Abstract
Background and aims
Soil cadmium (Cd) contamination threatens food safety and human health. Increasing Cd phytoextraction efficiency by hyperaccumulators and growing safe products during remediation remain challenges.
Methods
A pot experiment was conducted to explore the effects of different rates of sulfur (S) associated with alternating drying and wetting on a Sedum plumbizincicola-Oryza sativa rotation in Cd-contaminated neutral and calcareous soils.
Results
The oxidation of added S under aerobic conditions significantly decreased soil solution pH and increased soluble sulfate (SO42−), Cd and iron (Fe) concentrations in both soils. During the rice growing season the soil solution redox (Eh) decreased to < −200 mV and the solution pH increased to neutral during the first few days of flooding. Soluble SO42− and Cd in the S treatments decreased significantly with increasing duration of flooding. Sulfur addition promoted shoot Cd concentrations of S. plumbizincicola by 1.7–5.5 times on calcareous soil and 1.7–2.3 times on neutral soil compared to the controls. Rice yields increased but Cd concentrations decreased at suitable S addition rates.
Conclusions
Appropriate sulfur amendment combined with water management can be a feasible strategy to enhance the Cd remediation efficiency of the hyperaccumulator and reduce the accumulation of Cd in the rice grains in this rotation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Elevated cadmium (Cd) concentrations in Chinese arable soils due to industrial and agricultural activities represent an important threat to human and animal health (Teng et al. 2014; Chen et al. 2015; Xue et al. 2017). Many Cd-polluted soils urgently require remediation. A range of options have been proposed including the identification of the contaminant sources in agricultural systems, minimization of contaminant inputs, reduction of the phytoavailability of potentially toxic elements in soils by liming or the application of other metal immobilizing materials, selection and breeding of crop cultivars with low metal accumulation ability, adoption of appropriate water and fertilizer management practices, bioremediation, and changes in land use to grow non-food crops (Zhao et al. 2015). One technique, phytoextraction using hyperaccumulators, is an in-situ, environmental friendly and low-cost approach to the remediation of metal contaminated agricultural soils (Van der Ent et al. 2013). Rotation or intercropping of hyperaccumulators with crops is now widely used to remove metals from contaminated soils and continue agricultural production (Yang et al. 2017; Zhan et al. 2016; Deng et al. 2016). However, technologies for increasing metal phytoextraction efficiency and minimizing remediation time need to be developed (Li et al. 2018). Growing safe products during the period of remediation by hyperaccumulators remains a major challenge (Wan et al. 2016; Shen et al. 2010).
Synthetic or natural metal chelating agents such as EDTA (ethylene diamine tetraacetic acid), EDDS (ethylene diamine disuccinic acid), and citric acid are often used to increase phytoextraction efficiency (Anwar et al. 2017; Freitas et al. 2013; Attinti et al. 2017). Acidifying compounds such as elemental sulfur (S) have been proposed to enhance metal uptake by phytoextraction crops grown in polluted soils (Kayser et al. 2000; Wang et al. 2006; Iqbal et al. 2012). Sulfur is an essential element to all living organisms because it forms part of important life sustaining molecules such as cysteine, thioredoxin metallothionein, enzymes and vitamins (Hell 1997; Saito 2000). These compounds can increase plant tolerance to metals through complexation and/or sequestration into vacuoles (de Oliveira et al. 2014). It is well known that sulfur changes its valence state due to moisture-driven soil oxidation status. In aerobic conditions S application can decrease soil pH because the oxidation of S to sulfate (SO42−) by sulfur-oxidizing bacteria or chemical process releases protons (H+) (Cui et al. 2004). After sulfur application the soil pH decreases significantly, thus increasing the solubility of soil Cd and plant Cd accumulation (Wang et al. 2007). In anaerobic environments, HS− and S2− produced by sulfate reduction can precipitate with thiophilic elements (Cd, Cu, Fe) or co-precipitate with FeS (Khaokaew et al. 2011; Hu et al. 2013; Fulda et al. 2013; Hashimoto et al. 2016). However, there is little information on the effects of S on Cd solubility in drying-wetting rotation systems.
Sedum plumbizincicola, a native Cd hyperaccumulator found in east China, has shown a considerable capacity to extract Cd from soils (Wu et al. 2013; Hu et al. 2015; Li et al. 2014b). Several modes have been developed using this plant for remediation practice, for example rotation with rice and intercropping with maize or aubergine (Deng et al. 2016). A recent study shows that the optimum pH for phytoextraction with S. plumbizincicola was ~5.5 because of high Cd bioavailability (Wu et al. 2018). However, the bioavailability of Cd in neutral and calcareous soils is usually poor, and phytoextraction takes longer times (Li et al. 2014a, 2016; Wu et al. 2018). It is therefore necessary to develop techniques to increase the phytoextraction efficiency of S. plumbizincicola and to inhibit Cd accumulation in associated rice.
In the present study a pot experiment was conducted to explore the effects of different addition rates of S associated with drying-wetting rotation between S. plumbizincicola and rice in Cd-contaminated neutral and calcareous soils. We hypothesized that S oxidation in aerobic conditions decreases soil pH and increases Cd bioavailability and thus increases Cd phytoextraction by S. plumbizincicola. Furthermore, sulfate reduction in flooded conditions can precipitate Cd and restrict Cd accumulation by rice. The aim was to develop a rotation system that would optimize phytoextraction and produce safe rice on Cd-contaminated agricultural soils.
Materials and methods
Soil characterization
Two test soils were collected from the top 15 cm of the soil profile of agricultural fields. One is classified as a Calcaric Purpli-Orthic Primosols from Lanping County, Yunnan Province (designated L-soil) with a pH of 8.0 and a total Cd concentration of 4.44 mg kg−1. The other is classified as a Typic Hapli-Stagnic Anthrosols from Taicang City, Jiangsu Province (designated T-soil) with pH 6.5 and total Cd 1.33 mg kg−1. Selected physicochemical properties of the test soils are listed in Table 1.
Design of pot experiment
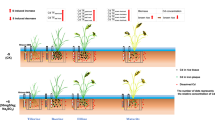
A glasshouse pot experiment was conducted with two consecutive crops comprising S. plumbizincicola (days 0 to 90) followed by rice (days 120 to 210). There were five rates of S addition, namely controls (L0 and T0), 0.5 g kg−1 (L0.5 and T0.5), 1.0 g kg−1 (L1 and T1), 2.0 g kg−1 (L2 and T2) and 4.0 g kg−1 (L4 and T4). Sulfur was added to the soils before S. plumbizincicola was transplanted. Four cuttings of S. plumbizincicola were transplanted into each 15-cm-diameter plastic pot containing 1.5 kg (oven dry basis) of 2-mm sieved air dry soil. There were four replicate pots of each treatment giving a total of 40 pots. Fertilizers were applied at rates of 0.3 g CO(NH2)2 and 0.3 g KH2PO4 kg−1 soil 30 days after S. plumbizincicola was transplanted. Deionized water was added daily to maintain soil moisture at about 60% of soil water holding capacity (WHC) during the S. plumbizincicola growth period. Shoots of the hyperaccumulator were harvested after growth for 90 days. The remaining soil was used for the subsequent rice crop (Oryza sativa cv. Xiangzaoxian 24) which grew from days 120 to 210. The soil in each pot was mixed with basal fertilizer composed of 0.4 g CO(NH2)2 kg−1 and 0.4 g KH2PO4 kg−1. Then 0.6 g CO(NH2)2 and 0.6 g KH2PO4 kg−1 were top dressed at the tillering and grain filling stages, respectively. A water layer about 4 cm deep above the soil surface was maintained during the rice growing period. The pots were fully randomized and then re-randomized once a month.
One Rhizon soil moisture sampler (Rhizon-MOM, Wageningen Research Products, Wageningen, The Netherlands) was installed in the middle of the soil in each pot at an angle of 45° during the growth of both plant species. Soil solution samples of ~15 ml were extracted from each pot on days 1, 10, 20, 30, 50, 70, 90, 121, 125, 150, 175 and 200. The soil water content was adjusted to 80% of the WHC with deionized water 12 h before sampling during the S. plumbizincicola growing period.
At harvest, S. plumbizincicola shoots and rice grains were collected and washed with running tap water and rinsed twice with deionized water, oven dried at 75 °C, weighed, and ground. Fresh soil samples were collected at the same time and immediately used for the determination of soil extractable Cd.
Chemical analysis
Soil pH, organic matter content, texture, and available nitrogen, available phosphorus and available potassium contents were determined following the methods of Lu (2000). Soil available sulfur was extracted with Ca(H2PO4)2 and determined by ion chromatography (ICS3000, Thermo Dionex, Sunnyvale, CA). Soil total Cd, Zn, Fe and Mn concentrations were determined by digestion of 0.20-g samples with 5 mL HCl and 5 mL HNO3. Available Cd in fresh soil samples was extracted with 0.01 M CaCl2 at a soil:water ratio of 1:10 (w/v). Plant samples (0.20 g) were digested with 6 mL HNO3 and 2 mL H2O2. Metal concentrations in the digest solutions and extract solutions were determined by inductively coupled plasma-mass spectrometry (ICP-MS, Ultramass, Varian, Palo Alto, CA). Plant S concentrations were determined by inductively coupled plasma-optical emission spectrometry (ICP-OES, Perkin Elmer Optima 8000, Waltham, MA). Replicates, blanks and a certified reference material (GBW07406) were included for analytical quality control.
The pH and Eh values of soil solutions were determined immediately after sampling. Soil solutions were acidified (1% (v/v) of concentrated HNO3) and stored at −20 °C for metal and SO42− determination (Fulda et al. 2013). Metal concentrations in solutions were determined by ICP-MS or ICP-OES and SO42− concentration was determined by ion chromatography as described above.
Statistical analysis
All data are expressed as mean ± standard error (SE). The data were tested by one-way analysis of variance and mean values were compared by least significant difference (LSD) at the 5% protection level. Data processing was conducted using Microsoft Excel 2013 and the SPSS 21.0 for Windows software package (SPSS Inc., Chicago, IL).
Results
Soil solution pH, Eh and dissolved SO4 2− at different plant growth stages
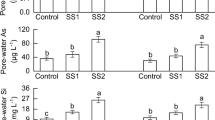
The dynamics of pH, Eh, SO42−, Cd, Fe, and Mn in the soil solution during aerobic (the first 90 days, S. plumbizincicola) and anaerobic (days 120–210, rice) culture under the different rates of S amendment are shown in Fig. 1. In the L-soil control treatment solution pH was maintained between 6.6 and 7.6 in the early part of the aerobic stage but showed a decreasing trend after 30 d. Soil solution pH increased and then remained between 7.5 and 8.2 during the flooded stage and similar trends occurred in the T-soil. Sulfur treatments led to lower pH than in the control in both soils. A rapid decrease in soil solution pH occurred during the first 30 days. On day 30 the solution pH in L2 (5.68), L4 (5.03), T2 (3.78) and T4 (3.23) decreased by 1.82, 2.47, 2.62 and 3.17 units compared with the controls L0 (7.50) and T0 (6.40), respectively. On day 90 the solution pH of L2 (4.63), L4 (3.98), T2 (3.19) and T4 (2.77) decreased by 1.95, 2.60, 2.09 and 2.81 units compared with the controls L0 (6.58) and T0 (5.58), respectively. During the growth of the rice the soil solution pH changed from acid to neutral with the exception of treatments L4, T2 and T4.
Soil solution Eh of all treatments was between 200 mV and 400 mV during the growth of S. plumbizincicola and decreased to −200 mV five days after flooding and then remained between −200 mV and − 300 mV until the rice harvest. Dissolved SO42− in the controls was maintained at 50.8–103 mg L−1 in the L-soil and 83.1–189 mg L−1 in the T-soil during the S. plumbizincicola growth period. Dissolved SO42− in the S treatments increased significantly within the first 30 days in both soils. Dissolved SO42− reached a maximum of 1151 mg L−1 after 50 days in the T4 treatment. On day 90 the concentrations of SO42− were 461 and 646 mg L−1 in treatments L2 and L4, respectively, values 4.48 and 6.27 times that of the control L0 (103 mg L−1). The SO42− of treatments T2 and T4 were 445 and 876 mg L−1, respectively, 2.35 times and 4.63 times that of the control T0 (189 mg L−1). After one day of flooding (day 121) the soil solution SO42− concentrations in all treatments increased above those on day 90. During flooded rice growth, dissolved SO42− declined in general. After 200 days the concentrations of SO42− in treatments L0 and L4 were 0.47 and 360 mg L−1, respectively, and the SO42−concentrations in treatments T0 and T4 were 0.67 and 154 mg L−1, respectively.
Soil solution ion concentrations and CaCl2-extractable Cd concentrations at different growth stages
During the growth of S. plumbizincicola the solution Cd in both soils increased with increasing S addition rate from 0 to 30 days. Solution Cd declined slightly from day 30 to day 90 in the controls and some of the S treatments. On day 90 the solution Cd concentrations in L2 and L4 were 21.7 and 184 μg L−1, respectively, values 9.0 and 76.3 times that of L0 (2.41 μg L−1). In T-soil the 0.5 g kg−1 S treatment (L0.5) increased the solution Cd concentration to 7.4–24.8 μg L−1, and higher S application led to higher solution Cd concentrations. During flooding (days 120 to 210) the soil solution Cd showed a maximum value after 5 days of flooding (on day 125). After flooding for 30 days (day 150) the concentration rapidly decreased to ~0.1 μg L−1 and was maintained until the harvest in the control and lower S treatments, e.g. 0.5–2 g kg−1 in L-soil and 0.5 g kg−1 in T-soil. The high S treatments also reached minimum concentrations but did so over a longer time period.
Soil CaCl2-extractable Cd concentrations in both soils were determined at the S. plumbizincicola and rice harvests (Table 2). They increased with increasing S application rate at the S. plumbizincicola harvest. Soil CaCl2-extractable Cd concentrations decreased substantially after flooding compared to aerobic conditions. Furthermore, the S treatments at 0.5–2 g kg−1 in L-soil and 0.5–1 g kg−1 in T-soil led to significantly lower extractable Cd than in the controls. However, S application at 4 g kg−1 in L-soil and 2–4 g kg−1 in T-soil produced significantly higher extractable Cd than in the controls.
The dynamic changes in soil solution Fe and Mn followed similar trends. From 0 to 90 days Fe and Mn increased with plant growth time and S rate. Fe showed a decrease after flooding for 5 days (on day 125) and then increased rapidly. Sulfur treatments showed higher solution Fe and Mn concentrations during the rice growing period compared to the controls.
Plant biomass and elemental accumulation
Compared with the controls the growth of S. plumbizincicola was significantly affected when the S rate was ≥ 2 g kg−1 in L-soil and ≥ 1 g kg−1 in T-soil (Table 3). The S. plumbizincicola shoot Cd concentrations in the S treatments were significantly higher than in the controls in both soils. For example, the shoot Cd concentrations in L2 and T0.5 were 106 and 96.8 mg kg−1, respectively, values 4.16 and 1.67 times those of L0 and T0. Soil Cd removal rate increased after S treatment. For example, the soil Cd removal rates in L2 and T0.5 treatments were 14.6 and 44.7%, respectively, values significantly higher than in L0 (4.03%) and T0 (32.0%). Sulfur treatments significantly increased brown rice yields in L4, T1 and T2 treatments compared to the controls (Table 4). Brown rice Cd concentrations at 0.5–2 g kg−1 S treatments in both soils were significantly lower than the controls. Sulfur treatments also significantly increased S concentrations in S. plumbizincicola shoots and brown rice (Tables 3 and 4).
Discussion
Dynamics of soil chemical processes affected by sulfur addition and water management
Our experiment indicates that S addition combined with alternating drying and wetting of the soil significantly affected soil chemical processes in the S. plumbizincicola and rice rotation system. The behaviour of S is closely linked to paddy soil redox conditions because of the transformation of inorganic species between valence −2 and valence +6 and oxidation of organic S compounds. In aerobic conditions with S. plumbizincicola a dramatic increase in soil soluble sulfate indicates the oxidation of sulfur which was very pronounced for the first 30 days (Fig. 1). This was the period of rapid sulfur oxidation (Wen et al. 2001; Wang et al. 2007). The sulfur oxidation process under aerobic conditions resulted in a decrease in soil pH. Generally, soil pH determines the processes of dissolution-precipitation and adsorption-desorption of metals in soils. The decrease in soil solution pH therefore further increased the soil solution Cd, Mn and Fe concentrations (Fig. 1). These effects depended on both the sulfur addition rate and soil properties. In general, the degree of change is proportional to the sulfur rate. However, similar amounts of S had significantly different effects in the two soils. For example, on day 30 the soil solution pH values in treatments L4 and T4 were 5.03 and 3.23, respectively. This is because of the different properties of the two soils including the initial pH and clay concentrations (Table 1). It is notable that soil solution pH and solution Cd decreased slightly in some treatments from 30 to 90 days and this can be explained by rhizosphere acidification and Cd accumulation by S. plumbizincicola (Sun et al. 2019).
Upon flooding, O2 in the soil is depleted quickly and this is followed by reduction of nitrate, manganese oxides and then iron oxides/hydroxides. After the reduction of Fe, sulfate is reduced to sulfide mainly due to microbial activity (Borch et al. 2010; Hashimoto et al. 2016). One day after flooding (day 121) in the current study the concentration of soil solution SO42− in all treatments increased compared to day 90. This was due to the competitive effects of anions e.g. CO2, acidic carbonates and organic acids resulting from the decomposition of organic matter (Ding and Xu 2011). Subsequently, soil solution SO42− showed an overall downward trend (Fig. 1) which indicates reduction of SO42− (Burton et al. 2013, 2014). When flooding was prolonged the soil solution Eh decreased from −200 mV to −300 mV and soil SO42− was reduced to generate reducing substances such as HS−/S2− which formed sulfide precipitates with various trace metals, e.g. Cu, Cd, Fe, and Zn (Fulda et al. 2013; Hashimoto et al. 2016). On the other hand, the reduction reaction consumes protons, thus the soil pH of all treatments changed to neutral (Burton et al. 2013). However, the pH in treatment T4 remained low, possibly because the sulfur rate exceeded the buffering capacity of the soil.
It is notable that at day 125 (five days after flooding) the soil solution Cd concentration increased but Fe and Mn concentrations decreased (Fig. 1). One possible explanation is that Fe/Mn oxides underwent a reduction and dissolution process, releasing Cd2+ (Kocar et al. 2010; Stroud et al. 2011). The soil solution Fe concentration is higher than that of Cd and therefore Fe readily combines with S2− to form FeS precipitate. This resulted in a temporary decline in solution Fe concentration. After prolonged submergence the continuous reduction of Fe/Mn oxides provided soluble metals while soil solution Cd directly formed CdS or replaced Fe in FeS (Ditoro et al. 1990). Moreover, the continuous flooding within 30 days in appropriate S treatments eliminated elevated soluble Cd resulting from S oxidation, and maintained soluble Cd at a low level (~0.1 μg L−1) until the rice harvest. This is consistent with previous studies (de Livera et al. 2011; Fan et al. 2010). It has been reported that moderate sulfur-containing substances in flooded soils change the diversity of the bacterial community and this accelerates the reduction of sulfate and facilitates the precipitation of metal sulfides (Wu et al. 2019; Sebastian and Prasad 2014; Kelly and Wood 2000). The 0.5–2 g kg−1 S treatments in L-soil and 0.5–1 g kg−1 S treatments in T-soil showed significantly lower extractable Cd concentrations than the controls (Table 2). More Cd would be expected to form CdS precipitate which would be stable and non-extractable by weak extracting agents such as CaCl2 (Furuya et al. 2016). However, the excessive S treatments, e.g. L4, T2 and T4, took a long time to reduce soluble Cd to the minimum level, and the concentration of CaCl2-extractable Cd after the rice harvest was also maintained at a high level (Fig. 1, Table 2). One possible explanation is the inhibition of Cd precipitation under extremely low soil pH conditions.
Effects of sulfur application and water management on phytoextraction and accumulation of Cd by S. plumbizincicola and rice
Sulfur addition and water management also affected plant growth and metal accumulation. Appropriate S treatment had no significant negative effect on S. plumbizincicola biomass but significantly increased the shoot Cd concentrations because of the increase in soil Cd bioavailability both in neutral and calcareous soils. For example, shoot Cd concentrations in the S treatment in L-soil were 1.74–5.45 times and in T-soil were 1.67–2.67 times higher than in the controls. Moreover, the soil Cd removal rate in L2 and T0.5 reached 14.6 and 44.7%, respectively, values significantly higher than in L0 (4.03%) and T0 (32.0%). The increase in Cd removal rate with S treatment will markedly shorten the duration of remediation. For example, we assume that the Cd removal rate by S. plumbizincicola is consistent in subsequent remediation, costing three crops in T0.5 but four crops in T0 to decease the soil Cd (1.33 mg kg−1, pH 6.5) to below the Chinese risk screening value (0.4 mg kg−1, 5.5 < pH ≤ 6.5, GB15618–2018). Similarly, the cost is around 42 crops in L0 but only 11 crops in L2 to decrease soil Cd (4.44 mg kg−1, pH 8.0) to below the Chinese risk screening value (0.8 mg kg−1, pH > 7.5, GB15618–2018). Previous studies also report that phytoextraction efficiency in S treatments was enhanced by 50% during microbial S oxidation in the rhizosphere of willow (Iqbal et al. 2012). The addition of S had positive effects on the accumulation of Cd and Zn in Sedum alfredii because of a “sulfur-induced Cd requirement” (Li et al. 2008). In our study the S treatments at 2 g kg−1 in L-soil and 0.5 g kg−1 in T-soil produced relatively high Cd removal rates by S. plumbizincicola (Table 3). Further increase in S treatment did not promote Cd removal or even decrease Cd removal because of the toxic effects on plant growth. Excessive S over-acidifying the soil during the first few weeks might inhibit new root development and induce aluminium toxicity (Rao et al. 2016).
Furthermore, a novel finding was that appropriate S treatments significantly increased yields and reduced total Cd concentrations of brown rice from both test soils. There are several possible contributory factors. Firstly, as discussed above the continuous flooding and reduction of SO42− maintained soil soluble Cd at a very low level during the intermediate and later growth stages of rice (Fig. 1 and Table 2). Secondly, S might enhance the formation of iron plaque in the rhizosphere which would suppress Cd accumulation by rice (Hu et al. 2007). Thirdly, S treatment increased rice S concentrations and led to the formation of glutathione and chlorophyll that alleviated Cd stress (Sarwar et al. 2010; Dixit et al. 2015). This type of defence mechanism involving Cd-induced photosynthetic inhibition appears to be more effective when the soil is treated with the optimum rate of S rather than excessive S (Masood et al. 2012). However, it is important to note that excessive levels of S in T-soil increased rice Cd accumulation and thus increased the Cd risk (Table 4).
Taking into account the effects of S addition on soil chemical processes, plant growth and Cd uptake and potential environmental risk, S addition at 2 g kg−1 to the calcareous soil (L-soil) and 0.5 g kg−1 to the neutral soil (T-soil) are the optimum treatments to simultaneously increase the soil Cd removal rate by S. plumbizincicola and depress the Cd concentration in the brown rice. Excessive levels of S should be avoided because of growth inhibition of S. plumbizincicola and increased Cd uptake by rice. This may also increase the potential risk of Cd leaching and induce S toxicity to the plants.
Strategy for phytoextraction and safe rice production in Cd-contaminated soils
This study indicates a new strategy for phytoextraction together with the safe production of food in Cd-contaminated soils. This involves the rotation of the Cd hyperaccumulator S. plumbizincicola with a low Cd accumulating cultivar of rice. Appropriate application rates of S are recommended during the S. plumbizincicola growing season especially in neutral and calcareous soils to activate soil Cd through S oxidation to increase Cd phytoextraction and shorten the duration of the remediation. Then, during the rice season, flooding should be maintained to promote the reduction of sulfate and the formation of CdS precipitate to ensure low Cd availability and thus minimize the risk of Cd contamination in the rice. In the next rotation, there is no need to add S again. Soil pH will decline in oxidised conditions and the re-oxidation of sulfide compounds will release Cd again (Fulda et al. 2013; Furuya et al. 2016) which is beneficial for phytoextraction by S. plumbizincicola. Soil total Cd concentrations can be removed gradually with safe agricultural production until the soil remediation goal is finally achieved. In addition, it is notable that Cd release with S oxidation is a rapid process compared to plant uptake, and this may increase the Cd leaching risk in field practice. The future development of slow release S fertilizers and relevant agronomic measures, e.g. low dosage with suitable frequency, may help to overcome this limitation (Mann et al. 2019).
Conclusions
Sulfur addition combined with water management significantly affected soil chemical processes and plant Cd uptake in neutral and calcareous soils. The oxidation of applied S in aerobic conditions significantly acidified the soil and increased soluble SO42−, Cd and Fe concentrations and promoted Cd phytoextraction by S. plumbizincicola. In the rice season continuous flooding decreased soil Eh and increased pH. Appropriate rates of S application induced the reduction of SO42− and facilitated the stabilization of Cd. This rapidly eliminated elevated soluble Cd resulting from S oxidation in the former season and also maintained available Cd at lower levels than in the controls until the rice harvest. This increased rice yields and reduced rice Cd uptake. Therefore, appropriate sulfur amendment associated with alternating drying and wetting of the soil can enhance soil Cd remediation efficiency by the hyperaccumulator and control Cd accumulation in rice grains in the rotation system. This study thus proposes a new strategy for phytoextraction and continued safe production of rice in Cd-contaminated neutral and calcareous soils.
References
Anwar S, Khan S, Ashraf MY, Noman A, Zafar S, Liu LJ, Ullah S, Fahad S (2017) Impact of chelator-induced phytoextraction of cadmium on yield and ionic uptake of maize. Int J Phytoremediat 19:505–513
Attinti R, Barrett KR, Datta R, Sarkar D (2017) Ethylenediaminedisuccinic acid (EDDS) enhances phytoextraction of lead by vetiver grass from contaminated residential soils in a panel study in the field. Environ Pollut 225:524–533
Borch T, Kretzschmar R, Kappler A, Van Cappellen P, Ginder-Vogel M, Voegelin A, Campbell K (2010) Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23
Burton ED, Johnston SG, Kraal P, Bush RT, Claff S (2013) Sulfate availability drives divergent evolution of arsenic speciation during microbially mediated reductive transformation of schwertmannite. Environ Sci Technol 47:2221–2229
Burton ED, Johnston SG, Kocar BD (2014) Arsenic mobility during flooding of contaminated soil: the effect of microbial sulfate reduction. Environ Sci Technol 48:13660–13667
Chen HY, Teng YG, Lu SJ, Wang YY, Wang JS (2015) Contamination features and health risk of soil heavy metals in China. Sci Total Environ 512:143–153
Cui YS, Wang QR, Dong YT, Li HF, Christie P (2004) Enhanced uptake of soil Pb and Zn by Indian mustard and winter wheat following combined soil application of elemental sulphur and EDTA. Plant Soil 261:181–188
de Livera J, McLaughlin MJ, Hettiarachchi GM, Kirby JK, Beak DG (2011) Cadmium solubility in paddy soils: effects of soil oxidation, metal sulfides and competitive ions. Sci Total Environ 409:1489–1497
de Oliveira LM, Ma LQ, Santos JAG, Guilherme LRG, Lessl JT (2014) Effects of arsenate, chromate, and sulfate on arsenic and chromium uptake and translocation by arsenic hyperaccumulator Pteris vittata L. Environ Pollut 184:187–192
Deng L, Li Z, Wang J, Liu HY, Li N, Wu LH, Hu PJ, Luo YM, Christie P (2016) Long-term field phytoextraction of zinc/cadmium contaminated soil by Sedum plumbizincicola under different agronomic strategies. Int J Phytorem 18:134–140
Ding CP, Xu RK (2011) Oxidation-reduction processes of soils and their research methods. Science Press, Beijing, pp 197–220
Ditoro DM, Mahony JD, Hansen DJ, Scott KJ, Hicks MB, Mayr SM, Redmond MS (1990) Toxicity of cadmium in sediments: the role of acid volatile sulfide. Environ Toxicol Chem 9:1487–1502
Dixit G, Singh AP, Kumar A, Singh PK, Kurnar S, Dwivedi S, Trivedi PK, Pandey V, Norton GJ, Dhankher OP, Tripathi RD (2015) Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J Hazard Mater 298:241–251
Fan JL, Hu ZY, Ziadi N, Xia X, Wu CYH (2010) Excessive sulfur supply reduces cadmium accumulation in brown rice (Oryza sativa L.). Environ Pollut 158:409–415
Freitas EV, Nascimento CW, Souza A, Silva FB (2013) Citric acid-assisted phytoextraction of lead: a field experiment. Chemosphere 92:213–217
Fulda B, Voegelin A, Kretzschmar R (2013) Redox-controlled changes in cadmium solubility and solid-phase speciation in a paddy soil as affected by reducible sulfate and copper. Environ Sci Technol 47:12775–12783
Furuya M, Hashimoto Y, Yamaguchi N (2016) Time-course changes in speciation and solubility of cadmium in reduced and oxidized paddy soils. Soil Sci Soc Am J 80:870–877
Hashimoto Y, Furuya M, Yamaguchi N, Makino T (2016) Zerovalent iron with high sulfur content enhances the formation of cadmium sulfide in reduced paddy soils. Soil Sci Soc Am J 80:55–63
Hell R (1997) Molecular physiology of plant sulfur metabolism. Planta 202:138–148
Hu ZY, Zhu YG, Li M, Zhang LG, Cao ZH, Smith EA (2007) Sulfur (S)-induced enhancement of iron plaque formation in the rhizosphere reduces arsenic accumulation in rice (Oryza sativa L.) seedlings. Environ Pollut 147:387–393
Hu PJ, Li Z, Yuan C, Ouyang YN, Zhou LQ, Huang JX, Huang YJ, Luo YM, Christie P, Wu LH (2013) Effect of water management on cadmium and arsenic accumulation by rice (Oryza sativa L.) with different metal accumulation capacities. J Soils Sediments 13:916–924
Hu PJ, Wang YD, Przybylowicz WJ, Li Z, Barnabas A, Wu LH, Luo YM, Mesjasz-Przybylowicz J (2015) Elemental distribution by cryo-micro-PIXE in the zinc and cadmium hyperaccumulator Sedum plumbizincicola grown naturally. Plant Soil 388:267–282
Iqbal M, Puschenreiter M, Oburger E, Santner J, Wenzel WW (2012) Sulfur-aided phytoextraction of Cd and Zn by Salix smithiana combined with in situ metal immobilization by gravel sludge and red mud. Environ Pollut 170:222–231
Kayser A, Wenger K, Keller A, Attinger W, Felix HR, Gupta SK, Schulin R (2000) Enhancement of phytoextraction of Zn, Cd, and Cu from calcareous soil: the use of NTA and sulfur amendments. Environ Sci Technol 34:1778–1783
Kelly DP, Wood AP (2000) Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. Nov., Halothiobacillus gen. Nov and Thermithiobacillus gen. Nov. Int J Syst Evol Microbiol 50:511–516
Khaokaew S, Chaney RL, Landrot G, Ginder-Vogel M, Sparks DL (2011) Speciation and release kinetics of cadmium in an alkaline paddy soil under various flooding periods and draining conditions. Environ Sci Technol 45:4249–4255
Kocar BD, Borch T, Fendorf S (2010) Arsenic repartitioning during biogenic sulfidization and transformation of ferrihydrite. Geochim Cosmochim Acta 74:980–994
Li HH, Hu MH, Li TQ, Yang XE (2008) Effects of sulfur on growth, cadmium absorption and accumulation in hyperaccumulator Sedum alfredii Hance. J Soil Water Conserv 22(6):71–74 (in Chinese with English abstract)
Li Z, Wu LH, Hu PJ, Luo YM, Zhang H, Christie P (2014a) Repeated phytoextraction of four metal-contaminated soils using the cadmium/zinc hyperaccumulator Sedum plumbizincicola. Environ Pollut 189:176–183
Li Z, Wu LH, Luo YM, Christie P (2014b) Dynamics of plant metal uptake and metal changes in whole soil and soil particle fractions during repeated phytoextraction. Plant Soil 374:857–869
Li Z, Jia MY, Wu LH, Christie P, Luo YM (2016) Changes in metal availability, desorption kinetics and speciation in contaminated soils during repeated phytoextraction with the Zn/Cd hyperaccumulator Sedum plumbizincicola. Environ Pollut 209:123–131
Li JT, Gurajala HK, Wu LH, Van der Ent A, Qiu RL, Baker AJM, Tang YT, Yang XE, Shu WS (2018) Hyperaccumulator plants from China: a synthesis of the current state of knowledge. Environ Sci Technol 52:11980–11994
Lu RK (2000) Analytical methods for soil and agrochemistry. Agricultural science and technology press. Beijing, China
Mann M, Kruger JE, Andari F, McErlean J, Gascooke JR, Smith JA, Worthington MJH, McKinley CCC, Campbell JA, Lewis DA, Hasell T, Perkins MV, Chalker JM (2019) Sulfur polymer composites as controlled-release fertilisers. Org Biomol Chem 17:1929–1936
Masood A, Iqbal N, Khan NA (2012) Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ 35:524–533
Rao IM, Miles JW, Beebe SE, Horst WJ (2016) Root adaptations to soils with low fertility and aluminium toxicity. Ann Bot-London 118:593–605
Saito K (2000) Regulation of sulfate transport and synthesis of sulfur-containing amino acids. Curr Opin Plant Biol 3:188–195
Sarwar N, Saifullah MSS, Zia MH, Naeem A, Bibi S, Farid G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 90:925–937
Sebastian A, Prasad MNV (2014) Cadmium minimization in rice. A review. Agron Sustain Dev 34:155–173
Shen LB, Wu LH, Tan WN, Han XR, Luo YM, Ouyang YN, Jin QY, Jiang YG (2010) Effects of Sedum plumbizincicola-Oryza sativa rotation and phosphate amendment on Cd and Zn uptake by O. sativa. Chin J Appl Ecol 21:2952–2958 (in Chinese with English abstract)
Stroud JL, Norton GJ, Islam MR, Dasgupta T, White RP, Price AH, Meharg AA, McGrath SP, Zhao FJ (2011) The dynamics of arsenic in four paddy fields in the Bengal delta. Environ Pollut 159:947–953
Sun X, Li Z, Wu LH, Christie P, Luo YM, Fornara DA (2019) Root-induced soil acidification and cadmium mobilization in the rhizosphere of Sedum plumbizincicola: evidence from a high-resolution imaging study. Plant Soil 436:267–282
Teng YG, Wu J, Lu SJ, Wang YY, Jiao XD, Song LT (2014) Soil and soil environmental quality monitoring in China: a review. Environ Int 69:177–199
Van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334
Wan XM, Lei M, Chen TB (2016) Cost-benefit calculation of phytoremediation technology for heavy metal-contaminated soil. Sci Total Environ 563:796–802
Wang AS, Angle JS, Chaney RL, Delorme TA, Reeves RD (2006) Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 281:325–337
Wang GQ, Koopmans GF, Song J, Temminghoff EJM, Luo YM, Zhao QG, Japenga J (2007) Mobilization of heavy metals from contaminated paddy soil by EDDS, EDTA, and elemental sulfur. Environ Geochem Hlth 29:221–235
Wen G, Schoenau JJ, Yamamoto T, Inoue M (2001) A model of oxidation of an elemental sulfur fertilizer in soils. Soil Sci 166:607–613
Wu LH, Liu YJ, Zhou SB, Guo FG, Bi D, Guo XH, Baker AJM, Smith JAC, Luo YM (2013) Sedum plumbizincicola X.H. Guo et S.B. Zhou ex L.H. Wu (Crassulaceae): a new species from Zhejiang Province, China. Plant Syst Evol 299:487–498
Wu LH, Zhou JW, Zhou T, Li Z, Jiang JP, Zhu D, Hou JY, Wang ZY, Luo YM, Christie P (2018) Estimating cadmium availability to the hyperaccumulator Sedum plumbizincicola in a wide range of soil types using a piecewise function. Sci Total Environ 637:1342–1350
Wu C, Shi LZ, Xue SG, Li WC, Jiang XX, Rajendran M, Qian ZY (2019) Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. Sci Total Environ 647:1158–1168
Xue SG, Shi LZ, Wu C, Wu H, Qin YY, Pan WS, Hartley W, Cui MQ (2017) Cadmium, lead, and arsenic contamination in paddy soils of a mining area and their exposure effects on human HEPG2 and keratinocyte cell-lines. Environ Res 156:23–30
Yang JX, Yang J, Huang J (2017) Role of co-planting and chitosan in phytoextraction of As and heavy metals by Pteris vittata and castor bean: a field case. Ecol Eng 109:35–40
Zhan FD, Qin L, Guo XH, Tan JB, Liu NN, Zu YQ, Li Y (2016) Cadmium and lead accumulation and low-molecular-weight organic acids secreted by roots in an intercropping of a cadmium accumulator Sonchus asper L. with Vicia faba L. RSC Adv 6:33240–33248
Zhao FJ, Ma YB, Zhu YG, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 49:750–759
Acknowledgments
This study was funded by the National Key Research and Development Program of China (Nos. 2016YFE0106400 and 2016YFD0801104), the Science and Technology Project of Jiangsu Province (Nos. BE2017778 and BE2016812), and the Institute of Soil Science, Chinese Academy of Sciences (No. ISSASIP1613).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Antony Van der Ent.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, G., Hu, P., Zhou, J. et al. Sulfur application combined with water management enhances phytoextraction rate and decreases rice cadmium uptake in a Sedum plumbizincicola - Oryza sativa rotation. Plant Soil 440, 539–549 (2019). https://doi.org/10.1007/s11104-019-04095-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04095-w