Abstract
The present study showed that osmopriming or pretreatment with low H2O2 doses (2 mM) for 6 h alleviated salt-reduced seed germination. The NADPH oxidase activity was the main source, and superoxide dismutase (SOD) activity might be a secondary source of H2O2 generation during osmopriming or H2O2 pretreatment. Hematin pretreatment similar to osmopriming improved salt-reduced seed germination that was coincident with the enhancement of heme oxygenase (HO) activity. The semi-quantitative RT-PCR confirmed that osmopriming or H2O2 pretreatment was able to upregulate heme oxygenase HO-1 transcription, while the application of N,N-dimethyl thiourea (DMTU as trap of endogenous H2O2) and diphenyleneiodonium (DPI as inhibitor of NADPHox) not only blocked the upregulation of HO but also reversed the osmopriming-induced salt attenuation. The addition of CO-saturated aqueous rescued the inhibitory effect of DMTU and DPI on seed germination and α-amylase activity during osmopriming or H2O2 pretreatment, but H2O2 could not reverse the inhibitory effect of ZnPPIX (as HO inhibitor) or Hb (as CO scavenger) that indicates that the CO acts downstream of H2O2 in priming-driven salt acclimation. The antioxidant enzymes and proline synthesis were upregulated in roots of seedlings grown from primed seeds, and these responses were reversed by adding DMTU, ZnPPIX, and Hb during osmopriming. These findings for the first time suggest that H2O2 signaling and upregulation of heme oxygenase play a crucial role in priming-driven salt tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity is a devastating environmental stress for seed germination and plant growth. It can affect seed germination and stand establishment through osmotic stress, ion toxicity, and oxidative stress. The salinity delays or prevents seed germination through reducing water availability, changing the mobilization of stored reserves, and affecting the structural organization of proteins. Thus, various techniques are applied to improve seed germination and stand establishment under salt conditions. Among these, seed priming is considered as a simple procedure that not only enhances seed germination but also mitigates the adverse effects of environmental stresses (Ibrahim 2015; Jisha et al. 2013).
Seed priming is a presowing technique that exposes seeds to a certain solution for a certain period and promotes partial hydration and physiological process during early phase of germination called “pregerminative metabolism,” but prevents from radicle emergence and complement of seed germination (Jisha et al. 2013; Amooaghaie et al. 2010). Seed priming enhances germination rate and seedling establishment by weakening endosperm and modulating the activity of many enzymes involved in the stored reserves mobilization (such as α and β amylases) prior to the emergence of the radicle (Anese et al. 2011; Amooaghaie et al. 2010; Varier et al. 2010). These enzymes are involved in seed storage degradation that is essential process for the development and growth of the embryo, and thus they contribute to early and higher seedling emergence (Farooq et al. 2006; Varier et al. 2010). The priming is also triggering DNA repair and epigenetic changes as well as activating transcription factors that led to better seed germination (Paparella et al. 2015; Tanou et al. 2012).
Seed priming also enhances defense responses including the activities of antioxidant enzymes and proline accumulation in seedlings grown from primed seeds under stress condition (Amooaghaie 2011; Khodabakhsh et al. 2014). It has been suggested that seed priming increases stress tolerance by inducing “stress imprint” (Bruce et al. 2007). The primary source of stress could be the priming strategy itself. For example, hydropriming that is shortening the hydration duration and osmopriming, matric priming, or drum priming that are exposing seeds to relatively low external water potential; subject seeds to partial drought or osmotic stress. Polyethylene glycol (PEG), as a usual osmopriming agent, not only triggers osmotic stress but can also impose anoxia (Hardegree and Emmerich 1994) and the oxidative stress on primed seeds (Balestrazzi et al. 2011). H2O2 priming directly induces oxidative injuries in cells. Halopriming causes both osmotic stress and ionic toxicity. Thus, the priming itself can cause moderate abiotic stresses (such as osmotic, drought, and oxidative). Furthermore, seeds were exposed to stress during the post priming drying. Drying of primed seeds that have partially lost desiccation tolerance constitutes a stress (Chen and Arora 2012). These experiences activate an array of defense processes in primed seeds that establishes resistance to various environmental stresses in subsequent exposures (Amooaghaie 2011; Chen and Arora 2011; Khodabakhsh et al. 2014; Ibrahim 2015). In other words, seed priming acts as a pregermination stress exposure and creates a “stress memory” in cells that facilitates the expression of stress responses in subsequent exposures (Bruce et al. 2007; Chen and Arora 2011; Paparella et al. 2015; Pastor et al. 2013). From this point of view, priming is similar to acclimation phenomena during early seed imbibition.
Recently, much evidence has documented that in many plant species, H2O2, NO, and CO play role of important signal molecules in the perception of environmental factors in normal or stress conditions (Amooaghaie et al. 2015; Amooaghaie and Nikzad 2013; Amooaghaie and Roohollahi 2016; Bailly et al. 2008). Moreover, several lines of evidence exhibited that these signal molecules mediate the phenomena of acclimation and cross-tolerance, in which previous exposure to low level of one stress can lead to tolerance in subsequent exposure to higher level of the same or different stresses (Neill et al. 2002). Therefore, it is possible that the first step in activating molecular and cellular responses by priming can be stress experience and relaying stress-related information through signal transduction pathways.
It has been shown that reactive oxygen species (ROS) accumulate during seed germination, and the exogenous application of H2O2 can improve seed germination in many plant species. These results emphasize the requirement for the crucial levels of ROS for seed germination proposed by the model of the “oxidative window” (Bailly et al. 2008). The interplay between ROS and hormone signaling pathways that induces changes in gene expression or in cellular redox status has an important role in the perception of environmental factors by seeds during their germination (Ishibashi et al. 2012; Sarath et al. 2007). Several lines of evidence suggest that H2O2 pretreatment of seeds increases the tolerance of plants to abiotic stresses and H2O2 plays role of a signal molecule in triggering plant responses to stresses (Gondim et al. 2010; Wahid et al. 2007). On the other hand, the high dose of H2O2 which is generated in adverse environmental conditions in seeds is deleterious and ROS-induced oxidative damage inhibits or delays seed germination (Bailly et al. 2008). However, there is very little knowledge concerning on how priming affects H2O2 levels in seeds and ultimately leads to the change of the developmental and physiological programs of plants in response to stresses.
Recently, a number of studies on plants have also demonstrated that endogenous H2O2 production can enhance abiotic stress tolerance via the upregulation of heme oxygenase (Chen et al. 2009; Wei et al. 2012). Heme oxygenase (HO) is one of enzymes involved in phytochrome chromophore biosynthesis (Muramoto et al. 2002) that catalyzes the cleavage of heme to biliverdin-IXα, iron (Fe2+) and carbon monoxide (CO). In addition, HO-1, as an inducible form of heme oxygenase and the potential source of CO production in plants, has a strong antioxidant enzyme against different abiotic stresses (Xu et al. 2006; Liu et al. 2007). For example, the application of the exogenous hematin as HO-1 inducer and a CO aqueous solution dose dependently alleviated the inhibition of wheat seed germination and seedling growth under osmotic stress (Liu et al. 2010). Several authors confirmed the role of HO in acclimation to stresses (Chen et al. 2009; Wei et al. 2012; Xie et al. 2011). According to our literature review, no information exists on the changes of HO gene expression or HO activity in seeds after priming.
Proteomic analyses of primed seeds of Medicago sativa L. has emphasized that metabolic and biochemical processes are involved in the increment of seed vigor of primed seeds (Yacoubi et al. 2013). The global proteome and transcriptome expression profiling of PEG-primed seeds of Brassica napus L. also demonstrated abundant genes and numerous proteins were differentially expressed during the main phases of priming technique and final germination of primed seeds (Kubala et al. 2015). However, which signal transduction pathways are involved in activating pregerminative metabolism and gene expression, by priming, is still unknown. Therefore, we investigated assumption that priming similar to acclimation process may act to improve seed germination and salt tolerance of alfalfa, through ROS signaling and HO/CO upregulation.
Materials and methods
Pretreatment with various concentrations of H2O2
Seeds of alfalfa (M. sativa, Hamedani cv.) were sterilized in 2% NaClO for 5 min and washed in distilled water. In the first experiment, seeds were treated with different H2O2 concentrations (0, 1, 2, 4, 8 mM) for 6 h. Then, laboratory seed germination tests were conducted in 9-cm Petri dishes (50 seeds per each plate and five replicates for each treatment) on a layer of filter paper moistened with distilled water at 25 °C with a continuous light intensity of 300 μmol m−2 s−1. The seeds treated with distilled water under the same conditions were considered as controls. Seed germination was counted daily for 5 days. Seeds were considered germinated when radicles were protruded for more than 2 mm.
Osmopriming and other treatments
For osmopriming, seeds were primed in polyethylene glycol solution (PEG 6000; Shanghai Chemical Reagent Co. Ltd., Shanghai, China) at 300 g L−1 with continuous aeration. After 6 h at 25 °C, the seeds were removed and rinsed in distilled water and wiped free of water and air dried at 25 °C for 24 h. It is worth to note that in the preliminary study, 150 mM NaCl was chosen as optimal stress conditions and measurements with concentration gradients of PEG revealed this PEG concentration was the best treatment for improving seed germination under 150 mM salt stress (data not shown).
Carbon monoxide aqueous solution was prepared according to the method described by Han et al. (2008). For the preparation of CO-saturated aqueous solution, CO gas was bubbled into 500 mL of distilled water by a glass tube for 50 min. Then, the required concentration of CO aqueous solution was prepared by the dilution of this saturated stock solution (100% of saturation) with distilled water.
Preliminary studies using various concentrations (1, 10, 20, 40, and 80% of saturation) of CO aqueous solution showed that 10% CO-saturated aqueous solution was the suitable treatment due to its better effect on improving seed germination under 150 mM salt stress (data not shown).
Hematin (H: C34H33N4O5Fe) and zinc protoporphyrin IX (ZnPPIX), were purchased from Sigma and applied as a CO donor and inhibitor of HO-1, respectively (Liu et al. 2010). The seeds were treated with various concentrations (0, 2.5, 5, 10, and 20.0 μM) of hematin, and the data showed that 2.5 μM hematin was the best treatment. ZnPPIX was used at 100 μM concentration. Furthermore, hemoglobin (Hb) as the scavenger of CO and N,N-dimethylthiourea (DMTU) as the scavenger of H2O2 (Chen et al. 2009) were purchased from Fluka. Additionally, the remaining chemicals were of analytical grade from Chinese companies.
In various experiments, osmoprimed seeds or seeds pretreated with 100 μM H2O2, as well as control seeds, directly were exposed with 1 mM DMTU, 1 mM DPI, AT, 5 μM hematin, 100 μM ZnPPIX, 10% CO-saturated aqueous solution, and 0.1 g L−1 Hb singly or combination of two or three of the previous chemicals for 6 h then transferred to Petri dishes containing of distilled water or 150 mM NaCl and seed germination was evaluated as mentioned previously. The seeds were treated with distilled water under the same conditions considered as controls.
After various treatments, biochemical parameters (NADPHox, SOD, HO activity, H2O2 content) were measured 6 h after salt exposure in germinating seeds before any radicle protrusion occurred or 24 h after salt exposure as described in the “Results” section. Also, H2O2 content and the activities of SOD, CAT, APX, proline content, and SOD, CAT, APX, and P5CS (d-1-pyrroline-5-carboxylate synthetase enzyme) transcripts were assessed in 5-day-old seedlings. The biochemical parameters and gene expressions were evaluated as described in following sections.
H2O2 content determination
The H2O2 content of seeds was determined according to method described by Oracz et al. (2007). The extracts of seed obtained from the homogenization of seed with perchloric acid were centrifuged at 10,000×g at 4 °C for 15 min, and supernatant was used for H2O2 determination. The assay mixture (1.5 ml final volume) contained 12 mM 3-dimethylaminobenzoic acid in 0.375 M phosphate buffer (pH 6.5), 1.3 mM 3- methyl-2-benzothiazolidone hydrazone, 20 μl (0.25 U) horseradish peroxidase (Sigma), and 70 μl of the collected supernatant. The spectrophotometric absorbance at 590 nm was read after 5 min at 25 °C, and results are reported as nmol H2O2 g−1DW.
Assays of enzyme activities
Heme oxygenase activity was measured as described by Muramoto et al. (2002), with minor modifications. The concentration of biliverdin IX (BV) was determined using a molar absorption coefficient at 650 nm of 6.25 mM−1 cm−1 in 0.1 M HEPES–NaOH buffer (pH 7.2). One unit of activity (U) was defined by calculating the quantity of the enzyme to produce 1 nmol BV per 30 min.
Total SOD activity was assayed on the basis of its ability to reduce nitroblue tetrazolium (NBT) by the superoxide anion generated by the riboflavin system under illumination. One unit of SOD (U) was expressed as the amount of crude enzyme extract required to inhibit the reduction rate of NBT by 50%.
The activities of CAT, SOD, and APX in roots of seedlings were determined as described by Amooaghaie and Roohollahi (2016).
NADPH oxidase were analyzed using the method described by Van Gestelen et al. (1997) using nitroblue tetrazolium (NBT) and NADPH. NADPH-ox activity was calculated from the difference in NBT reduction using an extinction coefficient of 12.8 mM−1 cm−1 in the absence or presence of 50 U mL−1 superoxide dismutase (bovine erythrocytes, Sigma-Aldrich, St. Louis, MO, USA).
Protein was evaluated by the method of Bradford (1976), using bovine serum albumin (BSA) as a standard.
Semi-quantitative RT-PCR analysis
Samples were homogenized with mortar and pestle in liquid nitrogen and after total RNA isolation; cDNA was amplified by PCR using the following primers: amplifying a 476-bp fragment for HO-1 (accession number HM212768), forward (5′-TACATACAAAGGACCAGGCTAAAG-3′ ) and reverse (5′-GTCCCTCACATTCTGCAACAACTG-3′), for P5CS gene (accession number: CAA67069.1) forward (5′-CATCCCTGTTTCTCTCCACC-3′) and reverse (5′- CCATCTCGCGTACATCAACC-3′), for Cu-Zn SOD gene (accession numbers: XM003626314.2, XM013565057.1) forward (AATGTCACCGTCGGTGATGATG) and reverse (GTTCATCCTTGCAAACCAATAATACC), for CAT gene (accession numbers: XM013606824.1, XM013606823.1) forward (CCTATTTGATGATGTGGGTGTCC ) and reverse (GTCTTGAGTAGCATGGCTGTGGT) and for APX gene (accession numbers: XM003615115.2, CU928858.2) forward (ACCAACCTCGTTCAGTGTCCAT) and reverse (AGAGCGCTGTCTGCGTTCTATT) and finally amplifying a 505-bp fragment; for actin, forward 5′- GTGACAATGGAACTGGAATGG-3′ and reverse 5′-AGACGGAGGATAGCGTGAGG-3′ designed based on primary sequence with accession number NM_001316010.1 in the NCBI genomic data bank.
To standardize the results, the relative intensity of electrophoretic bands belong to genes PCR products against ones of actin gene as internal control were calculated in exponential phase of PCR reactions (22–28 cycles). The aliquots of the PCR reactions were loaded on 1.5% agarose gels with the use of ethidium bromide. Ethidium bromide-stained gels were scanned and analyzed using the Total Lab v1.10 software (Nonlinear Dynamics, Newcastle-upon-Tyne, UK).
Proline measurement
The proline content of roots was determined as described by Bates et al. (1973). Briefly, root samples were homogenized in 1 mL of 3% sulphosalicylic acid solution on an ice bath and were filtrated. The test tubes including the filtrate, 2.5% ninhidrine solution, and glacial acetic acid were laid in a water bath at 100 °C for 50 min and then were cooled in ice bath and toluene was added. The absorbance of the pink-red upper phase was recorded at 520 nm, against toluene blank. The proline concentration was calculated using a standard curve and expressed as micromole proline per gram fresh weight.
Statistical analysis
Some experiments were carried out as factorial experiment with a completely randomized design, and other trials were conducted as completely randomized design. For all tests, five replications were utilized. The differences among treatments were analyzed by one-way ANOVA using SPSS version 13, and Duncan’s multiple range tests at P < 0.05 were used to compare the treatment means.
Results
Effects of pretreatment at various concentrations of H2O2 on seed germination
As shown in Fig. 1, pretreatment with lower H2O2 doses (1, 2 mM) mitigated the salt inhibition of seed germination and 2 mM H2O2 was the most effective concentrations, which was then used to investigate the role of H2O2 in acclimation to salt stress in subsequent experiments. However, higher concentrations (4 and 8 mM) reduced seed germination not only under salt stress but also in normal condition. For example, under salinity stress, seed germination in seeds pretreated with 2 mM H2O2 increased approximately 30%, but the pretreatment with 8 mM H2O2 not only did not show any positive effect but also damaged seeds.
Effects of pretreatment at various concentrations of H2O2 on alfalfa seed germination under salinity stress. Seeds were pretreated with various H2O2 concentrations (0, 1, 2, 4, 8 mM) for 6 h and then exposed to 150 mM salt solution or distilled water for 5 days. Values are means ± SE of five replications. Different letters above the bars indicate significant differences (P < 0.05) according to Duncan’s multiple range test
Effects of osmopriming and exogenous H2O2 pretreatment on seed germination
In the second experiment, seeds were pretreated with 2 mM H2O2 or osmoprimed with PEG and then exposed to 150 mM NaCl solution.
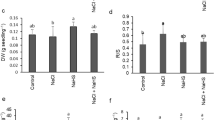
Figure 2 shows that 150 mM NaCl alone (without osmopriming or H2O2 pretreatment) significantly decreased seed germination in comparison with salt-free control (C). Both osmopriming and H2O2 pretreatment were able to rescue the salt-induced inhibition of seed germination partly (Fig. 2). The stimulatory effect of conferred by osmopriming or H2O2 pretreatment on seed germination could be fully repressed by N,N′-dimethylthiourea (DMTU), as scavenger of H2O2 or with DPI as an inhibitors of NADPHox, while the responses induced by osmopriming or H2O2 pretreatment only briefly were reversed by the addition of AT as the inhibitor of SOD (Fig. 2).
Effect of osmopriming and exogenous H2O2 pretreatment singly or in combination with DMTU, DPI, and AT on alfalfa seed germination under salinity stress. Seeds pretreated with 2 mM H2O2 or primed in polyethylene glycol solution (PEG 6000) after preincubation with 1 mM DMTU, DPI, and AT for 6 h were exposed to water (C) or 150 mM salinity for 5 days. Values are means ± SE of five replications. Different letters above the bars indicate significant differences (P < 0.05) according to Duncan’s multiple range test. P osmopriming, S salinity, C control
To examine whether the acclimation to salinity caused by H2O2 pretreatment or osmopriming is due to the increase of internal H2O2 in the plants, endogenous H2O2 levels were measured in the seeds of each treatment after 6 and 24 h of imbibition in the presence of water or a 150 mM NaCl solution.
Data showed that H2O2 contents significantly increased in germinating alfalfa seeds under salt stress as compared to the control (Fig. 3). Both osmopriming and H2O2 pretreatment significantly increased endogenous H2O2 levels after 6 h of imbibition. When DMTU, DPI, and AT were combined with the osmopriming or H2O2 pretreatment, endogenous H2O2 levels were dramatically declined, reaching to a level lesser than the treatment of salt stress alone. These results indicate that an increase in endogenous H2O2 could be part of the mechanism triggered by H2O2 pretreatment or osmopriming for salt acclimation.
Effect of osmopriming and H2O2 pretreatment singly or in combination with DMTU, DPI, and AT on endogenous levels of H2O2 in germinating seeds of alfalfa under salinity stress. Seeds pretreated with 2 mM H2O2 or primed in polyethylene glycol solution (PEG 6000) after preincubation with 1 mM. DMTU, DPI, and AT for 6 h were exposed to water (C) or 150 mM salinity, The endogenous H2O2 levels were measured in germinating seeds of alfalfa after 6- and 24-h exposure to 150 mM salinity. Values are means ± SE of five replications. Different letters above the bars indicate significant differences (P < 0.05) according to Duncan’s multiple range test. P osmopriming, S salinity, C control
Interestingly, both osmopriming and H2O2 pretreatment significantly reduced endogenous levels of H2O2 after 24 h of imbibition. In other words, these treatments induced a fast burst of endogenous H2O2 production at 6 h followed by a sharp decrease at 24 h of imbibition. Conversely, in salinity treatment alone, endogenous H2O2 levels increased after 24 h when they were compared to it at 6-h imbibition (Fig. 3).
The assessment of two H2O2 producing enzymes revealed that salinity stress after 6-h imbibition had no significant effect on SOD activity but increased NADPH oxidase activity significantly. Conversely, after 24-h imbibition, SOD activity increased and NADPH oxidase activity decreased sharply (Fig. 4). H2O2 pretreatment or osmopriming after 6-h imbibition markedly increased NADPH oxidase activity in germinating seeds under salt stress. The combination of DPI with the osmopriming and H2O2 pretreatment repressed this increment. In contrast, SOD activity after 6-h imbibition was increased only by H2O2 pretreatment significantly (Fig. 1) and osmopriming had no significant effect on SOD activity.
Effect of osmopriming and H2O2 pretreatment singly or in combination with DPI and AT on NADPH oxidase (NADPHox) and superoxide dismutase (SOD) activities in germinating seeds of alfalfa under salinity stress. Seeds pretreated with 2 mM H2O2 or primed in polyethylene glycol solution (PEG 6000) after preincubation with 1 mM. DPI and AT for 6 h were exposed to water (C) or 150 mM salinity and then enzyme activity levels were measured in germinating seeds of alfalfa after 6- and 24-h exposure to 150 mM salinity. Values are means ± SE of five replications. Different letters indicate significant differences (P < 0.05) according to Duncan’s multiple range test. P osmopriming, S salinity, C control
After 6-h imbibition, AT partly suppressed the effect of H2O2 pretreatment and also reduced SOD activity and production of endogenous H2O2 in germinating seeds of alfalfa subjected to osmopriming or H2O2 pretreatment. Thus, SOD activity might be a secondary potential source of H2O2 production during osmopriming.
Interestingly, both osmopriming and H2O2 pretreatment reduced activities of NADPH oxidase and SOD after 24 h of imbibition. In other words, these treatments induced a fast burst of NADPH oxidase activity at 6 h of imbibition followed by a sharp decrease after 24 h of imbibition.
Role of HO/CO system in responses conferred by osmopriming on seed germination
Figure 5 shows that the pretreatment of hematin improved seed germination under salt stress in a dose-dependent manner, and 2.5 μM hematin was the best concentrations that could rescue the inhibition of seed germination under salt stress, which was then used in subsequent tests. However, high concentration (≥5 μM) reduced seed germination under both normal and salt stress condition. For example, under salinity stress, seeds pretreated in 2.5 μM hematin germinated 25% further than non-pretreated seeds, but the pretreatment with 20 μM hematin not only did not show any protective effect but also damaged the seeds.
Effects of pretreatment at various concentrations of hematin on alfalfa seed germination under salinity stress. Seeds were pretreated at various hematin concentrations (0, 2.5, 5, 10, 20 μM) for 6 h and then exposed to water or 150 mM salinity for 5 days. Values are means ± SE of five replications. Different letters above the bars indicate significant differences (P < 0.05) according to Duncan’s multiple range test. P osmopriming, S salinity, C control
To evaluate whether HO-1 is involved in the salt acclimation induced by osmopriming, the effects of hematin pretreatment and osmopriming on HO activity and seed germination under salt stress were compared. The results showed that both osmopriming and hematin pretreatment significantly increased HO activity and seed germination. Interestingly, the elevation of HO activity and seed germination, conferred by osmopriming or hematin pretreatment, could be completely repressed by ZnPPIX, as an inhibitor of heme oxygenase (Fig. 6).
Effect of osmopriming and hematin pretreatment singly or in combination with ZnPPIX on seed germination (a) and heme oxygenase activity (b) in germinating seeds of alfalfa under salinity stress. Seeds pretreated with 2.5 mM hematin or primed in polyethylene glycol solution (PEG 6000) after preincubation with 100 μM ZnPPIX for 6 h were exposed to water or 150 mM salinity for 5 days. Enzyme assays were performed at germinating seeds of alfalfa after 6-h imbibition. Values are means ± SE of five replications. Different letters above the bars indicate significant differences (P < 0.05) according to Duncan’s multiple range test. P osmopriming, S salinity, C control
Interplay HO-1 and H2O2 in responses conferred by osmopriming on seed germination
To assess whether interplay HO-1 activity and H2O2 is involved in the acclimation to salt stress induced by osmopriming, the effects of H2O2 pretreatment and osmopriming on HO activity and HO-1 transcripts were investigated. As expected, both osmopriming and H2O2 pretreatments significantly increased HO activity and HO-1 transcripts in germinating alfalfa seeds under salt stress as compared to the control (Fig. 7). Application of DMTU and DPI significantly reversed effects of H2O2 pretreatment and osmopriming on HO activity and HO-1 transcripts. The results also demonstrated a positive correlation between HO enzyme activity and HO-1 transcript level (Fig. 7).
Effects of osmopriming and H2O2 pretreatment singly or in combination with DMTU and DPI on HO activity (a) and HO-1 transcripts (b, c) in seeds under salt treatment. Seeds were pretreated with 2 mM H2O2 or primed in polyethylene glycol solution (PEG 6000) after preincubation with 1 mM DMTU and DPI for 6 h were exposed to water or 150 mM salinity. HO activity and HO-1 transcripts were measured after 6-h imbibition. HO-1 transcripts were analyzed by semi-quantitative reverse transcription-polymerase chain reaction. The relative intensity of electrophoretic bands belong to HO-1 transcripts against ones of actin gene (as internal control) was calculated as relative abundance of transcripts. Values are means ± SE of five replications. Different letters above the bars indicate significant differences (P < 0.05) according to Duncan’s multiple range test. P osmopriming, S salinity, C control
The results shown in Fig. 8 revealed that the alleviation of the salt-induced inhibition of seed germination, conferred by osmopriming, could be reversed by DMTU, DPI, ZnPPIX, and Hb. Interestingly, the addition of CO aqueous solution mitigated the negative effects conferred by DMTU and DPI during osmopriming (Fig. 8). In contrast, the addition of H2O2 could not alleviate the inhibitory effects of ZnPPIX or Hb on osmopriming-induced seed germination completely.
Effects of osmopriming, N,N′-dimethylthiourea (DMTU), DPI and ZnPPIX, Hb and CO saturated aqueous solution, and H2O2 singly or in combinations on germination of alfalfa seeds under salinity stress. Seeds primed in polyethylene glycol solution (PEG 6000) after preincubation with 1 mM DCMU and DPI, 100 μM ZnppIX and 0.1 g L−1 Hb, 10% CO-saturated aqueous solution, H2O2 or a combination of treatments for 6 h were exposed 150 mM salinity for 5 days. The α-amylase activity was measured after 6-h imbibition. Values are means ± SE of five replications. The different letters above the bars indicate statistically significant differences (P < 0.05) according to Duncan’s multiple range test. P osmopriming, S salinity, C control
Role of H2O2 and HO/CO system in salt tolerance of seedlings grown from the primed seeds
An increase of H2O2 content following salinity stress was observed in the control plants. The H2O2 content was lower in primed seedlings when compared to controls (Table 1).
Data also showed that salinity upregulated antioxidant enzymes and proline biosynthesis in seedlings slightly. In addition, osmopriming, as well as pretreatment with hematin or H2O2, considerably enhanced antioxidant enzymes at both levels of activity and transcription and also increased P5CS (d-1-pyrroline-5-carboxylate synthetase) transcripts and proline accumulation in the roots of seedlings grown from the primed seeds. Adding DMTU, ZnPPIX, and Hb during osmopriming, reversed the previous responses in seedlings grown from the primed seeds (Table 1 and Fig. 9).
Effects of osmopriming, H2O2 or hematin or osmopriming singly or in combination with DMTU, ZnPPIX, and Hb on relative abundance of SOD, APX, CAT, and P5CS transcripts in roots of 5-day-old seedlings under salinity stress. Seeds primed in polyethylene glycol solution (PEG 6000) or pretreated with hematin or 2 mM H2O2 after preincubation with 1 mM DMTU and ZnPPIX or Hb for 6 h were exposed to water (control) or 150 mM salinity. The transcripts were analyzed by semi-quantitative reverse transcription-polymerase chain reaction in roots of 5-day-old seedlings grown from seeds. The relative intensity of electrophoretic bands belong to gene PCR products against ones of actin gene (as internal control) was calculated as relative abundance of transcripts were indicated above each band. Values are means of five replications. P osmopriming, S salinity, H hematin, Hb hemoglobin
Discussion
Results clearly confirmed that H2O2 pretreatment caused oxidative stress at high concentrations, while low levels of H2O2 conferred a protective effect (Fig. 1). These results support a dual role of H2O2 in seed physiology, as signal messenger at lower doses and as toxic and detrimental molecule at high doses that accumulates under biotic and abiotic stress conditions (Bailly et al. 2008).
The present study confirmed that osmopriming as well as lower doses of H2O2 increased seed germination percentage under salt stress (Fig. 2) and led to the acclimation to salt stress. The improvement of seed germination and salt acclimation by pretreatment with lower doses of H2O2 (Wahid et al. 2007; Gondim et al. 2010) and osmopriming of seeds (Farooq et al. 2006; Pradhan et al. 2014; Sivritepe et al. 2008) has been reported in many plants. Pandolfi et al. (2012) described that plant exposure to low-level stress activates multiple defense responses leading to an improvement of plant stress tolerance. Chen et al. (2009) also demonstrated that H2O2 is involved in the acclimation tolerance against oxidative stress in wheat seedling leaves.
As expected, salt stress reduced seed germination and increased H2O2 accumulation. Salt stress is deleterious for plant cells through inducing oxidative stress (Sivritepe et al. 2008). Interestingly, a burst of endogenous H2O2 in seeds subjected to both osmopriming and H2O2 pretreatment occurred after 6 h of imbibition (Fig. 3). In consistent with these results, Chen et al. (2009) illustrated that 0.5 mM H2O2 pretreatment triggered the biphasic production of H2O2 during a 24-h period. Naglreiter et al. (2005) also reported that the combined priming of PEG and GA induced the accumulation of free radicals in the seeds of Scotch pine (Pinus sylvestris) and larch (Larix deciduas). It seems that the pretreatment with lower doses of H2O2 elevated endogenous ROS levels and induced a moderate oxidative stress by the disruption of cellular ROS homeostasis. Similarity, seed priming can be considered as a brief stress since water is not provided in a sufficient amount to allow radicle protrusion through the seed coat (Chen and Arora 2012) and reduction of water availability can lead to producing excessive ROS. It is known that plants mediate their developmental patterns to reprogram their gene expression in response to ROS level in cells for acclimation to their environment. Moreover, data of this survey illustrated the application of DMTU as a scavenger of H2O2 and DPI as a NADPH oxidase inhibitor could repress the positive effects of osmopriming and H2O2 pretreatment on seed germination under salt stress (Fig. 2) that coincided with less content of H2O2 in these treatments (Fig. 3). These findings suggest that the success of osmopriming as well as H2O2 pretreatment largely depends on H2O2 signaling.
Interestingly, the induced burst of endogenous H2O2 production after 6 h of imbibition by both osmopriming and H2O2 pretreatment was followed by a sharp decrease after 24 h of imbibition (Fig. 3). Although ROS burst during priming known to be a certainty, only Naglreiter et al. (2005) have reported such increase, while the decrease of ROS content during post priming in mature plants has been reported more frequently (Khodabakhsh et al. 2014; Chiu et al. 2006; Sivritepe et al. 2008). The decrease of H2O2 content after 24 h of imbibition may be the result of rapid response of antioxidant system that scavenges ROS. This assumption further supported by these findings that the reduction of H2O2 content in seedlings grown from the primed seeds was coincident to more upregulation of antioxidant enzymes and proline synthesis in them (Table 1 and Fig. 9). The similar results have been reported about effect of priming on the elevation of antioxidant system and declining ROS content in many plants (Chiu et al. 2006; Chen and Arora 2011; Khodabakhsh et al. 2014; Li and Zhang 2012). Wahid et al. (2007) also reported that the pretreatment of wheat seed with H2O2 reduced H2O2 content in the seedlings under saline conditions in comparison with H2O2 content in seedlings grown from control seeds. Overall, these findings suggest that early ROS burst during osmopriming can be considered as mild stress exposure at the pregermination stage, which may activate cellular antioxidant system and enhance the detoxification of ROS in subsequent developmental stages and induce cross-tolerance during early seedling establishment.
Data showed that the alterations of H2O2 content in germinating seeds were coincident with the effect of osmopriming and H2O2 pretreatment on the activity of NADPHox and partly SOD. The activities of these enzymes increased at early 6 h of imbibition and reduced after 24 h of imbibition (Fig. 4). NADPH oxidase (NADPHox), a plasma membrane-bound enzyme, catalyzes the production of superoxide that is quickly converted to H2O2 (Sarath et al. 2007), and SOD is one other of enzymes responsible for the generation of H2O2 (Hossain et al. 2015). Results exhibited the osmopriming-induced accumulation of H2O2 and is further mediated through the activation of NADPH oxidase in alfalfa germinating seeds. Xie et al. (2011) also observed the exposure with mild salt stress caused biphasic increases in NADPHox-dependent ROS production that led to the improvement of tolerance to higher salt concentration in seedlings. Despite a briefly and non-significant enhancement of SOD activity in following osmopriming, the significant effect of AT as an inhibitor of SOD on the germination of primed seeds (Fig. 4) suggests that it might be the secondary source of H2O2 production in beside of NADPHox enzyme. The elevation of SOD activity, endogenous H2O2 content and the improvement of growth by H2O2 pretreatment and alleviation these acclamatory responses by DMTU, has been also reported for wheat seedlings grown under oxidative stress (Chen et al. 2009). According to the literature review, this is the first report in regard to role of the NADPHox-dependent production of H2O2 in salt acclamatory responses induced by osmopriming.
Data showed that lower doses of hematin enhanced seed germination under salt stress, while high doses of hematin had deleterious effects (Fig. 5). Although several lines of evidence confirmed the antioxidative effects of heme compounds against various stresses (Amooaghaie et al. 2015; Cui et al. 2011; Liu et al. 2010; Xu et al. 2011), it has been also reported that heme compounds act primarily as a prooxidant in vivo, which can promote the production of oxygen radicals and thus cause an oxidative damage of biologically active molecules (Schmitt et al. 1993).
Hematin pretreatment similar to osmopriming was able to alleviate the inhibition of salt-driven seed germination (Fig. 6a). The similar role of hematin in salt acclimation has been reported in wheat (Xu et al. 2006) and rice (Liu et al. 2007) seeds. As expected, results showed that both osmopriming and hematin pretreatment enhanced HO activity, and ZnPPIX, as an inhibitor of HO, could repress their stimulatory effects on seed germination (Fig. 6a) and HO activity under salt stress (Fig. 6b). These findings suggest that HO could act as an antioxidative barrier against salt induced oxidative damage in seeds, and endogenous HO activity might play a pivotal role in salt-protective effects induced by osmopriming or hematin pretreatment.
The salinity increased H2O2 accumulation (Fig. 3) and enhanced HO activity and HO-1 messenger RNA (mRNA) levels moderately (Fig. 7). Both osmopriming and H2O2 pretreatment induced the considerable elevation of HO activity and HO-1 mRNA levels (Fig. 7). Importantly, these changes were correlated with seed germination responses (Fig. 2). It has been also reported that H2O2, as a downstream regulator of HO, participated in regulating rice seed germination under salinity stress (Wei et al. 2012). These findings suggest that osmopriming and lower dose of H2O2 act as slight stressors that induce H2O2 generation, and H2O2 signaling triggers the elevation of HO activity and shortens the time to achieve the required HO activity levels for subsequent seed germination and seedling growth under severe salinity stress. This conclusion was further supported by observations that the increments of HO activity and HO-1 mRNA levels as well as seed germination induced by osmopriming and H2O2 pretreatment could be blocked by DPI and DMTU (Fig. 7).
These findings are consistent with the report of Xie et al. (2011) who suggested the participation of NADPHox-driven ROS is necessary for HO upregulation and HO plays an important role in salt acclimation. Chen et al. (2009) also reported that SOD-driven H2O2 induced the upregulation of HO activity and HO-1 expression in wheat leaves. However, to the best of our knowledge, the present paper is the first report that is suggesting NADPHox-driven H2O2 might be involved in the upregulation of HO by osmopriming. Certainly, the application of mutants and other molecular techniques underlying their gene expression must provide more evidence to confirm this hypothesis.
On the other hand, the application of 10% CO-saturated aqueous solution was able to mimic the effect of osmopriming on the alleviation of the negative effect of salt on seed germination and α-amylase activity (Fig. 8). The priming-mediated response on improving seed germination and α-amylase activity appeared to be CO dependent, as it was completely blocked by treatment of the scavenger of CO. In other words, hemoglobin (Hb as scavenger of CO) was able to mimic the effects of DMTU on the reversal of priming effects (Fig. 8). These results again suggested that HO and its product, i.e., CO were involved in cytoprotective effects induced by osmopriming. Wu et al. (2012) also found that CO mediates Amyc2 expression and activates amylase activity in seeds. Similar results about effect of HO and CO on stress tolerance were also reported previously (Amooaghaie et al. 2015; Liu et al. 2007; Han et al. 2008; Xie et al. 2008). The enhancement of amylase activity by H2O2 (Ishibashi et al. 2012), hematin (Amooaghaie et al. 2015), hemin (Wu et al. 2012), CO (Liu et al. 2010), and priming (Amooaghaie and Nikzad 2013; Farooq et al. 2006) has been reported by other authors. However, our paper is the first report on the role of heme oxygenase activity and CO signaling in responses induced by osmopriming.
Interestingly, the addition of CO-saturated aqueous solution after osmopriming alleviated the inhibitory effect of DMTU or DPI on salt acclimatory responses. Conversely, H2O2 could not reverse the inhibitory effect of ZnPPIX and Hb on seed germination and α-amylase activity. This pharmacological evidence again proposed that the maintenance of homeostatic H2O2 and CO is necessary for the cytoprotective effects of osmopriming and probably CO acts in the downstream of H2O2 in the signal transduction network promoted by osmopriming. These results suggest that osmopriming induced H2O2 production and H2O2 signaling triggered HO upregulation. CO, as the product of HO activity, promoted the enhancement of amylase activity and consequently resulted in a higher rate of seed germination under salt stress.
It is known that enhancing the activity of d-1-pyrroline-5-carboxylate synthetase enzyme, (P5CS catalyze the rate-limiting step of proline biosynthesis) and also antioxidant enzymes contribute to stress tolerance in plants. Therefore, to explore the role of signal molecules (H2O2 and CO) in priming-induced salt tolerance, we examined the changes of antioxidant enzymes and proline content. Our results revealed that osmopriming as well as pretreatment with hematin or H2O2 upregulated genes of SOD, APX, CAT, and P5CS (Fig. 9) that led to the increase of proline content and elevating the activities of antioxidant enzymes (Table 1) in seedlings grown from the primed and hematin- or H2O2- pretreated seeds. Importantly, these effects were reversed when DMTU, ZnPPIX, and Hb were added after osmopriming (Table 1 and Fig. 9). These results are indicating that H2O2 and HO/CO burst during osmopriming are essential for establishing subsequent stress tolerance in seedlings grown from the primed seeds.
In conclusion, our data suggest that osmopriming as a mild stress induced H2O2 production and HO activity; signal molecules (H2O2 and CO) might shared a pathway that led to creating stress memory in cells and primed cells using this stress imprint could upregulate defense responses more effectively than unprimed cells under subsequent exposure with salt stress.
References
Amooaghaie R (2011) The effect of hydro- and osmoprimng on alfalfa seed germination and antioxidant defense under high salt concentration stress. Afr J Biotech 33:6269–6275
Amooaghaie R, Nikzad K (2013) The role of nitric oxide in priming induced low temperature tolerance in two genotypes of tomato. Seed Sci Res 23:123–131. doi:10.1017/S0960258513000068
Amooaghaie R, Roohollahi Sh (2016) Effect of sodium nitroprusside on responses of Melissa officinalis to bicarbonate exposure and direct Fe deficiency stress. Photosynthetica 54: doi:10.1007/s11099-016-0240-8
Amooaghaie R, Nikzad K, Shareghi B (2010) The effect of priming on emergence and biochemical changes of tomato seeds under suboptimal temperatures. Seed Sci Technol 38:508–512
Amooaghaie R, Tabatabaei F, Ahadi AM (2015) Role of hematin and sodium nitroprusside in regulating Brassica nigra seed germination under nanosilver and silver nitrate stresses. Eco Environ Safe 113:259–270
Anese S, da Silva EAA, Davide AC, Faria JMR, Soares GCM, Matos ACB, Toorop PE (2011) Seed priming improves endosperm weakening, germination, and subsequent seedling development of Solanum lycocarpum St. Hill. Seed Sci Technol 39:125–139
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331:806–814
Balestrazzi A, Confalonieri M, Macovei A, Carbonera D (2011) Seed imbibition in Medicago truncatula Gaertn.: expression profiles of DNA repair genes in relation to PEG-mediated stress. J Plant Physiol 168:706–713
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant and Soil 39 (1):205–207
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful memories of plants: evidence and possible mechanisms. Plant Sci 173:603–608
Chen K, Arora R (2011) Dynamic of the antioxidant system during seed osmopriming, post-priming germination and seedling establishment in spinach (Spinacia oleracea). Plant Sci 180:212–220
Chen K, Arora R (2012) Priming-memory invokes seed stress-tolerance. Environ Exp Bot. doi:10.1016/j.envexpbot.2012.03.005
Chen XY, Ding X, Xu S, Wang R, Xuan W, Cao ZY, Chen J, Wu HH, Ye MB, Shen WB (2009) Endogenous hydrogen peroxide plays a positive role in the upregulation of heme oxygenase and acclimation to oxidative stress in wheat seedling leaves. J Integ Plant Biol 51(10):951–960
Chiu KY, Chuang SJ, Sung JM (2006) Both antioxidant and lipid-carbohydrate conversion enhancements are involved in priming-improved emergence of Echinacea purpurea seeds that differ in size. Sci Hort 108:220–226
Cui W, Fu G, Wu H, She W (2011) Cadmium-induced heme oxygenase-1 gene expression is associated with the depletion of glutathione in the roots of Medicago sativa. Biometals 24:93–103
Farooq M, Basra SMA, Hafeez K (2006) Seed invigoration by osmohardening in coarse and fine rice. Seed Sci Technol 34:181–187
Gondim F, Gomes-filho E, Lacerda CF, Prisco JT, Azevedo Neto AD, Marques EC (2010) Pretreatment with H2O2 in maize seeds: effects on germination and seedling acclimation to salt stress. Braz J Plant Physiol 22(2):103–112
Han Y, Zhang J, Chen X, Gao Z, Xuan W, Xu S, Ding X, Shen W (2008) Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol 177:155–166
Hardegree SP, Emmerich WE (1994) Seed germination response to polyethylene glycol solution depth. Seed Sci Technol 22:1–7
Hossain MA, Bhattacharjee S, Armin S-M, Qian P, Xin W, Li H-Y, Burritt DJ, Fujita M, Tran L-SP (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420. doi:10.3389/fpls.2015.00420
Ibrahim EA (2015) Seed priming to alleviate salinity stress in germinating seeds. J Plant Physiol. doi:10.1016/j.jplph.2015.12.011
Ishibashi Y, Tawaratsumida T, Kondo K, Kasa S, Sakamoto M, Aoki N, Zheng SH, Yuasa T, Iwaya-Inoue M (2012) Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol 158:1705–1714
Jisha KC, Vijayakumari K, Puthur JT (2013) Seed priming for abiotic stress tolerance: an overview. Acta Physiol Plant 35:1381–1396
Khodabakhsh F, Amooaghaie R, Mostajeran A (2014) Effect of hydro- and osmopriming on membrane deterioration, proline accumulation and H2O2 scavenging enzymes in two salt stressed chickpea cultivars. Environ Eng Manag J 13(3):619–626
Kubala S, Garnczarska M, Wojtyla L, Rucinska-Sobkowiak R, Kubala S, Garnczarska M (2015) Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant Sci 231:94–113
Li X, Zhang L (2012) SA and PEG-induced priming for water stress tolerance in rice seedling. Inf Tech Agr 134:881–887
Liu KL, Xu S, Xuan W, Ling TF, Cao ZY, Huang BK, Sun YG, Fang L, Liu ZY, Zhao N, Shen WB (2007) Carbon monoxide counteracts the inhibition of seed germination and alleviates oxidative damage caused by salt stress in Oryza sativa. Plant Sci 172:544–555
Liu YH, Xu S, Ling TF, Xu LL, Shen WB (2010) Heme oxygenase/carbon monoxide system participates in regulating wheat seed germination under osmotic stress involving the nitric oxide pathway. J Plant Physiol 167:1371–1379
Muramoto T, Tsurui N, Terry MJ, Yokota A, Kohchi T (2002) Expression and biochemical properties of a ferredoxin-dependent heme oxygenase required for phytochrome chromophore synthesis. Plant Physiol 130:1958–1966
Naglreiter C, Reichenauer TG, Goodman BA, Bolhàr-Nordenkampf HR (2005) Free radical generation in Pinus sylvestris and Larix deciduasseeds primed with polyethylene glycol or potassium salt solutions. Plant Physiol Biochem 43:117–123
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5:388–395
Oracz K, El Maarouf-Bouteau H, Farrant JM, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C (2007) ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J 50:452–465
Pandolfi C, Mancuso S, Shabala S (2012) Physiology of acclimation to salinity stress in pea (Pisum sativa). Environ Exp Bot 84:44–51
Paparella S, Araújo SS, Rossi G, Wijayasinghe M, Carbonera D, Balestrazzi A (2015) Seed priming: state of the art and new perspectives. Plant Cell Rep 34(8):1281–1293
Pastor V, Luna E, Mauch-Mani B, Ton J, Flors V (2013) Primed plants do not forget. Environ Exp Bot 94:46–56. doi:10.1016/j.envexpb
Pradhan N, Prakash P, Tiwari SK, Manimurugan C, Sharma RP, Singh PM (2014) Osmopriming of tomato genotypes with polyethylene glycol 6000 induces tolerance to salinity stress. Trends Biosci 7:4412–4417
Sarath G, Hou G, Baird LM, Mitchell RB (2007) Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm season C4-grasses. Planta 226:697–708
Schmitt TH, Frezzatti WA, Schreier S (1993) Hemin-induced lipid membrane disorder and increased permeability: a molecular model for the mechanism of cell lysis. Arch Biochem Biophys 307:96–103
Sivritepe N, Sivritepe HO, Turkan I, Bor M, Ozdemir F (2008) NaCl pre-treatments mediate salt adaptation in melon plants through antioxidative system. Seed Sci Technol 36:360–370
Tanou G, Fotopoulos V, Molassiotis A (2012) Priming against environmental challenges and proteomics in plants: update and agricultural perspectives. Frontiers in Plant Sci 3:216. doi:10.3389/fpls.2012.00216
Van Gestelen P, Asard H, Caubergs RJ (1997) Solubilization and separation of a plant plasma membrane NADPH-O2 ، synthase from other NAD(P)H oxidoreductases. Plant Physiol 115:543–550
Varier A, Vari AK, Dadlani M (2010) The subcellular basis of seed priming. Current Sci 99:450–456
Wahid A, Perveen M, Gelani S, Basra SM (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:283–294
Wei MY, Chao YY, Kao CH (2012) NaCl-induced heme oxygenase in roots of rice seedlings is mediated through hydrogen peroxide. Plant Growth Regul DOI. doi:10.1007/s10725-012-9762-7
Wu M, Wang F, Zhang C, Xie Y, Han B, Huang J, Shen W (2012) Heme oxygenase-1 is involved in nitric oxide- and cGMP-induced Amy2/54 gene expression in GA-treated wheat aleurone layers. Plant Mol Biol. doi:10.1007/s11103-012-9979-x
Xie YJ, Ling TF, Han Y, Liu KL, Zheng QS, Huang LQ, Yuan XX, He ZY, Hu B, Fang L, Shen ZG, Yang Q, Shen WB (2008) Carbon monoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defense in wheat seedling roots. Plant Cell Environ 31:1864–1881
Xie YJ, Xu S, Han B, Wu MZ, Yuan XX, Han Y, Gu Q, Xu DK, Yang Q, Shen WB (2011) Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. The Plant J. doi:10.1111/j.1365-313X.2011.04488.x
Xu S, Sa ZS, Cao ZY, Xuan W, Huang BK, Ling TF, Hu QY, Shen WB (2006) Carbon monoxide alleviates wheat seed germination inhibition and counteracts lipid peroxidation mediated by salinity. J Integr Plant Biol 48:1168–1176
Xu S, Lou T, Zhao N, Gao Y, Dong L, Jiang D, Shen W, Huang L, Wang R (2011) Presoaking with hemin improves salinity tolerance during wheat seed germination. Acta Physiol Plant 33:1173–1183
Yacoubi R, Job C, Belghazi M, Chaibi W, Job D (2013) Proteomic analysis of the enhancement of seed vigor in osmoprimed alfalfa seeds germinated under salinity stress. Seed Sci Res 23:99–110
Acknowledgements
This study was supported by a financial grant of Shahrekord University, Iran. Author thank Dr. Hashemi for his help in the semi-quantitative RT-PCR performance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bhumi Nath Tripathi
Rights and permissions
About this article
Cite this article
Amooaghaie, R., Tabatabaie, F. Osmopriming-induced salt tolerance during seed germination of alfalfa most likely mediates through H2O2 signaling and upregulation of heme oxygenase. Protoplasma 254, 1791–1803 (2017). https://doi.org/10.1007/s00709-016-1069-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-1069-5