Abstract
Background and aims
This study investigated the detailed mechanism underlying the alleviation of osmotic stress by exogenous hydrogen (H2) in Medicago sativa.
Methods
By using biochemical and molecular approaches, the experiments were performed with the analyses of biomass, relative water content (RWC), lipid peroxidation, abscisic acid (ABA) content, antioxidant activities, and related gene expression profiles.
Results
H2 application stimulated ABA production, which was accompanied by the regulation of ABA biosynthesis and deactivation/activation genes. Elevated H2-induced ABA synthesis was sensitive to tungstate, an inhibitor of ABA synthesis. Meanwhile, H2-alleviated osmotic stress, which was supported by the increases in biomass and RWC, and the reduction of lipid peroxidation, was impaired by the inhibition of ABA synthesis. Consistently, tungstate blocked H2-induced antioxidant defense. Molecular evidence revealed that miR528 was down-regulated by H2, showing a negative correlation with its target gene POD2. When tungstate was added together, the decreased miR528 and increased POD2 transcripts were respectively blocked. Transcriptional factor genes involved in ABA signaling, including MYB102, MYC2, and ABF/AREB2, were differentially upregulated by H2, but further impaired by the co-incubation with tungstate.
Conclusions
Collectively, our results suggested the possible role of ABA signaling in exogenous H2-mediated tolerance against osmotic stress in alfalfa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water deficit stress is a common adverse environmental condition that has damaging effects on plant metabolic processes. These include the reduction in nutrient uptake, stomatal aperture, and photosynthetic assimilates, thus causing crop losses (Neumann 2008). In response to stress, plants employ various mechanisms against osmotic stress, including metabolism regulation and morphological changes. Often, the osmoticum polyethylene glycol (PEG6000) is used to mimic osmotic stress in scientific research (Srinath and Jabeen 2013), even though soil drying induces both water and mechanical stress (Jin et al. 2013a).
The best-identified trigger of drought tolerance is phytohormone abscisic acid (ABA) (Hu et al. 2006; Lu et al. 2009). Evidence revealed that zeaxanthin epoxidase (ZEP; also named as ABA1), 9-cis epoxycarotenoid dioxygenase (NCED), MoCo sulfurase (ABA2), aldehyde oxidase (AAO3), β-D-glucosidase (βG1), and ABA glucosyltransferase (AOG), regulate ABA biosynthesis and deactivation/activation (Fujii 2014). The expression of these genes is induced either by exogenous ABA or osmotic stress (Iuchi et al. 2001; Xiong et al. 2001, 2002; Seo and Koshiba 2002; Xiong and Zhu 2003; Tan et al. 2003).
Although reactive oxygen species (ROS) are considered to be critical signaling molecules in sensing stresses, their toxic effects, including protein denaturation, nucleotides degradation, and lipids peroxidation (Hu et al. 2008; Miller et al. 2010; Vaseem et al. 2017), especially under osmotic stress conditions (Jiang and Zhang 2001, 2002), were discovered. The tight regulation is therefore needed to balance ROS production, and to scavenge and preserve cellular redox poise (Bailly et al. 2008; Miller et al. 2010). It is well established that antioxidant defense against ROS includes the enzymatic system, such as superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase (POD), and catalase (CAT) (Foyer and Noctor 2005). Above antioxidant enzymes are regulated at transcriptional and post-transcriptional levels (Carrington and Ambros 2003; Bartel 2004). For example, previous results revealed that drought in maize seedlings downregulated miR528, while its target transcript POD was upregulated (Wei et al. 2009). MiR398 targets two closely related Cu/Zn SODs (CSD1 and CSD2) that are involved in the detoxification of oxidative stress (Mittler 2002; Wei et al. 2009).
Besides, the targets of microRNAs (miRNAs) include transcriptional factors (TFs) (Guo et al. 2005). It is well-known that TFs represent the critical molecular switches composing the regulation of plant developmental processes in response to a variety of stresses (Joshi et al. 2016). A large number of genes in the plant genome (up to 10%) possibly encode TFs (Franco-Zorrilla et al. 2014), which were classified into different families, such as ABA-responsive element (ABRE)-binding proteins (AREB), DEHYDRATION-RESPONSIVE ELEMENT BINDING (DREB), myeloblastosis (MYB), NAM (NO APICAL MERISTEM), ATAF1/2 (ARABIDOPSIS TRANSCRIPTION ACTIVATING FACTOR1/2), and CUC2 (CUP-SHAPED COTYLEDON2) (NAC), and the BASIC LEUCINE ZIPPER (bZIP). Above classification is according to their distinct DNA-binding domain structure (Golldack et al. 2011; Cai et al. 2017). The modulation of above TFs genes in response to osmotic stress is via ABA-dependent or -independent pathway (Todaka et al. 2012).
Hydrogen gas (H2) is an odorless, colorless, tasteless, and flammable gas. Although several reports discovered H2 evolution and uptake in illuminated leaves and germinating seeds about fifty years ago (Renwick et al. 1964), there is little evidence about the specific mechanism of its biosynthesis and even physiological roles in plants (Dong et al. 2003). Since the direct application of H2 gas is not appropriate in practice and may be dangerous due to its inflammable and explosive feature (Zheng et al. 2011), hydrogen-rich water (HRW) in vitro experiments is used to mimic the functions of endogenous H2 in plants. By using this approach, related results showed that H2 could act as a significant gaseous molecule with numerous biological functions in plant responses against oxidative stress triggered by drought, salinity, and paraquat exposure in alfalfa and Arabidopsis (Xie et al. 2012, 2014; Jin et al. 2013b, 2016). It was further suggested that exogenous H2 might enhance cold tolerance and tolerance against aluminum in plants, at least partially, by the restoration of redox homeostasis via modulating the expression of miRNAs like miR398 and miR528 (Xu et al. 2017a, 2017b). The induction of lateral root and adventitious rooting was also discovered (Lin et al. 2014; Cao et al. 2017). Interestingly, previous results (Xie et al. 2014; Jin et al. 2016) demonstrated that exogenously applied ABA increased endogenous H2 production under the normal growth conditions and upon drought stress. By manipulating endogenous nitric oxide (NO) level with its synthetic inhibitor and scavengers, the requirement of NO in hydrogen-alleviated osmotic stress was confirmed (Su et al. 2018). Since NO was found to be involved in brassinosteroid-induced ABA biosynthesis and -alleviated oxidative stress in maize leaves (Zhang et al. 2011), the detailed molecular mechanism underlying the functions of H2 in the enhancement of plant tolerance against osmotic stress, especially the possible crosstalk between H2 and ABA, remains to be fully elucidated.
This study aims to address how hydrogen gas enables alfalfa seedlings to cope with osmotic stress. H2 application increased endogenous ABA metabolism and antioxidants defense in alfalfa seedlings. Afterward, it led to the reestablishment of redox balance. Gene expression analyses of miRNAs, TFs, and antioxidant genes supported H2 action during osmotic stress, which was differentially abolished by the addition of ABA synthetic inhibitor tungstate (Jiang and Zhang 2002; Liu et al. 2012). The inhibition in ABA synthesis triggered by tungstate was confirmed in our experimental conditions. The alleviation of osmotic stress by H2 was blocked as well. Together, our results support the idea that ABA, at least partially, operates downstream of H2 alleviating osmotic stress through the reestablishment of redox balance. Combined with the previous results (Xie et al. 2014; Jin et al. 2016), it was further deduced that H2-triggered ABA synthesis could be an amplification loop in H2 signaling.
Materials and methods
Plant material and growth conditions
Alfalfa (Medicago sativa L. cv. Victoria) seeds were surface-sterilized with 5% NaClO for 10 min, and rinsed extensively in distilled water then germinated for 1 day at 25 °C in the darkness. Uniform seedlings were selected and transferred to the plastic chambers and cultured in quarter-strength Hoagland’s solution: 0.97 mM Ca(NO3)2, 0.66 mM KNO3, 0.5 mM MgSO4, 25 μM KH2PO4, 12.5 μM Fe-EDTA, 0.12 μM H3BO3, 0.14 μM ZnSO4, 0.23 μM MnSO4, 0.26 μM CuSO4, and 0.04 μM Na2MoO4. The pH was adjusted to 6.0. Seedlings were grown in the illuminating incubator (14 h light with a light intensity of 200 mol m−2 s−1, 25 ± 1 °C, and 10 h dark, 23 ± 1 °C). Five-day-old seedlings were then incubated in quarter-strength Hoagland’s solution with or without 0.39 mM H2, 0.1 mM ABA, 1 mM tungstate (Tu; an inhibitor of ABA synthesis; Jiang and Zhang 2002; Liu et al. 2012), alone or their combinations for 12 h, then exposed to 20% (w/v) polyethylene glycol (PEG; MW 6000; osmotic potential −0.5 MPa) stress for the indicated time points. The sample without chemicals was the control (Con). The pH of both nutrient medium and treatment solutions was adjusted to 6.0. After various treatments, plants were photographed, and shoot tissues were sampled for use immediately or flash-frozen in liquid nitrogen, and stored at −80 °C for further analysis.
Preparation of hydrogen-rich water (HRW)
Purified hydrogen gas (99.99%, v/v) generated from a hydrogen gas generator (SHC-300; Saikesaisi Hydrogen Energy Co., Ltd. Shandong, China) was bubbled into 1000 ml quarter-strength Hoagland’s solution (pH 6.0, 25 °C) at a rate of 150 ml min−1 for 60 min (Jin et al. 2013b). Then, the corresponding HRW was immediately diluted to the required 50% saturation (v/v), which contains 0.39 mM H2 for at least 12 h, determined by gas chorography (Su et al. 2018).
Analysis of water status and phenotypic analysis
Water status of tissues, measured as relative water content (RWC), was determined as previous method (García-Mata and Lamattina 2001). Fresh weight (FW), dry weight (DW), and root elongation were measured as previously described (Xie et al. 2016; Xu et al. 2017b).
Determination of thiobarbituric acid reactive substances (TBARS) and ABA content
Lipid peroxidation was estimated by measuring the amount of TBARS using an extinction coefficient of 155 mM−1 cm−1 and expressed as nmol g−1 dry weight (DW) (Wang et al. 2012; Xu et al. 2017b).
The extraction and purification of ABA were carried out following the previous method (Guinn et al. 1986). ABA contents were analyzed by high performance liquid chromatography (HPLC) (Shimadzu, D-2000, Hitachi Ltd., Tokyo, Japan) using ultraviolet detector, and C18 column. Pure ABA (Sigma-Aldrich, St Louis, MO, USA) was used as a standard. ABA was identified and quantified by retention time.
Enzymatic activities assays
Fresh samples (about 0.3 g) were homogenized in 5 ml of 50 mM cold phosphate buffer (pH 7.0), containing 1 mM EDTA and 1% (w/v) polyvinylpolypyrrolidone (PVP) for superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), and guaiacol peroxidase (POD, EC 1.11.1.7) activity assays, or the combinations with 1 mM ascorbic acid (ASA) in the case of ascorbate peroxidase (APX, EC 1.11.1.11) determination. The homogenates were centrifuged at 15000 g for 20 min at 4 °C, and the supernatants were used for assays of enzymatic activity.
Total SOD activity was assayed according to the previous method (Beauchamp and Fridovich 1971), and one unit of SOD (U) was defined as the amount of crude enzyme extract required to inhibit the reduction rate of nitroblue tetrazolium (NBT) by 50%. The CAT activity was analyzed by monitoring the consumption of H2O2 (extinction coefficient 39.4 mM−1 cm−1) at 240 nm for at least 2 min (Xu et al. 2017b). POD activity was determined by measuring the oxidation of guaiacol (extinction coefficient 26.6 mM−1 cm−1) at 470 nm (Han et al. 2008). APX activity was determined by monitoring the decrease at 290 nm (extinction coefficient 2.8 mM−1 cm−1) (Xu et al. 2017b). Protein was determined by the method previously described (Bradford 1976), using bovine serum albumin (BSA) as a standard.
Quantitative reverse transcription polymerase chain reaction (qPCR) analysis
Total RNA in samples was extracted by using Trizol reagent (Invitrogen, Gaithersburg, MD, USA), and then digested with RNase-free DNase to eliminate genomic DNA contamination. First-strand cDNA was synthesized with oligo(dT) primers using SuperScript™ reverse transcriptase (Invitrogen, Carlsbad, CA, USA). qPCR was performed using a Mastercycler® ep realplex real-time PCR system (Eppendorf, Hamburg, Germany) with SYBR® Premix Ex Taq™ (TaKaRa Bio Inc., Dalian, China). The accession numbers (GenBank/miRBase) and oligonucleotide primers were shown in Supplemental Table S1. Melting curves were analyzed at the dissociation step to examine the specificity of amplification. Relative expression levels were presented as values, relative to that of the corresponding control samples, after normalization with Actin2 and ELF2 transcript levels (Gu et al. 2017; Mei et al. 2017). Three independent experiments with three replicates were obtained for each data.
Statistical analysis
Results were expressed as the means ± SE of three independent experiments with at least three replicates for each. Statistical analysis was performed using SPSS 17.0 software. Data was analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests, and P values <0.05 were considered as statistically significant.
Results
The beneficial role of H2 against osmotic stress was blocked by the ABA synthetic inhibitor
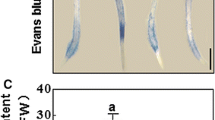
To assess the role of ABA in H2-alleviated osmotic stress, tungstate (1 mM; the inhibitor of ABA synthesis) was used together with H2 and ABA (as a positive control), followed by PEG stress. Similar to the response of ABA, H2 was able to alleviate osmotic stress (Fig. 1a). This was further correlated with the alleviation of seedling growth inhibition, including the changes in fresh weight and dry weight (Fig. 1b), relative water content (Fig. 1c), and the root elongation (Fig. 1d). A correlation analysis between fresh weight and relative water content further revealed that H2 promoted plant tolerance against osmotic stress. Above H2 responses were differentially blocked by the addition of 1 mM tungstate. Comparatively, weaker or no significant changes were observed when ABA was supplemented with or without tungstate, followed by stress. Above results thus indicated the possible link between ABA and H2 in the alleviation of osmotic stress in alfalfa seedlings.
Exogenous H2-alleviated osmotic stress was sensitive to the inhibitor of ABA synthesis. Five-day-old alfalfa seedlings were grown in nutrient solutions containing 0.39 mM H2, 0.1 mM ABA, 1 mM tungstate (Tu; an inhibitor of ABA synthesis), alone or their combinations for 12 h, then exposed to 20% PEG for an additional 48 h. Afterward, the representative phenotype (a), fresh weight and dry weight (b), the relative water content in shoot parts (c), and root elongation (d) were provided or measured. The plants without chemicals were regarded as the control sample (Con)
Changes in ABA concentration in response to H2 and tungstate
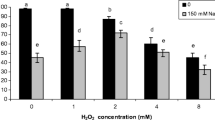
To confirm above hypothesis, we measured the ABA concentrations in shoot tissue of alfalfa plants. As expected, compared to the control plants, the ABA concentrations were increased in response to PEG stress for both 12 and 24 h (Fig. 2a). Meanwhile, supplemented H2 and ABA obviously increased endogenous ABA production under stressed and non-stressed conditions. In the presence of tungstate at 1 mM, above H2-induced ABA production was significantly blocked, which was different from the response of exogenously applied ABA. The decreased ABA concentration was also observed when tungstate was applied alone. Above results indicated that 1 mM tungstate is effective in the inhibition of ABA synthesis, at least under our experimental conditions. Combined with the corresponding phenotypic parameters (Fig. 1), we deduced that the beneficial roles of H2 in the alleviation of osmotic stress might be dependent on ABA.
Changes in ABA and TBARS contents. Five-day-old alfalfa seedlings were grown in nutrient solutions containing 0.39 mM H2, 0.1 mM ABA, 1 mM tungstate (Tu; an inhibitor of ABA synthesis), alone or their combinations for 12 h, then exposed to 20% PEG. Afterward, the shoot tissues were collected at 12 and 24 h for the determination of ABA content using HPLC (a), or at 48 h for TBARS content (b). The plants without chemicals were regarded as the control sample (Con)
H2-alleviated lipid peroxidation was sensitive to tungstate
To further assess the molecular mechanism of H2 governing the alleviation of osmotic stress, TBARS production, an indicator of lipid peroxidation, was investigated. As expected, the increased TBARS level triggered by osmotic stress was obviously alleviated by H2, which was reversed by the addition of tungstate (Fig. 2b). However, the combination of tungstate and ABA in the stressed condition brought about a contrasting response. These results obtained from the above suggested that H2-alleviated lipid peroxidation was, at least partially, in ABA-dependent fashion, although the possibility of ABA-independent pathway could not be easily ruled out. Consistently, we noticed that H2 alone did not influence lipid peroxidation, in comparison with the control plants.
Changes in ABA metabolism gene expression
ABA metabolism gene expression was increased by osmotic stress (Fujii 2014). To confirm the role of ABA in above H2 responses, several major genes responsible for ABA biosynthesis (ABA2 and AAO3) and deactivation/activation (AOG and βG1) were analyzed (a simple ABA metabolism pathway was shown in supplemental Fig. S1). As expected, the transcripts of above ABA biosynthesis genes (in particular) and deactivation/activation genes were elevated after osmotic stress with (especially) or without ABA supplementation (Fig. 3). Subsequent results revealed that in the presence of osmotic stress, the expression of ABA2 was significantly enhanced, but AOG and βG1 were obviously inhibited, by H2 pretreatment. However, AAO3 transcripts were similarly induced by PEG regardless of H2. By contrast, the addition of tungstate was able to differentially reverse the effects of H2 on the transcript levels of ABA2, AAO3, AOG, and βG1 under the stressed conditions. The up-regulation of ABA2 and AAO3 mRNA was observed by H2 alone. We also discovered the similar changes in AOG and βG1 transcripts under the identical treatments, suggesting that deactivation/activation of ABA might be not important factor(s) responsible for H2-triggered ABA production in osmotic stress. Consequently, the ability of H2 to trigger ABA metabolism genes further strengthens its reliance on ABA under osmotic stress.
Changes in ABA biosynthesis and deactivation/activation gene expression. Five-day-old alfalfa seedlings were grown in nutrient solutions containing 0.39 mM H2, 0.1 mM ABA, 1 mM tungstate (Tu; an inhibitor of ABA synthesis), alone or their combinations for 12 h, and then exposed to 20% PEG. Afterwards, the shoot tissues were collected at 24 h for determining ABA2 (a), AAO3 (b), AOG (c), and βG1 (d) gene expression analyzed by qPCR, normalized against two internal reference genes Actin2 and ELF2, which were stably expressed. The plants without chemicals were regarded as the control sample (Con)
Changes in antioxidant enzyme activities, transcripts of antioxidant genes, and miR528
To further evaluate the contribution of antioxidant defence in above H2 responses, the SOD, POD, APX, and CAT enzymatic activities and their transcripts were analyzed. As anticipated, in shoot parts of alfalfa plants, PEG exposure led to significant decreases in above mentioned antioxidant enzyme activities (Fig. 4). Treatment with H2 significantly blocked the decreases in the activities of these antioxidant enzymes induced by osmotic stress. Further experiments revealed that the co-incubation with tungstate differentially blocked the enhancement in the activities of antioxidant enzymes triggered by H2. Meanwhile in the stressed conditions, no significant differences were observed in ABA-treated plants either in the presence or absence of tungstate. Comparatively, molecular evidence revealed that changes in CuZnSOD, APX1, CAT, and POD2 transcripts exhibited the similar tendencies (except MnSOD; Fig. 5a-d).
Changes in antioxidant enzyme activities. Five-day-old alfalfa seedlings were grown in nutrient solutions containing 0.39 mM H2, 0.1 mM ABA, 1 mM tungstate (Tu; an inhibitor of ABA synthesis), alone or their combinations for 12 h, then exposed to 20% PEG. The shoot tissues were collected at 24 h for determining SOD (a), POD (b), APX (c), and CAT (d) activities. The plants without chemicals were regarded as the control sample (Con)
Changes in antioxidant gene expression and miRNA. Five-day-old alfalfa seedlings were grown in nutrient solutions containing 0.39 mM H2, 0.1 mM ABA, 1 mM tungstate (Tu; an inhibitor of ABA synthesis), alone or their combinations for 12 h, then exposed to 20% PEG. The shoot tissues were collected at 12 h for CuZnSOD and MnSOD (a), APX1 (b), CAT (c), POD2 (d), and miR528 (e) gene expression analyzed by qPCR. The plants without chemicals were regarded as the control sample (Con)
Subsequently, miR528 transcript was investigated (Fig. 5e), and compared with POD2 mRNA (Fig. 5d), to assess their roles in osmotic stress tolerance elicited by H2. The expression levels of miR528 were upregulated upon osmotic stress, compared to the control sample. The addition of H2 or ABA further obviously reduced the transcript levels of miR528, compared to the PEG-treated alone. Above response of H2, other than that of ABA, was significantly blocked by tungstate, in stressed conditions. Interestingly, above changes in miR528 displayed a negative correlation with its target gene, POD2 (Fig. 5d). These results indicated the requirement of ABA in the induction of antioxidant defence elicited by H2 in the stressed conditions, at least partly.
Expression profiles of transcriptional factors associated with osmotic stress
TFs have been reported to respond to osmotic stress via ABA-dependent or -independent pathways (Joshi et al. 2016). The gene expression of some representative TFs involved in ABA signaling in response to H2, ABA, and tungstate application was studied. As expected, osmotic stress resulted in the significant decreases in MYB102, MYC2, and ABF/AREB2 in alfalfa seedling shoots, compared to the untreated control samples (Fig. 6). The addition of exogenous H2 upregulated the above transcripts compared to the PEG-treated alone samples. By contrast, the inclusion of tungstate in above H2 treatment impaired MYB102, MYC2, and ABF/AREB2 transcripts. Furthermore, we observed that exogenously applied ABA followed by osmotic stress significantly increased above mentioned gene expression, which were not altered by the co-incubation with tungstate.
Gene expression of transcriptional factors involved in ABA signaling. Five-day-old alfalfa seedlings were grown in nutrient solutions containing 0.39 mM H2, 0.1 mM ABA, 1 mM tungstate (Tu; an inhibitor of ABA synthesis), alone or their combinations for 12 h, then exposed to 20% PEG. The shoot tissues were collected at 12 h. Afterward, gene profiles of MYB102 (a), MYC2 (b), and ABF/AREB2 (c), were analyzed by qPCR, and normalized against two internal reference genes Actin2 and EIF2, which were stably expressed. The plants without chemicals were regarded as the control sample (Con)
Discussion
During the recent years, the interaction between H2 and phytohormones has attracted a lot of attention. In our previous work (Xie et al. 2014; Jin et al. 2016), we discovered that ABA and osmotic stress significantly induced H2 production in both alfalfa and Arabidopsis plants, and the role of H2 in alfalfa stomata sensitivity to ABA was partly associated with its effect on the modification of leaf apoplastic pH (Jin et al. 2016). Here, by using inhibitor (tungstate, an inhibitor of ABA biosynthesis; Jiang and Zhang 2002; Liu et al. 2012) test and molecular approach, we discovered that H2 could increase ABA synthesis (Fig. 2a). Importantly, an increase in the concentration of ABA might be critical for exogenous H2-mediated plant adaptive responses to osmotic stress via the upregulation of transcriptional factor genes and antioxidant defense, and above study extended the former results (Fig. 7; Xie et al. 2014; Jin et al. 2016; Su et al. 2018).
Model summarizing the interaction of H2 and ABA in plant tolerance against osmotic stress. Exogenous H2 could increase ABA synthesis, and an increase in the concentration of ABA might be critical for exogenous H2-mediated plant adaptive responses to osmotic stress (This study). H2 accumulates in response to ABA (Xie et al. 2014) and drought stress (Jin et al. 2016), thus forming an amplification loop in osmotic stress tolerance signaling. The role of nitrate reductase-dependent nitric oxide is also suggested (Su et al. 2018). Additionally, the involvement of ABA-independent pathway is not easily ruled out, and illustrated with dash line. The above pathways might be mediated by modulating gene expression of miRNA, TFs, and antioxidant defence
Collectively, we further deduced that osmotic stress-triggered ABA might increase H2 production (Xie et al. 2014; Jin et al. 2016), which enhances ABA accumulation, thus forming an amplification loop in osmotic stress tolerance signaling (Fig. 7). Similarly, the existence of positive amplification loops in ROS signaling has previously been discovered (Zhang et al. 2006). Certainly, we can not exclude the possibility that tungstate (also regarded as the inhibitor of nitrate reductase; Tossi et al. 2009; Xiong et al. 2012) used in the present study, may not specifically target ABA. However, combined with the changes in ABA metabolism (Figs. 2a, 3), our results collectively point to the fact that there might be existing, not only a linear pathway, but also a crosstalk between H2 and ABA (synthesis and/or sensitivity) in higher tissue water status and biomass when plants are exposed to osmotic stress.
Experimental evidence supported the involvement of H2 in plant tolerance against abiotic stresses, including salinity (Xie et al. 2012), paraquat exposure (Jin et al. 2013b), cadmium stress (Cui et al. 2013), and mercury stress (Cui et al. 2014). The mechanism underlying above H2 in plants is largely associated with the enhancement of antioxidant defence. Meanwhile, ample evidence confirmed that ABA plays vital roles in plant tolerance to stresses (Hu et al. 2006; Lu et al. 2009). To better characterize the mechanism of H2 governing plant tolerance against osmotic stress, alfalfa plants were treated with tungstate since it can block the formation of ABA from abscisic aldehyde by impairing abscisic aldehyde oxidase (Hansen and Grossmann 2000) and its encoding gene (AAO3; Fig. 3b). First, the results presented here demonstrated that upon osmotic stress, a higher level of ABA induced by H2 (Fig. 2a), correlates with the enhancement of plant tolerance against osmotic stress (Fig. 1). Combined with results shown in Fig. 2a and Fig. 3, we confirmed that H2 increased the leaf ABA concentration by upregulating the ABA biosynthesis gene (especially ABA2) expression. This finding is critical because one of the best-identified triggers of the cascade of drought signaling is ABA (Iuchi et al. 2001; Seo and Koshiba 2002; Hu et al. 2006; Lu et al. 2009). In view of the changes in ABA levels (Fig. 2a), we deduced that ABA metabolism is influenced by H2. These behaviors might be because, under osmotic stress, H2 requires endogenous ABA action to alleviate stress. Similar beneficial responses of ABA, when exogenously applied, were thus provided as positive controls. For example, when the ABA concentration in vivo is high in response to exogenous H2 or ABA, osmotic stress-triggered seedling growth inhibition and the loss of water content were differentially improved (Fig. 1). By contrast, when ABA synthesis is inhibited by tungstate (Fig. 2a), osmotic stress-triggered growth stunt and the loss of water content were blocked (Fig. 1). In agreement with these results, the beneficial roles of H2 were also impaired by the co-incubation with tungstate. These data thus supported the hypothesis that ABA might mediate H2-induced plant tolerance against osmotic stress.
Ample evidence portrays that avoidance of oxidative stress and reestablishment of redox homeostasis are essential determinants of alleviating osmotic stress in plants (Jiang and Zhang 2002; Mittler et al. 2004). Meanwhile, the ability of a plant to regulate a series of genes, including antioxidant defence genes, that further alter plant physiology and morphology, gives it the ability to avoid or escape osmotic stress (Jiang and Zhang 2002; Lu et al. 2009). Our further experiment revealed that mimicking the effects of ABA, H2 was able to alleviate PEG-triggered lipid peroxidation in shoots of alfalfa seedlings (Fig. 2b), both of which were supported by the enhanced activities of SOD, POD, APX, and CAT (Fig. 4), and the upregulation of corresponding transcripts (Fig. 5). These may be beneficial for the improvement of alfalfa seedling growth under osmotic stress, since the over-expression of Arabidopsis APX gene in tobacco chloroplast could enhance plant tolerance against water deficit and salinity stress (Badawi et al. 2004). Consistently, tobacco plants overexpressing a maize E3 ubiquitin ligase gene exhibited drought tolerance by regulating antioxidant defence and stomatal closure (Liu et al. 2013). However, the inclusion of the ABA synthetic inhibitor tungstate reversed this alleviating effect given by H2 (Fig. 2b). Changes in enzymatic activities (Fig. 4) and gene transcripts (Fig. 5) showed the similar tendencies. These results of Fig. 2b and Fig. 4 revealed that the decrease of TBARS concentration was attributed to the increased antioxidant enzyme activity induced by H2 in a ABA-dependent fashion. Additionally, ABA-alleviated lipid peroxidation might be partially used to explain the reason why H2 alleviates osmotic stress.
MicroRNAs (miRNAs), including miR528 and miR398, have been implicated in plant tolerance against osmotic stress, particularly in the regulation of ABA (Wang et al. 2011; Ding et al. 2013). Previous results showed that osmotic stress modulated miR528 transcripts in maize (Wei et al. 2009) and M. truncatula (Wang et al. 2011). Also, miRNAs have been reported to be involved in plant response to oxidative stress and regulating ROS balance (Zhu et al. 2011; Ma et al. 2015). In this study, we observed that miR528 was increased by osmotic stress in alfalfa seedlings, which was sensitive to the administration with H2 (Fig. 5e), showing a negative correlation with its target gene POD2 (Fig. 5d; Wei et al. 2009). The reversing effects were observed when tungstate was added together. Combined with the changes in endogenous ABA levels (Fig. 2a), above results suggested that in the presence of PEG stress, H2-regulated the expression of miRNAs was associated with ABA, and similar down-regulation of miR398 elicited by H2 have been discovered in rice plants upon cold stress (Xu et al. 2017b) and aluminum stress (Xu et al. 2017a).
Transcriptional factors contain two distinct domains, a DNA-binding domain and a transcriptional activation/repression domain, that regulate diverse cellular processes through controlling the transcriptional rates of target genes (Guo et al. 2005; Golldack et al. 2011; Todaka et al. 2012; Franco-Zorrilla et al. 2014; Joshi et al. 2016; Cai et al. 2017). In this study, osmotic stress downregulated the expression of three transcriptional factor genes, including MYB102, MYC2, and ABF/AREB2 (Fig. 6). By contrast, the addition of H2 and ABA reversed above changes. Contrasting results were observed when tungstate was added together with H2, suggesting that these transcriptional factors might be targeted by H2 in an ABA-dependent fashion.
On the other hand, ample evidence revealed that osmotic stress-responsive gene expression is regulated by ABA-dependent and ABA-independent pathways (Yoshida et al. 2014). Meanwhile, ABA-independent cold- and drought-responsive gene expression is respectively modulated by CBF/DREB1 and DREB2 proteins (Liu et al. 1998; Haake et al. 2002). In our experimental conditions, PEG-induced alfalfa DREB1 transcripts were intensified by H2 (supplemental Fig. S2). Since transgenic soybean overexpressing alfalfa DREB1 gene displayed drought tolerance (Li et al. 2017), we further deduced that H2-triggered tolerance against osmotic stress might be partially mediated by ABA-independent pathway. Certainly, this possibility should be carefully investigated.
In response to abiotic stress, plants normally rely on a complex network of signaling transduction pathways. Combined with the previous results (Xie et al. 2014; Jin et al. 2016; Su et al. 2018), our data support the model (Fig. 7) involving ABA signaling during H2-enhanced plant response to counterbalance the deleterious effects of osmotic stress, and H2-triggered ABA synthesis might be an amplification loop in H2 signaling. The participation of representative miRNAs, TFs, and antioxidant defense were also suggested. Certainly, the role of ABA-independent pathway is not easily ruled out.
Conclusions
In summary, our physiological and molecular data demonstrate that upon osmotic stress, H2 could trigger an increase in ABA production, thus maintaining cell homeostasis and attenuate osmotic stress-derived redox imbalance and seedling growth inhibition. Further genetic evidence should be provided to confirm above results, which will provide insights into H2 signaling and regulation of its bioactivity in agriculture, including the enhancement of crop production in osmotic stress environment.
References
Badawi GH, Kawano N, Yamauchi Y, Shimada E, Sasaki R, Kubo A, Tanaka K (2004) Over-expression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol Plant 121:231–238. https://doi.org/10.1111/j.0031-9317.2004.00308.x
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. CR Biol 331:806–814. https://doi.org/10.1016/j.crvi.2008.07.022
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. https://doi.org/10.1016/S0092-8674(04)00045-5
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cai S, Chen G, Wang Y, Huang Y, Marchant DB, Wang Y, Yang Q, Dai F, Hills A, Franks PJ, Nevo E, Soltis DE, Soltis PS, Sessa E, Wolf PG, Xue D, Zhang G, Pogson BJ, Blatt MR, Chen ZH (2017) Evolutionary conservation of ABA signaling for stomatal closure. Plant Physiol 174:732–747. https://doi.org/10.1104/pp.16.01848
Cao Z, Duan X, Yao P, Cui W, Cheng D, Zhang J, Jin Q, Chen J, Dai T, Shen W (2017) Hydrogen gas is involved in auxin-induced lateral root formation by modulating nitric oxide synthesis. Int J Mol Sci 18:2084. https://doi.org/10.3390/ijms18102084
Carrington JC, Ambros V (2003) Role of microRNAs in plant and animal development. Science 301:336–338. https://doi.org/10.1126/science.1085242
Cui W, Fang P, Zhu K, Mao Y, Gao C, Xie Y, Wang J, Shen W (2014) Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol Environ Saf 105:103–111. https://doi.org/10.1016/j.ecoenv.2014.04.009
Cui W, Gao C, Fang P, Lin G, Shen W (2013) Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J Haz Mat 260:715–724. https://doi.org/10.1016/j.jhazmat.2013.06.032
Ding Y, Tao Y, Zhu C (2013) Emerging roles of microRNAs in the mediation of drought stress response in plants. J Exp Bot 64:3077–3086. https://doi.org/10.1093/jxb/ert164
Dong Z, Wu L, Kettlewell B, Caldwell CD, Layzell DB (2003) Hydrogen fertilization of soils - is a benefit of legumes in rotation? Plant Cell Environ 26:1875–1879. https://doi.org/10.1046/j.1365-3040.2003.01103.x
Foyer CH, Noctor G (2005) Oxidant and antioxidant signaling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071. https://doi.org/10.1111/j.1365-3040.2005.01327.x
Franco-Zorrilla JM, López-Vidriero I, Carrasco JL, Godoy M, Vera P, Solano R (2014) DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc Natl Acad Sci U S A 111:2367–2372. https://doi.org/10.1073/pnas.1316278111
Fujii H (2014) Abscisic acid implication in plant growth and stress responses. Phytohormones: A window to metabolism, signaling and biotechnological applications. Springer, New York, NY 37–54. 10.1007/978-1-4939-0491-4_2
García-Mata C, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126:1196–1204. https://doi.org/10.1104/pp.126.3.1196
Golldack D, Lüking I, Yang O (2011) Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep 30:1383–1391. https://doi.org/10.1007/s00299-011-1068-0
Gu Q, Chen ZP, Yu XL, Cui WT, Pan JC, Zhao G, Xu S, Wang R, Shen WB (2017) Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci 261:28–37. https://doi.org/10.1016/j.plantsci.2017.05.001
Guinn G, Brummett DL, Beier RC (1986) Purification and measurement of abscisic acid and indoleacetic acid by high performance liquid chromatography. Plant Physiol 81:997–1002. https://doi.org/10.1104/pp.81.4.997
Guo HS, Xie Q, Fei JF, Chua NH (2005) MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell 17:1376–1386. https://doi.org/10.1105/tpc.105.030841
Haake V, Cook D, Riechmann JL, Pineda O, Thomashow MF, Zhang JZ (2002) Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol 130:639–648. https://doi.org/10.1104/pp.006478
Han Y, Zhang J, Chen XY, Gao ZZ, Xuan W, Xu S, Ding X, Shen WB (2008) Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol 177:155–166. https://doi.org/10.1111/j.1469-8137.2007.02251.x
Hansen H, Grossmann K (2000) Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol 124:1437–1448. https://doi.org/10.1104/pp.124.3.1437
Hu H, You J, Fang Y, Zhu X, Qi Z, Xiong L (2008) Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol Biol 67:169–181. https://doi.org/10.1007/s11103-010-9598-3
Hu X, Zhang A, Zheng J, Jiang M (2006) Abscisic acid is a key inducer of hydrogen peroxide production in leaves of maize plants exposed to water stress. Plant Cell Physiol 47:1484–1495. https://doi.org/10.1093/pcp/pcl014
Iuchi S, Kobayashi M, Taji T, Nakamoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27:325–333. https://doi.org/10.1046/j.1365-313x.2001.01096.x
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273. https://doi.org/10.1093/pcp/pce162
Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53:2401–2410. https://doi.org/10.1093/jxb/erf090
Jin K, Shen J, Ashton RW, Dodd IC, Parry MA, Whalley WR (2013a) How do roots elongate in a structured soil? J Exp Bot 64:4761–4777. https://doi.org/10.1093/jxb/ert286
Jin Q, Zhu K, Cui W, Li L, Shen W (2016) Hydrogen-modulated stomatal sensitivity to abscisic acid and drought tolerance via the regulation of apoplastic pH in Medicago sativa. J Plant Growth Regul 35:565–573. https://doi.org/10.1007/s00344-015-9561-2
Jin Q, Zhu K, Cui W, Xie Y, Han B, Shen W (2013b) Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of the heme oxygenase-1 signaling system. Plant Cell Environ 36:956–969. https://doi.org/10.1111/pce.12029
Joshi R, Wani SH, Singh B, Bohra A, Dar ZA, Lone AA, Pareek A, Singla-Pareek SL (2016) Transcription factors and plants response to drought stress: current understanding and future directions. Front Plant Sci 7:1029. https://doi.org/10.3389/fpls.2016.01029
Li DH, Zheng WN, Jiang BS, Li HY (2017) Drought tolerance analysis of transgenic soybean with overexpressed MsDREB1 gene from alfalfa. Plant Physiol J 53:1479–1488. https://doi.org/10.13592/j.cnki.ppj.2016.0459
Lin Y, Zhang W, Qi F, Cui W, Xie Y, Shen W (2014) Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J Plant Physiol 17:1–8. https://doi.org/10.1016/j.jplph.2013.08.009
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406. https://doi.org/10.1105/tpc.10.8.1391
Liu P, Sun F, Gao R, Dong H (2012) RAP2.6L overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity. Plant Mol biol 79:609–622. https://doi.org/10.1007/s11103-012-9936-8
Liu J, Xia Z, Wang M, Zhang X, Yang T, Wu J (2013) Overexpression of a maize E3 ubiquitin ligase gene enhances drought tolerance through regulating stomatal aperture and antioxidant system in transgenic tobacco. J Physiol Biochem 73:114–120. https://doi.org/10.1016/j.plaphy.2013.09.006
Lu G, Gao C, Zheng X, Han B (2009) Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta 229:605–615. https://doi.org/10.1007/s00425-008-0857-3
Ma C, Burd S, Lers A (2015) miR408 is involved in abiotic stress responses in Arabidopsis. Plant J 84:169–187. https://doi.org/10.1111/tpj.12999
Mei Y, Chen H, Shen W, Shen W, Huang L (2017) Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol 17:162. https://doi.org/10.1186/s12870-017-1110-7
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33:453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. https://doi.org/10.1016/j.tplants.2004.08.009
Neumann PM (2008) Coping mechanisms for crop plants in drought-prone environments. Annal Bot 101:901–907. https://doi.org/10.1093/aob/mcn018
Renwick GM, Giumarro C, Siegel SM (1964) Hydrogen metabolism in higher plants. Plant Physiol 39:303. https://doi.org/10.1104/pp.39.3.303
Seo M, Koshiba T (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci 7:41–48. https://doi.org/10.1016/S1360-1385(01)02187-2
Srinath R, Jabeen FTZ (2013) In vitro selection and characterization of polyethylene glycol (PEG) tolerant callus lines and regeneration of plantlets from the selected callus lines in sugarcane (Saccharum officinarum L.). Physiol Mol Biol Plants 19:261–268. https://doi.org/10.1007/s12298-013-0162-x
Su J, Zhang Y, Nie Y, Cheng D, Wang R, Hu H, Chen J, Zhang J, Du Y, Shen W (2018) Hydrogen-induced osmotic tolerance is associated with nitric oxide-mediated proline accumulation and reestablishment of redox balance in alfalfa seedlings. Environ Exp Bot 147:249–260. https://doi.org/10.1016/j.envexpbot.2017.12.022
Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35:44–56. https://doi.org/10.1046/j.1365-313X.2003.01786.x
Todaka D, Nakashima K, Shinozaki K, Yamaguchi-Shinozaki K (2012) Toward understanding transcriptional regulatory networks in abiotic stress responses and tolerance in rice. Rice 5:6. https://doi.org/10.1186/1939-8433-5-6
Tossi V, Lamattina L, Cassia R (2009) An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol 181:871–879. https://doi.org/10.2307/30224735
Vaseem R, Umer M, Hunseung K, Khursheed IA, Riffat J (2017) Abiotic stress: interplay between ROS, hormones and MAPKs. Environ Exp Bot 137:142–157. https://doi.org/10.1016/j.envexpbot.2017.02.010
Wang T, Chen L, Zhao M, Tian Q, Zhang WH (2011) Identification of drought-responsive microRNAs in Medicago truncatula by genome-wide high throughput sequencing. BMC Genet 12:367. https://doi.org/10.1186/1471-2164-12-367
Wang YQ, Li L, Cui WT, Xu S, Shen WB, Wang R (2012) Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 351:107–119. https://doi.org/10.1007/s11104-011-0936-2
Wei L, Zhang D, Xiang F, Zhang Z (2009) Differentially expressed miRNAs potentially involved in the regulation of defense mechanism to drought stress in maize seedlings. Int J Plant Sci 170:979–989. https://doi.org/10.1086/605122
Xie Y, Mao Y, Duan X, Zhou H, Lai D, Zhang Y, Shen W (2016) Arabidopsis HY1-modualted stomatal movement: an integrative hub for functionally associated with ABI4 in dehydration-induced ABA responsiveness. Plant Physiol 170:1699–1713. https://doi.org/10.1104/pp.15.01550
Xie Y, Mao Y, Lai D, Zhang W, Shen W (2012) H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS One 7:e49800. https://doi.org/10.1371/journal.pone.0049800
Xie Y, Mao Y, Zhang W, Lai D, Wang Q, Shen W (2014) Reactive oxygen species-dependent nitric oxide production contributes to hydrogen promoted stomatal closure in Arabidopsis. Plant Physiol 165:759–773. https://doi.org/10.1104/pp.114.237925
Xiong J, Fu G, Yang Y, Zhu C, Tao L (2012) Tungstate: is it really a specific nitrate reductase inhibitor in plant nitric oxide research? J Exp bot 63:33–41. https://doi.org/10.1093/jxb/err268
Xiong L, Ishitani M, Lee H, Zhu JK (2001) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13:2063–2083. https://doi.org/10.2307/3871428
Xiong L, Lee H, Ishitani M, Zhu JK (2002) Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Bio Chem 277:8588–8596. https://doi.org/10.1074/jbc.M109275200
Xiong L, Zhu JK (2003) Regulation of abscisic acid biosynthesis. Plant Physiol 133:29–36. https://doi.org/10.1104/pp.103.025395
Xu D, Cao H, Fang W, Pan J, Chen J, Zhang J, Shen W (2017a) Linking hydrogen-enhanced rice aluminum tolerance with the reestablishment of GA/ABA balance and miRNA-modulated gene expression: a case study on germination. Ecotoxicol Environ Saf 145:303–312. https://doi.org/10.1016/j.ecoenv.2017.07.055
Xu S, Jiang Y, Cui W, Jin Q, Zhang Y, Bu D, Fu J, Wang R, Zhou F, Shen W (2017b) Hydrogen enhances the adaptation of rice seedlings to cold stress via the reestablishment of redox homeostasis mediated by miRNA expression. Plant Soil 414:53–67. https://doi.org/10.1007/s11104-016-3106-8
Yoshida T, Mogami J, Yamaguchi-Shinozaki K (2014) ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr Opin Plant Biol 21:133–139. https://doi.org/10.1016/j.pbi.2014.07.009
Zhang A, Jiang M, Zhang J, Tan M, Hu X (2006) Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol 141:475–487. https://doi.org/10.1104/pp.105.075416
Zhang AY, Zhang J, Zhang JH, Ye NH, Zhang H, Tan MP, Jiang MY (2011) Nitric oxide mediates brassinosteroid-induced ABA biosynthesis involved in oxidative stress tolerance in maize leaves. Plant Cell Physiol 52:181–192. https://doi.org/10.1093/pcp/pcq187
Zheng XF, Sun XJ, Xia ZF (2011) Hydrogen resuscitation, a new cytoprotective approach. Clin Exp Pharmacol Physiol 38:155–163. https://doi.org/10.1111/j.1440-1681.2011.05479.x
Zhu C, Ding Y, Liu H (2011) MiR398 and plant stress responses. Plant Physiol 143:1–9. https://doi.org/10.1111/j.1399-3054.2011.01477.x
Acknowledgments
This research was supported by Foshan Agriculture Science and Technology Project (Foshan City Budget No. 140, 2019.), the Fundamental Research Funds for the Central Universities (Grant number KJQN201640), the National Natural Science Foundation of China (31371546), and the Funding from Center of Hydrogen Science, Shanghai Jiao Tong University, China.
Availability of data and materials
All relevant data are within this article and its supporting information files.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: WS. Acquisition of data for the study: KF, JS, GZ, WC, and HM. Analysis of data for the work: KF, JS, and GZ. Interpretation of data for the work: KF, JS, LR, GZ, RW, HM, JC, and WS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Ian Dodd.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 136 kb)
Rights and permissions
About this article
Cite this article
Felix, K., Su, J., Lu, R. et al. Hydrogen-induced tolerance against osmotic stress in alfalfa seedlings involves ABA signaling. Plant Soil 445, 409–423 (2019). https://doi.org/10.1007/s11104-019-04328-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04328-y