Abstract
Aims
This study of a maize-white lupin model cropping system was conducted to investigate the effects of rhizosphere-sharing of white lupin, a P-efficient plant, on growth and P accumulation of maize under different P rates and forms in two contrasting soils.
Methods
With Regosol and Andosol, a 42-day pot experiment was conducted for 0P (no P addition), 50Pi, 100Pi (50 and 100 mg P kg−1 soil by NaHPO4⋅2H2O respectively), and 100Po (100 mg P kg−1 soil by phytate). Plant growth, P uptake, rhizosphere pH, and different P fractions were investigated.
Results
Complementary effects of intercropping for maize were observed in Regosol, but not in Andosol. Total P uptake by intercropped maize in 0P, 50Pi, and 100Po was elevated by 46, 37, and 65 %, respectively, compared to when it was grown as a monoculture. White lupin mobilized P from sparingly soluble forms. Thereby, maize plant enhanced its P accumulation as a result of access to these two fractions in mixed culture in Regosol, where strong root intermingling occurred among intercropped plants.
Conclusions
Results suggest that the P mobilization strategy of white lupin from sparingly soluble P pools in soil can enhance the P acquisition efficiency of coexisting maize with P facilitation in this intercropping occurring in the direction of white lupin to maize. Achieving enhanced growth and P uptake by P-inefficient species in intercropping with white lupin is dependent on the type of soil in which those plants are grown.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although phosphorus (P) plays a vital role in the energy metabolism and in biosynthesis of nucleic acid and membranes, it is regarded as the second most frequently limiting major nutrient for plant growth (Raghothama 1999). In most soils, the P concentration in soil solution is inadequate for optimal growth of many crop plants (Hinsinger 2001; Raghothama 1999). Consequently, P-deficiency is a major yield-limiting factor for plants, especially in acidic and calcareous soils, where P retention is high (Hinsinger 2001). In this context, it is necessary to apply P fertilizers continually to sustain crop production. However, increasing the fertilizer P usage further is not an option to improve agricultural production to meet the global food demand (Hinsinger et al. 2011; Vance 2001) because phosphate rocks that are used to manufacture fertilizer P are a finite resource (Cordell et al. 2009; Dawson and Hilton 2011) that is expected to be exhausted by the end of the century (Gilbert 2009). It is therefore important to use alternatives such as P-efficient genotypes and alternative management strategies to exploit soil P resources better through increasing P bioavailability in agro-ecosystems (Lambers et al. 2006; Vance 2001).
Richardson et al. (2011) reported three strategies for plants to increase production in P-deficient soils and to reduce the amounts of P fertilizers that are necessary to enhance production: (i) ‘root foraging strategies’, which enable plant to explore large volumes of soil and thereby acquire more P from soil; (ii) ‘P mining strategies’, which increase desorption and mineralization of sparingly available P pools and organic P pools in soil by root exudates, organic anions, and phosphatases; and (iii) ‘improved internal P-utilization efficiency’, which might be helpful for plants to produce a higher yield per unit of P uptake. Plants that are able to use any of these strategies would use soil P and fertilizer P efficiently, irrespective of the application of fertilizer P to soil (Simpson et al. 2011).
Several legume species that exude organic anion to access sparingly available P have been examined. Those crop species include white lupin (Cu et al. 2005; Gardner and Boundy 1983; Hocking and Randall 2001), pigeon pea (Cajanus cajan L. Millsp.; Ae et al. 1990), faba bean (Vicia faba L.; Li et al. 2007), and chickpea (Cicer arietinum L.; Veneklaas et al. 2003). Traits of some lupins, such as formation of cluster roots and exudation of vast amounts of phosphate-mobilizing substances, have made them ideal plants to thrive in soils where higher amounts of P remain in poorly available forms for most plants (Lambers et al. 2013). White lupin has these desirable traits to a greater degree. Formation of cluster roots (proteoid roots) in response to low P supply (Gardner et al. 1981; Keerthisinghe et al. 1998) and exudation of organic acids and phosphatase from these specific root structures have been demonstrated in earlier studies (Dinkelaker et al. 1989; Gardner et al. 1983). Organic anion exudation into the rhizosphere increases the mobilization of sparingly soluble soil P (Gardner et al. 1983; Gerke et al. 1994; Li et al. 1997), whereas phosphatase secretion by white lupin helps it to use organic P fractions in addition to inorganic P in soil (Adams and Pate 1992; Lambers et al. 1998). Enhanced ability of white lupin to secrete acid phosphatase under P-deficient conditions has also been described by Tadano and Sakai (1991) and by Wasaki et al. (2003). Lupins can mobilize sparingly available nutrients, mainly P and micronutrients, not only for themselves but also for interplanted or subsequent crops (Lambers et al. 2013). Therefore, the ability of white lupin to secrete these types of P-mobilizing substances and to access more recalcitrant sources of P in soil highlights its potential applicability to improve P inefficient species through sharing of rhizosphere functions in mixed cultures.
For a particular crop plant, P benefit can be achieved by integrating plant species that use sparingly available phosphate and organic P in intercropping systems. In this context, P-mobilizing species can enhance the growth and P uptake by companion cereals (Simpson et al. 2011). Enhanced P acquisition and growth have been demonstrated previously for different cereals in mixed culture systems with legumes. Evidence of positive responses by interplanted cereals with legumes includes that of wheat intercropped with chickpea (Li et al. 2003), white lupin (Cu et al. 2005; Kamh et al. 1999), and faba bean (Song et al. 2007). Other cereals include maize (Li et al. 2004) and barley (Gunes et al. 2007) intercropped with chickpea and sorghum intercropped with pigeon pea (Ae et al. 1990).
In cereal legume intercropping systems, the P acquisition of cereal is enhanced because of the legumes’ ability to exude large amounts of P-mobilizing compounds, such as carboxylates, that can mobilize sparingly available P (Neumann and Römheld 1999; Pearse et al. 2006; Veneklaas et al. 2003), phosphatases that can mineralize organic P (Nuruzzaman et al. 2006), and protons from N2 fixation (Hinsinger et al. 2003; Tang et al. 1997) that ultimately increase P availability. Soil pH is known to be a crucially important parameter determining the P availability in soil (Hinsinger 2001). Acidification of the rhizosphere is expected in cereal–legume mixed cultures as a result of proton release by roots of N2-fixing legumes (Cu et al. 2005; Li et al. 2008). This acidification can be expected to benefit plants grown in alkaline soils. However, few studies to date have examined the potential role of rhizosphere pH in intercropping systems.
Most studies of P mobilization for a main crop plant by a P-efficient plant species in mixed cultures have been confined to soils with low P availability because the P mobilization capacity is enhanced under P-deficient conditions (Neumann et al. 1999). Crop cultivators apply fertilizer P to cropping lands to reduce the risk of P-deficiency during crop growth either by organic or inorganic fertilizer sources. To date, positive effects of intercropping have been reported mainly in alkaline soils. Facilitation of P uptake in the intercropping of P-inefficient and efficient plant species under different rates and forms of P supply have been found only in a few studies. Given these circumstances, the P uptake of main crop plants from different P pools as influenced by interplanted P-efficient plant species has yet to be investigated. Consequently, this study addressed this knowledge gap by assessing the growth and P-advantages of maize as main crop plant intercropped with white lupin as P-efficient plant under different P rates and forms in two contrasting acidic soils. This study also evaluated the various soil P fractions in the rhizosphere of monocropped and intercropped plants species. Our study fundamentally examined the early stage growth of the two plants. White lupin was selected as the P-efficient plant species for this study because of its enhanced ability to secrete P mobilizing substances, by which it accesses less-labile P pools in soil, which are not available to other plants.
Material and methods
Soils
Two soil types, Regosol and Andosol, were collected respectively from a field at Hiroshima University at Higashi-Hiroshima and a forest at Shobara, Hiroshima, Japan. Soil was air-dried and passed through a 2-mm sieve. Table 1 presents physical and chemical parameters of the soils. Both soils were acidic with contrasting P adsorption capacities.
Plant growth conditions
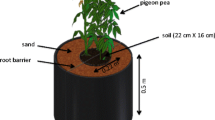
A pot experiment was conducted in a naturally lit glass house at Hiroshima University. The two plant species examined were white lupin (Lupinus albus L. cv. Energy) and maize (Zea mays L. cv. Snow-dent 125 ‘Wakaba’) either as monocropping or mixed cropping. Four P fertilizer treatments were no P addition (0P), 50 mg P kg−1 soil as NaHPO4⋅2H2O (50Pi), 100 mg P kg−1 soil as NaHPO4⋅2H2O (100Pi), and 100 mg P kg−1 soil as phytic acid, dodecasodiumsalt (100Po). Regosol was mixed with 10 % (w/w) peat moss. Then, to adjust the pH, CaCO3 was added to a rate of 2 g kg−1 of soil because the addition of peat moss can lower pH in Regosol. The two soils were mixed carefully with relevant P rates either as inorganic P or organic P. Both soils were supplied with 100 mg N kg−1 soil as NH4NO3 and 100 mg K kg−1 soil as K2SO4. Soil moisture was adjusted to 70 % of field capacity. Pots were filled with 1 kg of prepared soil. Seeds of white lupin and maize were surface-sterilized using 70 % ethanol and were soaked in a polystyrene box under running tap water over 48 h. After radicle emergence, four evenly sized seeds per pot were planted in a monocropping system. In mixed culture, 2:2 combinations from either species were planted per pot. After germination, seedlings were thinned to leave two plants per pot in a monoculture system and a 1:1 combination in mixed culture. Pots were arranged in complete randomized design with three replicates. The arrangements were re-randomized on every seventh days. The experiment also included a pot of unplanted control soil for each P supply for both soils. These pots were examined under the same conditions and were kept for the same duration as the cultivated pots. Soil moisture was maintained at 70 % field capacity by adding deionized water by weight every day. Plants were grown for 42 days.
Plant and soil analysis
At harvest, plants were lifted carefully out of the soil. The root system was shaken gently to remove loosely adhering soil. Then the rhizosphere soil was collected by vigorous shaking of the root system, followed by gentle brushing without damaging the root system. For mixed cropping pots, the root systems of the two species were separated carefully to avoid excessive breakage. Then rhizosphere soil was collected separately for the two species. For sole cropping pots, we collected the rhizosphere soil of two plants together. Immediately after the collection, rhizosphere soil was sieved through 2-mm holes to remove roots. It was then stored at −20 °C until analysis. Shoots and roots were separated after washing the root system with water. The cluster roots formed by white lupin were counted. Then both roots and shoots were oven-dried at 70 °C for 3 days. The dry weights of shoots and roots were recorded to estimate the plant growth. All dried roots and shoots were then ground. A sample of approximately 50 mg was digested using H2SO4-H2O2. The phosphorus concentration in the digested solution was quantified using vanadomolybdate blue method (Murphy and Reley 1962).
For the determination of soil P pools, sequential fractionation was conducted using the method described by Hedley et al. (1982). Briefly, 0.5 g of rhizosphere and unplanted control soil was weighed and extracted sequentially by shaking overnight (16 h) with a solution. (1) First, 30 mL deionized water was used with two resin strips (Selemion™ ion exchange membrane; Asahi Glass Co. Ltd., Japan). Then P in these resins was extracted by shaking with 20 mL of 0.5 M HCl for 2 h (resin P). (2) Second, 30 mL of 0.5 M NaHCO3 was used after adjustment to pH 8.5 (NaHCO3–P). (3) Subsequently, 30 mL of 0.1 M NaOH (NaOH–P) was used, followed by (4) 20 mL of 1 M HCl (HCl–P). The inorganic P (Pi) concentration in all extracts was determined using the molybdenum blue method (Murphy and Reley 1962). The total P (Pt) in 0.5 M NaHCO3 and 0.1 M NaOH fractions were measured by digestion of the extract with 10 mL of 1.8 N H2SO4, and respectively with 0.5 and 0.6 g of (NH4)2S2O8. Organic P (Po) in these fractions was calculated by subtracting the Pi from Pt. Total P of rhizosphere soil was determined separately using vanadomolybdate blue method after digestion of the sample with H2SO4-H2O2. Residual P was measured as the difference between total P and the sum of all organic and inorganic P fractions. The P adsorption capacity was determined according to the method by Sekiya (1970). Briefly, 5 g of air-dried soil was mixed with 10 mL of 5.869 g-P L−1solutionand was kept for 24 h. Then the solution was centrifuged (8000×g, 1 min) and filtered. The P concentration of filtered solution was measured using molybdenum yellow color method. The P adsorption capacity of each soil type was calculated based on the difference between the P concentration of the original solution added to the soil and the filtered solution. The pH of the rhizosphere soil and unplanted control soil was determined in a water suspension (soil water ratio of 1:2.5) after shaking for one hour.
Statistical analysis
All analyses were conducted using software (SAS ver. 9.1; SAS Institute Inc., Cary, NC, USA). Biomass of shoot, root and whole plant, P concentration of shoot and root, and plant P content data were subjected to two-way ANOVA using the P supply (0P, 50Pi, 100Pi, and 100Po) and cropping system (monocrop and intercrop) as the treatment effects for each crop. This analysis was done separately for each soil. Rhizosphere pH and all P fraction data were subjected to one-way ANOVA with cropping treatments including unplanted control soil (UP, unplanted control soil; MM, monocropped maize; IM, intercropped maize; ML, monocropped lupin; and IL, intercropped lupin) for each P supply under each soil. Significance of the difference between means was assessed using Tukey’s Studentized Range Test at the 0.05 probability level. Means are presented with the standard error.
Results
Plant growth and P uptake
This study investigated the rhizosphere-sharing of white lupin, a P-efficient plant, on growth and P acquisition of intercropped maize under different P rates and forms in two soils with contrasting P dynamics. Biomass and P accumulation of both maize and white lupin differed between the two soil types and the P rates used for the study (Tables 2 and 3). Both types of plants recorded higher growth and P uptake in Regosol than in Andosol. For both maize and white lupin, P supply had a significant effect on all plant parameters measured in both soils (Tables 2 and 3). White lupin responded strongly to added phytate in Regosol (Table 2). Neither maize nor white lupin responded to added phytate in Andosol (Table 3). Shoot and root P concentrations were higher in white lupin than in maize under 0P and 100Po in both soils. However, maize plants were somewhat larger than white lupin in each P supply (Tables 2 and 3). Positive effects of intercropped white lupin on growth and P accumulation of maize were observed only in Regosol where mixed culture increased dry weight of shoot and whole plant, P concentration of root and shoot, and plant P content of maize. This effect was significant in 0P, 50Pi, and 100Po treatments (Table 2). The respective plant P contents of intercropped maize in 0P, 50Pi, and 100Po were 46, 37, and 65 % greater than those in monoculture systems in Regosol. In the same soil for the same P dosages, the plant dry matter increments were, respectively, 12, 10, and 17 % (Table 2). In Andosol, cropping had no effect on maize (Table 3).
Cluster root formation by white lupin and rhizosphere pH changes in two soils
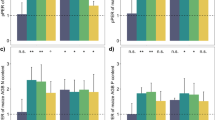
White lupin produced cluster roots irrespective of the soil type and addition of soluble or organic P sources except for the 100Pi supply in Regosol (Fig. 1). Cluster root formation was much more pronounced in Andosol than in Regosol. Each soil type exhibited a tendency to decrease the cluster root formation with increasing added soluble P dosage. Under 100Pi treatment, cluster root formation was suppressed completely in Regosol, but not in Andosol (Fig. 1).
Cluster root formation by white lupin under different rates and forms of P in two soils. Black columns represent white lupin in an intercropping system with maize, whereas others show data for sole cropping. Different letters denote significant differences between treatments (P ≤ 0.05) by Tukey’s Studentized Range Test. Error bars represent the standard error (S.E.; n = 3). 0P no P addition; 50Pi 50 mg P kg−1 soil by NaH2PO4⋅2H2O; 100Pi 100 mg P kg−1 soil by NaH2PO4⋅2H2O; 100Po 100 mg P kg−1 soil by phytate. (N.D: Cluster root formation was not observed under 100Pi treatment in Regosol)
Irrespective of the soil type and cropping system, white lupin decreased its rhizosphere pH markedly under all P rates (Table 4). Reduction of rhizosphere pH of white lupin was low with the application of 100Pi as soluble P compared to no P addition, 50Pi, and 100Po dosages, where this effect was observed especially more in Regosol than in Andosol (Table 4). In the case of maize, no significant change of pH was observed in the rhizosphere of a monoculture system compared to bulk soil. Incorporation of white lupin as a P-efficient companion plant into the maize cropping system lowered the pH in the maize rhizosphere compared to sole maize cropping. The reduction of pH in intercropped maize rhizosphere was intermediate between monocropped maize and monocropped or intercropped white lupin (Table 4). It is noteworthy that this effect was pronounced in Regosol, but in Andosol, rhizosphere pH reduction of intercropped maize was negligible (Table 4).
Rhizosphere P fractions
Compared to a lack of P addition, the addition of soluble P as NaH2PO4•2H2O increased the inorganic pools, particularly those of resin P and NaHCO3–Pi, both in Regosol and Andosol (Tables 5 and 6), although it increased NaOH–Pi only in Andosol (Table 6). The application of phytate as an organic P source increased mainly NaOH–Po and residual P in both soils (Tables 5 and 6), although it produced a shift in NaHCO3–Po only in Regosol (Table 5). The increment of resin P with the addition of soluble P was greater in Regosol than in Andosol (Tables 5 and 6).
Inorganic labile pools, resin P, and NaHCO3–Pi were significantly lower in the rhizosphere of maize and white lupin in both soils, irrespective of the cropping system, with the stronger reduction in maize rhizosphere rather than in white lupin rhizosphere (Tables 5 and 6). NaHCO3–Po, which is regarded as a labile pool, was significantly lower in the maize rhizosphere in both soils (Tables 5 and 6). Surprisingly, this labile organic P pool was augmented in the white lupin rhizosphere in Regosol (Table 5), although its concentration was lower in Andosol (Table 6).
No apparent change of NaOH–Pi and NaOH–Po fractions was noted in maize rhizosphere alone in any soil type (Tables 5 and 6). In contrast, concentration of these sparingly soluble P fractions in the rhizosphere of white lupin was lower in 0P, 50Pi, and 100Po than in their unplanted control soils in Regosol (Table 5). However, in Andosol, only the NaOH–Po pool decreased, without apparent reduction of the NaOH–Pi fraction in its rhizosphere (Table 6). In the intercropping system, white lupin influenced the maize rhizosphere to alter the concentration of sparingly soluble P pools in 0P, 50Pi, and 100Po applications only in Regosol. Because of the white lupin’s effect, maize showed lower concentrations of both NaOH–Pi and NaOH–Po in its rhizosphere in that intercropping system compared to sole cropping (Table 5). The respective degrees of reduction of NaOH–Pi in maize rhizosphere in intercropping systems with the application of 0P, 50Pi, 100Pi, and 100Po in Regosol were 7.6, 6.7, 1.8, and 9.2 mg P kg−1 soil. In the case of NaOH–Po, the respective degrees of reduction were 7.7, 7.8, 2.7 and 15.5 mg P kg−1 soil. A tendency to increase the NaOH–Po concentration in maize rhizosphere was noted in Andosol, irrespective of the cropping system (Table 6). The HCl-P fraction was accumulated significantly in white lupin rhizosphere in both soils, where the augmentation appeared to be greater in Andosol (Tables 5 and 6). Maize plants also tended to increase HCl-P fraction in the rhizosphere in Andosol (Table 6). No significant change of residual P fraction was found in maize rhizosphere in either soil (Tables 5 and 6), although a substantial reduction of this pool in lupin rhizosphere in Regosol was noted. The change was not significant (Table 5). The soil total P, which was determined separately, was significantly lower in the rhizospheres of both plants than in unplanted control soil irrespective of the P rate and form,, cropping system, and soil type (Tables 5 and 6).
Discussion
Effects of intercropped white lupin on growth and P accumulation of maize plant
This study demonstrates the effect of white lupin on early stage growth and P uptake of maize plant under different P rates and forms in two contrasting soils. The soil type was a major factor determining the beneficial effects of intercropping because in this study, a complementary effect of mixed cropping was observed only in Regosol (Table 2). This positive effect of intercropping is apparent both in low (0P) and moderate (50Pi) levels of P supply, as well as with organic P application (100Po). In this soil, growth and P accumulation of intercropped maize were higher than those of monocropped maize, suggesting that white lupin increases the availability of P for maize to uptake in mixed culture. Intercropping affected none of these parameters for white lupin, suggesting that P facilitation occurs from white lupin to maize and white lupin gains no advantage or disadvantage from intercropping. This result also underscores the fact that, in mixed cultures white lupin can obtain adequate P while enhancing P supply for the companion plant.
An interesting finding is that the positive effect of intercropping was evident even under organic P supply as phytate. Our results complement the findings of Li et al. (2003) and Li et al. (2004), who found that chickpea could facilitate P uptake of wheat and maize from the same organic P source; phytate. This study demonstrates the ability of white lupin to facilitate P uptake of companion plant from an organic P source, similar to the facilitation of P to wheat and maize by chickpea. Regarding plant growth, it might be argued that the difference of plant size between maize and white lupin affected the growth and P uptake of maize in both cropping systems through alteration of the competitive relationship between plants because, in this study, maize is somewhat larger than white lupin. This phenomenon suggests that a maize plant grown with a smaller white lupin in a pot filled with 1 kg soil would have ability to explore more resources in soil than a maize plant grown with another maize plant of equal size in the same pot. However, our study did not specifically examine intra-specific competition for soil P between two maize plants and inter-specific competition between maize and white lupin. Consequently, a future experiment that includes a same size pot of single maize plant as a cropping treatment with the other two cropping combinations used in our study must be undertaken to address the potential issue of competitive relation on P acquisition by maize in different cropping systems.
Cluster root formation and rhizosphere acidification by white lupin
Cluster root formation of white lupin (Fig. 1) is consistent with that of a study by Hassan et al. (2012) who reported the formation of many cluster roots by white lupin in a soil culture experiment conducted with the addition of soluble P to a rate of 80 mg-P kg−1 soil. Li and Liang (2005), Shane et al. (2003), and Shen et al. (2005) have reported that the formation of cluster roots decreased concomitantly with increasing P concentration in shoots and phloem sap of white lupin at higher P supply. Consequently, the complete suppression of cluster root formation in 100Pi in Regosol occurs because of high shoot P concentration of white lupin. White lupin had a lower shoot P concentration in Andosol than in Regosol, which might have affected differential capacities to form cluster roots in two soils. In the rhizosphere, pH change can be brought about by several processes and rhizosphere acidification caused by carboxylates exudation has been reported from earlier studies. Nuruzzaman et al. (2006) demonstrated the acidification of the rhizosphere of white lupin, which secreted a more substantial amount of citrate than the amounts from other plant species used in that study. Rhizosphere acidification of white lupin was also reported from a study by Cu et al. (2005) in which white lupin reduced the citric-acid-leachable soil P fraction. Therefore, by comparing those previous results with the rhizosphere pH changes in our study, we infer that the observed pH reduction in the rhizosphere of white lupin in this study would have occurred as a result of carboxylate exudation from roots.
P fractions in the rhizosphere
Resin P and NaHCO3–Pi pools are regarded as labile soil P fractions. They are known to be the most available for plant growth (Bowman and Cole 1978; Tiessen and Moir 1993). These two labile pools were reduced in rhizosphere of maize and white lupin in both soils, with the greatest reduction in the maize rhizosphere (Tables 5 and 6). The NaHCO3–Po fraction also declined in the rhizosphere of both plants in Andosol, despite the different rates and forms of P supply. However, in Regosol, this reduction was evident only under organic P application (100Po), although this fraction was augmented in the white lupin rhizosphere. Although the NaHCO3–Po fraction is regarded as labile and available to plants (Tiessen et al. 1992), its availability might be limited by binding to metal oxides in soil (Stewart and Tiessen 1987).
The NaOH extractable Pi and Po, acid extractable (HCl) P and residual P are regarded as less labile pools (Hedley et al. 1982; Tiessen and Moir 1993). Both NaOH–Pi that is P-associated with Al and Fe hydrous oxide (Hedley et al. 1982) and NaOH–Po were decreased significantly in the rhizosphere of white lupin in Regosol, but only NaOH-Po was decreased in Andosol (Tables 5 and 6). Reportedly, P in sparingly soluble fractions can be solubilized by carboxylates (George et al. 2002b; Gerke 1992; Richardson et al. 2001; Wang et al. 2006, 2012). This phenomenon is likely to be relevant to our study for observed rhizosphere pH reduction. Although we did not analyze the organic acid or acid phosphatase activity in rhizosphere soil, the pH decline in white lupin rhizosphere suggests the secretion of organic acids from white lupin and their role in increasing P availability in soil. For organic P to be available for plants, it must be hydrolyzed by phosphatase (Richardson et al. 2001). Cluster root formation can further enhance the P uptake of white lupin attributable to the secretion of extracellular acid phosphatases (Adams and Pate 1992; Neumann et al. 1999, 2000; Ozawa et al. 1995; Wasaki et al. 2003). In our study, we infer that lower concentration of NaOH-Po in lupin rhizosphere compared to unplanted control might have occurred by acid phosphatase secretion, as demonstrated by George et al. (2006), who found a positive correlation between activity of acid phosphatase and reduction of NaOH–Po in the rhizosphere of Tithonia and transgenic clover. Reduction of NaOH–Pi and Po in maize rhizosphere was noted only in mixed culture with white lupin in Regosol, but not in monoculture maize. This fact emphasizes the effect of companion white lupin of changing the concentration of sparingly soluble P pools in maize rhizosphere because, in the same soil, these two particular P pools were decreased in white lupin rhizosphere, irrespective of the cropping system. This decrease also suggests the contribution of organic acids and acid phosphatase secreted by white lupin to increase P availability for maize in intercropping system through rhizosphere-sharing. The tendency of maize to increase the NaOH–Po concentration in its rhizosphere in Andosol might reflect the high microbial activity in Andosol and the build-up of more stable organic P. This tendency is consistent with that described in an earlier report by George et al. (2002a), who found augmentation of NaOH–Po in maize rhizosphere in acidic soil.
Not in maize but in white lupin rhizosphere, a tendency of substantial reduction of stable residual P fraction was apparent, which presumably consists of P that is bound strongly with Al and Fe oxides (Hedley et al. 1982), although the reduction of this pool in lupin rhizosphere is not significant (Table 5). The absolute reduction of residual P in lupin rhizosphere in 0P in Regosol was approximately 25 mg P kg−1. For comparison, under the same P supply and soil, reduction of NaOH-Pi and NaOH-Po were, respectively 20 and 10.4 mg P kg−1. However, considering the concentration of a particular P pool in unplanted control soil under the P supply and soil conditions described above, residual P recorded the highest concentration of 125.8 mg P kg−1 whereas the concentrations of NaOH-Pi and NaOH-Po were, respectively 38.6 and 18.5 mg P kg−1 (Table 5). Consequently, this high base value and low proportion of the depletion of residual P compared to other P pools, probably coupled with low number of replication (n = 3) would have caused this change insignificant in our study. Acid phosphatase by white lupin might have influenced this reduction in lupin rhizosphere.
Enhanced growth and P benefits for maize from intercropping with white lupin differ among contrasting soils and different P rates and forms
Our findings of positive effect of intercropped white lupin on maize in Regosol, an acidic soil show agreement with results reported by Cu et al. (2005) in alkaline soil. However, our study has produced the novel finding that maize can enhance growth and P uptake from intercropped white lupin only in Regosol: not in Andosol. The differences observed in intercropping effect on maize in two contrasting soils are explainable mainly by three factors: (1) difference in P availability between two soils as affected by their P adsorption capacities; (2) contrasting root growth behavior of maize and root intermingling of maize with white lupin between two soils; and (3) differential capacity of white lupin to increase P availability for intercropped maize to uptake between two soils. In the intercropping system, roots of maize and white lupin can mix together. For that reason, strong root intermingling complements companion maize plant to uptake more P, which is made available by white lupin. Strong root intermingling is evident from the rhizosphere pH reduction of intercropped maize in Regosol. Full root intermingling between two plants grown in a small pot filled with 1 kg soil can be expected in any soil type if crop growth is normal. Both high bulk density and low water holding capacity of Regosol compared to Andosol suggest that Regosol is a heavier and more compacted soil than Andosol is (Table 1). Although root elongation of maize could be easier under these conditions, we observed poorer root growth in Andosol (Table 3). Therefore, this contrasting root growth behavior in two soils highlights the fact that, the Andosol itself has limited the root growth and development of maize. The root architecture of plants can undergo several changes in response to P deficiency. Effects of soil P availability for the root growth of maize have been demonstrated previously. Hajabbasi and Schumacher (1994) have reported a significant negative effect of soil P availability on the rate of appearance of axile roots of maize. Mollier and Pellerin (1999) showed reduction in both lateral root elongation and emergence of new axile roots of maize under low P availability. This phenomenon is clear from comparison of P availability of two soils used in present study, where in Andosol, resin P, which is regarded as readily available fraction for plants is extremely low (Table 1). For comparison, resin-P concentrations in unfertilized crop and pasture soils are in the range of 20–40 mg P kg−1 dry soil (Hedley et al. 1982). Ten times higher phosphorus adsorption capacity of Andosol than Regosol would explain the high fixation of applied P in less labile pools. Many Andosols are rich in allophanes, containing active aluminum and show a high retention of P, which makes the utilization of applied fertilizer P particularly low (Lukito et al. 1998) because accumulated P exists mainly in non-labile forms (Hirata et al. 1999). This phenomenon is expected to have led to the poorer root growth of maize in Andosol.
The concentrations of three less labile pools, NaOH–Pi, NaOH–Po, and residual P were lower in lupin rhizosphere in Regosol (Table 5) compared to Andosol, where the notable reduction was occurred only from one less labile pool, NaOH–Po (Table 6). P pools can decrease by root uptake but can also increase or decrease by shifts of P from one pool to another, through changing rhizosphere chemistry, induced by root exudates. Microbial processes that are effective in directly solubilizing and mineralizing P from organic P can also change the concentrations of P pools in soil (Richardson et al. 2009). Most probably, exudation of P-mobilizing compounds of white lupin affects this reduction and part of the P declined from above pools would have transferred to labile P pools as an increment of NaHCO3-Po pool in lupin rhizosphere in Regosol. If this happens, it suggests that the ability of white lupin to increase P availability is higher in Regosol than in Andosol, which constitutes another contributing factor that complements intercropped maize to uptake more P in Regosol, creating the observed difference between two soil types used in the study.
Although positive effects of intercropped white lupin for main crop plant are achieved in a soil, the application of high doses of soluble P to that particular soil can eliminate this complementary effect because the ability of white lupin to increase P availability apparently declines with increasing soluble P supply. Moreover, the main crop plant would be able to uptake the required amount of P under high soluble P supply without the supportive effect of white lupin. This phenomenon is readily apparent with the absence of positive effects for intercropped maize under 100Pi in Regosol, although, in the same soil, enhanced growth and P acquisition by intercropped maize with white lupin were found for 0P, 50Pi supply and with the addition of P as an organic form: phytate.
Conclusions
Results of this study demonstrate that interspecific P facilitation in the intercropping of maize and white lupin is unidirectional, occurring from white lupin to maize, where white lupin increases the availability of P and companion maize uptake part of those, giving no benefit to white lupin. Enhanced growth and P accumulation by maize with the effect of intercropped white lupin can be dependent on the soil type where they are grown and rates and forms of P applied to particular soil. Findings also highlight the ability of white lupin to improve growth and P acquisition of maize in mixed culture in Regosol without detrimentally affecting its own growth or P accumulation. Nevertheless, white lupin is not the ideal P-efficient plant to improve companion P-inefficient plant species in some soils. Our results open the door to additional detailed exploration of this model cropping system in actual field conditions in Regosol, where crops are grown to maturity and where translocation of P to the grain occurs, to ascertain whether white lupin can benefit companion maize plant in the latter stage of growth, as observed in the early stage of growth in our study. Before a definitive conclusion can be made, more field studies conducted with widely various soil types are warranted to confirm the applicability of white lupin as a P-efficient plant to improve P-inefficient plants in mixed cropping systems.
References
Adams MA, Pate JS (1992) Availability of organic and inorganic forms of phosphorus to lupins (Lupinus spp.). Plant Soil 145:107–113
Ae N, Arihara J, Okada K, Yoshihara T, Johansen C (1990) Phosphorus uptake by pigeon pea and its role in cropping systems of the Indian subcontinent. Science 248:477–480
Bowman RA, Cole CV (1978) Transformation of organic phosphorus substrates in soil as evaluated by NaHCO3 extractions. Soil Sci 125:49–54. doi:10.1097/00010694-197801000-00008
Cordell D, Drangert JO, White S (2009) The story of phosphorus: global food security and food for thought. Global Environ Chang 19:292–305
Cu S, Hutson J, Schuller KA (2005) Mixed culture of wheat (Triticum aestivum L.) with white lupin (Lupinus albus L.) improves the growth and phosphorus nutrition of the wheat. Plant Soil 272:143–151. doi:10.1007/s11104-004-4336-8
Dawson CJ, Hilton J (2011) Fertiliser availability in a resource-limited world: production and recycling of nitrogen and phosphorus. Food Policy 36:14–22
Dinkelaker B, Romheld V, Marschner H (1989) Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant Cell Environ 12:285–292. doi:10.1111/j.1365-3040.1989.tb01942.x
Gardner WK, Boundy KA (1983) The acquisition of phosphorus by Lupinus albus L. IV. The effect of interplanting wheat and white lupin on the growth and mineral composition of the two species. Plant Soil 70:391–402
Gardner WK, Parbery DG, Barber DA (1981) Proteoid root morphology and function in Lupinus albus. Plant Soil 60:143–147
Gardner WK, Barber DA, Parbery DG (1983) The acquisition of phosphorus by Lupinus albus L. III. The probable mechanism by which phosphorus movement in the soil/root interface is enhanced. Plant Soil 70:107–124
George TS, Grogory PJ, Robinson JS, Buresh RJ (2002a) Changes in phosphorus concentrations and pH in the rhizosphere of some agroforestry and crop species. Plant Soil 246:65–73
George TS, Gregory PJ, Wood M, Read D, Buresh RJ (2002b) Phosphatase activity and organic acids in the rhizosphere of potential agroforestry species and maize. Soil Biol Biochem 34:1487–1494
George TS, Turner BL, Gregory PJ, Cade-Menun BJ, Richardson AE (2006) Depletion of organic phosphorus from oxisols in relation to phosphatase activities in the rhizosphere. Eur J Soil Sci 57:47–57. doi:10.1111/j.1365-2389.2006.00767.x
Gerke J (1992) Phosphate, aluminium and iron in the soil solution of three different soils in relation to varying concentration of citric acid. Z PflansBodenkunde 155:339–343. doi:10.1002/jpln.19921550417
Gerke J, Romer W, Junk A (1994) The excretion of citric and malic acid by proteoid roots of Lupinusalbus L.: effects of soil solution concentration of phosphate, iron, and aluminium in the proteoid rhizosphere samples of an oxisol and luvisol. Z PflansBodenkunde 157:289–294. doi:10.1002/jpln.19941570408
Gilbert N (2009) The disappearing nutrient. Nature 461:716–718. doi:10.1038/461716a
Gunes A, Bagci EG, Inal A (2007) Interspecific facilitative root interactions and rhizosphere effects on phosphorus and iron nutrition between mixed grown chickpea and barley. J Plant Nutr 30:1455–1469. doi:10.1080/01904160701555648
Hajabbasi MA, Schumacher TE (1994) Phosphorus effects on root growth and development in two maize genotypes. Plant Soil 158:39–46
Hassan HM, Marschner P, McNeill A, Tang C (2012) Growth P uptake in grain legumes and changes in rhizosphere soil P pools. Biol Fertil Soils 48:151–159. doi:10.1007/s00374-011-0612-y
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions by cultivation practices and by laboratory incubation. Soil Sci Soc Am J 46:970–976. doi:10.2136/sssaj1982.03615995004600050017x
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Hinsinger P, Betencourt E, Bernard L, Brauman A, Plassard C, Shen J, Tang X, Zhang F (2011) P for two, sharing a scarce resource - soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol 156:1078–1086
Hirata H, Watanabe K, Fukushima K, Aoki M, Imamura R, Takahashi M (1999) Effect of continues application of farmyard manure and inorganic fertilizer for 9 years on changes in phosphorus compounds in plow layer of an upland Andosol. Soil Sci Plant Nutr 45:577–590
Hocking PJ, Randall PJ (2001) Better growth and phosphorus nutrition of sorghum and wheat following organic acid secreting crops. In: Horst WJ, Schenk MK, Burkert A, Claassen N, Flessa H, Frommer WB, Goldbach H, Olfs HW, Romheld V, Sattlemacher B, Schmidhalter U, Schubert S, Wiren NV, Wittenmayer L (eds) Plant nutrition food security and sustainability of agro-ecosystems through basic and applied research. Kluwer, Dordrecht, pp 548–549
Kamh M, Horst WJ, Amer F, Mostafa H, Maier P (1999) Mobilization of soil and fertilizer phosphate by cover crops. Plant Soil 211:19–27
Keerthisinghe G, Hocking PJ, Ryan PR, Delhaize E (1998) Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.). Plant Cell Environ 21:467–478. doi:10.1046/j.1365-3040.1998.00300.x
Lambers H, Chapin FS III, Pons TL (1998) Plant physiological ecology. Springer, New York
Lambers H, Shane MW, Cramer MD, Pearse S, Veneklaas E (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713. doi:10.1093/aob/mcl114
Lambers H, Clements JC, Nelson MN (2013) How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am J Bot 100:263–288. doi:10.3732/ajb.1200474
Li CJ, Liang RX (2005) Root cluster formation and citrate exudation of white lupin (Lupinus albus L.) as related to phosphorus availability. J Integr Plant Biol 47:172–177. doi:10.1111/j.1744-7909.2005.00012.x
Li M, Shinano T, Tadano T (1997) Distribution of exudates of lupin roots in the rhizosphere under phosphorus deficient conditions. Soil Sci Plant Nutr 43:237–245. doi:10.1080/00380768.1997.10414731
Li L, Tang C, Rengel Z, Zhang F (2003) Chickpea facilitates phosphorus uptake by intercropped wheat from an organic phosphorus source. Plant Soil 248:297–303
Li SM, Li L, Zhang FS, Tang C (2004) Acid phosphatase role in chickpea/maize intercropping. Ann Bot 94:297–303
Li L, Li S, Sun J, Zhou L, Bao X, Zhang H, Zhang F (2007) Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc Natl Acad Sci 104:11192–11196
Li H, Shen J, Zhang F, Clairotte M, Drevon JJ, Le Cadre E, Hinsinger P (2008) Dynamics of phosphorus fractions in the rhizosphere of common bean (Phaseolus vulgaris L.) and durum wheat (Triticum turgidum durum L.) grown in monocropping and intercropping systems. Pant Soil 312:139–150. doi:10.1007/s11104-007-9512-1
Lukito HP, Kouno K, Ando T (1998) Phosphorus requirements of microbial biomass in a Regosol and an Andosol. Soil Biol Biochem 30:865–872
Mollier A, Pellerin S (1999) Maize root system growth and development as influenced by phosphorus deficiency. J Exp Bot 50:487–497
Murphy J, Reley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Neumann G, Römheld V (1999) Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 211:121–130
Neumann G, Massonneau A, Martinoia E, Römheld V (1999) Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 208:373–382
Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Römheld V, Martinoia E (2000) Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Ann Bot 85:909–919. doi:10.1006/anbo.2000.1135
Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ (2006) Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 281:109–120. doi:10.1007/s11104-005-3936-2
Ozawa K, Osaki M, Matsui H, Honma M, Tadano T (1995) Purification and properties of acid phosphatase secreted from lupin roots under phosphorus-deficiency conditions. Soil Sci Plant Nutr 41:461–469. doi:10.1080/00380768.1995.10419608
Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MDA, Lambers H (2006) Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant Soil 288:127–139. doi:10.1007/s11104-006-9099-y
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50:665–693
Richardson AE, Hadobas PA, Hayes JE (2001) Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate. Plant J 25:641–649
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339. doi:10.1007/s11104-009-9895-2
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349:121–156. doi:10.1007/s11104-011-0950-4
Sekiya K (1970) Phosphoric acid. In: Ishizawa S (ed) Analysis methods for measuring soil fertility. Yokendo Co. Ltd, Tokyo, pp 251–253
Shane MW, De Vos M, De Roock S, Lambers H (2003) Shoot P status regulates cluster-root growth and citrate exudation in Lupinus albus grown with a divided root system. Plant Cell Environ 26:265–273. doi:10.1046/j.1365-3040.2003.00957.x
Shen J, Li H, Neumann G, Zhang F (2005) Nutrient uptake, cluster root formation and exudation of protons and citrate in Lupinus albus as affected by localized supply of phosphorus in a split-root system. Plant Sci 168:837–845
Simpson RJ, Oberson A, Culvenor RA, Ryan MH, Veneklaas EJ, Lambers H, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Richardson AE (2011) Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant Soil 349:89–120. doi:10.1007/s11104-011-0880-1
Song YN, Zhang FS, Marschner P, Fan FL, Gao HM, Bao XG, Sun JH, Li L (2007) Effect of intercropping on crop yield and chemical and microbiological properties in rhizosphere of wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L.). Biol Fertil Soils 43:565–574. doi:10.1007/s00374-006-0139-9
Stewart JWB, Tiessen H (1987) Dynamics of soil organic phosphorus. Biogeochemistry 4:41–60
Tadano T, Sakai H (1991) Secretion of acid phosphatase by the roots of several crop species under phosphorus-deficient conditions. Soil Sci Plant Nutr 37:129–140. doi:10.1080/00380768.1991.10415018
Tang C, Barton L, Rapheal C (1997) Pasture legume species differ in their capacity to acidify a low-buffer soil. Aust J Agric Res 49:53–58
Tiessen H, Moir JO (1993) Characterization of available P by sequential extraction. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis, Boca Raton, pp 104–107
Tiessen H, Salcedo IH, Sampio EVSB (1992) Nutrient and soil organic matter dynamics under shifting cultivation in semi arid northeastern Brazil. Agric Ecosyst Environ 38:139–151
Vance CP (2001) Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol 127:390–397
Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248:187–197
Wang Z, Shen J, Zhang F (2006) Cluster roots formation, carboxylate exudation and proton release of Lupinus pilosus Murr. as affected by medium pH and P deficiency. Plant Soil 287:247–256. doi:10.1007/s11104-006-9071-x
Wang Y, Marschner P, Zhang F (2012) Phosphorus pools and other soil properties in the rhizosphere of wheat and legumes growing in three soils in monoculture or as a mixture of wheat and legume. Plant Soil 354:283–298. doi:10.1007/s11104-011-1065-7
Wasaki J, Yamamura T, Shinano T, Osaki M (2003) Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency. Plant Soil 248:129–136
Acknowledgments
This research was partly supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Ministry of Agriculture, Forestry and Fisheries (MAFF), Japan through a Grant-in-Aid for Young Scientists (23688010) and a research project entitled: “Development of technologies for mitigation and adaptation to climate change in Agriculture, Forestry and Fisheries.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Hammond.
Rights and permissions
About this article
Cite this article
Dissanayaka, D.M.S.B., Maruyama, H., Masuda, G. et al. Interspecific facilitation of P acquisition in intercropping of maize with white lupin in two contrasting soils as influenced by different rates and forms of P supply. Plant Soil 390, 223–236 (2015). https://doi.org/10.1007/s11104-015-2392-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2392-x