Abstract
Aims and methods
The molecular mechanisms and signal transduction pathways of hydrogen sulfide (H2S) in plant biology are still unclear. Here, by using pharmacological and biochemical approaches, we report that H2S promotes germination and alleviates salinity damage involving nitric oxide (NO) pathway.
Results
Upon 100 mM NaCl treatment, both H2S donor sodium hydrosulfide (NaHS) and NO donor sodium nitroprusside (SNP) at 100 μM could significantly attenuate the inhibition of alfalfa (Medicago sativa) seed germination and thereafter seedling growth inhibition. Meanwhile, the ratio of potassium (K) to sodium (Na) in the root parts was increased. Total, isozymatic activities or corresponding transcripts of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (POD), or ascorbate peroxidase (APX) were activated differentially, thus resulting in the alleviation of oxidative damage. The above protective roles of NaHS might be related to the induction of endogenous NO, because the addition of the specific scavenger of NO 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (cPTIO) reversed above effects. Meanwhile, NaHS-triggered NO production was confirmed.
Conclusions
Our observations indicate that H2S enhances plant responses against salinity stress by reducing oxidative damage, which might have a possible interaction with NO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In animals, the group of gaseous messengers continues to expand and now includes nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), and several molecules from the broad category of reactive oxygen species (ROS). Like NO and CO, it has been confirmed that H2S at physiologically relevant levels affects the structures and functions of the human body at molecular, cellular, tissue, and systemic levels. During the last decade, the development in the identification of H2S as an important gasotransmitter becomes the focus of biology systems (Wang 2003; Lefer 2007; Baskar and Bian 2011). Meanwhile, recent studies confirmed that H2S was able to induce adventitious rooting (Zhang et al. 2009a). The role of H2S is also extended to the regulation of diverse aspects of plant responses against abiotic stresses. For example, it has been shown that H2S plays a role in plant adaptive responses to boron (Wang et al. 2010), aluminum (Zhang et al. 2010b), and heavy metal exposure (Zhang et al. 2008), stomatal movement (Lisjak et al. 2010; García-Mata and Lamattina 2010), osmotic and drought stresses (Zhang et al. 2009b, 2010a). However, although H2S plays a role in germination under stressful conditions (Zhang et al. 2008, 2010b), its precise role and the corresponding molecular mechanisms involved have not been fully elucidated.
It was well known that salt stress led to various negative influences on seed germination, seedling growth, and even plant productivity. Excess of salinity exerts adverse effects such as ion toxicity, osmotic strain, nutritional imbalances, and the inhibition of plant growth (Zhu 2001). Other important consequence of salinity stress is the overproduction of ROS, which shows toxicity to the metabolic functions in plants. In response to various stressful conditions, numerous studies have demonstrated that the modulation of seed germination of different plant species occurred when some growth hormones (auxin, GA, ABA, and ethylene), or other signaling substances (salicylic acid, hydrogen peroxide, NO, and CO, etc.) were applied (Arasimowicz and Floryszak-Wieczorek 2007; Wahid et al. 2007; Chen et al. 2008; Liu et al. 2010; Lee et al. 2010; Molassiotis et al. 2010). Therefore, knowledge of how these second messengers or modulators interact is crucial in gaining a better understanding of fundamental plant adaptive against various abiotic stresses.
Alfalfa (Medicago sativa L.) is the most widely grown perennial pasture in the world and its productivity in saline soils is considerably decreased due to improper nutrition of plants as well as osmotic and drought stresses. In this study, we carried out a set of germination assays to systematically examine the effect of H2S on alfalfa seed germination. Germination parameters were measured either under normal growth conditions or in the presence of salinity stress. We found that exogenous H2S donor sodium hydrosulfide (NaHS), could mimic the well-known NO donor sodium nitroprusside (SNP) effects on the alleviation of salinity-induced seed germination and seedling growth inhibition by the up-regulation of antioxidative system. Additionally, a possible cross-talk between H2S and NO, which has been found in animals (Hosoki et al. 1997; Ondrias et al. 2008), was assessed by the addition of the specific NO scavenger, control chemicals of SNP and the detection of NO release upon various treatments.
Materials and methods
Chemicals
Sodium hydrosulfide (NaHS) and sodium nitroprusside (SNP), purchased from Sigma (St Louis, MO, USA), were used as the H2S or HS- and NO donor, respectively (Zhao et al. 2001; Bethke et al. 2006a, b; Xie et al. 2008; Wang et al. 2010). 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (cPTIO) was used as the specific NO scavenger (Xuan et al. 2008; Liu et al. 2010). NO −2 /NO −3 and K3Fe(CN)6/K4Fe(CN)6 were used as the degradation product of SNP and SNP analogue (Bethke et al. 2006a, b; Shi et al. 2007; Xie et al. 2008).
Plant material, growth condition and experimental design
Commercially available alfalfa (Medicago sativa L., Victoria) seeds were surface-sterilized with 5% NaClO for 15 min, rinsed extensively in distilled water and then dried. These seeds were transferred to Petri dishes containing 4 ml of distilled water (Con), varying concentrations of NaCl (S) and NaHS, 100 μM SNP, 100/100 μM NO −2 /NO −3 , 100/100 μM K3Fe(CN)6/K4Fe(CN)6, 200 μM cPTIO, 100 μM NaHSO4, 100 μM NaHSO3, 100 μM Na2S, 100 μM Na2SO4, 100 μM Na2SO3, and 100 μM NaAc alone, or containing two or three of the above chemicals, and kept at 25°C in a growth chamber in darkness for 2 or 4d of incubation. These solutions were renewed for every 2 days. All experiments were repeated for at least three times. After 2 or 4d of salinity treatment, the samples were harvested, growth parameters were determined, and material was frozen at −80°C for further analysis.
Germination and growth analysis
Germination tests were carried out on three replicates of 150 seeds each. There were 50 seeds in each Petri dish. Germination percentage or rate (%) was recorded every day for 2 or 4d, respectively, and seeds were considered to have germinated when the emerging shoot was approximately half the length of the seeds. Seed germination energy (GE,%) = (number of germinating seeds/number of total seeds per treatment after germination for 2 days) × 100, according to the method described by Hu et al. (2004). Germination index (GI,%) was calculated as described in the Association of Official Seed Analysis (1983), using the following formulae:
The measurements of root length and shoot length were carried out at 4d after various treatments.
Determination of ion content
Na and K contents of root part were measured by an atomic emission spectrophotometer (TAS-986; Beijing Purkinje General Instrument Co., Ltd., Beijing, China).
Thiobarbituric acid reactive substances (TBARS) determination
Oxidative damage was estimated by measuring the concentration of TBARS as described by Han et al. (2008).
Antioxidant enzyme assays
Frozen alfalfa plants (approximately 200 mg) were homogenized in 10 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 1% polyvinylpyrrolidone (PVP) for CAT, guaiacol POD, and SOD assay, or the combination with the addition of 1 mM ascorbic acid (ASC) in the case of APX assay. The homogenate was centrifuged at 12,000 g in a rotor (model Avanti J-25, Beckman) for 20 min at 4°C and the supernatant was immediately desalted by Sephadex G-25 gel filtration to remove interfering materials and used as the crude enzyme extract.
Catalase (CAT) activity was spectrophotometrically measured by monitoring the consumption of H2O2 (ε = 39.4 M−1 cm−1) at 240 nm for at least 3 min (Aebi 1984; Watanabe et al. 2003). Determination of guaiacol POD activity was performed by measuring the oxidation of guaiacol (ε = 26.6 mM−1 cm−1). The reaction mixture contained 50 mM potassium phosphate buffer (pH 6.4), 8 mM guaiacol, and 2.75 mM H2O2. The increase in absorbance was recorded at 470 nm within 2 min (linear phase) after the addition of H2O2 (Ruan et al. 2002). APX activity was measured by monitoring the decrease in absorbance at 290 nm as ASC was oxidized (ε = 2.8 mM−1 cm−1) for at least 1 min in 3 ml reaction mixture, as described by Nakano and Asada (1981). Total superoxide dismutase (SOD) activity was measured on the basis of its ability to inhibit the reduction of nitroblue tetrazolium (NBT) by superoxide anion generated by the riboflavin system under illumination. One unit of SOD (U) was defined as the amount of crude enzyme extract required to inhibit the reduction rate of NBT by 50% (Beauchamp and Fridovich 1971).
Gel electrophoresis
SOD isozymes were separated on native PAGE (5–20%). Proteins were electrophoresed for 20 h at 4°C at a constant voltage of 120 V in Tris-Gly buffer, pH 8.3. For each lane, 50 μg of protein extract was applied. SOD isozymatic activity on the gel was visualised by activity staining combined with the inhibitor test according to the procedure described by Beauchamp and Fridovich (1971) and Xie et al. (2008). All staining procedures were carried out at room temperature, and the reaction mixture containing gels was shaken at 75 rpm until the colorless bands of SOD activity in a purple-stained gel were visible. For the relative activity of different SOD isozymatic determination, gels were scanned in the transmission black-and-white mode and the intensity of bands was calculated by using the Quantity One v4.4.0 software (Bio-Rad, Hercules, California, USA). Then the band intensities of the individual isozymes were expressed as % of the control (Con) value.
Real-time quantitative RT-PCR analysis
Total RNA from 100 mg of fresh-weight alfalfa plants was isolated by grinding with mortar and pestle in liquid nitrogen until a fine powder appeared and using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. DNA-free total RNA (5 μg) from different treatments was used for first-strand cDNA synthesis in a 20-μL reaction volume containing 2.5 units of avian myeloblastosis virus reverse transcriptase XL (TaKaRa) and 2.5 μM random primer.
Real-time quantitative RT-PCR reactions were performed with Mastercycler® realplex2 real-time PCR system (Eppendorf, Hamburg, Germany) using the SYBR® Premix Ex Taq™ (TaKaRa Bio Inc., China) according to the user manual. The cDNA was amplified using the following primers: APX-1 (accession number DQ122791), forward 5′-TCCTCTTATGCTCCGTTTG-3′ and reverse 5′-GTTCCACCCAGTAATCCCA-3′; APX-2 (accession number AY054988), forward 5′-GTCCTTTCGGAACCATCAA-3′ and reverse 5′- GCAACAACACCAGCCAACT-3′; Cu/Zn-SOD (accession number AF056621), forward 5′-TAATTGCTGATGCCAACG-3′ and reverse 5′-ACCACAGGCTAATCTTCCAC-3′; and MSC27 (accession number X63872, Calderini et al. 2007), forward 5′-TGTCAGACTCTTACCCATAC-3′ and reverse 5′-CCTCATCTTCTCCACCTT-3′. The PCR program consisted of an initial denaturation and Taq activation step of 5 min at 95°C, followed by 40 cycles of 15 s at 95°C and 20 s at 50°C. A melting curve analysis was performed after every PCR reaction to confirm the accuracy of each amplified product. All reactions were set up in duplicate. Relative expression levels were presented as values relative to that of corresponding control sample at the indicated time, after normalization to MSC27 transcript levels.
NO production determined by using greiss reagent
NO production was determined using the previous methods (Ding 1998; Xu et al. 2005; Zhou et al. 2005) with some modifications. Samples were ground in a mortar and pestle in 3 ml of 50 mM cool acetic acid buffer (pH 3.6, containing 4% zinc diacetate). The homogenates were centrifuged at 10 000 g for 15 min at 4°C. The supernatant was collected. The pellet was washed by 1 ml of extraction buffer and centrifuged as before. The two supernatants were combined and 0.1 g of charcoal was added. After vortex and filtration, the filtrate was leached and collected. The mixture of 1 ml of filtrate and 1 ml of the Greiss reagent was incubated at room temperature for 30 min. Meanwhile, identical filtrate which was pretreated with 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (cPTIO), the specific scavenger of NO, for 15 min, was used as blanks. Absorbance was assayed at 540 nm. NO content was calculated by comparison to a standard curve of NaNO2.
Statistical analysis
Where indicated, results are expressed as mean values ± SE of at least three independent experiments. For statistical analysis, Duncan’s multiple test was used as appropriate, after testing for data normality. A value of P < 0.05 was considered significant for mean differences.
Results
The NaCl-induced inhibition of seed germination and seedling growth restrain were rescued by H2S
To assess the sensitivity of alfalfa seed germination to salinity stress, the effect of varying NaCl concentrations on seed germination was investigated. As expected, seed germination was decreased approximately in a dose-dependent fashion when exposed to 0–150 mM NaCl (Fig. 1a). Results further showed that in comparison with 100 mM NaCl-stressed alone sample, concentrations between 0.01 and 1.0 mM sodium hydrosulfide (NaHS), an effective H2S or HS- donor used both in animal and plant researches (Zhao et al. 2001; Kimura and Kimura 2004; Wang et al. 2010; Zhang et al. 2010a), alleviated alfalfa seed germination inhibition with a maximal response at 0.1 mM (Fig. 1b, Supplementary Information Fig. S1). Similarly, the cytoprotective roles of 0.1 mM NaHS were confirmed by the measurement of other germination and seedling growth parameters, such as germination energy (GE, 2d) and germination index (GI, 4d), root and shoot length (Table 1). However, no significant differences were observed when various concentrations of NaHS were used alone, comparing with the control sample. To verify the role of H2S in the promotion of seed germination induced by NaHS, 0.1 mM Na2S, Na2SO4, Na2SO3, NaHSO4, NaHSO3 and NaAc were used as the controls for Na+- and sulfur-containing compounds. Interestingly, we noticed that these compounds were unable to exhibit the similar cytoprotective roles (Fig. 1c). Together, we suggested that H2S or HS−, rather than other compounds directly or indirectly derived from the decomposing of NaHS, were responsible for the promotive effects of NaHS on the amelioration of seed germination inhibition and seedling growth restrain triggered by NaCl exposure.
Hydrogen sulfide (H2S) or HS−, but not other compounds derived from sodium hydrosulfide (NaHS), are contributed to the alleviation of seed germination inhibition caused by salinity stress for 2 days of incubation. Dose-dependent seed germination inhibition upon NaCl (S) treatments as indicated concentrations (a). Effects of NaHS at the indicated concentrations with or without 100 mM NaCl on the seed germination (b). Seeds were treated with either H2O (Con), 100 mM NaCl (S), 100 μM NaHS, 100 μM NaHSO4, 100 μM NaHSO3,100 μM Na2S, 100 μM Na2SO4, 100 μM Na2SO3, and 100 μM NaAc, or containing two of the above chemicals for 2 days of incubation (c). Values are the mean ± SE of at least three independent experiments. Different letters indicate significant differences (P < 0.05) according to Duncan’s multiple test
Above H2S-triggered responses were sensitive to the specific scavenger of NO
In animals and recently in plants, most H2S responses are similar to or mediated by NO (Ondrias et al. 2008; Zhang et al. 2009a; Wang et al. 2010). Endogenous or exogenous NO has been confirmed to induce seed germination under various abiotic stresses or reduce dormancy (Delledonne 2005). In the following experiments, our results confirmed that SNP, a well-known NO donor, was able to alleviate the salt-induced alfalfa seed germination and seedling growth inhibition (P < 0.05; Table 1, Fig. 2a). To further confirm a specific role of NO in above responses, several controls such as NO −2 /NO −3 and K3Fe(CN)6/K4Fe(CN)6 were employed. Contrary to the effects of SNP, no significant differences or negative responses were observed when NO -2 /NO −3 or K3Fe(CN)6/K4Fe(CN)6 was added together with NaCl treatment (Table 1, Fig. 2a). Meanwhile, the control chemicals exhibited the similar or negative response in comparison with the control sample (Table 1). To further assess whether endogenous NO was involved in responses induced by NaHS, the specific NO scavenger cPTIO was applied. As shown in Fig. 2a, the NaHS- and SNP-driven alleviation of salinity stress-induced alfalfa seedling growth inhibition was significantly prevented when cPTIO was added, correlating those data from seed germination parameters (Table 1, Supplementary Information Fig. S2). Meanwhile, plants exposed to cPTIO treatment alone exhibited a slight but no significant decrease of the above parameters in comparison with the NaCl-free control sample.
Effects of cPTIO on NaHS- or SNP-induced the alleviation of growth constrains (a) and reestablishment of ion homeostasis (b) in alfalfa seedlings and seedling roots upon 100 mM NaCl exposure. The inset shows the effect of K3Fe(CN)6/K4Fe(CN)6 or NO −2 /NO −3 on NaCl-induced inhibition of seedling growth. Seeds were treated with either H2O (Con), 100 mM NaCl (S), 100 μM NaHS, 100 μM SNP, 200 μM cPTIO, K3Fe(CN)6/K4Fe(CN)6 (100/100 μM, Fe), NO −2 /NO −3 (100/100 μM, NO −x ), or containing two or three of the above chemicals for 4 days of incubation. Values are the mean ± SE of at least three independent experiments. Different letters indicate significant differences (P < 0.05) according to Duncan’s multiple test
Reestablishment of ion homeostasis
The results of Fig. 2b illustrated that the inducible changes of K/Na ratio were observed in root tissues of NaHS- and SNP-treated samples, which is mainly caused by the accumulation of K+ (data not shown). However, when exogenous cPTIO was added, above inducible responses were blocked significantly. Thus, the above results suggested that both NaHS and SNP treatment could enhance salinity tolerance by reestablishing ion homeostasis.
Lipid peroxidation
As expected (Bailly 2004), salinity stress brought about the obvious increase of TBARS level (Fig. 3). However, treatment with NaHS or SNP at 100 μM had the significant decreasing effects on salinity-induced accumulation of TBARS content. Generation of TBARS content was decreased by 59.4% and 50.7% in the alfalfa plants treated with NaHS or SNP plus NaCl compared with the NaCl-treated alone sample. By contrast, no significant difference was observed when NO −2 /NO −3 or K3Fe(CN)6/K4Fe(CN)6 was added together with NaCl treatment. Meanwhile, we demonstrated that the addition of cPTIO together with NaHS or SNP resulted in the obvious increases of TBARS content in salinity-stressed samples (P < 0.05). In comparison with the control sample, the increase of TBARS content was also observed in cPTIO-treated alone sample.
Effects of cPTIO on NaHS- or SNP-induced the alleviation of lipid peroxidation in alfalfa seedlings upon 100 mM NaCl exposure. The inset shows the effect of K3Fe(CN)6/K4Fe(CN)6 or NO -2 /NO -3 on NaCl-induced lipid peroxidation. Seeds were treated with either H2O (Con), 100 mM NaCl (S), 100 μM NaHS, 100 μM SNP, 200 μM cPTIO, K3Fe(CN)6/K4Fe(CN)6 (100/100 μM, Fe), NO -2 /NO -3 (100/100 μM, NO -x ), or containing two or three of the above chemicals for 2 days of incubation. Values are the mean ± SE of at least three independent experiments. Different letters indicate significant differences (P < 0.05) according to Duncan’s multiple test
Transcripts and activities of antioxidant enzymes
Salinity-induced oxidative stress in plants was associated with ROS overproduction (Hernandez et al. 2010; Miller et al. 2010). Thus, it was necessary to examine the antioxidative enzymes responsible for scavenging of ROS. The transcript levels of several antioxidant genes, such as APX-1, APX-2, and Cu/Zn-SOD, were down-regulated by salinity stress (Fig. 4). Similar decreased tendency was observed in total activities of APX, SOD, POD, and CAT (Fig. 5). Whereas, NaHS or SNP could significantly prevent salt-induced decreases of the transcripts and activities of these enzymes. Comparatively, the treatment in combined with cPTIO treatment produced a decrease tendency in the mRNA and activity levels compared with the values obtained when only NaHS or SNP was added together with NaCl. In addition, cPTIO alone produced the down-regulation of gene expression (except Cu/Zn-SOD) or activities (except CAT) of these antioxidant enzymes.
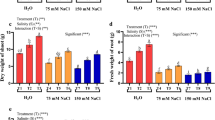
Effects of cPTIO, NaHS and SNP on the transcripts of ascorbate peroxidase (APX, a and b), and Cu/Zn-superoxide dismutase (Cu/Zn-SOD, c) in alfalfa seedlings upon 100 mM NaCl exposure. Seeds were treated with either H2O (Con), 100 mM NaCl (S), 100 μM NaHS, 100 μM SNP, 200 μM cPTIO, or containing two or three of the above chemicals for 24 h of incubation. The corresponding mRNA expression was analysed by real-time quantitative RT-PCR. The relative abundance of the corresponding genes is presented as values relative to the control sample. Values are the mean ± SE of at least three independent experiments
Effects of cPTIO, NaHS and SNP on the activities of ascorbate peroxidase (APX, a), superoxide dismutase (SOD, b), guaiacol peroxidase (POD, c), and catalase (CAT, d) in alfalfa seedlings upon 100 mM NaCl exposure. Seeds were treated with either H2O (Con), 100 mM NaCl (S), 100 μM NaHS, 100 μM SNP, 200 μM cPTIO, or containing two or three of the above chemicals for 24 h of incubation. Then, enzyme activities were determined. Values are the mean ± SE of at least three independent experiments. Different letters indicate significant differences (P < 0.05) according to Duncan’s multiple test
To confirm the responses of SOD, a native gradient polyacrylamide gel electrophoresis (PAGE, 5–20%) for SOD isozymatic analysis was performed. At least four SOD isoforms were detected in alfalfa plants (Fig. 6). As expected (Samis et al. 2001), isoforms I, II, III and IV, belonging to Mn-SOD, Fe-SOD, and Cu/Zn-SOD respectively (inhibitor test, data not shown), exhibited decreased activities in responses to salinity stress, whereas those with NaHS or SNP (especially) brought about the obvious induction. Interestingly, the application of cPTIO weaken the above responses, consisting with changes of lipid peroxidation (Fig. 3), ROS-scavenging enzymes or corresponding transcripts (Figs. 4, 5 and 6).
Effects of cPTIO, NaHS and SNP on the isozymatic activities of superoxide dismutase (SOD) in alfalfa seedlings upon 100 mM NaCl exposure. Seeds were treated with either H2O (Con), 100 mM NaCl (S), 100 μM NaHS, 100 μM SNP, 200 μM cPTIO, or containing two or three of the above chemicals. The gradient PAGE analysis of SOD isozymes (a) from salt-stressed alfalfa seeds for 24 h was determined. Meanwhile, relative activity of different SOD isozymes was also shown in (b). Band intensities of the individual isozymes were expressed as% of the control samples. Values are the mean ± SE of at least three independent experiments
NO production triggered by NaHS
Above results suggested that NaHS-induced responses were sensitive to the scavenger of NO. To further verify whether above NaHS-driven responses were related to endogenous NO signal, we checked the endogenous NO level in our experimental conditions. As expected (Xie et al. 2008), NaCl stress for 24 h induced NO level moderately, as demonstrated by 21.5% increase in alfalfa seed tissues (Fig. 7). Meanwhile, NaHS (especially) and SNP treatment produced an increase of NO, being 26.9% and 8.1% higher than that of sample under salinity stress alone. By contrast, when cPTIO, the specific NO scavenger, was incubated with NaHS or SNP, the rate of NO generation was completely inhibited, reaching a lower level in comparison with those of the salt stressed alone sample. However, no significant difference was observed when cPTIO was applied alone in comparison with the control plants. Also, no significant changes of NO content was discovered when NO -2 /NO -3 or K3Fe(CN)6/K4Fe(CN)6 was added together with NaCl treatment.
NaHS-dependent NO generation. Seeds were treated with either H2O (Con), 100 mM NaCl (S), 100 μM NaHS, 100 μM SNP, 200 μM cPTIO, K3Fe(CN)6/K4Fe(CN)6 (100/100 μM, Fe), NO -2 /NO -3 (100/100 μM, NO -x ), or containing two or three of the above chemicals. NO contents of alfalfa seeds were detected by using Greiss reagent after 24 h of treatment. The inset shows the effect of K3Fe(CN)6/K4Fe(CN)6 or NO -2 /NO -3 on NaCl-induced NO generation. Values are the mean ± SE of at least three independent experiments. Different letters indicate significant differences (P < 0.05) according to Duncan’s multiple test
Discussion
H2S is a water-soluble colorless molecule that easily penetrates biological membranes and exerts its effects independent of membrane receptors (Li et al. 2009). Until now, ample evidences have shown that H2S acts as a signaling molecule in animals (Mancuso et al. 2010; Baskar and Bian 2011; Yong et al. 2011). In view of the fact that H2S is produced in response to oxidative stress in yeast, it was deduced that H2S functions as an antioxidant (Kimura and Kimura 2004). Given that plants can produce H2S when subjected to relatively low concentrations of SO2 and L-cysteine in the field and laboratory conditions (Hällgren and Fredriksson 1982; Sekiya et al. 1982), it is important to examine whether H2S also plays a role in modulating of physiological processes in plants. More recently, investigations on the function of H2S in plants have created some interesting discovers. For example, H2S was shown to participate in the protection against copper toxicity (Zhang et al. 2008), stomatal movement (Lisjak et al. 2010; García-Mata and Lamattina 2010), adventitious rooting (Zhang et al. 2009a), and seed germination under osmotic stress by protecting these plants from oxidative damage (Zhang et al. 2009b). In the present study, we found that exposure of alfalfa seeds to increasing concentrations of NaCl treatment inhibited seed germination in a dose-dependent fashion (Fig. 1a). By using pharmacological approaches, we further demonstrated that the salinity-induced inhibitory effect on seed germination and changes in seedling growth could be alleviated by NaHS (Figs. 1b, 2a, Table 1, Supplementary Information Fig. S1), the well-known H2S and HS- donor both in the animal and plant kingdoms (Zhao et al. 2001; Xie et al. 2008; Wang et al. 2010), by the regulation of antioxidant enzymes expression or establishment of ion homeostasis, all of which might be partially mediated by the NaHS-driven NO production. As expected (Delledonne 2005), similar cytoprotective roles of NO were confirmed by the use of NO donor SNP compared with no significant or slight negative responses conferred by its control chemicals (Table 1, Figs. 2a and 3).
In view of the fact that NaHS dissolves in water and dissociates to produce Na+ and HS-; HS- associated with H+ and produce H2S, we further used other chemicals, such as S2-, SO 2-4 , SO 2-3 , HSO -4 , HSO -3 , and Na+, as controls for NaHS, but discovered that none were able to exhibit the similar cytoprotective role of NaHS in comparison with the NaCl stressed alone sample (Fig. 1c), Thus, above results confirmed that H2S or HS-, rather than other compounds derived from NaHS, play a role in the enhancement of salinity tolerance in alfalfa plants.
Ample evidence has confirmed that ROS overproduction in plants when upon various abiotic stresses, led to oxidative stress and cellular damage in plant tissues (Bailly 2004; Asada 2006; Hernandez et al. 2010; Miller et al. 2010). In response to salt stress, it is well established that antioxidant enzyme activities reveal stimulation, no-effect and suppression depending on different plant species, organ analyzed as well as the concentration and exposure duration of NaCl treatment (Yoshimura et al. 2000; Kacperska 2004; Hamed et al. 2007). Despite the lack of distinct patterns, above responses reflect the modified redox status of the plant cells induced by salinity stress. On the other hand, evidence has confirmed that plant tolerance to salinity stress is partially correlated with a more efficient antioxidant system (Miller et al. 2010). For example, the transformed tobacco over-expressing Arabidopsis APX showed more salinity and water stresses tolerance than the wild type (Badawi et al. 2004). In the subsequent experiment, our results further suggested that to cope with the elevation of lipid peroxidation triggered by salinity stress (Fig. 3), NaHS treatment differentially prevented the salt-induced decreases of APX1/2 and Cu/Zn-SOD transcripts (Fig. 4) and APX, SOD, POD, and CAT total (Fig. 5) or SOD isozymatic (Fig. 6) activities in alfalfa seedlings upon salinity stress. We also noticed that there was a strong correlation among lipid peroxidation (Fig. 3), antioxidant enzyme and their expression (Figs. 4, 5 and 6), and corresponding responses of alfalfa seed germination and seedling growth parameters (Table 1, Fig. 2a) subjected to salinity stress. These results suggested that cytoprotective role of H2S might be contributed to the increased activity of antioxidant enzymes (Figs. 5 and 6), which might be partially contributed to the up-regulation of their gene expression (Fig. 4), and thereafter decreased TBARS accumulation (Fig. 3). Above results correspondingly demonstrated the similar effects of H2S in plants as that found in animal researches (Kimura and Kimura 2004). Other explanation of H2S response might be attributed to its capability to exhibit antioxidant behavior (Li et al. 2009; Yonezawa et al. 2007). It was confirmed that H2S was able to protect neurons against glutamate-mediated oxidative stress, or oxytosis, through the pleiotropic effects of maintaining the activities of γ-glutamylcysteine synthetase (γ-GCS) and cysteine transport, leading to an increase in the well-known antioxidant glutathione (GSH) levels (Kimura and Kimura 2004). Similarly, the levels of cysteine and GSH were significantly increased by fumigation with H2S in Arabidopsis (Riemenschneider et al. 2005).
When plants are exposed to NaCl, cellular ion homeostasis may be impaired. Thus, a high ratio of K/Na is a key factor of cellular adaptation to salt stress (Zhu 2003). In the subsequent test, we found that NaHS treatment resulted in a higher K/Na ratio in root parts of alfalfa seedlings 4 days after salt stress (Fig. 2b). This result illustrated that NaHS may help maintain the ion balance between K+ and Na+ in alfalfa seedlings upon salinity stress. Thus, ion homeostasis is reestablished so as to adapt to salt stress.
NO is a bioactive molecule mediating responses to biotic and abiotic stresses in the plant kingdom. It could induce germination instead of red light (Beligni and Lamattina 2000), affect growth and development of plant tissues (Durner and Klessig 1999), and be involved in the responses to drought stress, salinity exposure, and disease resistance (Delledonne 2005). In the subsequent experiment, another explanation for the above beneficial actions triggered by H2S involved the NaHS-driven NO production (Fig. 7), which was consistent with the result reported by Jeong et al. (2006), showing that H2S enhances NO production and inducible NO synthase (iNOS) expression by potentiating IL-1 ß-induced NF-κB activation through a mechanism involving extracellular signal-regulated kinase 1/2 (ERK1/2) signaling cascade in rat vascular smooth muscle cells (VSMCs). For example, the changes of germination parameters conferred by NaHS and SNP were partially reversed by treatment together with cPTIO (Table 1, Supplementary Information Fig. S2), a specific scavenger of NO, and reestablishment of ion homeostasis (Fig. 2b) and induction of antioxidative behaviors (Figs. 3, 4, 5 and 6) were also blocked. Taken together, all of these results suggested that endogenous NO might play a key role in H2S-mediated cytoprotective roles, although we have not provided the potential source of NO triggered by NaHS treatment.
In conclusion, H2S attenuated salinity-induced inhibition of alfalfa seed germination and seedling growth, and was partially due to the induction of antioxidant metabolism as well as the reestablishment of ion homeostasis. Interestingly, all these events might have a possible interaction with NO, one of the gaseoustransmitter recently confirmed in the plant kingdom (Delledonne 2005). Thus, this data further deepened our knowledge of the relationship between H2S and NO in the enhancement of salinity tolerance in plants.
Abbreviations
- APX:
-
Ascorbate peroxidase
- ASC:
-
Ascorbic acid
- CAT:
-
Catalase
- cPTIO:
-
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt
- H2S:
-
Hydrogen sulfide
- NaHS:
-
Sodium hydrosulfide
- NO:
-
Nitric oxide
- POD:
-
Guaiacol peroxidase
- SNP:
-
Sodium nitroprusside
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thiobarbituric acid reactive substances
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Arasimowicz M, Floryszak-Wieczorek J (2007) Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci 172:876–887. doi:10.1016/j.plantsci.2007.02.005
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396. doi:10.1104/pp.106.082040
Association of Official Seed Analysis (AOSA) (1983) Seed vigor testing handbook. Contribution No. 32 to the handbook on Seed Testing. Association of Official Seed Analysis Springfield. IL
Badawi GH, Kawano N, Yamauchi Y, Shimada E, Sasaki R, Kubo A, Tanaka K (2004) Overexpression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol Plant 121:231–238. doi:10.1111/j.1399-3054.2004.00308.x
Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14:93–107. doi:10.1079/ssr2005159
Baskar R, Bian J (2011) Hydrogen sulfide gas has cell growth regulatory role. Eur J Pharmacol 656:5–9. doi:10.1016/j.ejphar.2011.01.052
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. doi:10.1016/0003-2697(71)90370-8
Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light inducible responses in plants. Planta 210:215–221. doi:10.1007/pl00008128
Bethke PC, Libourel IG, Jones RL (2006a) Nitric oxide reduces seed dormancy in Arabidopsis. J Exp Bot 57:517–526. doi:10.1093/jxb/erj060
Bethke PC, Libourel IG, Reinöhl V, Jones RL (2006b) Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 223:805–812. doi:10.1007/s00425-005-0116-9
Calderini O, Bovone T, Scotti C, Pupilli F, Piano E, Arcioni S (2007) Delay of leaf senescence in Medicago sativa transformed with the ipt gene controlled by the senescence-specific promoter SAG12. Plant Cell Rep 26:611–615. doi:10.1007/s00299-006-0262-y
Chen H, Zhang JY, Neff MM, Hong SW, Zhang H, Deng XW, Xiong L (2008) Integration of light and abscisic acid signaling during seed germination and early seedling development. Proc Natl Acad Sci USA 105:4495–4500. doi:10.1073/pnas.0710778105
Delledonne M (2005) NO news is good news for plants. Curr Opin Plant Biol 8:390–396. doi:10.1016/j.pbi.2005.05.002
Ding AH (1998) Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophage. J Immunol 141:2407–2412
Durner J, Klessig DF (1999) Nitric oxide as a signal in plants. Curr Opin Plant Biol 2:369–374. doi:10.1016/s1369-5266(99)00007-2
García-Mata C, Lamattina L (2010) Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol 188:977–984. doi:10.1111/j.1469-8137.2010.03465.x
Hällgren JE, Fredriksson SA (1982) Emission of hydrogen sulfide from sulfur dioxide-funigated pine trees. Plant Physiol 70:456–459. doi:10.1104/pp.70.2.456
Hamed KB, Castagna A, Salem E, Ranieri A, Abdelly C (2007) Sea fennel (Crithmum maritimum L.) under salinity conditions: a comparison of leaf and root antioxidant responses. Plant Growth Regul 53:185–194. doi:10.1007/s10725-007-9217-8
Han Y, Zhang J, Chen XY, Gao ZZ, Xuan W, Xu S, Ding X, Shen WB (2008) Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol 177:155–166. doi:10.1111/j.1469-8137.2007.02251.x
Hernandez M, Fernandez-Garcia N, Diaz-Vivancos P, Olmos E (2010) A different role for hydrogen peroxide and the antioxidative system under short and long salt stress in Brassica oleracea roots. J Exp Bot 61:521–535. doi:10.1093/jxb/erp321
Hosoki R, Matsuki N, Kimura H (1997) The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237:527–531. doi:10.1006/bbrc.1997.6878
Hu XK, Jiang XL, Hwang HM, Liu SL, Guan HS (2004) Promotive effects of alginate-derived oligosaccharide on maize seed germination. J Appl Phycol 16:73–76. doi:10.1023/b:japh.0000019139.35046.0c
Jeong SO, Pae HO, Oh GS, Jeong GS, Lee BS, Lee S, du Kim Y, Rhew HY, Lee KM, Chung HT (2006) Hydrogen sulfide potentiates interleukin-1β-induced nitric oxide production via enhancement of extracellular signal-regulated kinase activation in rat vascular smooth muscle cells. Biochem Biophys Res Commun 345:938–944. doi:10.1016/j.bbrc.2006.05.002
Kacperska A (2004) Sensor types in signal transduction pathways in plant cells responding to abiotic stressors: do they depend on stress intensity? Physiol Plant 122:159–168. doi:10.1111/j.0031-9317.2004.00388.x
Kimura Y, Kimura H (2004) Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18:1165–1167. doi:10.1096/fj.04-1815fje
Lee S, Kim SG, Park CM (2010) Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol 188:626–637. doi:10.1111/j.1469-8137.2010.03378.x
Lefer DJ (2007) A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc Natl Acad Sci USA 104:17907–17908. doi:10.1073/pnas.0709010104
Li X, Bazer FW, Gao H, Jobqen W, Johnson GA, Li P, McKnight JR, Satterfield MC, Spencer TE, Wu G (2009) Amino acids and gaseous signaling. Amino Acids 37:65–78. doi:10.1007/s00726-009-0264-5
Lisjak M, Srivastava N, Teklic T, Civale L, Lewandowski K, Wilson I, Wood ME, Whiteman M, Hancock JT (2010) A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol Biochem 48:931–935. doi:10.1016/j.plaphy.2010.09.016
Liu YH, Xu S, Ling TF, Xu LL, Shen WB (2010) Heme oxygenase/carbon monoxide system participates in regulating wheat seed germination under osmotic stress involving the nitric oxide pathway. J Plant Physiol 167:1371–1379. doi:10.1016/j.jplph.2010.05.021
Mancuso C, Navarra P, Preziosi P (2010) Roles of nitric oxide, carbon monoxide, and hydrogen sulfide in the regulation of the hypothalamic-pituitary-adrenal axis. J Neurochem 113:563–575. doi:10.1111/j.1471-4159.2010.06606.x
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467. doi:10.1111/j.1365-3040.2009.02041.x
Molassiotis A, Tanou G, Diamantidis G (2010) NO says more than ‘YES’ to salt tolerance: Salt priming and systemic nitric oxide signaling in plants. Plant Signal Behav 5:209–212. doi:10.4161/psb.5.3.10738
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Ondrias K, Stasko A, Cacanyiova S, Sulova Z, Krizanova O, Kristek F, Malekova L, Knezl V, Breier A (2008) H2S and HS− donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells. Pflugers Arch 457:271–279. doi:10.1007/s00424-008-0519-0
Riemenschneider A, Nikiforova V, Hoefgen R, De Kok LJ, Papenbrock J (2005) Impact of elevated H2S on metabolite levels, activity of enzymes and expression of genes involved in cysteine metabolism. Plant Physiol Biochem 43:473–483. doi:10.1016/j.plaphy.2005.04.001
Ruan HH, Shen WB, Ye MB, Xu LL (2002) Protective effects of nitric oxide on salt stress-induced oxidative damages to wheat (Triticum aestivum) leaves. Chin Sci Bull 47:677–681. doi:10.1360/02tb9154
Samis K, Bowley S, McKersie B (2001) Pyramiding Mn-superoxide dismutase transgenes to improve persistence and biomass production in alfalfa. J Exp Bot 53:1343–1350. doi:10.1093/jexbot/53.372.1343
Sekiya J, Schmidt A, Wilson LG, Filner P (1982) Emission of hydrogen sulfide by leaf tissue in response to L-cysteine. Plant Physiol 70:430–436. doi:10.1104/pp.70.2.430
Shi Q, Ding F, Wang X, Wei M (2007) Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiol Biochem 45:542–550. doi:10.1016/j.plaphy.2007.05.005
Wahid A, Perveen M, Gelani S, Basra SM (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:283–294. doi:10.1016/j.jplph.2006.01.005
Wang R (2003) The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal 5:493–501. doi:10.1089/152308603768295294
Wang BL, Shi L, Li YX, Zhang WH (2010) Boron toxicity is alleviated by hydrogen sulfide in cucumber (Cucumis sativus L.) seedlings. Planta 231:1301–1309. doi:10.1007/s00425-010-1134-9
Watanabe N, Iwamoto T, Bowen KD, Dickinson DA, Torres M, Forman HJ (2003) Bio-effectiveness of Tat-catalase conjugate: a potential tool for the identification of H2O2-dependent cellular signal transduction pathways. Biochem Biophys Res Commun 303:287–293. doi:10.1016/S0006-291X(03)00335-8
Xie YJ, Ling TF, Han Y, Liu K, Zheng Q, Huang L, Yuan X, He Z, Hu B, Fang L, Shen Z, Yang Q, Shen W (2008) Carbon monoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defence in wheat seedling roots. Plant Cell Environ 31:1864–1881. doi:10.1111/j.1365-3040.2008.01888.x
Xu MJ, Dong JF, Zhu MY (2005) Nitric oxide mediates the fungal elicitor-induced hypericin production of hypericum perforatum cell suspension cultures through a jasmonic-acid-dependent signal pathway. Plant Physiol 139:991–998. doi:10.1104/pp.105.066407
Xuan W, Zhu FY, Xu S, Huang BK, Ling TF, Ye MB, Shen WB (2008) The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol 148:881–893. doi:10.1104/pp.108.125567
Yonezawa D, Sekiguchi F, Miyamoto M, Taniquchi E, Honjo M, Masuko T, Nishikawa H, Kawabata A (2007) A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology 241:11–18. doi:10.1016/j.tox.2007.07.020
Yong QC, Cheong JL, Hua F, Deng LW, Khoo YM, Lee HS, Perry A, Wood M, Whiteman M, Bian JS (2011) Regulation of heart function by endogenous gaseous mediators—crosstalk between nitric oxide and hydrogen sulfide. Antioxid Redox Signal 14:2081–2091. doi:10.1089/ars.2010.3572
Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S (2000) Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol 123:223–233. doi:10.1104/pp.123.1.223
Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo JP (2008) Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50:1518–1529. doi:10.1111/j.1744-7909.2008.00769.x
Zhang H, Tang J, Liu XP, Wang Y, Yu W, Peng WY, Fang F, Ma DF, Wei ZJ, Hu LY (2009a) Hydrogen sulfide promotes root organogenesis in ipomoea batatas, salix matsudana and glycine max. J Integr Plant Biol 51:1086–1094. doi:10.1111/j.1744-7909.2009.00885.x
Zhang H, Ye YK, Wang SH, Luo JP, Tang J, Ma DF (2009b) Hydrogen sulfide counteracts chlorophyll loss in sweetpotato seedling leaves and alleviates oxidative damage against osmotic stress. Plant Growth Regul 58:243–250. doi:10.1007/s10725-009-9372-1
Zhang H, Jiao H, Jiang CX, Wang SH, Wei ZJ, Luo JP, Jones RL (2010a) Hydrogen sulfide protects soybean seedlings against drought-induced oxidative stress. Acta Physiol Plant 32:849–857. doi:10.1007/s11738-010-0469-y
Zhang H, Tan ZQ, Hu LY, Wang SH, Luo JP, Jones RL (2010b) Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J Integr Plant Biol 52:556–567. doi:10.1111/j.1744-7909.2010.00946.x
Zhao WM, Zhang J, Lu YJ, Wang R (2001) The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20:6008–6016. doi:10.1093/emboj/20.21.6008
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228. doi:10.1093/jxb/er319
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71. doi:10.1016/s1360-1385(00)1838-0
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445. doi:10.1016/s1369-5266(03)00085-2
Acknowledgements
This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Education Department of Jiangsu (grant no. 200910), the Technology Support Program in Jiangsu Province, China (grant no. BE2010382), and the Fundamental Research Funds for the Central Universities (KYZ200905).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Frans J.M. Maathuis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Effects of sodium hydrosulfide (NaHS) at the indicated concentrations on the seed germination inhibition conferred by 100 mM NaCl (S) for 2 days. Germination tests were carried out on three replicates of 150 seeds each. There were 50 seeds in each Petri dish. The photograph was taken after 2 days of incubation. In comparison with 100 mM NaCl-stressed alone sample, concentrations between 0.01 and 1.0 mM NaHS alleviated alfalfa seed germination inhibition with a maximal response at 0.1 mM. Bar, 1 cm. (DOC 3386 kb)
Figure S2
Effects of NaHS (100 μM), SNP (100 μM), and cPTIO (200 μM) treatments on the inhibition of alfalfa seed germination caused by 100 mM NaCl stress (S). Germination tests were carried out on three replicates of 150 seeds each. There were 50 seeds in each Petri dish. The photograph was taken after 2 days of incubation. Bar, 1 cm. (DOC 3902 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Li, L., Cui, W. et al. Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 351, 107–119 (2012). https://doi.org/10.1007/s11104-011-0936-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0936-2