Abstract
Sedum plumbizincicola X.H. Guo et S.B. Zhou ex L.H. Wu (Crassulaceae), a new species restricted to lead–zinc mining areas in Zhejiang Province, China, is described and illustrated. This taxon belongs to sect. Sedum (H. Ohba) S.H. Fu based on the adaxially gibbous carpels and follicles. It superficially resembles S. alfredii Hance and three other Sedum species found in the same area, but differs from these other taxa in bearing 4-merous flowers. Differences in geographical distribution, growth habit, phenology, macromorphology, leaf and stem anatomy, as well as seed micromorphology among S. plumbizincicola, S. alfredii and other related taxa in the genus Sedum are also reported. nrDNA internal transcribed spacers (ITS) sequences from seven populations of S. plumbizincicola support the recognition of this as a taxonomic entity distinct from S. alfredii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sedum L. is the largest genus in the family Crassulaceae and as currently circumscribed, includes about 430 species, with major centres of diversity in eastern Asia, the Mediterranean basin, and northern America and Mexico (’t Hart and Bleij 2003; Thiede and Eggli 2007). The genus appears to be paraphyletic and is consequently taxonomically rather problematic, and a large number of sectional names and segregate genera have been published (’t Hart and Bleij 2003; Carrillo-Reyes et al. 2009). Approximately 121 species (91 endemics) occur in China, which on the basis of morphological characters (gibbous carpels, spurred leaf bases and petal colour) have been assigned to three sections: sect. Filipes (Fröderström) S.H. Fu, sect. Oreades (Fröderström) K.T. Fu, and sect. Sedum (H. Ohba) S.H. Fu (Fu and Ohba 2001). Section Filipes includes eight species and is distributed in Bhutan, China, Japan, Myanmar, Nepal and Sikkim Province in India, with three species endemic to China; section Oreades contains about 67 species distributed in Bhutan, China, India, Myanmar, Nepal and Pakistan, with 64 species (54 endemic) in China; and section Sedum comprises more than 60 species, occurring mainly in Asia and Europe, with 49 species (34 endemics) in China.
In 2005, the senior author undertook extensive field research in Zhejiang Province and collected many specimens in a search for metal hyperaccumulator plants. Some unusual and isolated populations that superficially resembled S. alfredii Hance, but which produced 4-merous flowers, were found in Lin’an and Chun’an counties, together with four other species, S. alfredii, S. emarginatum Migo, S. hangzhouense K.T. Fu and G.Y. Rao and S. bailey Praeger, that were identified from the same areas. After comparison with the taxonomic accounts of Sedum in the Flora of China (Fu and Ohba 2001), the Flora of Zhejiang (He 1993), the Flora of Jiangsu (Jiangsu Institute of Botany 1982), the Flora of Anhui (Xue 1986), and the Flora of Jiangxi (Jiangxi Institute of Botany 2004), the authors found that the populations were distinctly different from S. alfredii and proposed that they should be recognized as a new species, Sedum plumbizincicola X.H. Guo et S.B. Zhou, in a brief report (without Latin or English description) in the Chinese journal Soils (Wu et al. 2006). The specimens were lodged in the Herbarium of Anhui Normal University, China (ANU!).
The identification of Sedum is traditionally based on macromorphological characters of vegetative and generative organs (Fu and Ohba 2001). However, micromorphological characters such as pollen morphology (Zheng 1997), leaf epidermis (Zheng and Gong 1999), stem anatomy (Zheng et al. 2001) and seed morphology (Jin et al. 2008) have also proved useful, whilst DNA characters have been used to reveal the relationships among Sedum species (Wu et al. 2008; Carrillo-Reyes et al. 2009; Li et al. 2010). The aim of this paper is to formally describe the new species S. plumbizincicola X.H. Guo et S.B. Zhou ex L.H. Wu, and to clarify the affinities between S. plumbizincicola and closely related taxa on the basis of geographical distribution, growth habit, phenology, macromorphological characters, stem and leaf anatomical features, seed micromorphology and nrDNA internal transcribed spacers (ITS) sequence data.
Materials and methods
Sixteen populations comprising S. hangzhouense (one population), S. alfredii (three populations), S. emarginatum (three populations), S. bailey (two populations) and S. plumbizincicola (seven populations) from Zhejiang Province, China were sampled during the summer of 2011 (Table 1), and more than ten individuals were sampled per population for morphological, anatomical and molecular research. Voucher specimens are deposited in the Herbarium of Anhui Normal University (ANU!), China. Fifty-six ITS sequences were downloaded from GenBank (http://www.ncbi.nlm.nih.gov/: Table 2).
Eighteen quantitative macromorphological characters were measured in the sampled taxa (Table 3). Ten measurements were made for each population and then averaged at species level. Eight qualitative macromorphological characters, together with four habit, habitat and phenology characters were also determined. Seeds of S. plumbizincicola and S. alfredii were collected from mature capsules of living specimens. Dried seeds were arranged on stubs and sputter-coated with gold for observation by scanning electron microscopy (SEM). Seed micromorphology was photographed and morphometry of seeds was examined using an FEI Quanta 200 ESEM scanning electron microscope at Nanjing Forestry University. Ten measurements were made for each population, and then averaged at species level.
Ten fresh leaves and stem sections of S. alfredii and S. plumbizincicola were fixed and preserved in formaldehyde–acetic acid–alcohol (FAA) solution (Stern and Judd, 2002). They were dehydrated in a graded ethanol series, embedded in paraffin and sectioned transversely to 8 μm with a KD-2508 Rotary Microtome (Zhejiang Jinhua Kedi Instrument Equipment Co., China). These sections were stained with methylene blue and fixed in neutral resin. Finally, all specimens were observed and photographed under an Olympus stereomicroscope (DP71) to compare the main anatomical characters.
Genomic DNA from 160 individuals belonging to 16 populations of five Sedum species (Table 1) was extracted from silica gel-dried leaves using the modified 2× hexadecyltrimethylammonium bromide (CTAB) method (Hellwig et al. 1999). ITS-F (TGAACCTGCGGAAGGATCAT) and ITS-R (GGTAGTCCCGCCTGACCTG) primers to the conserved domain of the ITS sequence were synthesized by the Beijing Genomics Institute. Each reaction solution contained 10 μl of 2× EasyTaq PCR SuperMix, 10 μmol μl−1 of each primer, and 1 μl of DNA template in a total volume of 20 μl. PCR amplifications were carried out on a TaKaRa PCR Thermal Cycler Dice using the following programme: 300 s of initial denaturation at 94 °C, followed by 34 cycles of 45 s denaturation at 94 °C, 45 s annealing at 58 °C and 90 s elongation at 72 °C, and finishing with 10 min elongation at 71 °C. PCR products were subcloned with the pEASY-T3 Cloning Kit (TransGen Biotech) according to the manufacturer’s instructions, and five colonies for each sample were screened. Products were sequenced at the Beijing Genomics Institute. The ITS sequence data for 16 populations, together with 56 ITS sequences downloaded from GenBank, were edited and aligned using Lasergene version 7.0 (Griffin and Griffin 1996) and DNAMAN version 6.0.40 (Altschul et al. 1990). Phylogenetic trees were inferred using maximum parsimony (MP) criteria as implemented in MEGA version 5.0 (Tamura et al. 2011). All positions containing gaps and missing data were eliminated. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) (Felsenstein 1985) was calculated. The MP tree was obtained using the Close-Neighbor-Interchange algorithm (Nei and Kumar 2000) with search level 3, in which the initial trees were obtained with the random addition of sequences (ten replicates).
Results and discussion
Description of the new species S. plumbizincicola
Type: China, Zhejiang Province, Hangzhou city, Chun’an county, Zitong town, 29°32′08″–29°37′00′′N, 118°34′48″–118°39′51″E, alt. 220 m, lead–zinc mining area, 10 June 2005, Bide05061028 (holotype ANU!; isotype IBK!).
Description: Sedum plumbizincicola X.H. Guo et S.B. Zhou ex L.H. Wu sp. nov. Perennial, light green, or yellowish green herb. Roots fibrous. Rhizomes slender and horizontal, yellowish brown or dark brown, to 7 cm long, ca. 4–8 mm diameter. Sterile stems several-branched, erect, 10–25 cm, densely caespitose. Leaves of foliage branches alternate, usually deciduous, crowded distally on stem; leaf blade ovate to obovate-spatulate, 1–5 × 0.5–1.5 cm, glabrous, apex blunted, base cuneate, pseudopetiolate; adaxial surface bright green, abaxial surface jade green, the midrib not convex abaxially. Inflorescence yellow, erect, much-branched, the peduncles up to 35 cm tall, ca. 0.8 cm in diameter. Cyme corymbiform, ca. 8 cm in diameter, many flowered, bracts linear to linear-lanceolate, 5–10 × 3–8 mm, apex blunted. Flowers sessile, unequally 4-merous. Sepals 4, narrowly triangulate, ca. 1–2 mm long apex blunted. petals 4, yellow, lanceolate, 4–6 × 1–1.5 mm, apex acute. Stamens 8, slightly shorter than the petals, antesepalous ones ca. 3.5–4.5 mm long, antepetalous ones ca. 3 mm long, inserted ca. 1 mm from petal base; filaments greenish, anthers oblong, yellow. Nectar scales inverted trapezia, ca. 0.3–1.0 × 0.8 mm. Carpels erect, ovoid-lanceolate, ca. 4–5 mm long, connate about one-third at base. Styles ca. 1 mm. Follicles split divergent, tetra-aristiform. Seeds numerous, brown, obovoid-oblong, ca. 0.7–1 mm long, mammillate. Flowering early June–August, fruiting July–September. See Figs. 1 and 2.
Sedum plumbizincicola in its vegetative (a) and flowering (b) states in the original habitat (Wu et al. 2006)
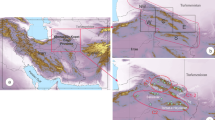
Ecology, distribution and importance: This new species is only known from the type locality, Zitong town (lead–zinc mining areas) northwest of Hangzhou city, in western Zhejiang Province of eastern China (see Fig. 3). Annual rainfall varies from 980 to 2,000 mm and occurs mainly in the summer, with a mean annual temperature of 15–18 °C. Soils in this area are usually sandy, acidic, highly leached and often shallow (http://baike.baidu.com/view/2341.htm). This species has a strong ability to hyperaccumulate zinc and cadmium, and is a promising taxon in the restoration of metal-polluted soils by phytoremediation.
Etymology: The specific epithet ‘plumbizincicola’ refers to the plant’s distribution in the lead and zinc mining areas of western Zhejiang Province, China.
Diagnosis: S. plumbizincicola is placed in sect. Sedum according to the adaxially gibbous carpels and follicles. S. alfredii, S. emarginatum, S. hangzhouense and S. bailey are also distributed in the same areas as S. plumbizincicola, but they produce 5-merous flowers while the latter bears 4-merous flowers. S. tetractinum Fröderström, S. hakonense Makino and S. dongzhiense D.Q. Wang and Y.L. Shi in sect. Sedum also bear 4-merous flowers, but they differ from S. plumbizincicola in leaf blade shape (oblanceolate in S. hakonense and S. dongzhiense, orbicular in S. tetractinum, and ovate to obovate-spathulate in S. plumbizincicola) and in their habitats.
Additional collections: China. Zhejiang Province: Chun’an county, Zitong town, alt. 250 m, 10 June 2011, Y.j.Liu 201106021(ANU!); ibid., 251 m, Y.j.Liu 201106022(ANU!); ibid., 234 m, Y.j.Liu 201106023(ANU!); ibid., 258 m, Y.j.Liu 201106024(ANU!); Zhejiang province: Chun’an county, Panjia town, alt. 134 m, Y.j.Liu 201106025(ANU!); ibid., 188 m, Y.j.Liu 201106026(ANU!); ibid., 199 m, Y.j.Liu 201106027(ANU!).
Affinities between S. plumbizincicola and closely related taxa
Because we found that S. plumbizincicola mostly resembled S. alfredii and that S. hangzhouense, S. emarginatum and S. baileyi were distributed in the same areas as these two species, our research work focused on the relationships among these five taxa.
Macromorphological, phenological and ecological characters of the five Sedum species are listed in Table 3. Macromorphological characters show that S. plumbizincicola is differentiated from S. alfredii and other similar Sedum species by its 4-merous flowers and thick sterile stems. Its restriction to metalliferous mining areas and low altitude in distribution also support recognition of S. plumbizincicola as a distinct taxonomic entity.
The leaf and stem anatomical characters of S. alfredii and S. plumbizincicola are shown in Table 4 and Fig. 4. Leaves of S. plumbizincicola are thicker than those of S. alfredii. The stems of S. alfredii are narrower than those of S. plumbizincicola, whilst S. alfredii stems have more cortical parenchyma cells and fewer pith cells than S. plumbizincicola. Sedum plumbizincicola produces more vascular bundles and has a higher ratio of xylem to phloem cross-sectional area than S. alfredii. The leaf and stem anatomical data support S. alfredii and S. plumbizincicola as separate taxa.
The seed micromorphological characters of S. alfredii and S. plumbizincicola are displayed in Fig. 5 and their comparisons with S. hangzhouense, S. emarginatum and S. baileyi are listed in Table 5. The epidermal cells of S. alfredii and S. hangzhouense seeds are uplifted, whereas those of the other three species are not. The surfaces of S. alfredii and S. plumbizincicola seeds are both loosely mammillate, but the mammillae differ in shape. Those of S. alfredii are spherica,l whereas those of S. plumbizincicola are prolate-spheroidal. The seed micromorphological characters also suggest that S. alfredii and S. plumbizincicola are different species.
The nrDNA ITS sequences of 16 populations of S. plumbizincicola, S. alfredii, S. emarginatum, S. hangzhouense and S. baileyi (Table 1) were determined. These ITS sequence data, together with 56 ITS sequence data of Sedum species and three outgroups downloaded from NCBI (Table 2), were used to reconstruct a nrDNA ITS phylogeny for these taxa using maximum parsimony. The sequence alignment consisted of 682 characters, of which 443 were variable sites and 352 were parsimony-informative sites. Twenty equally parsimonious trees were obtained (length = 1852, consistency index = 0.42, retention index = 0.78), of which one is shown in Fig. 6. The seven S. plumbizincicola accessions (top of Fig. 6) form a strongly supported monophyletic clade (99 % bootstrap support) sister to S. alfredii. Together, S. plumbizincicola and S. alfredii form a strongly supported clade (99 % bootstrap support) sister to a monophyletic S. emarginatum. Thus, the nrDNA ITS phylogeny supports the concept of S. plumbizincicola as a monophyletic entity.
At the infrageneric level, there is still uncertainty about taxonomic relationships within Sedum. Praeger (1921) recognized ten sections within the genus Sedum, whereas Berger (1930) recognized 22, of which ten have now been transferred to other genera (Carrillo-Reyes et al. 2009). Fu and Ohba (2001) assigned the Chinese species of Sedum to three sections, i.e. sect. Filipe and sect. Oreades (Fu 1965, 1974) and sect. Sedum, the largest in the genus (’t Hart 1991; Carrillo-Reyes et al. 2009). Figure 6 shows that the 69 accessions of 49 Sedum species can be split into two strongly supported clades (99 % bootstrap support): the upper one in Fig. 6 comprises 24 accessions of 7 species from China, 11 accessions of 11 species from Japan, and four accessions of four species from Nepal, whereas the lower one contains 30 accessions of 29 species from Mexico. This result suggests that Sedum species in East Asia are phylogenetically distinct from those in Mexico. Among the 39 East Asia accessions, S. oreades (Decaisne) Raym.-Hamet and S. trullipetalum J.D. Hooker and Thomson belong to sect. Oreades, while all the other accessions belong to sect. Sedum (Fu and Ohba 2001). Figure 6 does not support a sister-group relationship between S. oreades and S. trullipetalum, although relationships are not well resolved in this part of the tree. Nevertheless, this result lends weight to the view that the phylogeny of Sedum inferred from nucleotide sequence data does not accord with classifications of the genus based on morphological characters (Carrillo-Reyes et al. 2009). Greater taxon sampling and additional markers will be required to arrive at a more definitive consensus on infrageneric relationships within Sedum, but we place the new species S. plumbizincicola in sect. Sedum on the basis of its adaxially gibbous carpels and follicles.
In summary, the ecological, macromorphological, micromorphological and molecular data show that S. plumbizincicola should be recognized as a new species in sect. Sedum.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Berger A (1930) Crassulaceae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol 18A, 2nd edn. Verlag von WEngelmann, Leipzig, pp 352–483
Carrillo-Reyes P, Sosa V, Mort ME (2009) Molecular phylogeny of the Acre clade (Crassulaceae): dealing with the lack of definitions for Echeveria and Sedum. Mol Phylogenet Evol 53:267–276
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fu KT (1965) Species et combinaciones novae Crassulacearum sinicarum. Acta Phytotax Sin 10:111–128
Fu KT (1974) Revision of the section Oreades of Chinese Sedum. Acta Phytotax Sin 12:51–77
Fu KJ, Ohba H (2001) Crassulaceae. In: Wu ZY, Raven PH (eds) Flora of China, vol 8., Brassicaceae through SaxifragaceaeScience Press, Beijing and Missouri Botanical Garden Press, St Louis, pp 202–268
Griffin HG, Griffin AM (1996) Lasergene from DNAStar offers a comprehensive sequence analysis package. Mol Biotechnol 5:184
He YQ (1993) Sedum L. In: Flora of Zhejiang. vol 3, Science Press of Zhejiang, Hangzhou, pp 73–84
Hellwig FH, Nolte M, Ochsmann J, Wissemann V (1999) Rapid isolation of total cell DNA from milligram plant tissue. Haussknechtia 7:29–34
Jiangsu Institute of Botany (1982) Flora of Jiangsu, vol 2. Science Press of Jiangsu, Nanjing
Jiangxi Institute of Botany (2004) Flora of Jiangxi, vol 2. Science Press of Chinese, Nanchang
Jin XF, Qian L, Lu YH, Zhang HW, Wang HZ (2008) Seed micromorphology of Sedum (s.l.) from Zhejiang and its taxonomic implications. J Zhejiang Univ (Agric Life Sci) 34:409–417
Li N, Chen KL, Liu Z, Ye CJ, Chen SL (2010) Identification of medicinal plants from genus Sedum based on DNA bar coding. World Sci Technol Mod Tradit Chin Med Mater Medica 12:463–467
Nei M, Kumar S (2000) Molecular Evolution and Phylogenetics. Oxford University Press, New York
Praeger LR (1921) An account of the genus Sedum as found in cultivation. J R Hort Soc 46:1–314
Stern WL, Judd WS (2002) Systematic and comparative anatomy of Cymbidieae (Orchidaceae). Bot J Linn Soc 139:1–27
’t Hart H (1991) Evolution and classification of the European Sedum species. Fl Medit 1:31–61
’t Hart H, Bleij B (2003) Sedum. In: Eggli U (ed) Illustrated handbook of succulent plants: Crassulaceae. Springer, Berlin, pp 235–332
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thiede J, Eggli U (2007) Crassulaceae. In: Kubitzki K (ed) The families and genera of vascular plants, vol IX. Springer, Berlin, pp 83–118
Wu LH, Zhou SB, Bi D, Guo XH, Qin WH, Wang H, Wang CJ, Luo YM (2006) Sedum plumbizincicola, a new species of the Crassulaceae from Zhejiang. Soils 38:632–633
Wu HJ, Xu R, Wan DR, Chen Y (2008) Genetic diversity analysis on the medicinal plants of Sedum L. by RAPD. J Huazhong Agric Univ 27:782–786
Xue ZW (1986) Sedum L. In: Flora of Anhui, vol 2. Chinese Forecast Press, Anhui, pp 498–511
Zheng Y (1997) A pollen morphology study on twelve species of Sedum in Anhui. Bull Bot Res 17:158–162
Zheng Y, Gong J (1999) A leaf epidermis study on twelve species of Sedum in Anhui. Bull Bot Res 19:292–297
Zheng Y, Gong J, Liu DY, Jiang Y, Xu YM (2001) Anatomical studies on stem of Sedum from Anhui Province. J Anhui Normal Univ (Nat Sci) 24:239–242
Acknowledgments
This research was supported jointly by the International Scientific Collaborative Programme of the Ministry of Science and Technology of China (2010DFA92360), the National Natural Science Foundation of China (40821140539, 40871155), and the Program of Innovative Engineering of the Chinese Academy of Sciences (KSCX2-YW-G-053). A grant from Anhui Normal University is gratefully acknowledged. We extend special thanks to research scientists Xiao-quan Wang and Xian-zhao Kan. We thank Xia Pan, Jing Ren, Jie-xue Huang, Feng-jiao Nai, Li-ke Cheng, Li-qiang Zhou, Qi Luo, Ying Wang, Gui-jun Wang, Benqi Yu, Jinrong Hu, Jinghua Li and Naidong Chen for useful discussion regarding populations, habitat, and conservation. We also thank Dr S.A. Harris, Curator of the Oxford University Herbaria, UK, for helpful advice and discussions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, L.H., Liu, Y.J., Zhou, S.B. et al. Sedum plumbizincicola X.H. Guo et S.B. Zhou ex L.H. Wu (Crassulaceae): a new species from Zhejiang Province, China. Plant Syst Evol 299, 487–498 (2013). https://doi.org/10.1007/s00606-012-0738-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-012-0738-x