Abstract
Leaf rolling observed in some crops such as maize, rice, wheat and sorghum is an indicator of decreased water status. Moderate leaf rolling not tightly or early increases the photosynthesis and grain yield of crop cultivars under environmental stresses. Moreover, the effects of exogenous abscisic acid (ABA) on stomatal conductance, water status and synthesis of osmotic compounds are a well-known issue in plants subjected to water deficit. However, it is not clear how the cross-talk of ABA with H2O2 and osmolyte compounds affects the leaf rolling mechanism. Regulation mechanism of leaf rolling by ABA has been first studied in maize seedlings under drought stress induced by polyethylene glycol 6000 (PEG 6000) in this study. ABA treatment under drought stress reduced hydrogen peroxide (H2O2) content and the degree of leaf rolling (%) while the treatment-induced ABA synthesis, osmolyte levels (proline, polyamine and total soluble sugars) and some antioxidant enzyme activities in comparison to the plants that were not treated with ABA. Furthermore, exogenous ABA up-regulated the expression levels of arginine decarboxylase (ADC) and pyrroline-5-carboxylate synthase (P5CS) genes and down-regulated polyamine oxidase (PAO), diamine oxidase (DAO) and proline dehydrogenase (ProDH) gene expressions. When endogenous ABA content was decreased by the treatment of fluoridone (FLU) that is an ABA inhibitor, leaf rolling degree (%), H2O2 content and antioxidant enzyme activities increased, but osmolyte levels, ADC and P5CS gene expressions decreased. Finally, the treatment of ABA to maize seedlings exposed to drought stress resulted in the stimulation of the antioxidant system, osmotic adjustment and reduction of leaf rolling. We concluded that ABA can be a signal compound cross-talking H2O2, proline and polyamines and thus involved in the leaf rolling mechanism by providing osmotic adjustment. The results of this study can be used to provide data for the molecular breeding of maize hybrids with high grain yield by means of moderately rolled leaves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Being one of the important limiters for maize crops, drought is a major topic that attracts attention all over the world. Effects of drought stress on plants vary depending on plant type, ability of tolerance and adaptability (Madhova Rao et al. 2005; Kadioglu et al. 2011). Plants which are sessile organisms have developed defense mechanisms such as physiological, biochemical and molecular changes in response to drought. Some symptoms of tolerance to drought include stomatal closing, osmotic adjustment, higher water use efficiency (WUE), deposition of wax, increased root length, and leaf rolling (Kadioglu et al. 2012; Khazaei et al. 2013). Leaf rolling is indeed a response of maize plants to insufficient plant moisture content. When plant moisture content decreases, to protect itself from excessive plant moisture loss, maize plants frequently rolls its leaves (Kadioglu et al. 2012). However, moderate leaf rolling not tightly or early increases the photosynthesis and grain yield of rice cultivars (Zhang et al. 2009). Therefore, knowing how the leaf rolling phenomenon is regulated under drought stress is important. There are many different studies that were conducted to find out the possible signals and the effective genes regarding the leaf rolling phenomenon. For instance, maize ROLLED LEAF1 (RLD1) gene encoded a class III homeodomain-leucine zipper protein and controlled upward rolling of the leaf blade (Juarez et al. 2004a). Some microRNAs such as miRNA166 may form a movable signal to define the expression domain of RLD1 (Juarez et al. 2004b). Additionally, the phytohormones are well-known regulators of the rolling. One of these regulators, brassinosteroids (BRs) about which an accumulating evidence illustrated that BRs play a pivotal role in leaf unrolling (Chono et al. 2003; Asahina et al. 2014). Exogenous polyamines and ascorbic acid also decrease the rolling of the leaves (Kadioglu et al. 2002; Terzi et al. 2015). Decreased leaf rolling by salicylic acid has been reported (Kadioglu et al. 2011; Saruhan et al. 2012). Moreover, H2O2 applied at low concentration delayed leaf rolling as a signal compound by inducing the level of proline, polyamine and total soluble sugars (Terzi et al. 2013). However, the signal or regulator role of abscisic acid (ABA) on leaf rolling under drought stress conditions is not known.
ABA accumulation can initiate some response mechanisms, ultimately physiological changes such as the closure of stomata, synthesis of osmoprotectants and the induction of the antioxidant system (Akpinar et al. 2012). Induced antioxidant enzyme activity with ABA treatment was reported in maize under drought stress (Jiang and Zhang 2002). Exogenous ABA decreased reactive oxygen species (ROS) accumulation by inducing activities or increasing the expression levels of several antioxidant enzymes. For instance, exogenous ABA treatment under drought stress significantly increased the superoxide dismutase and peroxidase activities in wheat cultivars (Wei et al. 2015; Bano et al. 2012). Furthermore, exogenous ABA application remarkably increased the activities: guaiacol peroxidase, catalase, superoxide dismutase and ascorbate peroxidase (Wang et al. 2011). Moreover, accumulation of proline in wheat seedlings induced by ABA treatment (Marcińska et al. 2013) and up-regulated ADC2 expression in Arabidopsis under drought stress result in enhanced abiotic tolerance (Perez-Amador et al. 2002). However, to our knowledge, the role of exogenous ABA application in regulation of osmolyte levels and in stimulation of antioxidant enzymes is still unclear in plants, especially in crop cultivars.
We aimed, therefore, to measure the changes in some antioxidant enzyme activities, sugar contents, levels of proline and polyamines and relative expression levels of their biosynthesis genes, stomatal conductance, leaf water potential, membrane damage, internal H2O2 and ABA contents to reveal the mechanism by which ABA reduces leaf rolling. Here, we hypothesized that ABA may cross-talk with H2O2 and osmolyte compounds, stimulate antioxidant enzymes and function as a signal in regulation of the leaf rolling.
Materials and methods
Plant material, growth conditions, and ABA/stress treatments
Zea mays L. seeds (cv. Akpınar) were obtained from the Black Sea Agricultural Research Institute, Turkey for this study. The seeds were planted in plastic pots (25 × 18 × 12 cm) filled with soil, and six seedlings were maintained in each pot. The seedlings were grown at 22/18 °C (day/night) under a photoperiodic cycle of 16 h light and 8 h dark with 60 ± 5% relative humidity and about 400 µmol m−2 s−1 supplied with fluorescent lamps in a plant growth chamber. When the seedlings had four fully developed leaves (after 3 weeks), they were cut from 2 cm above the ground level and kept in distilled water for 1 h to minimize the damage of water deficit. To determine the effect of ABA concentration on leaf rolling, the excised seedlings were kept in different ABA concentrations ranging from 0 to 350 µM under drought stress (− 0.3 MPa) created by the addition of polyethylene glycol 6000 (PEG 6000). Additionally, different fluoridone (FLU) concentrations (0–40 µM) were applied to the seedlings and determined FLU concentration decreasing endogenous ABA levels in the leaves under the stress conditions. After ABA and FLU concentrations’ decreasing leaf rolling and ABA levels were determined respectively, the seedlings were exposed to four different treatments for 12 h: (1) only distilled water (mock), (2) drought stress treatment (− 0.3 MPa) created by PEG 6000 treatment (control) (3) 250 µM ABA with by PEG 6000, and (4) 30 µM FLU with by PEG 6000.
The leaves were used for the following experiments immediately after 12 h of treatments.
Leaf rolling degree (%)
The degree of leaf rolling was estimated according to the method described by Premachandra et al. (1993) as a percentage reduction in the width of the middle part of the leaf.
Determination of ABA content
Plant tissues (0.1 g) were powdered in liquid nitrogen, extracted in distilled water, and the samples were stored at 4 °C in the dark. Then, the extracted samples were centrifuged at 15,000g for 15 min at 4 °C. The ABA content was determined using a Phytodetek ABA ELISA kit (Agdia/Linaris) according to the manufacturer’s instructions.
Leaf water potential (Ψ leaf),
To measure Ψleaf, a thermocouple psychrometer (PSYPRO C-52, Wescor) was used. 6-mm-diameter discs were taken from the leaves of five plants. The samples were balanced for 1 h then the data were recorded.
Stomatal conductance (g s)
The gs values were taken by an AP4 dynamic diffusion porometer (Delta T Devices, UK). Calibration of device was performed with a standard calibration plate according to the manufacturer’s instructions.
Lipid peroxidation
Lipid peroxidation was determined as the content of malondialdehyde (MDA) according to the method by Heath and Packer (1968). The leaves (0.1 g) were homogenized in trichloroacetic acid (0.1%) and centrifuged at 14,000g for 15 min. The supernatant (1 ml) was mixed with 20% TCA including 0.5% thiobarbituric acid (4 ml). The mixture which was heated at 95 °C for 30 min was cooled on ice. The concentration of MDA was measured at 532 and 600 nm spectrophotometrically.
Hydrogen peroxide (H2O2)
The leaves (0.1 g) were homogenized TCA (0.1%) containing activated charcoal at 4 °C and centrifuged at 15,000g for 10 min. The reaction mixture comprised aliquot of the supernatant, 10 mM potassium phosphate buffer (pH 7.0) and 1 M KI. The H2O2 content was estimated using standard curve prepared with a varying concentration range of H2O2 from 0 to 100 µmol at 390 nm (Velikova et al. 2000).
3,3′-Diaminobenzidine (DAB) staining
For determination of H2O2, a DAB staining protocol was modified from the method by Daudi et al. (2012). The plant leaves were subjected to DAB with 0.05% v/v Tween 20 and 10 mM sodium phosphate buffer (pH 7.0). Then the leaves were placed in tubes and transferred into a standard laboratory shaker at 80–100 rpm for incubation. Following incubation, the leaves were fixed and then boiled in a water bath at 90–95 °C for 15 ± 5 min in a bleaching solution including ethanol:acetic acid:glycerol (3:1:1). The leaves were incubated in fresh bleaching solution for 30 min. The leaves could be monitored for DAB staining.
Enzyme extractions and assays
For superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX) enzyme and protein extractions, 0.1 g of the leaf tissues were homogenized with a 50 mM sodium phosphate buffer (pH 7.8) with 1 mM ethylenediaminetetraacetic acid (EDTA) and polyvinylpolypyrrolidone (1%). For ascorbate peroxidase (APX) activity determination, 2 mM of ascorbate was added into the sodium phosphate buffer. The samples were centrifuged at 15,000g for 15 min. The total soluble protein contents in enzyme extracts were determined using bovine serum albumin as a standard (Bradford 1976).
Activity of SOD (EC 1.15.1.1) was determined according to the method of Beauchamp and Fridovich (1971). Reaction was initiated by the addition of 2 μM riboflavin to 1 ml reaction medium containing 50 mM potassium phosphate buffer (pH 7.8), 0.1 mM EDTA, 13 Mm l-methionine, 75 μM nitro blue tetrazolium (NBT), and 50 μl extract the absorbance values at 560 nm were determined after the mixture was exposed to white light at 375 μmol m−2 s−1 for 10 min.
CAT (EC 1.11.1.6) activity was determined by measuring the decrease in reaction time of 1 ml at 240 nm for 5 min, containing 50 mM potassium phosphate buffer (pH 7.0), 30 mM H2O2 and 20 μl enzyme extract. Catalase activity was calculated using the 39.4 mM−1 cm−1 coefficient for H2O2 (Bergmeyer 1970).
APX (EC 1.11.1.11) activity was determined with decrease at 290 nm (Nakano and Asada 1987). APX activity was determined by measuring a 1 ml reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0), 250 μM ascorbate (ASC), 5 mM H2O2 and 20 μl enzyme extract. APX activity was calculated using the 2.8 mM−1 cm−1 epsilon coefficient for ASC.
GPX (EC 1.11.1.7) activity was measured increase in absorbance at 470 nm in a 100 mM potassium phosphate buffer (pH 7.0) containing 0.1 mM EDTA, 5 mM guaiacol, 15 mM H2O2 and 50 µl of enzyme extract (Urbanek et al. 1991).
Proline and total soluble sugar contents
Proline content was determined using the spectrophotometric method described by Bates et al. (1973). Dry leaves (0.1 g) were extracted in sulfosalicylic acid [5 ml, 3% (w/v)] and centrifuged at 10,000 rpm for 10 min. The supernatant (1 ml) was added into the glacial acetic acid (1 ml) and ninhydrin (1 ml) mixture and incubated in an oven at 100 °C for 1 h. To stop the reaction, samples were incubated in an ice bath, then, toluene was added into the reaction mixture. The toluene phase was measured at 520 nm. To determinate proline concentration, the calibration curve was prepared using standards of proline and expressed as µg g−1 dry weight.
For determination of total soluble sugar content, dried leaves (0.1 g) were homogenized in 5 ml 70% ethanol and boiled at 95 °C water bath for 10 min, the homogenized leaves were centrifuged at 5000g for 5 min. Phenol (5%), and H2SO4 (5 ml) were added to sample (1 ml). The absorbance of the sample was recorded at 490 nm (Dubois et al. 1956). The results were expressed as mg per 100 g of dry weight.
Polyamine content
Polyamines’ extraction and detection were performed by high performance liquid chromatography (HPLC) the method according to described by Ben-Gigirey et al. (1998). The leaf tissues (5 g) were extracted with 0.4 M perchloric acid (10 ml). The extract was centrifuged at 3000g at 4 °C for 12 min. The collected supernatants were adjusted with 25 ml of perchloric acid.
The extract samples (1 ml) were used for the HPLC analysis and derivatized with dansyl chloride. After derivatization, the samples were filtered through a 0.45 μm-pore-size syringe filter. The polyamine contents were assayed using a HPLC (Shimadzu, LC 20 AT/Prominence, Japan) which consisted of two pumps and a UV–VIS detector. Separation was achieved using a C18 Supelco column 5 μm (250 mm × 4.6 mm). Ammonium acetate was used as the mobile phase and acetonitrile at a flow rate of 1 ml min−1 with a gradient elution program for 35 min. The injected sample volumes were 10 μl. The samples were monitored at 254 nm.
RNA isolation and cDNA synthesis
The leaves that were kept at − 80 °C were used for total RNA isolation. Frozen leaf tissues (0.1 g) were powdered in a homogenizer with liquid nitrogen. Total RNA isolation was carried out using a total RNA isolation kit [Quiagen RNeasy Plant Mini Kit (Cat. No: 74904)]. The amount and purity of RNA samples were measured with a nanodrop spectrophotometer (Thermo Scientific, Nanodrop 2000, USA). The synthesis of cDNA was created using a high-capacity cDNA Reverse Transcription Kit 4368814, (Applied Biosystems) from the isolated total RNA samples (2000 ng RNA).
Quantitative real-time (qRT) PCR analysis
For each qPCR, 20 µl of total volume with gene specific primers was used, with 4 µl Supermix [5× HOT FIREPol Eva Green qPCR Supermix (08-36-00008, Solis Biodyne)], 1 µl of primers, 1 µl cDNA sample and added nuclease-free water by 20 µl. The analysis was performed on CFX Connect Real Time PCR System (BioRad). qRT PCR protocol was modified according to Solis Biodyne’s instructions; 95 °C for 12 min, 45 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s, and the melt curve was held in 0.5 °C increments from 60 to 95 °C. Each biological repeat was analyzed as three technical repeats and the average technical error was considered to be in the form of 0.5 (± 1) Cq values. The findings were normalized according to the Actin gene and relative gene expression was presented. The primers that were used in this study are listed in Table 1.
Statistical analysis
All experiments were repeated three times. Ten samples were used for each treatment group. Variance analysis of mean values was performed by Duncan multiple comparison test (one-way ANOVA) using the SPSS software for Microsoft Windows (Ver. 15.0, SPSS Inc., USA) and the significance level was determined as 5% (P < 0.05).
For qRT PCR analysis, the relative gene expression level was analyzed by assaying the Bio-Rad CFX Manager 3.1. Expression levels were assayed by SPSS software. Variance analysis of mean values was carried out by one-way ANOVA (P < 0.05).
Results
Leaf rolling degree (%)
As compared to the control group (54.0%), 250 µM of ABA caused a significant decrease in the leaf rolling degree (23.7%) while 10, 50 and 100 µM of ABA did not make any significant difference. However, 200, 300, 350 µM of ABA concentrations increased the leaf rolling degree (Fig. 1a). On the other hand, FLU treatments at 10, 20 and 30 µM concentrations increased the leaf rolling degree % as compared to the control (Fig. 1b). Additionally, the highest degree of leaf rolling (77.3%) was determined after the 30 µM FLU treatment.
Changes in degree of leaf rolling and ABA content. a Effect of different ABA concentrations on degree of leaf rolling. b Effect of different FLU concentrations on degree of leaf rolling. c Effect of different treatments on degree of leaf rolling (mock: dH2O, control: PEG, ABA: 250 µM ABA in PEG, FLU: 30 µM FLU in PEG). d Effect of different FLU concentrations on ABA content. e Effect of different treatments on ABA content. Data are means ± SD of three replicates. Different letters indicate significant differences according to a Duncan’s multiple range test (P < 0.05)
Leaf rolling degrees in mock, control, ABA and FLU were recorded as follows, respectively, 0, 54.4, 24.5 and 77% (Fig. 1c).
ABA content
The maximum reduction in the ABA content was detected at the seedlings where 30 µM of FLU was applied (Fig. 1d). There was a 1.6-fold difference between the control and 30 µM of FLU treatment. It decreased from 150 pmol g−1 DW to 96.1 pmol g−1 DW. The ABA contents at 10, 20 and 40 µM of the FLU-treated seedling were 1.2-, 1.2-, and 1.3-fold lower than the content in the control seedlings (Fig. 1d).
The ABA contents in mock, control, FLU and ABA-treated seedlings were also compared. A significantly high amount of ABA (733.3 pmol g−1 DW) was found in the ABA-treated seedlings as compared to the mock (61.64 pmol g−1 DW), control (154.06 pmol g−1 DW), and FLU-treated (96.0 pmol g−1 DW) seedlings (Fig. 1e).
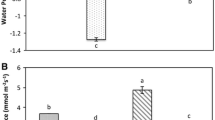
Leaf water potential
Ψ leaf decreased from − 0.5 MPa in mock to − 1.3 MPa in control under drought stress. Exogenously treated ABA caused a significant increase in leaf water potential of stressed plants (Ψleaf in ABA − 0.9 MPa). However, the Ψleaf value of the FLU-treated seedlings (− 1.4 MPa) was the lowest (Fig. 2a).
Changes in main stress parameters and antioxidant enzymes. a Leaf water potential, b stomatal conductance, c MDA content, d H2O2 content, e DAB staining, f SOD activity, g CAT activity, h APX activity, i GPX activity. Data are means ± SD of three replicates. Different letters indicate significant differences according to a Duncan’s multiple range test (P < 0.05)
Stomatal conductance
Control seedlings displayed a significantly lower stomatal conductance than the mock groups. It was found that FLU treatment had the highest stomatal conductivity, but ABA treatment was the lowest under drought stress conditions (Fig. 2b).
MDA content
Among the treatments, the highest MDA content (2.1 nmol g−1 DW) was observed in the FLU-treated seedlings. On the other hand, it was determined that MDA content decreased in the ABA-treated seedlings (1.2 nmol g−1 DW) in comparison with the controls (1.4 nmol g−1 DW) (Fig. 2c).
H2O2 content
The H2O2 content in leaves of maize is given in Fig. 2d. The H2O2 content was low in the ABA-treated seedlings (25.3 µmol g−1 DW) under drought stress conditions in comparison to the controls (51.5 µmol g−1 DW). However, the H2O2 content was the highest in the FLU-treated seedlings (Fig. 2d).
DAB staining
As shown in Fig. 2e, brown spots represented the presence of H2O2. The brown spots in the ABA-treated plants decreased in comparison to the control seedlings. However, the FLU-treated seedlings displayed higher numbers of brown spots in comparison to the controls.
Antioxidant enzyme activities
SOD activity
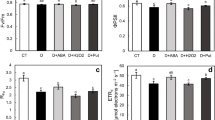
SOD activity was induced by drought stress (3.7 unit mg−1 of protein) compared to mock (1.53 unit mg−1 of protein) Fig. 2f. Exogenous ABA increased the activity as compared to the control seedlings. The activity rose to 7.8 unit mg−1 of protein in the ABA-treated seedlings. Whereas FLU treatment also increased the activity as compared to the controls, but the increase was the highest in the ABA-treated seedlings.
CAT activity
CAT activity increased under drought stress (control) as compared to mock treatment (Fig. 2g). The activity increased from 0.6 unit mg−1 of protein (mock) to 6.6 unit mg−1 of protein (control). The highest CAT activity was measured in the ABA-treated seedlings (10.8 unit mg−1 of protein). FLU treatment decreased the activity to 9.5 unit mg−1 of protein.
APX activity
APX activity was induced by drought stress compared to mock treatment (Fig. 2h). The activity increased from 542 unit mg−1 of protein to 917 unit mg−1 of protein. The highest activity was determined in the ABA-treated seedlings (1019 unit mg −1 of protein). On the other hand, FLU reduced enzyme activity (866 unit mg −1 of protein) in comparison to the controls and the ABA treatments.
GPX activity
The enzyme activity was enhanced by drought stress (control) compared to mock treatment (Fig. 2i). It increased from 51.5 unit mg−1 of protein (mock) to 120 unit mg−1 of protein (control). ABA treatment induced the GPX activity more than control (Fig. 2i). FLU treatment also increased the enzyme activity as compared to the controls (Fig. 2i).
Proline, total soluble sugar and polyamine contents
There was an increase in the proline content of the ABA-treated seedlings (1.7 µg g−1 DW) in comparison to the control seedlings (1.5 µg g−1 DW). However, the proline content in the FLU-treated seedlings decreased compared to the control (Fig. 3a). The H2O2 content decreased from 1.5 to 0.8 µg g−1 DW by FLU treatment.
There were significant differences among the treatments for total soluble sugar content (Fig. 3b). The seedlings treated with FLU under drought stress conditions showed the lowest levels of total soluble sugar. It was determined as 79 mg 100 g−1 DW, whereas in contrast, the ABA-treated seedlings had the highest amount of total soluble sugar during the drought stress. It was found as 153.2 mg 100 g−1 DW.
Under the drought stress conditions, ABA treatment significantly increased the Put, Spd and Spm contents in comparison to the controls. The putrescine content rose from 8.1 µg g−1 DW (control) to 15.3 µg g−1 DW (ABA) while FLU treatment decreased the Put content to 1.68 µg g−1 DW (Fig. 3c). The spermidine level in the control group was determined to be 1.1 µg g−1 DW. Following ABA treatment, this value was measured as 4.8 µg g−1 DW. FLU treatment reduced the Spd level to 0.3 µg g−1 DW. As to the Spm, it was also induced by the ABA treatment. The Spm content increased from 1.5 µg g−1 DW (control) to 1.9 µg g−1 DW (ABA). In similarity to Spd, the FLU treatment decreased the Spm content as well. It was decreased to 0.8 µg g−1 DW in comparison to the controls (1.48 ± 0.02 µg g−1 DW).
Expression levels of genes in proline metabolic pathway
Expression rates of the P5CS and ProDH genes were determined. The expression of these genes is summarized in Fig. 4. The expression of P5CS was up-regulated by water loss. The expression level of P5CS in the controls was 1.1-fold higher than that in the mock group. ABA also induced the expression (1.4) in comparison to the controls (1.1). P5CS expression was down-regulated by FLU treatment (Fig. 4a). The expression of the ProDH gene was significantly decreased among the controls as compared to the mock group (Fig. 4b). ABA treatment reduced the expression level in comparison to the controls. ProDH gene expression was induced by the FLU treatment. The expression level in FLU-treated seedlings was higher than those of ABA and control.
Expression levels of genes in polyamine metabolic pathway
To fully understand the accumulation of polyamines, gene expressions that play a part in polyamines’ biosynthesis and degradation were investigated. As shown in Fig. 5a, expression of the ADC gene was up-regulated by ABA treatment in comparison to the controls. The expression level in ABA treatment was 1.8-fold higher than that of the controls. On the other hand, FLU treatment down-regulated the ADC expression in comparison to the controls. The expression levels of the PAO and DAO genes were also increased in the ABA-treated seedlings in comparison to the controls. The expression levels of the DAO and PAO genes in the ABA-treated seedlings were two- and 1.6-fold higher than their controls. FLU treatment also up-regulated the DAO and PAO expressions. The expression levels were threefold higher than the control groups (Fig. 5b, c).
Discussion
A decrease in the leaf area and transpiration due to leaf rolling is accepted as an effective drought avoidance mechanism. However, a long-term rolling may result in loss of yield. Therefore, a reduction in degree of leaf rolling might be beneficial for plants under environmental stresses. Although there are some studies about how the application of certain substances such as brassinolides (Chen et al. 2015), salicylic acid (Kadioglu et al. 2011) and polyamines (Kadioglu et al. 2002) and ascorbic acid (Terzi et al. 2015) decreases leaf rolling, there is no study about the mechanism of exogenous ABA on leaf rolling so far. In this study, we tried to clarify how ABA decreased leaf rolling in Z. mays under drought stress conditions. We hypothesized that ABA may cross-talk with H2O2 and osmolyte compounds, stimulate antioxidant enzymes, and it may be a signal associated with the regulation of leaf rolling. Exogenously applied ABA increased endogenous ABA content under the drought stress conditions, similar to the previous reports in maize and wheat cultivars (Bano and Yasmeen 2010; Bano et al. 2012). Here, we tested whether the reduced leaf rolling was related to the ABA contents of the leaves. Fluoridone, which inhibits ABA biosynthesis (Perales et al. 2005), was applied to the leaves under drought stress. Inhibition of ABA biosynthesis by fluoridone led to an increase in the degree of leaf rolling which pointed out that leaf ABA content might be related to regulation of leaf rolling. Importance of stomata in regulating the water content of plants and the role of ABA in the control of stoma movements were recorded in the plants. Leaf rolling is closely related to leaf water content, and therefore, leaf water potential was measured under drought stress conditions in this study. Similar to our results, Eamus (1986) recorded that ABA-treated okra leaves had higher Ψleaf content than un-treated leaves under drought stress. Increased Ψleaf content was also determined in drought-stressed soybean plants that were treated with ABA in comparison to plants that were not (Hossain et al. 2015).
ABA is considered to be a major signal molecule involved in stomatal regulation (Akpinar et al. 2012). Treatment of exogenous ABA-induced stomatal closure, thus water loss from leaves, was inhibited in this study, thereby helping retain higher leaf water potential. Fluoridone treatment also supported ABA’s influence on water content. FLU treatment increased stomatal opening and alleviated water loss by reducing endogenous ABA content and the treatment caused an increase in leaf rolling. In similarity to this study, stomatal conductance was reported to decrease in ABA-treated wheat genotypes under drought stress (Saradadevi et al. 2017).
When the balance between ROS production and their scavenging activity by the antioxidant system deteriorates towards the ROS production pathway due to water scarcity, protein, lipid, DNA and membrane damages occur in plants (Gill and Tuteja 2010). Rising biosynthesis of ABA happens in plants as a response to drought stress mediated by changes in the levels of H2O2 (Phillips and Ludidi 2017). Similar to our study, Souza et al. (2014) showed the lowest H2O2 content in ABA-treated maize seedlings in the early periods of drought stress in comparison to plants that were not treated with ABA. Wei et al. (2015) recorded that ABA application remarkably enhanced the tolerance of common wheat seedlings subjected to 15% PEG-stimulated stress and decreased H2O2 and MDA. Our findings supported the result that the decrease of H2O2 production may be related to the triggering of antioxidant responses by ABA, which protects the plant from hazardous effects of oxidative stress. Alscher et al. (2002) suggested that membrane damages decreased in ABA-treated plants because of the decline in ROS production, which was indicated by lower levels of H2O2. Here, we found a decrease in MDA content of ABA-treated seedlings during the drought stress period. This finding is in compliance with lower leaf rolling degrees, and it supported other findings related to leaf water potential and stomatal conductance.
Moreover, Marcińska et al. (2013) demonstrated in drought resistant wheat cultivar that exposure to ABA decreased MDA content by inducing the antioxidant system and improved tolerance to drought. As based on our findings, we can suggest that the pretreatment of ABA-alleviated membrane damage thanks to scavenging of ROS by inducing the SOD, CAT, APX and GPX activities in maize seedlings and relieved oxidative stress. Indeed, in a similar study, FLU treatment enhanced MDA content in barley seedlings under drought stress (Popova 1998). Due to the idea that they are associated with water availability, changes in some osmolytes such as proline, soluble total sugar, and polyamine content were determined. Additionally, ABA can induce change in the biosynthesis of stress proteins, proline, sugar alcohol, soluble carbohydrate, glycine, betaine (Bagniewska-Zadworna et al. 2007). The accumulation of the osmolytes helps tolerance to dehydration by providing the continuity of the water balance (Chołuj et al. 2008; Costa et al. 2008). The accumulation of proline content after ABA treatment under drought stress conditions in our study was also similar to those found by Costa et al. (2011) and Marcińska et al. (2013) where ABA-induced biosynthesis of proline was reported. Genetic analyses of proline biosynthesis and degradation in plants were closely associated with biochemical analysis. For better understanding of proline accumulation in maize seedlings, we studied the relative gene expressions involved in its biosynthesis and degradation. In general, our results suggested that synthesis of proline in the ABA-treated seedlings increased as their degradation decreased under drought stress. Proline degradation by ProDH was reduced when the proline synthesis pathway was induced by ABA treatment in the plants. Thus, the proline content increased under drought stress conditions.
FLU-reverted biosynthesis of polyamines led this study to examine changes on the gene level. To obtain a better understanding of polyamine accumulation, the expression of genes, including in its biosynthesis and degradation were investigated. It was found that expressions of the PAO and DAO genes, responsible for degradation, and expression of ADC gene, responsible for polyamines biosynthesis was increased in ABA-treated seedlings as compared to control. However, ADC expression rate was higher than those of PAO and DAO. These results suggested that the synthesis rate of polyamines was higher than the degradation of them. As similar to our study, Alcazar et al. (2006) recorded that ABA up-regulated ADC2 expression in Arabidopsis thaliana and thus it regulated polyamine metabolism at transcriptional level in response to drought stress.
Conclusions
Here, we suggested for the first time that ABA treatment on maize seedlings under drought stress caused a reduction in leaf rolling by osmotic regulation orchestrated through the accumulation of proline, polyamine and total soluble sugars. ABA may be a signal compound that cross-talks with osmolytes such as soluble sugar, proline and polyamines. Moreover, the cross-talk of ABA with H2O2 may reduce the level of endogenous H2O2 by stimulating antioxidant enzyme activities. The cross-talk may modulate the expressions of metabolic genes of proline and polyamines, and antioxidant enzyme activities, and thus regulate the mechanism of leaf rolling and mitigate damages of oxidative stress. We may recommend investigation of how ABA affects the expression of leaf rolling genes such as RLD1 and SHALLOT-LIKE. Therefore, learning the signal compound and its cross-talking in the rolled leaves that occur by the effects of abiotic stress may supply researchers and farmers with an opportunity to improve crop cultivars with a high grain yield.
Author contribution statement
AS, AK and RT designed the research. AS, CA, AS and MD conducted experiments. AS, RT and AK analyzed all data. AS, AS and RT wrote the manuscript.
Abbreviations
- ABA:
-
Abscisic acid
- PEG:
-
Polyethylene glycol
- H2O2 :
-
Hydrogen peroxide
- ADC :
-
Arginine decarboxylase
- P5CS :
-
Pyrroline 5-carboxylate synthase
- PAO:
-
Polyamine oxidase
- DAO :
-
Diamine oxidase
- ProDH :
-
Proline dehydrogenase
- FLU:
-
Fluoridone
- MDA:
-
Malondialdehyde
- TCA:
-
Trichloroacetic acid
- NBT:
-
Nitroblue tetrazolium
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- GPX:
-
Guaiacol peroxidase
- SOD:
-
Superoxide dismutase
- HPLC:
-
High performance liquid chromatography
- BR:
-
Brassinosteroid
- EDTA:
-
Ethylenediaminetetraacetic acid
- DW:
-
Dry weight
- WUE:
-
Water use efficiency
- DAB:
-
3,3′-Diaminobenzidine
- ROS:
-
Reactive oxygen species
References
Akpinar BA, Avsar B, Lucas SJ, Budak H (2012) Plant abiotic stress signaling. Plant Signal Behav 7(11):1450–1455
Alcazar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Antonio FT, Teresa A (2006) Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett 28:1867–1876
Alscher RG, Ertürk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Asahina M, Tamaki Y, Sakamotob T, Shibata K, Nomura T, Yokota T (2014) Blue light-promoted rice leaf bending and unrolling are due to up-regulated brassinosteroid biosynthesis genes accompanied by accumulation of castasterone. Phytochemistry 104:21–29
Bagniewska-Zadworna A, Zenkteler E, Czaczyk K, Osinska M (2007) The effect of dehydration with or without abscisic acid pretreatment on buds regeneration from Polypodium vulgare L. rhizomes. Acta Physiol 29:47–56
Bano A, Yasmeen S (2010) Role of phytohormones under induced drought stress in wheat. Pak J Bot 42:2579–2587
Bano A, Ullah F, Nosheen A (2012) Role of abscisic acid and drought stress on the activities of antioxidant enzymes in wheat. Plant Soil Environ 58:181–185
Bates S, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Ben-Gigirey B, De Sousa JMVB, Villa TG, Barros-Velazquez J (1998) Changes in biogenic amines and microbiological analysis in albacore (Thunnus alalunga) muscle during frozen storage. J Food Prot 61:608–615
Bergmeyer N (1970) Methoden der Enzymatischen Analyse. Akademie Verlag, Berlin
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Chen Q, Xie Q, Gao J, Wang W, Sun B, Liu B, Zhu H, Peng H, Zhao H, Liu C, Wang J, Zhang J, Zhang G, Zhang Z (2015) Characterization of Rolled and Erect Leaf 1 in regulating leave morphology in rice. J Exp Bot 66:6047–6058
Chołuj D, Karwowska R, Ciszewska A, Jasińska M (2008) Influence of long-term drought stress on osmolyte accumulation in sugar beet (Beta vulgaris L.) plants. Acta Physiol Plant 30:679
Chono M, Honda I, Zeniya H, Yoneyama K, Saisho D, Takeda K, Takatsuto S, Hoshino T, Watanabe Y (2003) A semidwarf phenotype of barley uzu results from a nucleotide substitution in the gene encoding a putative brassinosteroid receptor. Plant Physiol 133:1209–1219
Costa RCL, Lobato AKS, Neto CFO, Maia PSP, Alves GAR, Laughinghouse HD (2008) Biochemical and physiological responses in two Vigna unguiculata (L.) walp cultivars under water stress. J Agron 7:98–101
Costa RCL, Lobato AKS, Silveira JAG, Laughinghouse HD (2011) ABA mediated proline synthesis in cowpea leaves exposed to water deficiency and rehydration. Turk J Agric For 35:309–317
Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24:275–287
Dubois M, Gilles KA, Rebers PA (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Eamus D (1986) The responses of leaf water potential and leaf diffusive resistance to abscisic acid, water stress and low temperature in Hibiscus esculentus: the effect of water stress and ABA pre-treatments. J Exp Bot 37:1854–1862
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hossain MM, Lam HM, Zhang J (2015) Responses in gas exchange and water status between drought-tolerant and -susceptible soybean genotypes with ABA application. Crop J 3:500–506
Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53:2401–2410
Juarez MT, Twigg RW, Timmermans CP (2004a) Specification of adaxial cell fate during maize leaf development. Development 131:4533–4544
Juarez MT, Kui JS, Thomas J, Heller BA, Timmermans MCP (2004b) MicroRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428:84–88
Kadioglu A, Turgut R, Palavan- Unsal N, Saruhan N (2002) Effect of polyamines on leaf rolling in Ctenanthe setosa. Isr J Plant Sci 50:19–23
Kadioglu A, Saruhan N, Saglam A, Terzi R, Acet T (2011) Exogenous salicylic acid alleviates effects of long term drought stress and delays leaf rolling by inducing antioxidant system. Plant Growth Regul 64:27–37
Kadioglu A, Terzi R, Saruhan N, Saglam A (2012) Current advances in the investigation of leaf rolling caused by biotic and abiotic stress factors. Plant Sci 182:42–48
Khazaei H, Street K, Bari A, Mackay M, Stoddard FL (2013) The FIGS (focused identification of germplasm strategy) approach identifies traits related to drought adaptation in Vicia faba genetic resources. PLoS One 8:e63107. https://doi.org/10.1371/journal.pone.0063107
Madhova Rao KV, Raghavendra AS, Janardhan Reddy K (2005) Physiology and molecular biology of stress tolerance in plants. Springer, Amsterdam
Marcińska I, Czyczyło-Mysza I, Skrzypek E, Grzesiak M, Janowiak F, Filek M, Dziurka M, Dziurka K, Waligorski P, Juzoń K, Cyganek K, Grzesiak S (2013) Alleviation of osmotic stress effects by exogenous application of salicylic or abscisic acid on wheat seedlings. Int J Mol Sci 14:13171–13193
Nakano Y, Asada K (1987) Purification of ascorbate peroxidase in spinach chloroplasts—its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol 28:131–140
Perales L, Arbona V, Gómez-Cadenas A, Cornejo MJ, Sanz A (2005) A relationship between tolerance to dehydration of rice cell lines and ability for ABA synthesis under stress. Plant Physiol Biochem 43:786–792
Perez-Amador MA, Leon J, Green PJ, Carbonell J (2002) Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in arabidopsis. Plant Physiol 130:1454–1463
Phillips K, Ludidi N (2017) Drought and exogenous abscisic acid alter hydrogen peroxide accumulation and differentially regulate the expression of two maize RD22-like genes. Sci Rep 7:8821
Popova LP (1998) Fluridone and light affected chloroplast ultrastructure and ABA accumulation in drought-stressed barley. Plant Physiol Biochem 36:313–319
Premachandra GS, Saneoka H, Fujita K, Ogata S (1993) Water stress and potassium fertilization in field grown maize (Zea mays L.): Effects on leaf water relations and leaf rolling. Agron Crop Sci 170:195–201
Saradadevi R, Palta JA, Siddique KHM (2017) ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01251
Saruhan N, Saglam A, Kadıoğlu A (2012) Salicylic acid pretreatment induces drought tolerance and delays leaf rolling by inducing antioxidant systems in maize genotypes. Acta Physiol Plant 34:97–106
Souza TC, Magalhães PC, Castro EM, Carneiro NP, Padilha FA, Júnior CCG (2014) ABA application to maize hybrids contrasting for drought tolerance: changes in water parameters and in antioxidant enzyme activity. Plant Growth Regul 73:205–217
Terzi R, Kadioglu A, Kalaycioglu E, Saglam (2013) Hydrogen peroxide pretreatment induces osmotic stress tolerance by influencing osmolyte and abscisic acid levels in maize leaves. J Plant Interact 9:559–565
Terzi R, Kalaycıoglu E, Demiralay M. Saglam A, Kadioglu A (2015) Exogenous ascorbic acid mitigates accumulation of abscisic acid, proline and polyamine under osmotic stress in maize leaves. Acta Physiol Plant 37:43–51
Urbanek H, Kuzniak-Gebarowska E, Herka K (1991) Elicitation of defense responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol Plant 13:43–50
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants, protective role of exogenous polyamines. Plant Sci 151:59–66
Wang Y, Ma F, Li M, Liang D, Zou J (2011) Physiological responses of kiwifruit plants to exogenous ABA under drought conditions. Plant Growth Regul 64:63–74
Wei L, Wang L, Yang Y, Wang P, Guo T, Kang G (2015) Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front Plant Sci 6:548
Zhang GH, Xu Q, Zhu XD, Qian Q, Xue HW (2009) SHALLOT-LIKE1 is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. Plant Cell 21:719–735
Acknowledgements
This work was supported by a Grant from TUBITAK (111T511).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Wojtaszek.
Rights and permissions
About this article
Cite this article
Sezgin, A., Altuntaş, C., Sağlam, A. et al. Abscisic acid cross-talking with hydrogen peroxide and osmolyte compounds may regulate the leaf rolling mechanism under drought. Acta Physiol Plant 40, 141 (2018). https://doi.org/10.1007/s11738-018-2716-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2716-6