4, Lal 2004). Species diversity enhancing SOC and total soil N have been shown in grasslands (Lange et al. 2015; Yang et al. 2019), forests (Diaz et al. 2009), and agroecosystems (Cong et al. 2015b). For example, in a long-term intercropping experiment, Cong et al. (2015b) found that SOC content in the topsoil was 4% greater in intercropping systems than in monocultures. In general, SOC storage represents the net outcome of C inputs and losses (Houghton 2007). In agroecosystems, root inputs are the major source of SOC, thus increased root biomass in intercropping systems compared with monocultures provides a possible mechanism for increased SOC content (Cong et al. 2015b). However, some studies also show that intercropping does not affect SOC (Wang et al. 2014b, 2015, 2020a). This may contribute to greater microbial activities, increased litter decomposition of mixed roots in intercropping and negatively affect C storage (Cong et al. 2015a). A recently published study also suggests that changes in SOC by intercropping depend on soil characteristics (Li et al. 2021b).
In addition to topsoil, plant diversity also affects SOC stocks in deeper soil which probably contributes to a deeper root distribution in diversified systems compared with monocultures (Chen et al. 2020). Soil organic carbon in deeper soils is more stable and difficult to access for microbes. Thus, root inputs in deeper soil are the major source of SOC. In addition, by using advanced equipment, the root-root interaction research is going deeper (Thorup-Kristensen et al. 2020). However, the effects of root- and microbe-driven changes on deep soil C and N processes remain largely unknown (Fig. 4, Lal 2018). Sequestration of SOC is a slow process; more long-term studies are needed to accurately estimate the relationships between crop diversity and SOC.
Greater species diversity increases soil N content (Cong et al. 2014; Fornara and Tilman 2008). In a long-term intercropping experiment, Cong et al. (2015b) observed that soil organic N content in the topsoil was 11% greater in intercropping systems than in monocultures, and lower soil δ15N values in intercropping systems with legumes indicated that increased biological N2 fixation probably contributed to the increased soil N content (Fig. 4). However, no studies have quantified the relative contribution of biological N2 fixation in changes in soil N sequestration in intercropping. In the intercropping system without legumes, mechanisms of N retention, e.g., complementary N-acquisition strategies of intercropped crops and reduced N leaching, might explain the increased total soil N content (Cong et al. 2015b). Specific species and cultivar may recruit distinct microbiomes via root exudates in multi-species and multi-cultivar combinations, shifting soil microbiome composition and function (Sasse et al. 2018). However, the effects of root exudates on soil microbiome and their linkage with soil C and N sequestration remain largely unknown in crop diversification (Fig. 4).
Soil C and N cycling are also tightly linked with greenhouse gas emissions. Less C emissions and a lower C footprint may result in more C sequestration in intercropping systems than in monocultures (Fig. 4, Chai et al. 2021; Hu et al. 2015, 2016; Wang et al. 2020a). Thus, we surmise intercropping has the potential to sequester more SOC and reduce CO2 emission to the atmosphere. In a four-year investigation, sugarcane (Saccharum officinarum L.)/soybean intercropping had an insignificant impact on N2O emissions, while reduced N fertilizer exhibited a greater effect on mitigating N2O emissions than intercropping (Luo et al. 2016b). Intercropping could save N fertilizer, and the reduced N fertilizer input by intercropping might enhance N-use efficiency and N storage, thus decreasing N2O emission and mitigating climate change. Thus, further research is necessary to determine the linkage between soil C and N sequestration and greenhouse gas emission in crop diversification systems (Fig. 4).
Biological properties of soil fertility in this review include soil enzyme activity, microbial biomass, composition, and function. Soil microbes increase soil fertility by enhancing nutrient availability, aggregation, and formation of SOC (Fig. 4, Bardgett and van der Putten 2014). Intercropping can shift soil microbial communities (Dai et al. 2013; Song et al. 2007a, b; Zheng et al. 2018). For example, sugarcane/peanut and sugarcane/soybean intercropping increases soil C-, N-, and P-related enzyme activities, soil bacterial and fungal biomass and unique microbe numbers compared with sole cropping, increasing nutrient uptake and soil fertility (Li et al. 2013; Tang et al. 2021a). A meta-analysis that focused on the effects of plant diversity on microbial biomass also showed that species mixtures enhance soil microbial biomass, fungal biomass, bacterial biomass, and the fungi: bacteria ratio (Chen et al. 2019a). This is because intercropping changes the type and amount of root exudates that can target control over soil microbial communities (Hartmann et al. 2009). Extracellular enzymes produced by soil microbes can decompose organic residues to release nutrients for plant production and microbial growth (Curtright and Tiemann 2021). A recent meta-analysis shows that soil enzyme activities related to C, N, and P cycling are significantly increased, and the intercropping effects are determined by plant types and environmental factors (Curtright and Tiemann 2021). Increased soil enzyme activities accounted for microbial biomass and microbial activity mediated by higher quality and quantity of plant residue inputs in intercropping (Curtright and Tiemann 2021). Although soil microbes produce extracellular enzymes, the links between the abundance of functional genes and extracellular enzyme production are often difficult to establish (Fig. 4, Burns et al. 2013). Future studies should focus on the effects of intercropping on microbial community composition and function, especially on the relationship of soil extracellular enzymes and enzyme-coding genes.
Belowground processes and sustainability in diverse agroecosystems under climate change contexts
Climate change threatens food security globally (Hasegawa et al. 2018; Ray et al. 2015; Rosenzweig and Parry 1994). Species diversity can stabilize productivity in both natural and agroecosystems via complementarity effects, while the selection effect may impair the stability (Gross et al. 2014; Isbell et al. 2009; Raseduzzaman and Jensen 2017; Renard and Tilman 2019; Río et al. 2017; Tilman and Downing 1994; Wang et al. 2021d). Case studies and synthesis studies show that temporal stability is regulated by complementarity effects (e.g., species asynchrony), compensatory effects (e.g., insurance hypothesis), the traits and stability of dominant species, and phylogenetic diversity, rather than diversity itself (Fig. 5, Bai et al. 2004; Craven et al. 2018; Ma et al. 2017; Sasaki and Lauenroth 2011).
Compared with the generally positive effects of species diversity on temporal stability, diversity-spatial stability relationships depend on functional trait diversity and small-scale heterogeneity (Weigelt et al. 2008). In this research, rooting depth and clonal growth form are important to determine spatial stability (Weigelt et al. 2008). However, another study indicates that diversity increases spatial stability across ecosystems at the global scale, with the greatest positive effects in the most productive communities (Wang et al. 2019).
The temporal and spatial stability in diverse agroecosystems has received little attention compared with that of natural ecosystems. A meta-analysis showed that intercropping enhances yield temporal stability compared with the corresponding monocultures, and cereal/legume intercropping exhibits greater yield stability than a combination without legumes (Raseduzzaman and Jensen 2017). Another meta-analysis showed that cultivar mixtures generally exhibit greater temporal stability than monocultures (Reiss and Drinkwater 2018). Our results also support the notion that diverse intercropping systems increase yield stability over a decade. This is probably related to the increased yield advantage and soil fertility via belowground interaction (Li et al. 2021b).
Compared with the positive BEF relationship in ambient environments, the relationship is usually greater under future climate conditions, indicating communities with higher diversity are more resistant and resilience to climate change, probably mediated by complementarity and facilitation (Hong et al. 2021; Isbell et al. 2015; Jourdan et al. 2019; Wright et al. 2017a, 2021). In the Jena Experiment, plant species are less negatively affected by flooding in more diverse plant communities. The positive BEF relationship is related to the enhanced soil aeration by species with more root aerenchyma, which facilitates the performance of flood-sensitive plant species (Wright et al. 2017a). A similar result was also found in positive BEF relationships in plant communities that experience drought stress (Wright et al. 2021).
Linking belowground processes and sustainability in intercropping. Climatic factors drive the shifts in soil properties and intra- and interspecific interactions affecting yield stability. The shifts of soil properties induced by climate change may also affect root traits of a given species, and the specific species combination then affects root-root interactions. The shift of root-root interaction may also help the plant community acclimate to soil environments (e.g., under flooding or drought), affecting sustainability in agroecosystems. Root-root interactions under climate change also affect yield stability through competition, complementarity or facilitation. “?” and dashed lines indicate that the processes are still unclear and in need of further study
Acclimation in response to climate change in diverse plant communities is associated with the plasticity of root traits and belowground processes in response to climate change (Fig. 5). For example, in a maize/soybean intercropping with plastic film mulching, intercropped maize modified root growth with a confined strategy in drought environments and inhibited roots with excessive growth in wet environments. The greater intercropping benefits under varied soil water availability are related to the plasticity of root traits which may stabilize yield advantages in response to drought (Zhang et al. 2021a).
A warming treatment increased fine root biomass and N concentration and decreased root C: N ratio and nonstructural carbohydrate concentrations (Wang et al. 2021b). The effect of warming on plant growth also depends on specific traits of plant species. For example, tolerant C4 grasses tend to be less limited under warming than C3 grasses (Ward et al. 1999), and legumes may increase N2 fixation at higher temperatures (Gundale et al. 2012; Whittington et al. 2012). Elevated CO2 promotes root morphological development and root system expansion (Nie et al. 2013), while the impact of competitive species on co-occurring less-aggressive species becomes more severe under elevated CO2, because of increased interspecific root competition (Arnone and Kestenholz 1997). Therefore, we surmise elevated CO2 may intensify belowground interspecific competition of maize in intercropping systems (Fig. 5). Nitrogen deposition may significantly increase the proportion of coarse roots, root turnover and root respiration, while decreasing fine root C: N ratio and AMF colonization (Li et al. 2015). However, how these climate change factors separately and interactively affect root-root interaction, and how root-root interaction mitigate future climatic conditions via acclimation and amelioration of soil properties in diverse agroecosystems remains largely unknown (Fig. 5, Calleja-Cabrera et al. 2020).
Perspectives and conclusions
The ancient agronomic approach of intercropping in China is receiving increasing interest from plant scientists, ecologists, and agronomists worldwide. Although scientists have made considerable advances in diverse agroecosystems, we raise several perspectives in this thriving field.
First, new methods and proxies are needed to better understand root-root interactions in intercropping and cultivar mixtures. Multi-species genotyping by sequencing and Fourier-transform infrared spectroscopy are promising tools to quantify the relative proportion of root biomass in multi-species and multi-genotype communities (Streit et al. 2019; Wagemaker et al. 2021). Coupling zymography, planar optodes and different “layers” can visually and quantitatively unravel belowground processes for nutrient acquisition in sole cropping (Ma et al. 2019b, 2021). The plasticity of root traits has also not been linked with ecosystem functioning in diverse plant communities due to the difficulty of distinguishing roots among species. Therefore, machine learning and convolutional neural network are urgently needed to separate roots of different plant species (Shen et al. 2020). Another promising alternative approach to study the highly variable root exudates is leaf [Mn], which has repeatedly been shown to be an easily-measurable proxy for P-acquisition strategies involving the release of carboxylates or other P-mobilizing compounds. Leaf [Mn] can be used to assess carboxylate release in both native plants and crop species (Lambers 2022; Lambers et al. 2015, 2021; Pang et al. 2018; Yu et al. 2020a, b).
Second, diverse plant communities maintain multiple ecosystem functions and services simultaneously, i.e. multifunctionality, such as food production, C and N storage, and pollination. Recent studies have shown that most crop diversification systems enhance multiple ecosystem services and functions, while the components may involve trade-offs and offset positive effects via diversity (Beillouin et al. 2021; Tamburini et al. 2020; Wittwer et al. 2021). In addition, the evaluation of multifunctionality usually includes averaging approaches and multiple threshold approaches, while agroecosystems usually include limited species, and the weight of different components may not be equal in agroecosystems in different areas (Manning et al. 2018). Therefore, the approach to weaken trade-offs in multifunctionality and the evaluation methods are vital to understand the net effects of intercropping on agroecosystem functions.
Third, we suggest combining cultivar diversity and species diversity is a promising approach to enhance intraspecific complementarity and interspecific facilitation and reduce intraspecific competition (Cong et al. 2020; Yu et al. 2021). When integrating cultivar diversity and species diversity, multiple facets of diversity may affect multifunctionality via functional diversity, trait identity, and trait metrics (e.g., community-weighted trait, variance, skewness and kurtosis) (dos Santos et al. 2021; Mahaut et al. 2019; Zhang et al. 2019a, b). A better understanding of above- and belowground trait-related processes may help us link complementarity and enhanced ecosystem functions via efficient use of resources. As we reviewed, selecting cultivar mixtures with the aim of exudate-mediated kin recognition may efficiently and economically inhibit weeds. However, combining cultivars that vary in nutrient use and root depth also enhances nutrient uptake and grain yield, but this may require genotypes to be distantly related (Cong et al. 2020). In addition, a mixture of glutinous rice and rice hybrids that are resistant to rice blast (which are distantly related) may reduce disease by 94% compared with monocultures (Zhu et al. 2000). Therefore, enhancing weed or disease inhibition (i.e. reducing the need for herbicides and pesticides which enhances environmental profits) without compromising yield needs further work in cultivar mixtures.
In the longer term, we need to explore the effects of soil microbial legacies on ecosystem functioning (e.g., productivity and disease control) to manipulate species assembly order in rotational intercropping systems or mixtures (Delory et al. 2019, 2021; Wang et al. 2021a). This soil microbiome-mediated yield advantage in intercropping through inhibition of the negative plant-soil feedbacks of pathogens requires more attention, especially in communities with high chemical inputs (Brooker et al. 2021; Wang et al. 2021a). Therefore, long-term studies are needed to accurately estimate the effects of plant-soil feedback on positive BEF relationships.
The effects of structured soil on root growth and root-root interaction are often overlooked. Structured soil offers a mechanical resistance or physical anchorage for root growth and induces temporal and spatial heterogeneity in water and nutrient distribution and availability (Jin et al. 2017; Wang et al. 2020b). Heterogeneity in structured soils could alter root architectural, morphological (e.g., root hairs) and physiological traits (e.g., root exudate composition); it may also shift root-associated microbial communities and functions, which may further shift the effects of root-soil and root-root interactions (Wang et al. 2020b). Root growth and root-root interaction response to structured soils (e.g., soil mechanical impedance) may also depend on nutrient availability (Wang et al. 2021e). Future belowground process research in crop diversification should aim to understand root-root interactions in an integrated manner in a real soil environment.
Finally, it is urgent to explore the effects of root mixtures on soil C and N sequestration and environmental costs to mitigate climate change (Fig. 4, Lal 2004). Recent reviews indicate that the root effect on SOC is a double-edged sword, which depends on the outcome of rhizodeposition, root turnover and nutrient-acquisition strategies (Dijkstra et al. 2021; Ding et al. 2021). For example, root inputs increase new C production and facilitate organo-mineral complexation and aggregate formation, enhancing SOC stabilization. In addition, root exudates also have opposite effects on the above processes which causes SOC destabilization (Dijkstra et al. 2021). The effects also depend on P-acquisition and -use strategies and soil heterogeneity (Ding et al. 2021; Wang et al. 2020b). For example, carboxylate release may act as a most important factor affecting soil N mineralization via SOC decomposition, which may enhance loss of C to the atmosphere. Arbuscular mycorrhizal fungi, however, may stabilize organic-mineral associations and aggregates and facilitate C sequestration (Ding et al. 2021). When growing crop species with different nutrient-use strategies, soil C and N sequestration may increase, not change, or even decrease. Therefore, we need to consider the effects of root-root interactions on nutrient mobilization, soil fertility and mitigation of climate change when selecting crop combinations.
In this review, we summarized widely-accepted methods to study belowground interactions in intercropping systems which may be further developed combined with new techniques to uncover the hidden half of root-root interactions in both agroecosystems and natural ecosystems. Then, we summarized the belowground competition-recovery theory, root-root interactions driven by allelochemicals, direct facilitation by resource enrichment, and indirect facilitation by disease suppression via the microbiome, which shed light on the promising approach to establish desirable crop combinations in future intercropping systems. We strongly recommend the elegance of and need for integrating multiple complementary methods and experimental designs to understand processes across scales, including long-term experiments and spatial scales, to illuminate root-root interactions. To better understand the potential advantages of intercropping in response to future climate change, we also reviewed the advances in soil fertility changes with crop diversification, especially in the long run. How intercropping mitigates climate changes via root-root interactions and shifts in soil microbiomes may be a hotspot under a changing climate. We also need to pay more attention to the potential publication bias of positive intercropping effects on ecosystem functions. Gray literature that reports neutral or negative effects needs to be analyzed in future meta-analyses, likely with the help of new tools (e.g., CorTexT Platform).
References
Abakumova M, Zobel K, Lepik A, Semchenko M (2016) Plasticity in plant functional traits is shaped by variability in neighbourhood species composition. New Phytol 211:455–463. https://doi.org/10.1111/nph.13935
Arnone JA, Kestenholz C (1997) Root competition and elevated CO2: Effects on seedling growth in Linum usitatissimum populations and Linum-Silene cretica mixtures. Funct Ecol 11:209–214. https://doi.org/10.1046/j.1365-2435.1997.00069.x
Bai Y, Han X, Wu J, Chen Z, Li L (2004) Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431:181–184. https://doi.org/10.1038/nature02850
Bardgett RD, van der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515:505–511. https://doi.org/10.1038/nature13855
Barry KE, Mommer L, van Ruijven J, Wirth C, Wright AJ, Bai Y, Connolly J, De Deyn GB, de Kroon H, Isbell F, Milcu A, Roscher C, Scherer-Lorenzen M, Schmid B, Weigelt A (2019) The future of complementarity: disentangling causes from consequences. Trends Ecol Evol 34:167–180. https://doi.org/10.1016/j.tree.2018.10.013
Bayala J, Prieto I (2019) Water acquisition, sharing and redistribution by roots: applications to agroforestry systems. Plant Soil 453:17–28. https://doi.org/10.1007/s11104-019-04173-z
Bechmann M, Schneider C, Carminati A, Vetterlein D, Attinger S, Hildebrandt A (2014) Effect of parameter choice in root water uptake models-the arrangement of root hydraulic properties within the root architecture affects dynamics and efficiency of root water uptake. Hydrol Earth Syst Sci 18:4189–4206. https://doi.org/10.5194/hess-18-4189-2014
Beillouin D, Ben-Ari T, Malezieux E, Seufert V, Makowski D (2021) Positive but variable effects of crop diversification on biodiversity and ecosystem services. Glob Chang Biol 27:4697–4710. https://doi.org/10.1111/gcb.15747
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83. https://doi.org/10.1023/a:1026290508166
Biedrzycki ML, Bais HP (2010) Kin recognition: another biological function for root secretions. Plant Signal Behav 5:401–402. https://doi.org/10.4161/psb.5.4.10795
Biedrzycki ML, Jilany TA, Dudley SA, Bais HP (2010) Root exudates mediate kin recognition in plants. Commun Integr Biol 3:28–35. https://doi.org/10.4161/cib.3.1.10118
Böhm W (2012) Methods of studying root systems. Springer Science & Business Media, Berlin
Brooker RW, Bennett AE, Cong W, Daniell TJ, George TS, Hallett PD, Hawes C, Iannetta PP, Jones HG, Karley AJ, Li L, McKenzie BM, Pakeman RJ, Paterson E, Schob C, Shen J, Squire G, Watson CA, Zhang C, Zhang F, Zhang J, White PJ (2015) Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. New Phytol 206:107–117. https://doi.org/10.1111/nph.13132
Brooker RW, George TS, Homulle Z, Karley AJ, Newton AC, Pakeman RJ, Schöb C, Wright A (2021) Facilitation and biodiversity-ecosystem function relationships in crop production systems and their role in sustainable farming. J Ecol 109:2054–2067. https://doi.org/10.1111/1365-2745.13592
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol Biochem 58:216–234. https://doi.org/10.1016/j.soilbio.2012.11.009
Caballero R, Goicoechea EL, Hernaiz PJ (1995) Forage yields and quality of common vetch and oat sown at varying seeding ratios and seeding rates of vetch. Field Crops Res 41:135–140. https://doi.org/10.1016/0378-4290(94)00114-r
Callaway RM (2007) Positive interactions and interdependence in plant communities. Springer, Dordrecht
Calleja-Cabrera J, Boter M, Onate-Sanchez L, Pernas M (2020) Root growth adaptation to climate change in crops. Front Plant Sci 11:544. https://doi.org/10.3389/fpls.2020.00544
Cardinale BJ, Wright JP, Cadotte MW, Carroll IT, Hector A, Srivastava DS, Loreau M, Weis JJ (2007) Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc Natl Acad Sci USA 104:18123–18128. https://doi.org/10.1073/pnas.0709069104
Ceulemans T, Bodé S, Bollyn J, Harpole S, Coorevits K, Peeters G, Van Acker K, Smolders E, Boeckx P, Honnay O (2017) Phosphorus resource partitioning shapes phosphorus acquisition and plant species abundance in grasslands. Nat Plants 3:16224. https://doi.org/10.1038/nplants.2016.224
Chai Q, Nemecek T, Liang C, Zhao C, Yu A, Coulter JA, Wang Y, Hu F, Wang L, Siddique KHM, Gan Y (2021) Integrated farming with intercropping increases food production while reducing environmental footprint. Proc Natl Acad Sci USA 118:e2106382118. https://doi.org/10.1073/pnas.2106382118
Chapagain T, Riseman A (2014) Barley-pea intercropping: Effects on land productivity, carbon and nitrogen transformations. Field Crops Res 166:18–25. https://doi.org/10.1016/j.fcr.2014.06.014
Chapagain T, Riseman A (2015) Nitrogen and carbon transformations, water use efficiency and ecosystem productivity in monocultures and wheat-bean intercropping systems. Nutr Cycl Agroecosyst 101:107–121. https://doi.org/10.1007/s10705-014-9647-4
Chen BJW, During HJ, Anten NPR (2012) Detect thy neighbor: Identity recognition at the root level in plants. Plant Sci 195:157–167. https://doi.org/10.1016/j.plantsci.2012.07.006
Chen C, Chen H, Chen X, Huang Z (2019) Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat Commun 10:1332. https://doi.org/10.1038/s41467-019-09258-y
Chen C, Liu W, Wu J, Jiang X, Zhu X (2019) Can intercropping with the cash crop help improve the soil physico-chemical properties of rubber plantations? Geoderma 335:149–160. https://doi.org/10.1016/j.geoderma.2018.08.023
Chen J, Arafat Y, Wu L, Xiao Z, Li Q, Khan MA, Khan MU, Lin S, Lin W (2018) Shifts in soil microbial community, soil enzymes and crop yield under peanut/maize intercropping with reduced nitrogen levels. Appl Soil Ecol 124:327–334. https://doi.org/10.1016/j.apsoil.2017.11.010
Chen X, Chen H, Chen C, Ma Z, Searle EB, Yu Z, Huang Z (2020) Effects of plant diversity on soil carbon in diverse ecosystems: a global meta-analysis. Biol Rev 95:167–183. https://doi.org/10.1111/brv.12554
Cipollini D, Rigsby CM, Barto EK (2012) Microbes as targets and mediators of allelopathy in plants. J Chem Ecol 38:714–727. https://doi.org/10.1007/s10886-012-0133-7
Cong W, Hoffland E, Li L, Janssen BH, van der Werf W (2015) Intercropping affects the rate of decomposition of soil organic matter and root litter. Plant Soil 391:399–411. https://doi.org/10.1007/s11104-015-2433-5
Cong W, van Ruijven J, Mommer L, De Deyn GB, Berendse F, Hoffland E (2014) Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J Ecol 102:1163–1170. https://doi.org/10.1111/1365-2745.12280
Cong W, Hoffland E, Li L, Six J, Sun J, Bao X, Zhang F, Van Der Werf W (2015) Intercropping enhances soil carbon and nitrogen. Glob Change Biol 21:1715–1726. https://doi.org/10.1111/gcb.12738
Cong W, Suriyagoda LDB, Lambers H (2020) Tightening the phosphorus cycle through phosphorus-efficient crop genotypes. Trends Plant Sci 25:967–975. https://doi.org/10.1016/j.tplants.2020.04.013
Craven D, Eisenhauer N, Pearse WD, Hautier Y, Isbell F, Roscher C, Bahn M, Beierkuhnlein C, Bonisch G, Buchmann N, Byun C, Catford JA, Cerabolini BEL, Cornelissen JHC, Craine JM, De Luca E, Ebeling A, Griffin JN, Hector A, Hines J, Jentsch A, Kattge J, Kreyling J, Lanta V, Lemoine N, Meyer ST, Minden V, Onipchenko V, Polley HW, Reich PB, van Ruijven J, Schamp B, Smith MD, Soudzilovskaia NA, Tilman D, Weigelt A, Wilsey B, Manning P (2018) Multiple facets of biodiversity drive the diversity-stability relationship. Nat Ecol Evol 2:1579–1587. https://doi.org/10.1038/s41559-018-0647-7
Curtright AJ, Tiemann LK (2021) Intercropping increases soil extracellular enzyme activity: A meta-analysis. Agr Ecosyst Environ 319:107489. https://doi.org/10.1016/j.agee.2021.107489
Dai C, Chen Y, Wang X, Li P (2013) Effects of intercropping of peanut with the medicinal plant Atractylodes lancea on soil microecology and peanut yield in subtropical China. Agrofor Syst 87:417–426. https://doi.org/10.1007/s10457-012-9563-z
Dai J, Qiu W, Wang N, Wang T, Nakanishi H, Zuo Y (2019) From leguminosae/gramineae intercropping systems to see benefits of intercropping on iron nutrition. Front Plant Sci 10:605. https://doi.org/10.3389/fpls.2019.00605
Dalal RC (1974) Effects of intercropping maize with pigeon peas on grain yield and nutrient uptake. Exp Agric 10:219–224. https://doi.org/10.1017/s0014479700000454
Dawson ET (1993) Hydraulic lift and water use by plants: implications for water balance, performance and plant-plant interactions. Oecologia 95:565–574. https://doi.org/10.2307/4220484
Delory BM, Schempp H, Spachmann SM, Storzer L, van Dam NM, Temperton VM, Weinhold A (2021) Soil chemical legacies trigger species-specific and context-dependent root responses in later arriving plants. Plant Cell Environ 44:1215–1230. https://doi.org/10.1111/pce.13999
Delory BM, Weidlich EWA, von Gillhaussen P, Temperton VM, Spasojevic M (2019) When history matters: The overlooked role of priority effects in grassland overyielding. Funct Ecol 33:2369–2380. https://doi.org/10.1111/1365-2435.13455
Detrey J, Cognard V, Djian-Caporalino C, Marteu N, Doidy J, Pourtau N, Vriet C, Maurousset L, Bouchon D, Clause J (2022) Growth and root-knot nematode infection of tomato are influenced by mycorrhizal fungi and earthworms in an intercropping cultivation system with leeks. Appl Soil Ecol 169:104181. https://doi.org/10.1016/j.apsoil.2021.104181
Diaz S, Hector A, Wardle DA (2009) Biodiversity in forest carbon sequestration initiatives: not just a side benefit. Curr Opin Env Sust 1:55–60. https://doi.org/10.1016/j.cosust.2009.08.001
Dijkstra FA, Zhu B, Cheng W (2021) Root effects on soil organic carbon: a double-edged sword. New Phytol 230:60–65. https://doi.org/10.1111/nph.17082
Ding W, Cong W, Lambers H (2021) Plant phosphorus-acquisition and -use strategies affect soil carbon cycling. Trends Ecol Evol 36:899–906. https://doi.org/10.1016/j.tree.2021.06.005
Dissanayaka DMS, Maruyama H, Masuda G, Wasaki J (2015) Interspecific facilitation of P acquisition in intercropping of maize with white lupin in two contrasting soils as influenced by different rates and forms of P supply. Plant Soil 390:223–236. https://doi.org/10.1007/s11104-015-2392-x
dos Santos D, Joner F, Shipley B, Teleginski M, Renata Rodrigues R, Siddique I (2021) Crop functional diversity drives multiple ecosystem functions during early agroforestry succession. J Appl Ecol 58:1718–1727. https://doi.org/10.1111/1365-2664.13930
Dudley SA, File AL (2007) Kin recognition in an annual plant. Biol Lett 3:435–438. https://doi.org/10.1098/rsbl.2007.0232
Fan F, Zhang F, Song Y, Sun J, Bao X, Guo T, Li L (2006) Nitrogen fixation of faba bean (Vicia faba L.) interacting with a non-legume in two contrasting intercropping systems. Plant Soil 283:275–286. https://doi.org/10.1007/s11104-006-0019-y
Fornara DA, Tilman D (2008) Plant functional composition influences rates of soil carbon and nitrogen accumulation. J Ecol 96:314–322. https://doi.org/10.1111/j.1365-2745.2007.01345.x
Fransen B, de Kroon H, Berendse F (2001) Soil nutrient heterogeneity alters competition between two perennial grass species. Ecology 82:2534–2546. https://doi.org/10.1890/0012-9658(2001)082[2534:SNHACB]2.0.CO;2
Freschet GT, Pages L, Iversen CM, Comas LH, Rewald B, Roumet C, Klimesova J, Zadworny M, Poorter H, Postma JA, Adams TS, Bagniewska-Zadworna A, Bengough AG, Blancaflor EB, Brunner I, Cornelissen JHC, Garnier E, Gessler A, Hobbie SE, Meier IC, Mommer L, Picon-Cochard C, Rose L, Ryser P, Scherer-Lorenzen M, Soudzilovskaia NA, Stokes A, Sun T, Valverde-Barrantes OJ, Weemstra M, Weigelt A, Wurzburger N, York LM, Batterman SA, de Moraes MG, Janecek S, Lambers H, Salmon V, Tharayil N, McCormack ML (2021) A starting guide to root ecology: strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol 232:973–1122. https://doi.org/10.1111/nph.17572
Gamboa CH, Vezzani FM, Kaschuk G, Favaretto N, Cobos JYG, da Costa GA (2020) Soil-root dynamics in maize-beans-eggplant intercropping system under organic management in a subtropical region. J Soil Sci Plant Nut 20:1480–1490. https://doi.org/10.1007/s42729-020-00227-9
Gao D, Pan X, Khashi u Rahman M, Zhou X, Wu F (2021) Common mycorrhizal networks benefit to the asymmetric interspecific facilitation via K exchange in an agricultural intercropping system. Biol Fert Soils 57:959–971. https://doi.org/10.1007/s00374-021-01561-5
Garland G, Bunemann EK, Oberson A, Frossard E, Six J (2017) Plant-mediated rhizospheric interactions in maize-pigeon pea intercropping enhance soil aggregation and organic phosphorus storage. Plant Soil 415:37–55. https://doi.org/10.1007/s11104-016-3145-1
Giles CD, Brown LK, Adu MO, Mezeli MM, Sandral GA, Simpson RJ, Wendler R, Shand CA, Menezes-Blackburn D, Darch T, Stutter MI, Lumsdon DG, Zhang H, Blackwell MS, Wearing C, Cooper P, Haygarth PM, George TS (2017) Response-based selection of barley cultivars and legume species for complementarity: Root morphology and exudation in relation to nutrient source. Plant Sci 255:12–28. https://doi.org/10.1016/j.plantsci.2016.11.002
Gould IJ, Quinton JN, Weigelt A, De Deyn GB, Bardgett RD (2016) Plant diversity and root traits benefit physical properties key to soil function in grasslands. Ecol Lett 19:1140–1149. https://doi.org/10.1111/ele.12652
Gross K, Cardinale BJ, Fox JW, Gonzalez A, Loreau M, Polley HW, Reich PB, van Ruijven J (2014) Species richness and the temporal stability of biomass production: A new analysis of recent biodiversity experiments. Am Nat 183:1–12. https://doi.org/10.1086/673915
Guderle M, Bachmann D, Milcu A, Gockele A, Bechmann M, Fischer C, Roscher C, Landais D, Ravel O, Devidal S, Roy J, Gessler A, Buchmann N, Weigelt A, Hildebrandt A (2018) Dynamic niche partitioning in root water uptake facilitates efficient water use in more diverse grassland plant communities. Funct Ecol 32:214–227. https://doi.org/10.1111/1365-2435.12948
Gundale MJ, Nilsson M, Bansal S, Jaderlund A (2012) The interactive effects of temperature and light on biological nitrogen fixation in boreal forests. New Phytol 194:453–463. https://doi.org/10.1111/j.1469-8137.2012.04071.x
Hartmann A, Schmid M, Tuinen Dv, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321:235–257. https://doi.org/10.1007/s11104-008-9814-y
Hasegawa T, Fujimori S, Havlík P, Valin H, Bodirsky BL, Doelman JC, Fellmann T, Kyle P, Koopman JFL, Lotze-Campen H, Mason-D’Croz D, Ochi Y, Pérez Domínguez I, Stehfest E, Sulser TB, Tabeau A, Takahashi K, van Takakura Jy H, van Zeist W-J, Wiebe K, Witzke P (2018) Risk of increased food insecurity under stringent global climate change mitigation policy. Nat Clim Chang 8:699–703. https://doi.org/10.1038/s41558-018-0230-x
Hauggaard-Nielsen H, Ambus P, Jensen ES (2001) Temporal and spatial distribution of roots and competition for nitrogen in pea-barley intercrops-a field study employing 32P technique. Plant Soil 236:63–74. https://doi.org/10.1023/A:1011909414400
Hendrick RL, Pregitzer KS (1996) Applications of minirhizotrons to understand root function in forests and other natural ecosystems. Plant Soil 185:293–304. https://doi.org/10.1007/bf02257535
Hobbie EA, Werner RA (2004) Intramolecular, compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: a review and synthesis. New Phytol 161:371–385. https://doi.org/10.1111/j.1469-8137.2004.00970.x
Hoekstra NJ, Finn JA, Buchmann N, Gockele A, Landert L, Prill N, Scherer-Lorenzen M, Lüscher A (2014) Methodological tests of the use of trace elements as tracers to assess root activity. Plant Soil 380:265–283. https://doi.org/10.1007/s11104-014-2048-2
Homulle Z, George TS, Karley AJ (2021) Root traits with team benefits: understanding belowground interactions in intercropping systems. Plant Soil 471:1–26. https://doi.org/10.1007/s11104-021-05165-8
Hong P, Schmid B, De Laender F, Eisenhauer N, Zhang X, Chen H, Craven D, De Boeck HJ, Hautier Y, Petchey OL, Reich PB, Steudel B, Striebel M, Thakur MP, Wang S (2021) Biodiversity promotes ecosystem functioning despite environmental change. Ecol Lett. https://doi.org/10.1111/ele.13936
Houghton RA (2007) Balancing the global carbon budget. Annu Rev Earth Planet Sci 35:313–347. https://doi.org/10.1146/annurev.earth.35.031306.140057
Hu F, Chai Q, Yu A, Yin W, Cui H, Gan Y (2015) Less carbon emissions of wheat-maize intercropping under reduced tillage in arid areas. Agron Sustain Dev 35:701–711. https://doi.org/10.1007/s13593-014-0257-y
Hu F, Gan Y, Cui H, Zhao C, Feng F, Yin W, Chai Q (2016) Intercropping maize and wheat with conservation agriculture principles improves water harvesting and reduces carbon emissions in dry areas. Eur J Agron 74:9–17. https://doi.org/10.1016/j.eja.2015.11.019
Hu H, Li H, Hao M, Ren Y, Zhang M, Liu R, Zhang Y, Li G, Chen J, Ning T, Kuzyakov Y (2021) Nitrogen fixation and crop productivity enhancements co-driven by intercrop root exudates and key rhizosphere bacteria. J Appl Ecol 58:2243–2255. https://doi.org/10.1111/1365-2664.13964
Isbell F, Craven D, Connolly J, Loreau M, Schmid B, Beierkuhnlein C, Bezemer TM, Bonin C, Bruelheide H, De Luca E (2015) Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526:574–577. https://doi.org/10.1038/nature15374
Isbell FI, Polley HW, Wilsey BJ (2009) Biodiversity, productivity and the temporal stability of productivity: patterns and processes. Ecol Lett 12:443–451. https://doi.org/10.1111/j.1461-0248.2009.01299.x
Iversen CM, Murphy MT, Allen MF, Childs J, Eissenstat DM, Lilleskov EA, Sarjala TM, Sloan VL, Sullivan PF (2012) Advancing the use of minirhizotrons in wetlands. Plant Soil 352:23–39. https://doi.org/10.1007/s11104-011-0953-1
Jiao N, Wang J, Ma C, Zhang C, Guo D, Zhang F, Jensen ES (2021) The importance of aboveground and belowground interspecific interactions in determining crop growth and advantages of peanut/maize intercropping. Crop J 9:1460–1469. https://doi.org/10.1016/j.cj.2020.12.004
Jiao N, Wang F, Ma C, Zhang F, Jensen ES (2021) Interspecific interactions of iron and nitrogen use in peanut (Arachis hypogaea L.)-maize (Zea mays L.) intercropping on a calcareous soil. Eur J Agron 128:11. https://doi.org/10.1016/j.eja.2021.126303
Jin K, White PJ, Whalley WR, Shen J, Shi L (2017) Shaping an optimal soil by root-soil interaction. Trends Plant Sci 22:823–829. https://doi.org/10.1016/j.tplants.2017.07.008
Jourdan M, Kunstler G, Morin X (2019) How neighbourhood interactions control the temporal stability and resilience to drought of trees in mountain forests. J Ecol 108:666–677. https://doi.org/10.1111/1365-2745.13294
Khashi u Rahman M, Wang X, Gao D, Zhou X, Wu F (2021) Root exudates increase phosphorus availability in the tomato/potato onion intercropping system. Plant Soil 464:45–62. https://doi.org/10.1007/s11104-021-04935-8
Kiaer LP, Weisbach AN, Weiner J, Gibson D (2013) Root and shoot competition: a meta-analysis. J Ecol 101:1298–1312. https://doi.org/10.1111/1365-2745.12129
Kong C, Zhang S, Li Y, Xia Z, Yang X, Meiners SJ, Wang P (2018) Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat Commun 9:3867. https://doi.org/10.1038/s41467-018-06429-1
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304:1623–1627. https://doi.org/10.1126/science.1097396
Lal R (2018) Digging deeper: A holistic perspective of factors affecting soil organic carbon sequestration in agroecosystems. Glob Chang Biol 24:3285–3301. https://doi.org/10.1111/gcb.14054
Lambers H (2022) Phosphorus acquisition and utilization in plants. Annu Rev Plant Biol 73. https://doi.org/10.1146/annurev-arplant-102720-125738
Lambers H, Hayes PE, Laliberté E, Oliveira RS, Turner BL (2015) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90. https://doi.org/10.1016/j.tplants.2014.10.007
Lambers H, Wright IJ, Guilherme Pereira C, Bellingham PJ, Bentley LP, Boonman A, Cernusak LA, Foulds W, Gleason SM, Gray EF, Hayes PE, Kooyman RM, Malhi Y, Richardson SJ, Shane MW, Staudinger C, Stock WD, Swarts ND, Turner BL, Turner J, Veneklaas EJ, Wasaki J, Westoby M, Xu Y (2021) Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant Soil 461:43–61. https://doi.org/10.1007/s11104-020-04690-2
Lange M, Eisenhauer N, Sierra CA, Bessler H, Engels C, Griffiths RI, Mellado-Vazquez PG, Malik AA, Roy J, Scheu S, Steinbeiss S, Thomson BC, Trumbore SE, Gleixner G (2015) Plant diversity increases soil microbial activity and soil carbon storage. Nat Commun 6:6707. https://doi.org/10.1038/ncomms7707
Li B, Li Y, Wu H, Zhang F, Li C, Li X, Lambers H, Li L (2016) Root exudates drive interspecific facilitation by enhancing nodulation and N2 fixation. Proc Natl Acad Sci USA 113:6496–6501. https://doi.org/10.1073/pnas.1523580113
Li C, Li Y, Yu C, Sun J, Christie P, An M, Zhang F, Li L (2011) Crop nitrogen use and soil mineral nitrogen accumulation under different crop combinations and patterns of strip intercropping in northwest China. Plant Soil 342:221–231. https://doi.org/10.1007/s11104-010-0686-6
Li C, Dong Y, Li H, Shen J, Zhang F (2016) Shift from complementarity to facilitation on P uptake by intercropped wheat neighboring with faba bean when available soil P is depleted. Sci Rep 6:18663. https://doi.org/10.1038/srep18663
Li C, Hoffland E, Kuyper TW, Yu Y, Li H, Zhang C, Zhang F, van der Werf W (2020a) Yield gain, complementarity and competitive dominance in intercropping in China: A meta-analysis of drivers of yield gain using additive partitioning. Eur J Agron 113. https://doi.org/10.1016/j.eja.2019.125987
Li C, Hoffland E, Kuyper TW, Yu Y, Zhang C, Li H, Zhang F, van der Werf W (2020) Syndromes of production in intercropping impact yield gains. Nat Plants 6:653–660. https://doi.org/10.1038/s41477-020-0680-9
Li L, Li S, Sun J, Zhou L, Bao X, Zhang H, Zhang F (2007) Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc Natl Acad Sci USA 104:11192–11196. https://doi.org/10.1073/pnas.0704591104
Li L, Liu Y, Li X (2021) Intercropping to maximize root–root interactions in agricultural plants: agronomic aspects. In: Rengel Z, Djalovic I (eds) The root systems in sustainable agricultural intensification. Wiley, New York, pp 309–328
Li L, Sun J, Zhang F, Guo T, Bao X, Smith FA, Smith SE (2006) Root distribution and interactions between intercropped species. Oecologia 147:280–290. https://doi.org/10.1007/s00442-005-0256-4
Li L, Sun J, Zhang F, Li X, Rengel Z, Yang S (2001) Wheat/maize or wheat/soybean strip intercropping II. Recovery or compensation of maize and soybean after wheat harvesting. Field Crop Res 71:173–181. https://doi.org/10.1016/s0378-4290(01)00157-5
Li L, Sun J, Zhang F, Li X, Yang S, Rengel Z (2001) Wheat/maize or wheat/soybean strip intercropping I. Yield advantage and interspecific interactions on nutrients. Field Crop Res 71:123–137. https://doi.org/10.1016/s0378-4290(01)00156-3
Li L, Tang C, Rengel Z, Zhang F (2003) Chickpea facilitates phosphorus uptake by intercropped wheat from an organic phosphorus source. Plant Soil 248:297–303. https://doi.org/10.1023/a:1022389707051
Li L, Tilman D, Lambers H, Zhang F (2014) Plant diversity and overyielding: insights from belowground facilitation of intercropping in agriculture. New Phytol 203:63–69. https://doi.org/10.1111/nph.12778
Li L, Yang S, Li X, Zhang F, Christie P (1999) Interspecific complementary and competitive interactions between intercropped maize and faba bean. Plant Soil 212:105–114. https://doi.org/10.1023/A:1004656205144
Li L, Zhang F, Li X, Christie P, Sun J, Yang S, Tang C (2003) Interspecific facilitation of nutrient uptake by intercropped maize and faba bean. Nutr Cycl Agroecosyst 65:61–71. https://doi.org/10.1023/a:1021885032241
Li L, Zhao H, Kong C (2020) (-)-Loliolide, the most ubiquitous lactone, is involved in barnyardgrass-induced rice allelopathy. J Exp Bot 71:1540–1550. https://doi.org/10.1093/jxb/erz497
Li M, Wei Z, Wang J, Jousset A, Friman VP, Xu Y, Shen Q, Pommier T (2019) Facilitation promotes invasions in plant-associated microbial communities. Ecol Lett 22:149–158. https://doi.org/10.1111/ele.13177
Li S, Li L, Zhang F, Tang C (2004) Acid phosphatase role in chickpea/maize intercropping. Ann Bot 94:297–303. https://doi.org/10.1093/aob/mch140
Li W, Jin C, Guan D, Wang Q, Wang A, Yuan F, Wu J (2015) The effects of simulated nitrogen deposition on plant root traits: A meta-analysis. Soil Biol Biochem 82:112–118. https://doi.org/10.1016/j.soilbio.2015.01.001
Li W, Li L, Sun J, Guo T, Zhang F, Bao X, Peng A, Tang C (2005) Effects of intercropping and nitrogen application on nitrate present in the profile of an Orthic Anthrosol in Northwest China. Agr Ecosyst Environ 105:483–491. https://doi.org/10.1016/j.agee.2004.07.008
Li X, Wang Z, Bao X, Sun J, Yang S, Wang P, Wang C, Wu J, Liu X, Tian X, Wang Y, Li J, Wang Y, Xia H, Mei P, Wang X, Zhao J, Yu R, Zhang W, Che Z, Gui L, Callaway RM, Tilman D, Li L (2021) Long-term increased grain yield and soil fertility from intercropping. Nat Sustain 4:943–950. https://doi.org/10.1038/s41893-021-00767-7
Li X, Mu Y, Cheng Y, Liu X, Nian H (2013) Effects of intercropping sugarcane and soybean on growth, rhizosphere soil microbes, nitrogen and phosphorus availability. Acta Physiol Plant 35:1113–1119. https://doi.org/10.1007/s11738-012-1148-y
Li Y, Ran W, Zhang R, Sun S, Xu G (2009) Facilitated legume nodulation, phosphate uptake and nitrogen transfer by arbuscular inoculation in an upland rice and mung bean intercropping system. Plant Soil 315:285–296. https://doi.org/10.1007/s11104-008-9751-9
Li Y, Yu C, Cheng X, Li C, Sun J, Zhang F, Lambers H, Li L (2009) Intercropping alleviates the inhibitory effect of N fertilization on nodulation and symbiotic N2 fixation of faba bean. Plant Soil 323:295–308. https://doi.org/10.1007/s11104-009-9938-8
Liu Y, Qin X, Xiao J, Tang L, Wei C, Wei J, Zheng Y (2017) Intercropping influences component and content change of flavonoids in root exudates and nodulation of Faba bean. J Plant Interact 12:187–192. https://doi.org/10.1080/17429145.2017.1308569
Liu Y, Sun J, Zhang F, Li L (2020) The plasticity of root distribution and nitrogen uptake contributes to recovery of maize growth at late growth stages in wheat/maize intercropping. Plant Soil 447:39–53. https://doi.org/10.1007/s11104-019-04034-9
Liu Y, Zhang W, Sun J, Li X, Christie P, Li L (2015) High morphological and physiological plasticity of wheat roots is conducive to higher competitive ability of wheat than maize in intercropping systems. Plant Soil 397:387–399. https://doi.org/10.1007/s11104-015-2654-7
Lopez-Raez JA, Shirasu K, Foo E (2017) Strigolactones in plant interactions with beneficial and detrimental organisms: the Yin and Yang. Trends Plant Sci 22:527–537. https://doi.org/10.1016/j.tplants.2017.03.011
Loreau M, Hector A (2001) Partitioning selection and complementarity in biodiversity experiments. Nature 412:72–76. https://doi.org/10.1038/35083573
Luo Y, Davis AS, Yannarell AC (2016) Interactions between allelochemicals and the microbial community affect weed suppression following cover crop residue incorporation into soil. Plant Soil 399:357–371. https://doi.org/10.1007/s11104-015-2698-8
Luo S, Yu L, Liu Y, Zhang Y, Yang W, Li Z, Wang J (2016) Effects of reduced nitrogen input on productivity and N2O emissions in a sugarcane/soybean intercropping system. Eur J Agron 81:78–85. https://doi.org/10.1016/j.eja.2016.09.002
Ma L, Li Y, Wu P, Zhao X, Chen X, Gao X (2019) Effects of varied water regimes on root development and its relations with soil water under wheat/maize intercropping system. Plant Soil 439:113–130. https://doi.org/10.1007/s11104-018-3800-9
Ma L, Li Y, Wu P, Zhao X, Gao X, Chen X (2020) Recovery growth and water use of intercropped maize following wheat harvest in wheat/maize relay strip intercropping. Field Crop Res 256:14. https://doi.org/10.1016/j.fcr.2020.107924
Ma X, Liu Y, Shen W, Kuzyakov Y (2021) Phosphatase activity and acidification in lupine and maize rhizosphere depend on phosphorus availability and root properties: Coupling zymography with planar optodes. Appl Soil Ecol 167. https://doi.org/10.1016/j.apsoil.2021.104029
Ma X, Mason-Jones K, Liu Y, Blagodatskaya E, Kuzyakov Y, Guber A, Dippold MA, Razavi BS (2019) Coupling zymography with pH mapping reveals a shift in lupine phosphorus acquisition strategy driven by cluster roots. Soil Biol Biochem 135:420–428. https://doi.org/10.1016/j.soilbio.2019.06.001
Ma Z, Liu H, Mi Z, Zhang Z, Wang Y, Xu W, Jiang L, He JS (2017) Climate warming reduces the temporal stability of plant community biomass production. Nat Commun 8:15378. https://doi.org/10.1038/ncomms15378
Macias FA, Mejias FJ, Molinillo JM (2019) Recent advances in allelopathy for weed control: from knowledge to applications. Pest Manag Sci 75:2413–2436. https://doi.org/10.1002/ps.5355
Mahaut L, Fort F, Violle C, Freschet GT (2019) Multiple facets of diversity effects on plant productivity: Species richness, functional diversity, species identity and intraspecific competition. Funct Ecol 34:287–298. https://doi.org/10.1111/1365-2435.13473
Manning P, van der Plas F, Soliveres S, Allan E, Maestre FT, Mace G, Whittingham MJ, Fischer M (2018) Redefining ecosystem multifunctionality. Nat Ecol Evol 2:427–436. https://doi.org/10.1038/s41559-017-0461-7
Mao L, Zhang L, Li W, van der Werf W, Sun J, Spiertz H, Li L (2012) Yield advantage and water saving in maize/pea intercrop. Field Crop Res 138:11–20. https://doi.org/10.1016/j.fcr.2012.09.019
Mariotti M, Masoni A, Ercoli L, Arduini I (2009) Above- and below-ground competition between barley, wheat, lupin and vetch in a cereal and legume intercropping system. Grass Forage Sci 64:401–412. https://doi.org/10.1111/j.1365-2494.2009.00705.x
Mei P, Gui L, Wang P, Huang J, Long H, Christie P, Li L (2012) Maize/faba bean intercropping with rhizobia inoculation enhances productivity and recovery of fertilizer P in a reclaimed desert soil. Field Crop Res 130:19–27. https://doi.org/10.1016/j.fcr.2012.02.007
Mommer L, Visser EJW, van Ruijven J, de Caluwe H, Pierik R, de Kroon H (2011) Contrasting root behaviour in two grass species: a test of functionality in dynamic heterogeneous conditions. Plant Soil 344:347–360. https://doi.org/10.1007/s11104-011-0752-8
Murphy GP, Swanton CJ, Van Acker RC, Dudley SA, Gibson D (2017) Kin recognition, multilevel selection and altruism in crop sustainability. J Ecol 105:930–934. https://doi.org/10.1111/1365-2745.12787
Murphy GP, Van Acker R, Rajcan I, Swanton CJ (2017) Identity recognition in response to different levels of genetic relatedness in commercial soya bean. R Soc Open Sci 4:160879. https://doi.org/10.1098/rsos.160879
Mwakilili AD, Mwaikono KS, Herrera SL, Midega CAO, Magingo F, Alsanius B, Dekker T, Lyantagaye SL (2021) Long-term maize-Desmodium intercropping shifts structure and composition of soil microbiome with stronger impact on fungal communities. Plant Soil 467:437–450. https://doi.org/10.1007/s11104-021-05082-w
Nassab ADM, Amon T, Kaul HP (2011) Competition and yield in intercrops of maize and sunflower for biogas. Ind Crop Prod 34:1203–1211. https://doi.org/10.1016/j.indcrop.2011.04.015
Nie M, Lu M, Bell J, Raut S, Pendall E (2013) Altered root traits due to elevated CO2: a meta-analysis. Global Ecol Biogeogr 22:1095–1105. https://doi.org/10.1111/geb.12062
Nyawade SO, Karanja NN, Gachene CKK, Gitari HI, Schulte-Geldermann E, Parker ML (2019) Short-term dynamics of soil organic matter fractions and microbial activity in smallholder potato-legume intercropping systems. Appl Soil Ecol 142:123–135. https://doi.org/10.1016/j.apsoil.2019.04.015
Oburger E, Jones DL (2018) Sampling root exudates-Mission impossible? Rhizosphere 6:116–133. https://doi.org/10.1016/j.rhisph.2018.06.004
Pang J, Bansal R, Zhao H, Bohuon E, Lambers H, Ryan MH, Ranathunge K, Siddique KH (2018) The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol 219:518–529. https://doi.org/10.1111/nph.15200
Pang J, Wang Y, Lambers H, Tibbett M, Siddique KH, Ryan MH (2013) Commensalism in an agroecosystem: hydraulic redistribution by deep-rooted legumes improves survival of a droughted shallow-rooted legume companion. Physiol Plant 149:79–90. https://doi.org/10.1111/ppl.12020
Peoples MB, Chalk PM, Unkovich MJ, Boddey RM (2015) Can differences in N-15 natural abundance be used to quantify the transfer of nitrogen from legumes to neighbouring non-legume plant species? Soil Biol Biochem 87:97–109. https://doi.org/10.1016/j.soilbio.2015.04.010
Peres G, Cluzeau D, Menasseri S, Soussana JF, Bessler H, Engels C, Habekost M, Gleixner G, Weigelt A, Weisser WW, Scheu S, Eisenhauer N (2013) Mechanisms linking plant community properties to soil aggregate stability in an experimental grassland plant diversity gradient. Plant Soil 373:285–299. https://doi.org/10.1007/s11104-013-1791-0
Pickles BJ, Wilhelm R, Asay AK, Hahn AS, Simard SW, Mohn WW (2017) Transfer of 13 C between paired Douglas-fir seedlings reveals plant kinship effects and uptake of exudates by ectomycorrhizas. New Phytol 214:400–411. https://doi.org/10.1111/nph.14325
Rabot E, Wiesmeier M, Schlueter S, Vogel HJ (2018) Soil structure as an indicator of soil functions: A review. Geoderma 314:122–137. https://doi.org/10.1016/j.geoderma.2017.11.009
Raseduzzaman M, Jensen ES (2017) Does intercropping enhance yield stability in arable crop production? A meta-analysis. Eur J Agron 91:25–33. https://doi.org/10.1016/j.eja.2017.09.009
Ravenek JM, Mommer L, Visser EJW, van Ruijven J, van der Paauw JW, Smit-Tiekstra A, de Caluwe H, de Kroon H (2016) Linking root traits and competitive success in grassland species. Plant Soil 407:39–53. https://doi.org/10.1007/s11104-016-2843-z
Ray DK, Gerber JS, MacDonald GK, West PC (2015) Climate variation explains a third of global crop yield variability. Nat Commun 6:5989. https://doi.org/10.1038/ncomms6989
Reiss ER, Drinkwater LE (2018) Cultivar mixtures: a meta-analysis of the effect of intraspecific diversity on crop yield. Ecol Appl 28:62–77. https://doi.org/10.1002/eap.1629
Renard D, Tilman D (2019) National food production stabilized by crop diversity. Nature 571:257–260. https://doi.org/10.1038/s41586-019-1316-y
Río M, Pretzsch H, Ruíz-Peinado R, Ampoorter E, Annighöfer P, Barbeito I, Bielak K, Brazaitis G, Coll L, Drössler L, Fabrika M, Forrester DI, Heym M, Hurt V, Kurylyak V, Löf M, Lombardi F, Madrickiene E, Matović B, Mohren F, Motta R, Ouden J, Pach M, Ponette Q, Schütze G, Skrzyszewski J, Sramek V, Sterba H, Stojanović D, Svoboda M, Zlatanov TM, Bravo-Oviedo A (2017) Species interactions increase the temporal stability of community productivity in Pinus sylvestris-Fagus sylvatica mixtures across Europe. J Ecol 105:1032–1043. https://doi.org/10.1111/1365-2745.12727
Rosenzweig C, Parry ML (1994) Potential impact of climate change on world food supply. Nature 367:133–138
Sasaki T, Lauenroth WK (2011) Dominant species, rather than diversity, regulates temporal stability of plant communities. Oecologia 166:761–768. https://doi.org/10.1007/s00442-011-1916-1
Sasse J, Martinoia E, Northen T (2018) Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci 23:25–41. https://doi.org/10.1016/j.tplants.2017.09.003
Schenk HJ (2006) Root competition: beyond resource depletion. J Ecol 94:725–739. https://doi.org/10.1111/j.1365-2745.2006.01124.x
Schneider HM, Lynch JP (2020) Should root plasticity be a crop breeding target? Front Plant Sci 11:546. https://doi.org/10.3389/fpls.2020.00546
Shen C, Liu L, Zhu L, Kang J, Wang N, Shao L (2020) High-throughput in situ root image segmentation based on the improved DeepLabv3 + method. Front Plant Sci 11:576791. https://doi.org/10.3389/fpls.2020.576791
Singh D, Mathimaran N, Boller T, Kahmen A (2019) Bioirrigation: a common mycorrhizal network facilitates the water transfer from deep-rooted pigeon pea to shallow-rooted finger millet under drought. Plant Soil 440:277–292. https://doi.org/10.1007/s11104-019-04082-1
Six J, Bossuyt H, Degryze S, Denef K (2004) A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Till Res 79:7–31. https://doi.org/10.1016/j.still.2004.03.008
Smit A, George E, Groenwold J (2000) Root observations and measurements at (transparent) interfaces with soil. Root methods. Springer, Dordrecht
Song Y, Marschner P, Li L, Bao X, Sun J, Zhang F (2007) Community composition of ammonia-oxidizing bacteria in the rhizosphere of intercropped wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L.). Biol Fert Soils 44:307–314. https://doi.org/10.1007/s00374-007-0205-y
Song Y, Zhang F, Marschner P, Fan F, Gao H, Bao X, Sun J, Li L (2007) Effect of intercropping on crop yield and chemical and microbiological properties in rhizosphere of wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L.). Biol Fert Soils 43:565–574. https://doi.org/10.1007/s00374-006-0139-9
Stomph T, Dordas C, Baranger A, de Rijk J, Dong B, Evers J, Gu C, Li L, Simon J, Jensen ES, Wang Q, Wang Y, Wang Z, Xu H, Zhang C, Zhang L, Zhang W, Bedoussac L, van der Werf W (2020) Designing intercrops for high yield, yield stability and efficient use of resources: Are there principles? Adv Agron 160:1–50. https://doi.org/10.1016/bs.agron.2019.10.002
Streit J, Meinen C, Nelson WCD, Siebrecht-Schöll DJ, Rauber R (2019) Above- and belowground biomass in a mixed cropping system with eight novel winter faba bean genotypes and winter wheat using FTIR spectroscopy for root species discrimination. Plant Soil 436:141–158. https://doi.org/10.1007/s11104-018-03904-y
Tamburini G, Bommarco R, Wanger TC, Kremen C, van der Heijden MGA, Liebman M, Hallin S (2020) Agricultural diversification promotes multiple ecosystem services without compromising yield. Sci Adv 6:eaba1715. https://doi.org/10.1126/sciadv.aba1715
Tan Y, Hu FL, Chai Q, Li G, Coulter JA, Zhao C, Yu AZ, Fan ZL, Yin W (2020) Expanding row ratio with lowered nitrogen fertilization improves system productivity of maize/pea strip intercropping. Eur J Agron 113:10. https://doi.org/10.1016/j.eja.2019.125986
Tang X, Bernard L, Brauman A, Daufresne T, Deleporte P, Desclaux D, Souche G, Placella SA, Hinsinger P (2014) Increase in microbial biomass and phosphorus availability in the rhizosphere of intercropped cereal and legumes under field conditions. Soil Biol Biochem 75:86–93. https://doi.org/10.1016/j.soilbio.2014.04.001
Tang X, Jiang J, Huang Z, Wu H, Wang J, He L, Xiong F, Zhong R, Liu J, Han Z, Tang R, He L (2021) Sugarcane/peanut intercropping system improves the soil quality and increases the abundance of beneficial microbes. J Basic Microbiol 61:165–176. https://doi.org/10.1002/jobm.202000750
Tang X, Zhang C, Yu Y, Shen J, van der Werf W, Zhang F (2021) Intercropping legumes and cereals increases phosphorus use efficiency; a meta-analysis. Plant Soil 460:89–104. https://doi.org/10.1007/s11104-020-04768-x
Tang X, Placella SA, Dayde F, Bernard L, Robin A, Journet EP, Justes E, Hinsinger P (2016) Phosphorus availability and microbial community in the rhizosphere of intercropped cereal and legume along a P-fertilizer gradient. Plant Soil 407:119–134. https://doi.org/10.1007/s11104-016-2949-3
Tang Y, Wu X, Chen C, Jia C, Chen Y (2019) Water source partitioning and nitrogen facilitation promote coexistence of nitrogen-fixing and neighbor species in mixed plantations in the semiarid Loess Plateau. Plant Soil 445:289–305. https://doi.org/10.1007/s11104-019-04301-9
Teste FP, Dixon KW, Lambers H, Zhou J, Veneklaas EJ (2020) The potential for phosphorus benefits through root placement in the rhizosphere of phosphorus-mobilising neighbours. Oecologia 193:843–855. https://doi.org/10.1007/s00442-020-04733-6
Thilakarathna MS, McElroy MS, Chapagain T, Papadopoulos YA, Raizada MN (2016) Belowground nitrogen transfer from legumes to non-legumes under managed herbaceous cropping systems. A review. Agron Sustain Dev 36:58. https://doi.org/10.1007/s13593-016-0396-4
Thorup-Kristensen K, Halberg N, Nicolaisen M, Olesen JE, Crews TE, Hinsinger P, Kirkegaard J, Pierret A, Dresbøll DB (2020) Digging deeper for agricultural resources, the value of deep rooting. Trends Plant Sci 25:406–417. https://doi.org/10.1016/j.tplants.2019.12.007
Tian J, Tang M, Xu X, Luo S, Condron LM, Lambers H, Cai K, Wang J (2020) Soybean (Glycine max (L.) Merrill) intercropping with reduced nitrogen input influences rhizosphere phosphorus dynamics and phosphorus acquisition of sugarcane (Saccharum officinarum). Biol Fert Soils 56:1063–1075. https://doi.org/10.1007/s00374-020-01484-7
Tian X, Wang C, Bao X, Wang P, Li X, Yang S, Ding GC, Christie P, Li L (2019) Crop diversity facilitates soil aggregation in relation to soil microbial community composition driven by intercropping. Plant Soil 436:173–192. https://doi.org/10.1007/s11104-018-03924-8
Tilman D, Downing JA (1994) Biodiversity and stability in grasslands. Nature 367:363–365. https://doi.org/10.1038/367363a0
Tisdall JM, Oades JM (1982) Organic-matter and water-stable aggregates in soils. J Soil Sci 33:141–163. https://doi.org/10.1111/j.1365-2389.1982.tb01755.x
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2011) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 341:75–87. https://doi.org/10.1007/s11104-010-0623-8
van der Heijden MGA, Horton TR (2009) Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J Ecol 97:1139–1150. https://doi.org/10.1111/j.1365-2745.2009.01570.x
van Ruijven J, Ampt E, Francioli D, Mommer L (2020) Do soil-borne fungal pathogens mediate plant diversity-productivity relationships? Evidence and future opportunities. J Ecol 108:1810–1821. https://doi.org/10.1111/1365-2745.13388
Vandermeer JH (1992) The ecology of intercropping. Cambridge University Press, Cambridge
von Felten S, Hector A, Buchmann N, Niklaus PA, Schmid B, Scherer-Lorenzen M (2009) Belowground nitrogen partitioning in experimental grassland plant communities of varying species richness. Ecology 90:1389–1399. https://doi.org/10.1890/08-0802.1
Wagemaker CAM, Mommer L, Visser EJW, Weigelt A, van Gurp TP, Postuma M, Smit-Tiekstra AE, de Kroon H (2021) msGBS: A new high-throughput approach to quantify the relative species abundance in root samples of multispecies plant communities. Mol Ecol Resour 21:1021–1036. https://doi.org/10.1111/1755-0998.13278
Wahbi S, Maghraoui T, Hafidi M, Sanguin H, Oufdou K, Prin Y, Duponnois R, Galiana A (2016) Enhanced transfer of biologically fixed N from faba bean to intercropped wheat through mycorrhizal symbiosis. Appl Soil Ecol 107:91–98. https://doi.org/10.1016/j.apsoil.2016.05.008
Wang B, Zhang W, Ahanbieke P, Gan Y, Xu W, Li L, Christie P, Li L (2014) Interspecific interactions alter root length density, root diameter and specific root length in jujube/wheat agroforestry systems. Agroforest Syst 88:835–850. https://doi.org/10.1007/s10457-014-9729-y
Wang G, Bei S, Li J, Bao X, Zhang J, Schultz PA, Li H, Li L, Zhang F, Bever JD, Zhang J, Hannula E (2021) Soil microbial legacy drives crop diversity advantage: Linking ecological plant-soil feedback with agricultural intercropping. J Appl Ecol 58:496–506. https://doi.org/10.1111/1365-2664.13802
Wang J, Defrenne C, McCormack ML, Yang L, Tian D, Luo Y, Hou E, Yan T, Li Z, Bu W, Chen Y, Niu S (2021) Fine-root functional trait responses to experimental warming: a global meta-analysis. New Phytol 230:1856–1867. https://doi.org/10.1111/nph.17279
Wang N, Kong C, Wang P, Meiners SJ (2021) Root exudate signals in plant-plant interactions. Plant Cell Environ 44:1044–1058. https://doi.org/10.1111/pce.13892
Wang S, Isbell F, Deng W, Hong P, Dee LE, Thompson P, Loreau M (2021) How complementarity and selection affect the relationship between ecosystem functioning and stability. Ecology 102:e03347. https://doi.org/10.1002/ecy.3347
Wang X, Feng Y, Yu L, Shu Y, Tan F, Gou Y, Luo S, Yang W, Li Z, Wang J (2020) Sugarcane/soybean intercropping with reduced nitrogen input improves crop productivity and reduces carbon footprint in China. Sci Total Environ 719:137517. https://doi.org/10.1016/j.scitotenv.2020.137517
Wang X, Shen J, Hedden P, Phillips AL, Thomas SG, Ge Y, Ashton RW, Whalley WR (2021) Wheat growth responses to soil mechanical impedance are dependent on phosphorus supply. Soil Till Res 205:104754. https://doi.org/10.1016/j.still.2020.104754
Wang X, Whalley WR, Miller AJ, White PJ, Zhang F, Shen J (2020) Sustainable cropping requires adaptation to a heterogeneous rhizosphere. Trends Plant Sci 25:1194–1202. https://doi.org/10.1016/j.tplants.2020.07.006
Wang Y, Cadotte MW, Chen Y, Fraser LH, Zhang Y, Huang F, Luo S, Shi N, Loreau M (2019) Global evidence of positive biodiversity effects on spatial ecosystem stability in natural grasslands. Nat Commun 10:3207. https://doi.org/10.1038/s41467-019-11191-z
Wang Y, Qin Y, Chai Q, Feng F, Zhao C, Yu A (2018) Interspecies interactions in relation to root distribution across the rooting profile in wheat-maize intercropping under different plant densities. Front Plant Sci 9:17. https://doi.org/10.3389/fpls.2018.00483
Wang Z, Bao X, Li X, Jin X, Zhao J, Sun J, Christie P, Li L (2015) Intercropping maintains soil fertility in terms of chemical properties and enzyme activities on a timescale of one decade. Plant Soil 391:265–282. https://doi.org/10.1007/s11104-015-2428-2
Wang Z, Jin X, Bao X, Li X, Zhao J, Sun J, Christie P, Li L (2014) Intercropping enhances productivity and maintains the most soil fertility properties relative to sole cropping. PLoS ONE 9:24. https://doi.org/10.1371/journal.pone.0113984
Ward JK, Tissue DT, Thomas RB, Strain BR (1999) Comparative responses of model C3 and C4 plants to drought in low and elevated CO2 Glob Change Biol 5:857–867. https://doi.org/10.1046/j.1365-2486.1999.00270.x
Weigelt A, Schumacher J, Roscher C, Schmid B (2008) Does biodiversity increase spatial stability in plant community biomass? Ecol Lett 11:338–347. https://doi.org/10.1111/j.1461-0248.2007.01145.x
Whittington HR, Deede L, Powers JS (2012) Growth responses, biomass partitioning, and nitrogen isotopes of prairie legumes in response to elevated temperature and varying nitrogen source in a growth chamber experiment. Am J Bot 99:838–846. https://doi.org/10.3732/ajb.1100283
Wittwer RA, Bender SF, Hartman K, Hydbom S, Lima RAA, Loaiza V, Nemecek T, Oehl F, Olsson PA, Petchey O, Prechsl UE, Schlaeppi K, Scholten T, Seitz S, Six J, van der Heijden MGA (2021) Organic and conservation agriculture promote ecosystem multifunctionality. Sci Adv 7. https://doi.org/10.1126/sciadv.abg6995
Wright AJ, de Kroon H, Visser EJ, Buchmann T, Ebeling A, Eisenhauer N, Fischer C, Hildebrandt A, Ravenek J, Roscher C, Weigelt A, Weisser W, Voesenek LA, Mommer L (2017) Plants are less negatively affected by flooding when growing in species-rich plant communities. New Phytol 213:645–656. https://doi.org/10.1111/nph.14185
Wright AJ, Mommer L, Barry K, van Ruijven J (2021) Stress gradients and biodiversity: monoculture vulnerability drives stronger biodiversity effects during drought years. Ecology 102:e03193. https://doi.org/10.1002/ecy.3193
Wright AJ, Wardle DA, Callaway R, Gaxiola A (2017) The overlooked role of facilitation in biodiversity experiments. Trends Ecol Evol 32:383–390. https://doi.org/10.1016/j.tree.2017.02.011
Wu J, Guo Y (2014) An integrated method for quantifying root architecture of field-grown maize. Ann Bot 114:841–851. https://doi.org/10.1093/aob/mcu009
Xia H, Wang Z, Zhao J, Sun J, Bao X, Christie P, Zhang F, Li L (2013) Contribution of interspecific interactions and phosphorus application to sustainable and productive intercropping systems. Field Crop Res 154:53–64. https://doi.org/10.1016/j.fcr.2013.07.011
Xia H, Zhao J, Sun J, Bao X, Christie P, Zhang F, Li L (2013) Dynamics of root length and distribution and shoot biomass of maize as affected by intercropping with different companion crops and phosphorus application rates. Field Crop Res 150:52–62. https://doi.org/10.1016/j.fcr.2013.05.027
Xia Z, Kong C, Chen L, Wang P, Wang S (2016) A broadleaf species enhances an autotoxic conifers growth through belowground chemical interactions. Ecology 97:2283–2292. https://doi.org/10.1002/ecy.1465
Xia Z, Yu L, He Y, Korpelainen H, Li C (2019) Broadleaf trees mediate chemically the growth of Chinese fir through root exudates. Biol Fert Soils 55:737–749. https://doi.org/10.1007/s00374-019-01389-0
Xiao Y, Li L, Zhang F (2004) Effect of root contact on interspecific competition and N transfer between wheat and fababean using direct and indirect 15 N techniques. Plant Soil 262:45–54. https://doi.org/10.1023/B:PLSO.0000037019.34719.0d
Xiao Z, Jiang L, Chen X, Zhang Y, Defossez E, Hu F, Liu M, Rasmann S (2019) Earthworms suppress thrips attack on tomato plants by concomitantly modulating soil properties and plant chemistry. Soil Biol Biochem 130:23–32. https://doi.org/10.1016/j.soilbio.2018.11.023
Xiong H, Kakei Y, Kobayashi T, Guo X, Nakazono M, Takahashi H, Nakanishi H, Shen H, Zhang F, Nishizawa NK, Zuo Y (2013) Molecular evidence for phytosiderophore-induced improvement of iron nutrition of peanut intercropped with maize in calcareous soil. Plant Cell Environ 36:1888–1902. https://doi.org/10.1111/pce.12097
Xu Y, Cheng H, Kong C, Meiners SJ (2021) Intra-specific kin recognition contributes to inter-specific allelopathy: A case study of allelopathic rice interference with paddy weeds. Plant Cell Environ 44:3709–3721. https://doi.org/10.1111/pce.14083
Xue Y, Xia H, Christie P, Zhang Z, Li L, Tang C (2016) Crop acquisition of phosphorus, iron and zinc from soil in cereal/legume intercropping systems: a critical review. Ann Bot 117:363–377. https://doi.org/10.1093/aob/mcv182
Yang H, Zhang W, Li L (2021) Intercropping: feed more people and build more sustainable agroecosystems. Front Agric Sci Eng 8:373–386. https://doi.org/10.15302/j-fase-2021398
Yang X, Qin J, Li J, Lai Z, Li H (2021) Upland rice intercropping with Solanum nigrum inoculated with arbuscular mycorrhizal fungi reduces grain Cd while promoting phytoremediation of Cd-contaminated soil. J Hazard Mater 406:124325. https://doi.org/10.1016/j.jhazmat.2020.124325
Yang X, Li L, Xu Y, Kong C (2018) Kin recognition in rice (Oryza sativa) lines. New Phytol 220:567–578. https://doi.org/10.1111/nph.15296
Yang Y, Tilman D, Furey G, Lehman C (2019) Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat Commun 10:718. https://doi.org/10.1038/s41467-019-08636-w
Yin W, Chai Q, Zhao C, Yu A, Fan Z, Hu F, Fan H, Guo Y, Coulter JA (2020) Water utilization in intercropping: A review. Agric Water Manag 241:13. https://doi.org/10.1016/j.agwat.2020.106335
Yu R, Lambers H, Callaway RM, Wright AJ, Li L (2021) Belowground facilitation and trait matching: two or three to tango? Trends Plant Sci 26:1227–1235. https://doi.org/10.1016/j.tplants.2021.07.014
Yu R, Li X, Xiao Z, Lambers H, Li L (2020) Phosphorus facilitation and covariation of root traits in steppe species. New Phytol 226:1285–1298. https://doi.org/10.1111/nph.16499
Yu R, Zhang W, Yu Y, Yu S, Lambers H, Li L (2020) Linking shifts in species composition induced by grazing with root traits for phosphorus acquisition in a typical steppe in Inner Mongolia. Sci Total Environ 712:136495. https://doi.org/10.1016/j.scitotenv.2020.136495
Yu Y, Stomph T-J, Makowski D, van der Werf W (2015) Temporal niche differentiation increases the land equivalent ratio of annual intercrops: A meta-analysis. Field Crop Res 184:133–144. https://doi.org/10.1016/j.fcr.2015.09.010
Yu Y, Stomph T-J, Makowski D, Zhang L, van der Werf W (2016) A meta-analysis of relative crop yields in cereal/legume mixtures suggests options for management. Field Crop Res 198:269–279. https://doi.org/10.1016/j.fcr.2016.08.001
Zarea MJ, Ghalavand A, Goltapeh EM, Rejali F, Zamaniyan M (2009) Effects of mixed cropping, earthworms (Pheretima sp.), and arbuscular mycorrhizal fungi (Glomus mosseae) on plant yield, mycorrhizal colonization rate, soil microbial biomass, and nitrogenase activity of free-living rhizosphere bacteria. Pedobiologia 52:223–235. https://doi.org/10.1016/j.pedobi.2008.10.004
Zhang C, Dong Y, Tang L, Zheng Y, Makowski D, Yu Y, Zhang F, van der Werf W (2019) Intercropping cereals with faba bean reduces plant disease incidence regardless of fertilizer input; a meta-analysis. Eur J Plant Pathol 154:931–942. https://doi.org/10.1007/s10658-019-01711-4
Zhang C, Postma JA, York LM, Lynch JP (2014) Root foraging elicits niche complementarity-dependent yield advantage in the ancient ‘three sisters’ (maize/bean/squash) polyculture. Ann Bot 114:1719–1733. https://doi.org/10.1093/aob/mcu191
Zhang D, Lyu Y, Li H, Tang X, Hu R, Rengel Z, Zhang F, Whalley WR, Davies WJ, Cahill JF Jr, Shen J (2020) Neighbouring plants modify maize root foraging for phosphorus: coupling nutrients and neighbours for improved nutrient-use efficiency. New Phytol 226:244–253. https://doi.org/10.1111/nph.16206
Zhang D, Peng Y, Li F, Yang G, Wang J, Yu J, Zhou G, Yang Y (2019) Trait identity and functional diversity co-drive response of ecosystem productivity to nitrogen enrichment. J Ecol 107:2402–2414. https://doi.org/10.1111/1365-2745.13184
Zhang D, Zhang C, Tang X, Li H, Zhang F, Rengel Z, Whalley WR, Davies WJ, Shen J (2016) Increased soil phosphorus availability induced by faba bean root exudation stimulates root growth and phosphorus uptake in neighbouring maize. New Phytol 209:823–831. https://doi.org/10.1111/nph.13613
Zhang F, Li L (2003) Using competitive and facilitative interactions in intercropping systems enhances crop productivity and nutrient-use efficiency. Plant Soil 248:305–312. https://doi.org/10.1023/a:1022352229863
Zhang H, Wang X, Gao Y, Sun B (2020) Short-term N transfer from alfalfa to maize is dependent more on arbuscular mycorrhizal fungi than root exudates in N deficient soil. Plant Soil 446:23–41. https://doi.org/10.1007/s11104-019-04333-1
Zhang L, van de Werf W, Bastiaans L, Zhang S, Li B, Spiertz JHJ (2008) Light interception and utilization in relay intercrops of wheat and cotton. Field Crop Res 107:29–42. https://doi.org/10.1016/j.fcr.2007.12.014
Zhang W, Ahanbieke P, Wang B, Gan Y, Li L, Christie P, Li L (2015) Temporal and spatial distribution of roots as affected by interspecific interactions in a young walnut/wheat alley cropping system in northwest China. Agroforest Syst 89:327–343. https://doi.org/10.1007/s10457-014-9770-x
Zhang W, Ahanbieke P, Wang B, Xu W, Li L, Christie P, Li L (2013) Root distribution and interactions in jujube tree/wheat agroforestry system. Agroforest Syst 87:929–939. https://doi.org/10.1007/s10457-013-9609-x
Zhang W, Li S, Shen Y, Yue S (2021a) Film mulching affects root growth and function in dryland maize-soybean intercropping. Field Crop Res 271. https://doi.org/10.1016/j.fcr.2021.108240
Zhang W, Gao S, Li Z, Xu H, Yang H, Yang X, Fan H, Su Y, Fornara D, Li L (2021) Shifts from complementarity to selection effects maintain high productivity in maize/legume intercropping systems. J Appl Ecol 58:2603–2613. https://doi.org/10.1111/1365-2664.13989
Zhang W, Liu G, Sun J, Fornara D, Zhang L, Zhang F, Li L, Niels A (2017) Temporal dynamics of nutrient uptake by neighbouring plant species: evidence from intercropping. Funct Ecol 31:469–479. https://doi.org/10.1111/1365-2435.12732
Zhang W, Liu G, Sun J, Zhang L, Weiner J, Li L (2015) Growth trajectories and interspecific competitive dynamics in wheat/maize and barley/maize intercropping. Plant Soil 397:227–238. https://doi.org/10.1007/s11104-015-2619-x
Zhao C, Chai Q, Zhao Y, Mu Y, Zhang Y, Yu A, Feng F, Liu C, Yin W, Hu F (2016) Interspecific competition and complementation is a function of N management in maize-pea intercropping systems. Crop Sci 56:3286–3294. https://doi.org/10.2135/cropsci2016.03.0204
Zheng C, Wang R, Zhou X, Li C, Dou X (2021) Effects of mulch and irrigation regimes on water distribution and root competition in an apple-soybean intercropping system in Loess Plateau, China. Agric Water Manag 246:13. https://doi.org/10.1016/j.agwat.2020.106656
Zheng W, Gong Q, Zhao Z, Liu J, Zhai B, Wang Z, Li Z (2018) Changes in the soil bacterial community structure and enzyme activities after intercrop mulch with cover crop for eight years in an orchard. Eur J Soil Biol 86:34–41. https://doi.org/10.1016/j.ejsobi.2018.01.009
Zhou L, Cao J, Zhang F, Li L (2009) Rhizosphere acidification of faba bean, soybean and maize. Sci Total Environ 407:4356–4362. https://doi.org/10.1016/j.scitotenv.2009.02.006
Zhu Y, Chen H, Fan J, Wang Y, Li Y, Chen J, Fan J, Yang S, Hu L, Leung H, Mew T, Teng P, Wang Z, Mundt CC (2000) Genetic diversity and disease control in rice. Nature 406:718–722. https://doi.org/10.1038/35021046
Zuo Y, Zhang F (2008) Effect of peanut mixed cropping with gramineous species on micronutrient concentrations and iron chlorosis of peanut plants grown in a calcareous soil. Plant Soil 306:23–36. https://doi.org/10.1007/s11104-007-9484-1
Zuo Y, Li X, Cao Y, Zhang F, Christie P (2003) Iron nutrition of peanut enhanced by mixed cropping with maize: Possible role of root morphology and rhizosphere microflora. J Plant Nutr 26:2093–2110. https://doi.org/10.1081/pln-120024267
Zuo Y, Liu Y, Zhang F, Christie P (2004) A study on the improvement iron nutrition of peanut intercropping with maize on nitrogen fixation at early stages of growth of peanut on a calcareous soil. Soil Sci Plant Nutr 50:1071–1078. https://doi.org/10.1080/00380768.2004.10408576
Zuo Y, Zhang F (2009) Iron and zinc biofortification strategies in dicot plants by intercropping with gramineous species. A review. Agron Sustain Dev 29:63–71. https://doi.org/10.1051/agro:2008055
Zuo Y, Zhang F, Li X, Cao Y (2000) Studies on the improvement in iron nutrition of peanut by intercropping with maize on a calcareous soil. Plant Soil 220:13–25. https://doi.org/10.1023/a:1004724219988
Acknowledgements
This review is written to celebrate the milestone that Hans Lambers has achieved by serving as Editor in Chief of Plant and Soil for 30 years, and to celebrate the friendship and the collaboration between Long Li and Hans Lambers for many years. Hans Lambers has collaborated with the intercropping research group at China Agricultural University to understand root-root interactions, especially provided leaf [Mn] as a proxy for carboxylate release, broadening the approach to explain interspecific P facilitation in multi-species community. This work was financially supported by the National Natural Science Foundation of China (Project Numbers: 32130067 and 31430014). Rui-Peng Yu was also financially supported by the National Natural Science Foundation of China (Project Numbers: 32101297). Hans Lambers received funding from the Deputy Vice Chancellor (Research) at the University of Western Australia.
Author information
Authors and Affiliations
Contributions
L.L. and R.P.Y. outlined the review. R.P.Y., H.Y. and Y.X. drafted the paper, and all authors contributed substantially to revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Ismail Cakmak.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, RP., Yang, H., Xing, Y. et al. Belowground processes and sustainability in agroecosystems with intercropping. Plant Soil 476, 263–288 (2022). https://doi.org/10.1007/s11104-022-05487-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05487-1