Abstract
Hydrogen gas (H2) was recently proposed as a novel gaseous signaling molecule. In our previous study, H2-mediated enhancement of plant tolerance to drought stress was preliminarily suggested. However, the detailed mechanisms of the action of H2 have not been fully explored. In this study, we observed that H2 production and hydrogenase activity were significantly induced by abscisic acid (ABA) and drought stress. Alfalfa seedlings pretreated with hydrogen-rich water (HRW) were hypersensitive to exogenous ABA. In response to ABA or water deficit, HRW-pretreated seedlings rapidly accumulated higher amounts of hydrogen peroxide (H2O2), and exhibited more tolerance to drought stress. By contrast, the inhibition or scavenging of H2O2 reduced HRW-induced drought tolerance. Further results showed that the apoplastic pH of leaves was significantly increased by HRW and/or drought stress. Cotreatment with the H+-ATPase inhibitor, however, could prevent the effects of H2 on the alkalinization of the apoplastic sap and stomatal sensitivity to exogenous ABA or water deficit. These responses were interpreted as an effect of H2 on sap pH and closure of stomata in alfalfa via an ABA-based mechanism. Overall, these results suggested a novel regulating mechanism of H2 in plant drought response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among different mechanisms of plants to avoid water deficit, reducing stomatal conductance is part of the systemic responses triggered by signals that originate in the root system (Jia and Davies 2007). Abscisic acid (ABA), pH, cytokinins, the precursor of ethylene, malate, and other unidentified factors have been implicated in root to shoot signaling under drought (Schachtman and Goodger 2008). Among them, ABA is considered the most important regulator of stomatal behavior and gas exchange in plants, particularly when water is in short supply (Wilkinson and Davies 2002). It was well accepted that roots in drying soil synthesize and/or transport ABA to the shoot via xylem (Davies and Zhang 1991). In the shoot, increasing and redistribution of ABA initiate an intricate network of signaling pathways in guard cells leading to stomatal closure (Desikan and others 2004). Hydrogen peroxide (H2O2) and nitric oxide (NO) have been identified as essential signals required for mediating ABA-induced stomatal closure in various species (Desikan and others 2004; Bright and others 2006).
pH is another important component of root to shoot signaling and may act synergistically with ABA. In many plant species, xylem and apoplastic sap pH becomes more alkaline when plants are water stressed (Jia and Davies 2007). Because ABA is a weak acid, it may become protonated or deprotonated in the pH range that occurred in the apoplasts of leaves. According to the anion-trap concept and the Henderson–Hasselbalch equation, drought-induced higher apoplastic sap pH could result in less ABA transported into the mesophyll cells and a build-up of ABA in the apoplast leading to enhanced stomatal closure (Slovik and others 1995; Hartung and others 2002). However, the mechanism involved in the regulation of plant sap pH is still unclear.
Hydrogen gas (H2) has recently been proposed as a new gasotransmitter. In animals, accumulating evidence suggested that H2 is a vital physiological regulatory molecule with antioxidant, anti-inflammatory, and antiapoptotic protective effects (Hong and others 2010). Particularly, there are some studies demonstrating that H2 does not act completely as an antioxidant and could also interact directly with specific signaling pathways (Ohsawa and others 2007; Hong and others 2010).
In plants, metabolism of H2 by algae has been reported by many investigators for the purposes of developing H2-based fuel cells (Melis and Happe 2001; Melis and Melnicki 2006). However, the physiological roles and detailed mechanisms of H2 production in higher plants remain elusive. More recently, there has been a renewed interest in the effects that H2 has on plant physiology, especially in stressed conditions (Xie and others 2012; Cui and others 2013, 2014; Jin and others 2013; Zeng and others 2013; Xie and others 2014). Some studies suggested that H2 might function as an important gaseous molecule in plant responses to environmental stimuli (Xie and others 2012; Jin and others 2013; Zeng and others 2013). H2 production was found to be induced by several plant hormones and abiotic stresses (Xie and others 2012; Cui and others 2013; Jin and others 2013; Zeng and others 2013; Xie and others 2014). When supplied with exogenous H2 in the form of hydrogen-rich water (HRW, which is safe, economical, and easily available), plants displayed enhanced tolerance to abiotic stresses (Xie and others 2012; Cui and others 2013; Jin and others 2013; Zeng and others 2013; Cui and others 2014). All these studies showed that the protective effect of H2 is partially mediated by enhancing the activities of antioxidant enzymes through various regulatory mechanisms (Xie and others 2012; Cui and others 2013; Jin and others 2013; Zeng and others 2013; Cui and others 2014). Moreover, H2 pretreatment could maintain ion homeostasis by regulating the antiporters and H+ pump responsible for Na+ exclusion and compartmentation under salt stress (Xie and others 2012). A recent work of Zeng and others (2013) also demonstrated the involvement of H2 produced by hydrogenase, in the plant hormone network and in the stress response. In our previous study of the physiology effect of H2, alfalfa seedlings pretreated with HRW could significantly enhance plant tolerance to drought stress (Jin and others 2013). Subsequent work also showed that hydrogen could promote stomatal closure in Arabidopsis by reactive oxygen species (ROS)-dependent nitric oxide (NO) production (Xie and others 2014). However, the interaction between H2 and ABA was not fully elucidated.
To investigate the relationship between H2 and ABA and test the existence of the unidentified mechanism of H2, we further explored the related mechanisms involved in the protected effects of H2 in the alfalfa drought response. Our evidence revealed a novel mechanism of H2 in alfalfa acting as a positive regulator of the ABA-dependent drought response: the modulation of apoplastic pH.
Materials and Methods
Preparation of Hydrogen-Rich Water (HRW)
Purified H2 (99.99 %, v/v) generated from a H2 generator (SHC-300, Saikesaisi Hydrogen Energy Co., Ltd, Shandong, China) was bubbled into 1000 ml nutrient medium (quarter-strength Hoagland’s solution) at a rate of 150 ml min−1 for 30 min (Cui and others 2013, 2014; Xie and others 2014). Then, according to our previous results (Jin and others 2013), the corresponding HRW was immediately diluted to the required concentration (50 % saturation). The pH of the HRW solution was adjusted to 6.1 before treatments.
Plant Materials and Growth Conditions
Alfalfa (Medicago sativa L. cv. Victoria) seeds were germinated for 1 day at 25 °C in darkness. Uniform seedlings were transferred to the plastic chambers and cultured in nutrient medium. Alfalfa seedlings were grown in the illuminating incubator at 25 °C, with a light intensity of 200 μmol m−2 s−1 and 14 h photoperiod. After growing for 7 days, seedlings were incubated in different pretreatment and treatment solutions as described in the corresponding figure legends. The pH for both nutrient medium and treatment solutions was adjusted to 6.1. For drought tolerance estimation, alfalfa seedlings from germinating seeds were irrigated with or without 50 % HRW for 7 days, and then for drought treatment.
Measurement of H2 Production
For analyzing H2 content, headspace sampling of gas followed by gas chromatography (GC), which was previously used to determine H2 production in plants, was adopted (Renwick and others 1964; Zeng and others 2013).
Measurement of Hydrogenase Activity
Hydrogenase activity of the alfalfa seedlings was measured according to the method of Zeng and others (2013). The extinction coefficient was 8.25 mM−1 × cm−1. One unit of enzyme is defined as the quantity of enzyme required to catalyze 1 μmol/l H2 releasing per minute (Frey 2002).
Stomatal Aperture Measurement
The adaxial surface of each leaf was peeled off. Epidermal strips were mounted on glass slides and observed with a light microscope (model Stemi 2000-C; Carl Zeiss, Germany). Images were captured and imported into Image Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). The ratio of width to length of the stomata was measured (Ryu and others 2010).
Detection of ROS Signals Using Fluorescent Probe
The epidermis was peeled carefully from the abaxial surface and incubated with 10 μM 2′,7′-dichlorofluorescin (H2DCFDA, Calbiochem, La Jolla, USA) probe for a further 15 min before images were visualized (Bright and others 2006). Fluorescence was imaged with a Zeiss LSM 710 confocal microscope (Carl Zeiss AG, Oberkochen, Germany).
Apoplastic pH Measurement
Apoplastic fluids were retrieved by the common infiltration–centrifugation technique (Cho and others 2012; Villiers and Kwak 2013). pH was determined using 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium (HPTS) fluorescent dye, and fluorescence was collected at 510 nm with a plate reader (excitation = 460 nm). The pH of the solution was determined against a standard curve made with the Britton–Robinson universal buffer (Cho and others 2012; Villiers and Kwak 2013).
Relative Water Content (RWC)
RWC was determined for alfalfa seedling leaves. For each experiment, RWC measurements were determined according to the method of García-Mata and Lamattina (2010).
3,3′-Diaminobenzidine (DAB) Staining
DAB staining was used to detect the accumulation of H2O2 (Thordal-Christensen and others 1997). Leaves were stained with 0.1 % DAB solution for 6 h, and then chlorophyll was removed with 95 % ethanol. After washing extensively, all the decolorized leaves were observed under a light microscope (model Stemi 2000-C; Carl Zeiss, Germany) and photographed.
Statistical Analysis
Data are the mean ± SE of three independent experiments with at least three replicates for each. For statistical analysis, either the t test (P < 0.05) or Tukey’s multiple test (P < 0.05) was selected where appropriate.
Result
Hydrogen Enhances Plant Sensitivity to Exogenous ABA
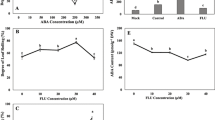
The production of H2 in alfalfa seedlings in response to drought and ABA treatment was analyzed. The results in Fig. 1a confirmed that H2 production in seedlings upon drought stress increased to 151.2 % of the control samples and 172.6 % in response to 50 μM ABA, after exposure for 6 h. The hydrogenase activity showed similar tendencies.
H2 production, hydrogenase activity, stomatal aperture, and ROS production of alfalfa seedlings with or without HRW pretreatment in response to ABA or drought stress. a 7-day-old seedlings were treated with or without 50 μM ABA or drought stress (by placing seedlings on Petri dishes at 25 °C and 60 % relative humidity) for 6 h, and then hydrogenase activity in alfalfa seedlings was analyzed. Afterwards, seedlings were transferred into a vial containing 2 ml H2O for 24 h. H2 concentration in head space of the vial was then measured. b–e 7-day-old seedlings were pretreated with or without 50 % HRW for 12 h. Then derooted seedlings were floated on MES-KCl (pH 6.1) solution under light for 2 h to induce stomatal opening. Subsequently, different concentrations of ABA (0, 1, 5, 10, and 20 μM) alone or in combination with 60 U/ml CAT were added. After treatments for 2 h, the epidermis of seedlings was peeled carefully from the abaxial surface for stomata observing and stomatal aperture measurement by light microscopy. After pretreatment, the epidermis of seedlings treated with 5 μM ABA alone or in combination with CAT was also incubated with H2DCFDA probe and observed with a laser confocal scanning microscope. Seedlings without treatment were used as the control (Con). Bar, 10 μm. Values are the mean ± SE of three independent experiments. Bars with different letters (a, e) or asterisks (d) are significantly different at P < 0.05 according to Tukey’s multiple test, or t test, respectively
We further provided plants with H2 in the form of HRW which could not only enhance plant H2 production, but also mimic the physiology effects of H2 according to previous studies (Xie and others 2012; Cui and others 2013, 2014; Jin and others 2013; Zeng and others 2013; Xie and others 2014). The results indicated that HRW-pretreated alfalfa seeds were hypersensitive to ABA with different concentrations at the germination stage when using the germination rate as an index (Supplemental Fig. 1a, b). Such hypersensitivity was confirmed at the postgermination stage, showing a slower growth rate of roots in HRW-pretreated plantlets than those in ABA alone (Supplemental Fig. 1c, d). To examine the role of H2 in relation to ABA response, we next examined phenotypes of ABA-dependent stomatal movement. As shown in Fig. 1b, in response to different concentrations of ABA (1, 5, 10, and 20 μM), stomatal closure in HRW-pretreated seedling leaves was markedly enhanced as compared with that in unpretreated controls (Fig. 1b, d). At 5 μM ABA treatment, for example, the average stomatal aperture in HRW-pretreated seedling leaves was approximately 21.6 % lower in comparison with leaves treated with ABA only. ROS production in guard cells was also monitored by a permeable fluorophore, H2DCFDA. Figure 1c, e shows that 5 μM ABA-induced ROS production and stomatal closure in seedling leaves were clearly enhanced by HRW pretreatment. The addition of catalase (CAT) confirmed that ABA-induced ROS was, at least partially, attributed to H2O2.
H2-Induced Modification of Leaf Apoplastic pH in Relation to Stomatal Sensitivity
Changes of apoplastic pH have long been considered as a means of regulating physiological processes including stomatal behavior. Previous studies suggested that ABA signaling can be modulated by pH in the apoplastic space (Jia and Davies 2007). To explore the mechanistic basis of the modification in ABA sensitivity of stomata, we further tested if H2 could influence leaf apoplastic pH and thereby potentially influence stomatal aperture. Apoplastic fluids were retrieved by centrifugation from leaves of different treatments and then subjected to pH measurement using the fluorescent dye HPTS, whose fluorescence is pH-dependent (Supplemental Fig. 2). Figure 2a shows that the apoplastic pH in control leaves was 5.13, whereas the apoplastic pH of HRW-pretreated seedling leaves was significantly higher (5.50; P < 0.05). The effect of the H+-ATPase inhibitor vanadate on apoplastic pH was then analyzed. As expected, vanadate prevents alkalinization of the apoplastic sap. The effect of HRW on enhancing the sensitivity of the stomatal response to ABA was also reversed by vanadate (Fig. 2b). It could be inferred that the effect of HRW on stomata sensitivity to ABA was partially due to its effects on the modification of leaf apoplastic pH, which was presumably by proton-ATPases associated with the xylem parenchyma and the distribution of ABA in the leaf.
Effects of HRW and vanadate on leaf apoplastic sap pH and stomatal aperture. a 7-day-old alfalfa seedlings were treated with 50 % HRW or 100 μM vanadate alone or in combinations for 12 h. The apoplastic pH was then measured. b Derooted seedlings were floated on MES-KCl (pH 6.1) solution under light for 2 h to induce stomatal opening. Subsequently, 5 μM ABA was added to the solution for another 2 h. Stomata on the abaxial surface were observed by light microscopy and stomatal aperture was measured. Samples without treatment were used as the control (Con). Values are the mean ± SE of three independent experiments. Bars with different letters are significantly different at P < 0.05 according to Tukey’s multiple test
Hydrogen Enhances Plant Tolerance to Drought Stress
The effects of external H2 on plant responses to drought stress were further estimated. Figure 3a shows that untreated plants were seriously wilted and impaired after dehydration stress, whereas seedlings treated with HRW appeared relatively healthy after drought. To further evaluate the responses to drought stress, water loss rates and stomatal response of the plants were estimated. The results indicated that leaves of unpretreated seedlings lost water continuously, and their RWC was approximately 62.5 % of their starting values, after 5-h incubation of drought (Fig. 3b). On the other hand, leaves of HRW-pretreated seedlings lost water more slowly than those of drought alone plants. Moreover, changes of stomatal aperture showed the similar trends (Fig. 3c).
Hydrogen enhances plant tolerance to drought stress. a Alfalfa seedlings from germinating seeds were irrigated with or without 50 % HRW for 7 days, and further grown for 15 days without watering. Afterwards, representative photographs were taken. b–d After pretreating with or without 50 % HRW under light for 12 h, 7-day-old alfalfa seedlings were treated with drought stress (by placing seedlings on Petri dishes at 25 °C and 60 % relative humidity) for 5 h. The RWC was determined after normal growth conditions or drought (b). Meanwhile, stomata on the abaxial surface were observed by light microscopy and stomatal aperture was measured (c). Also, apoplastic pH was measured at 0 and 5 h after the beginning of drought stress (d). Samples without chemical and drought stress treatment were used as the control (Con). Values are the mean ± SE of three independent experiments. Bars with asterisks (b, c) or different letters (d) are significantly different between the treatment Con → Drought and HRW → Drought at P < 0.05 according to t test, or Tukey’s multiple test, respectively
Meanwhile, we observed a significant change in apoplastic pH of the leaves in response to drought stress (Fig. 3d). HRW pretreatment alone could increase apoplastic pH. After drying for 5 h, it also resulted in alkalinization of the apoplast from 5.13 to 5.61, whereas feeding HRW to plants further increased the apoplastic pH to 5.85.
Hydrogen peroxide (H2O2) has been identified as an essential signal required for mediating ABA-induced stomatal closure in various species (Desikan and others 2004; Bright and others 2006; An and others 2008). To confirm that H2 enhanced plant drought stress tolerance by positively regulating the ABA pathway, we determined whether H2O2 is involved in H2-induced tolerance. Figure 4a shows that a higher level of H2O2 production in alfalfa seedling leaves was clearly detected in HRW-pretreated seedlings after 1 h of drought stress. HRW-induced H2O2 accumulation was sensitive to diphenyleneiodonium (DPI), a potent inhibitor of NADPH oxidase, and dimethylthiourea (DMTU), an H2O2 scavenger. Comparatively, in the absence of HRW, the addition of DPI or DMTU alone had no such obvious effect on staining patterns, compared to the stressed condition or controls (in particular). We therefore analyzed the effects of DPI and DMTU on HRW-induced tolerances to drought stress (Fig. 4b). As expected, the decrease in RWC caused by stress was slowed by HRW pretreatment, mimicking the physiological roles of H2O2 or ABA. Subsequent results demonstrated that DPI and DMTU could completely abolish the inducing effect of HRW on RWC (Fig. 4b). By contrast, a slight but not significant decrease of RWC was observed in response to DPI or DMTU alone. It was further revealed that changes in stomatal aperture could negatively match the previously mentioned changes of RWC upon different treatments (Fig. 4c).
Requirement of H2O2 for HRW-induced resistance to drought stress. a To in situ detect H2O2 in leaves, 7-day-old alfalfa seedlings were pretreated with or without 50 % HRW, 100 μM DPI, 5 mM DMTU, 10 μM H2O2, or 5 μM ABA alone or the combinations for 12 h under light, and then seedlings were treated with drought stress (by placing seedlings on Petri dishes at 25 °C and 60 % relative humidity) for 1 h. Afterwards, the seedling leaves were stained with DAB. Bar, 2 mm. b After drought for 5 h, the RWC was determined. c Stomata on the abaxial surface were observed by light microscopy and stomatal aperture was measured after drought for 3 h. Seedlings without treatment were used as the control (Con). Values are the mean ± SE of three independent experiments. Bars with different letters are significantly different at P < 0.05 according to Tukey’s multiple test
Discussion
It is accepted that chemical and gaseous signals are important for plant adaptation to water stress (Xie and others 2012; Cui and others 2013; Jin and others 2013; Zeng and others 2013). However, the identity of these signal pathways is less clear and new discoveries of signal components are still being made. H2 is such a new bioactive gas that we recently found could regulate stomatal movement via an ABA signaling cascade in which RbohF-dependent ROS and nitric reductase-associated NO production, and subsequent guard cell outward-rectifying K+ channel (GORK) activation, were causally involved (Xie and others 2014). Because the interaction between H2 and ABA was not fully elucidated, herein, we investigated the role of H2 in alfalfa drought response, such as whether the mechanisms found in Arabidopsis also existed in alfalfa plants, or most importantly, whether other mechanisms might be responsible for H2 responses. Interestingly, our results revealed a novel role for H2 in alfalfa drought response acting as a positive regulator of the ABA-dependent drought response: the involvement of the modification of apoplastic pH.
In this study, we confirmed that H2 might participate in an ABA-dependent drought stress tolerance in alfalfa based on the following reasons. First, H2 catalyzed by a hydrogenase-like enzyme (Zeng and others 2013) is obviously induced by drought stress and ABA treatment just for 6 h (Fig. 1a). Second, alfalfa plants pretreated with H2 in the form of HRW exhibited sensitivities to ABA in both germination and postgermination growth (Supplemental Fig. 1). Third, most importantly, H2 positively regulated ABA-promoted H2O2 production and stomatal closure, which may reduce transpirational water loss in response to dehydration stress (Fig. 1b–e). These results suggested that HRW-induced drought tolerance was, at least partially, via an H2O2-dependent pathway, further confirming the speculation that H2 positively regulated the ABA-dependent drought response (Xie and others 2014). In fact, the increased H2O2 levels, which might be derived from NADPH oxidase (Fig. 4a), in HRW-treated alfalfa seedlings were different from the selectively scavenging activity for hydroxyl radicals of H2 functioning in animals (Ohsawa and others 2007), indicating the complexities of molecular mechanisms of H2 functioning in animals and plants. Phenotypic analysis indeed demonstrated that HRW-pretreated seedlings were highly resistant to severe water stress, as they appeared relatively healthy after drought conditions (Fig. 3). Meanwhile, the changes of RWC and stomatal closure were consistent with the abovementioned phenotypes. Overall, these results led us to propose that H2 might be a positive regulator of an ABA-dependent response to drought stress in alfalfa.
There has been much recent interest in a role for plant apoplastic pH in modulating both guard cell functioning and cell expansion, as well as its impact on the effect exerted by ABA on both of these processes (Wilkinson and Davies 2002; Jia and Davies 2007). This study now showed for the first time, to our knowledge, that H2 could impact leaf apoplastic pH, determined directly with fluorescent pH-sensitive dyes. It was clear that H2 pretreatment could alkalinize the leaf apoplast (Fig. 2a). Importantly and consistently with the anion-trap mechanism outlined previously (Slovik and others 1995; Hartung and others 2002), H2 pretreatment to intact plants could influence the ABA response in stomata and the effects of mild soil drying on leaf water content (Figs. 1b–e, 3a–c). Accordingly, we further deduced that increased apoplastic pH might allow a given dose of ABA increased access to binding sites for ABA action on the guard cells.
The chemical agent responsible for the natural increase in sap pH under soil water deficits also remains unconfirmed. H+-ATPase was recently hypothesized to play a key role in controlling apoplastic pH (Cho and others 2012). Fromard and others (1995) showed that H+-ATPase was much more concentrated in the plasma membranes of xylem parenchyma cells than in any other plant cell types. They showed that these pumps were involved in the control of vascular sap pH. By using vanadate, an H+-ATPase inhibitor, Jia and Davies (2007) demonstrated that the alkaline of xylem sap could be prevented as moving through the plant to the shoot during drought stress or N treatment, presumably by inhibiting ATPases associated with the xylem parenchyma. In the subsequent study, we assessed if the H+-ATPase inhibitor could influence the effect of H2 on apoplastic pH. The results showed that vanadate prevented the effects of H2, on the alkalinization of the apoplastic sap and increasing sensitivity of stomatal response to ABA (Fig. 2). One of the implications of this is that H2-increased apoplastic pH may be through activation of ATPases in the xylem parenchyma. By then, the ATPases in xylem parenchyma could remove more protons from the xylem stream and therefore have higher xylem and apoplastic pHs. This detailed mechanism should be investigated in the near future.
Altogether, our results indicate a fundamental role of H2 in modifying apoplastic pH, likely via the regulation of ATPases in xylem parenchyma. Thus, H2 might alter ABA repartition and increase stomatal response to ABA. Based on these findings, we propose the following model (Supplemental Fig. 3). At least in alfalfa, soil drying will alkalinize the pH of the apoplastic sap. This effect can be enhanced by H2, allowing the stomata to become more sensitive to soil drying and low concentrations of ABA. These effects are consistent with repartitioning of ABA through the shoot according to pH gradients.
References
An Z, Jing W, Liu Y, Zhang W (2008) Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J Exp Bot 9:815–825
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Cho D, Villiers F, Kroniewicz L, Lee S, Seo YJ, Hirschi KD, Leonhardt N, Kwak JM (2012) Vacuolar CAX1 and CAX3 influence auxin transport in guard cells via regulation of apoplastic pH. Plant Physiol 160:1293–1302
Cui W, Gao C, Fang P, Lin G, Shen W (2013) Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J Hazard Mater 260:715–724
Cui W, Fang P, Zhu K, Mao Y, Gao C, Xie Y, Wang J, Shen W (2014) Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol Environ Saf 105:103–111
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot 55:205–212
Frey M (2002) Hydrogenases: hydrogen-activating enzymes. ChemBioChem 3:153–160
Fromard L, Babin V, Fleurat-Lessard P, Fromont JC, Serrano R, Bonnemain JL (1995) Control of vascular sap pH by the vessel-associated cells in woody species. Plant Physiol 108:913–918
García-Mata C, Lamattina L (2010) Hydrogen sulphide, a novel gasotransmitter involved in guard cell signaling. New Phytol 188:977–984
Hartung W, Sauter A, Hose E (2002) Abscisic acid in the xylem: where does it come from, where does it go to? J Exp Bot 53:27–32
Hong Y, Chen S, Zhang JM (2010) Hydrogen as a selective antioxidant: a review of clinical and experimental studies. J Int Med Res 38:1893–1903
Jia W, Davies WJ (2007) Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals. Plant Physiol 143:68–77
Jin Q, Zhu K, Cui W, Xie Y, Han B, Shen W (2013) Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ 36:956–969
Melis A, Happe T (2001) Hydrogen production. Green algae as a source of energy. Plant Physiol 127:740–748
Melis A, Melnicki MR (2006) Integrated biological hydrogen production. Int J Hydrog Energy 31:1563–1573
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S (2007) Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 13:688–694
Renwick GM, Giumarro C, Siegel SM (1964) Hydrogen metabolism in higher plants. Plant Physiol 39:303–306
Ryu MY, Cho SK, Kim WT (2010) The Arabidopsis C3H2C3-type RING E3 ubiquitin ligase AtAIRP1 is a positive regulator of an abscisic acid-dependent response to drought stress. Plant Physiol 154:1983–1997
Schachtman DP, Goodger JQ (2008) Chemical root to shoot signaling under drought. Trends Plant Sci 13:281–287
Slovik S, Daeter W, Hartung W (1995) Compartmental redistribution and long-distance transport of abscisic acid (ABA) in plants as influenced by environmental changes in the rhizosphere-a biomathematical model. J Exp Bot 46:881–894
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Villiers F, Kwak JM (2013) Rapid apoplastic pH measurement in Arabidopsis leaves using a fluorescent dye. Plant Signal Behav 8:e22587
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25:195–210
Xie Y, Mao Y, Lai D, Zhang W, Shen W (2012) H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS One 7:e49800
Xie Y, Mao Y, Zhang W, Lai D, Wang Q, Shen W (2014) Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol 165:759–773
Zeng J, Zhang M, Sun X (2013) Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS One 8:e71038
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31371546 and J1210056), the Fundamental Research Funds for the Central Universities (KYTZ201402), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Phenotypes of alfalfa seedlings pretreated with HRW in response to ABA during seed germination and early seedling growth (TIFF 4596 kb)
Supplementary Fig. 2
Fluorescence intensity of 8-hydroxypyrene-1,3,6-trisulfonic acid, trisodium salt (HTPS) as a function of pH (TIFF 190 kb)
Supplementary Fig. 3
A model detailing how H2 might be involved in the ABA-signal transduction network leading stomatal closure (TIFF 694 kb)
Rights and permissions
About this article
Cite this article
Jin, Q., Zhu, K., Cui, W. et al. Hydrogen-Modulated Stomatal Sensitivity to Abscisic Acid and Drought Tolerance Via the Regulation of Apoplastic pH in Medicago sativa . J Plant Growth Regul 35, 565–573 (2016). https://doi.org/10.1007/s00344-015-9561-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-015-9561-2