Abstract
Background and Aims
The inhibitory effect of selenium (Se) on cadmium accumulation in brown rice (BR-Cd) is controversial, which may be related to the Se application time. The optimum Se application time for reducing the BR-Cd is investigated.
Methods
A pot experiment was conducted to examine the changes in the dynamics of iron plaque on root surface and Cd accumulation in rice as Se was applied at different growth stages to two Cd-contaminated soils, and explore its mechanisms. Field trials were conducted to verify the results.
Results
Iron plaque formed mainly at the middle stage (tillering to booting stage) of rice and then decreased at the mature stage. Se application at the seeding, tillering, and booting stages reduced the BR-Cd by 10.6–18.4%, 14.9–23.7%, and 10.8–20.9% in the neutral soil, 18.3–32.4%, 40.3–63.0%, and 22.7–40.6% in the acid soil, respectively. Two field trials demonstrated that tillering application of Se caused significant reductions (27.1–35.1%) of BR-Cd.
Conclusions
Tillering stage is optimal for Se application to reduce BR-Cd, mainly because tillering application of Se causes the maximum amount iron plaque at the booting stage, maximumly inhibit Cd uptake by the root; meanwhile, the transport of Cd from the root to brown rice is inhibited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is one of the most highly toxic metals in the environment (Oono et al. 2014). It is widely distributed in farmland due to anthropogenic inputs such as the emission of industrial pollutants, application of phosphate fertilizers and sewage, and some organophosphorus pesticides such as amyl cyanoprene-malathion (Liu et al. 2009; Xiao et al. 2014). Cd ranks first in China in the percentage of sampling sites (7.0%) that exceed the soil quality standards, although 93.0% of the sites were classified as being slightly (0.3–0.9 mg kg−1 Cd in the soil with pH < 7.5, 0.6–1.8 mg kg−1 Cd in the soil with pH > 7.5) to moderately (0.9–1.5 mg kg−1 Cd in the soil with pH < 7.5, 1.8–3.0 mg kg−1 Cd in the soil with pH > 7.5) contaminated (the Ministry of Environmental Protection (MEP) and the Ministry of Land and Resources (MLR) of the People’s Republic of China 2014). Excess Cd in farmland severely threatens food safety (Sanita di Toppi and Gabbrielli 1999) and increases the health risk to human through the food chain. Rice (Oryza sativa L.), the main cereal crop in the world, is regarded as a major source of the dietary intake of Cd by humans (Meharg et al. 2013). The long-term consumption of grains with excessive Cd can lead to a number of incurable diseases (Baba et al. 2013; Moulis and Thevenod 2010; Skroder et al. 2015). In addition, rice has a higher Cd bio-accumulation factor compared to other cereals (Arao et al. 2009). Thus, efficient strategies should be studied to lower the risk of Cd consumption in brown rice.
Many strategies have been developed to reduce Cd accumulation in rice grain, such as screening and breeding low Cd-affinity cultivars (Uraguchi et al. 2011), applying alkaline-stabilized biosolids (Bolan et al. 2003; Wang et al. 2015), and adding beneficial elements such as selenium (Se) (Wan et al. 2016). Among these options, the application of beneficial elements is considered to be a convenient and effective way to treat slightly to moderately Cd-contaminated soil (Hu et al. 2014). Selenium has been proven to have an antagonistic effect on Cd uptake by many plants such as rice, wheat, and tobacco (Feng et al. 2013; Khan et al. 2015; Liu et al. 2015). In addition, Lin et al. (2012) found that Se can mitigate Cd toxicity in rice by depressing reactive oxygen species (ROS) such as O2• − and H2O2 induced by Cd stress. Therefore, the appropriate application of Se fertilizer is a potential way to remediate slightly to moderately Cd-contaminated soil, due to its multiple benefits to plants and humans.
Iron plaque is considered to be a barrier on the surface of rice roots involved in restricting many contaminants (Cheng et al. 2014; Yamaguchi et al. 2014). It has been reported that Se (IV) application increased the formation of iron plaque on the root surface at the mature stage and thus lowered Cd accumulation in rice (Chang et al. 2013). However, Hu et al. (2014) found that Se application did not stimulate Cd sequestration in iron plaque at the mature stage. The formation of iron plaque depends mainly on the level of radial oxygen loss (ROL) involved in the oxidation of Fe2+ in the rhizosphere (Wu et al. 2012; Yang et al. 2014). ROL has been found to increase rapidly from the tillering to the ear emergence stages but then greatly reduced at the grain-filling stage, resulting in a significant reduction in iron plaque at the mature stage (Wang et al. 2013). Thus, results that focus only on the mature stage do not reflect the real effects of Se (IV) on the formation of iron plaque and its sequestration of Cd. It is necessary to investigate the dynamic changes of iron plaque during the whole growth period of rice. In addition, selenite, the main form used in the previous studies, is generally applied as a base fertilizer in pot or hydroponic experiments (Hu et al. 2014; Lin et al. 2012). While the formation of iron plaque and accumulation of Cd in rice are more intensive in the middle growth stage (Wang et al. 2013). The effects of Se (IV) applied at different growth stages of rice are still unknown. In addition, since Se was found to be thermodynamically unstable in the soil (Zhu et al. 2009), the early stage of rice may not be the optimal application time for Se uptake and the enhancement of the formation of iron plaque. Therefore, it is of interest to study the effects of selenite applied at different growth stages.
Therefore, in this study, the pot experiment aimed to investigate the effects of selenite applied at different growth stages including the seeding, tillering and booting stages of rice on Cd accumulation in rice tissues at the mature stage and to investigate the dynamic changes in iron plaque and Cd sequestration at different stages and the transport characteristics of Cd within rice to clarify the underlying mechanisms. Field experiments were conducted during the following year to verify the effect of the optimal application time of Se on reducing Cd accumulation in brown rice.
Materials and methods
Soil preparation and pot experiment
A neutral paddy soil, was collected from Jingjiang, Jiangsu Province, China, and an acid paddy soil, was collected from Xiangyin, Hunan Province, China. The principal soil properties are shown in Table 1. The soils were air-dried and then passed through a 2-mm sieve. In order to study the performance of Se in the soil contaminated with different Cd levels, three Cd additions as 3CdSO4·8H2O to the soil (none (Cd1), 0.3 (Cd2), and 0.9 (Cd3) mg Cd [kg soil]−1) were designed, and then the soils were incubated at 80% of the field water-holding capacity for three months to simulate long term Cd-contaminated field soil (Alexander et al. 2006). After the aging process, the acid soils with 0.41, 0.68 and 1.28 mg kg−1 Cd and the neutral soils with 0.33, 0.62 and 1.21 mg kg−1 Cd were obtained after three Cd additions to the soils (none, 0.3, and 0.9 mg Cd [kg soil]−1). These soils were used for the pot experiment.
A pot experiment was conducted from May to October 2015 at the greenhouse of the Institute of Soil Science, Chinese Academy of Sciences, Nanjing, Jiangsu Province, China. All pots (30 cm diameter and 29 cm high) were filled with 8 kg soil, and base fertilizers were added to each pot as follows: 0.20 g N kg−1 DWsoil (dry weight of soil) (CO (NH2)2), 0.15 g P2O5 kg−1 DWsoil (CaH2PO4 H2O) and 0.20 g K2O kg−1 DWsoil (KCl). The soils were kept moist for seven days before the rice was planted. The treatments in each Cd-contaminated soil (6 Cd-contaminated soils in total) were designed as follows: the control treatment (no Se addition, CK1) with 12 replicates, 0.50 mg kg−1 DWsoil Se (Na2SeO3) applied to the soils at the seeding stage (SD-Se) with 12 replicates, the tillering stage (TL-Se, approximately 45 days) with 6 replicates and the booting stage (BT-Se, approximately 85 days) of rice with 3 replicates, respectively. Since the in situ immobilization using hydrated lime is the most commonly used method in the remediation of Cd-contaminated acid soil (Bolan et al. 2003), in order to compare the effects of Se application with respect to that of the immobilization method, 1 g kg−1 hydrated lime was mixed with the acid soils as another control (CK2) with 12 replicates. In total, there were 72 pots for the CK1, 72 pots for the CK2, 72 pots for the SD-Se, 36 pots for the TL-Se, 18 pots for the BT-Se. Se was applied to the soil in the form of solution, i.e., 8.76 mg pot−1 Na2SeO3 dissolved in 500 mL deionized water, and then evenly applied to each pot; while in the treatments of TL-Se and BT-Se, the pots were drained to keep approximately 1 cm water layer above the soil before Se application. Each sampling stage included three replicates.
Plant culture and collection
Suxiangjing 1 (obtained from the Suzhou Academy of Agricultural Sciences, China), one of the mainly planted cultivars in Jiangsu Province, was used as the experimental rice cultivar for the pot experiment described above. After being surface sterilized in 30% (v/v) H2O2 solution for 15 min, the seeds were rinsed with deionized water and soaked in deionized water at 25 °C in the dark for 24 h, then germinated in moist gauze for another 24 h. Afterwards, the healthy and uniform seeds were directly sown in the pots. Three holes were evenly set in each pot, and initially five seeds were placed in each hole. After emergence, the seedlings were thinned to two in each hole. Then, the soils were kept submerged (2–3 cm deep) throughout the whole rice-growing period.
The sampling time in this study varied between the different treatments (Table 2). The plants and soils were destructively sampled with three replicates at each sampling time. The roots were separated from the aerial part collected from each stage and then carefully rinsed three times with tap water followed by deionized water. After the iron plaque was extracted, the roots were oven-dried to a constant weight at 75 °C. The remaining samples collected at the mature stage were separated into shoots, husk, and brown rice. After rinsing with tap water and deionized water, these parts were also oven-dried to a constant weight at 75 °C. All parts were weighed after oven-drying.
Field trial
According to the results obtained from the pot experiment, the effect of Se application at the tillering stage on Cd accumulation in brown rice was verified through field trials. The field trials were carried out from May to October 2016 at Guixi County, Jiangxi Province, China (Site 1, N 28°01′, E 117°13′) and Tongling City, Anhui Province, China (Site 2, N 31°01′, E 117°53′). The soil at site 1 contained 1.11 ± 0.06 mg kg−1 Cd, and the pH value was 4.68 ± 0.19. The soil at site 2 contained 0.43 ± 0.05 mg kg−1 Cd, and its pH value was 6.01 ± 0.07. The local cultivar of rice was used in the field trials (Site 1: Zhongzao 39; Site 2: Wuyouhuazhan), the plot area was 20 m2 (4 m × 5 m). At the tillering stage, 0.50 mg kg−1 Se (0.25 g Na2SeO3 m−2) in a solution was applied to the topsoil (TL-Se), i.e., 5 g Na2SeO3 per plot was dissolved in 10 L water, and then applied to the topsoil using a sprinkling can (with a big hole nozzle); the water application was utilized as the control (CK1). The plots were drained to keep approximately 1 cm water layer above the soil before Se application, and flooded with approximately 5 cm water layer after 2 d, the plots were undrained at least for three weeks after Se application to prevent the loss of Se from drainage. Each treatment had three plots. At the mature stage, the brown rice was collected to determine the concentration of Cd.
Chemical analysis
The dithionite–citrate–bicarbonate (DCB) method (Otte et al. 1989; Taylor and Crowder 1983) was used to extract the iron plaques from the root surface, i.e., the fresh root were extracted in the mixed solution with 0.03 M sodium citrate (Na3C6H5O7·2H2O) and 0.125 M sodium bicarbonate (NaHCO3) solution, after 30 min, adding sodium dithionite (Na2S2O4) to the solution for another 70 min extraction. The detailed information referred to our previous description (Huang et al. 2017). The concentration of Fe and Cd in the extraction was determined utilizing atomic absorption spectroscopy (AAS) (SpectrAA 220Z, Varian, USA).
The plant samples were digested in HNO3-H2O2 solution (GR) using high pressure sealed digestion vessels (HTLAB, HR-25, Shanghai), the concentrations of Cd in the different parts of the rice and of Se in brown rice were determined using AAS and atom fluorescence spectroscopy (AFS-930, JiTian Instruments, Beijing), according to our previous study (Huang et al. 2017).
Water-extractable iron in the soil was extracted using ultrapure water (pH = 7.0) at a soil water ratio of 1:20 as described previously (Nishita and Haug 1974) and then determined utilizing AAS. The ferrous in the soil was extracted using 0.5 M HCl solution as described by Lovley and Phillips (1987), and determined using an MQX200 microplate reader (Bio-Tek Instrument, USA).
Data analysis
The total Cd in the plant was calculated by the equation as follows:
where Croot, Cshoot, Chusk and Cbrown rice represent the concentration of Cd in the root (without iron plaque), shoot, husk and brown rice, respectively. DWroot, DWshoot, DWhusk and DWbrown rice represent the dry weight of the root (without iron plaque), shoot, husk and brown rice, respectively.
Translocation factors (TFs) of Cd from the root to the shoot, the shoot to the husk, and the husk to brown rice were calculated as follows:
where a represents the root, shoot and husk, b represents the shoot, husk and brown rice. Ca and Cb represent the concentration of Cd in a and b, respectively.
Statistical analysis
The statistical relationship among all data was analyzed using one–way analysis of variance (ANOVA), and the least significant difference (LSD) was utilized to check the significant differences between the mean values (P < 0.05) utilizing SPSS 19.0. All data were expressed as the mean ± SD (n = 3). All of the figures were generated utilizing SigmaPlot 10.0 software.
Results
Dynamic formation of iron plaque on root surface at four growth stages
In the neutral soil, the DCB extractable Fe of roots was low at the seedling and tillering stages, quickly reached its peak at the booting stage, and finally decreased at the mature stage, as shown in Fig. 1. However, The DCB-extractable Fe increased rapidly as the rice grew, and almost peaked at the tillering stage before decreasing at the mature stage in the acid soil. The DCB-extractable Fe of the roots grown in the acid soil was much higher than that in the neutral soil (Fig. 1).
The dynamics of DCB-extractable Fe on the roots affected by Se application at different stages. Neutral soil contaminated with 0.33 (a), 0.62 (b), and 1.21 mg kg−1 (c) of Cd, and acid soil contaminated with 0.41 (d), 0.68 (e), and 1.28 mg kg−1 (f) of Cd; SL, TL, BT and MT mean seedlings, tillering, booting and mature stages of rice, respectively. SD-Se, TL-Se and BT-Se mean Se was applied at the seeding, tillering, and booting stages, respectively; CK1 and CK2 mean the blank control and lime treatment, respectively
Se application at the seeding stage (SD-Se) in the neutral soil significantly increased the concentration of DCB-extractable Fe by 23.9–26.7% and 38.7–57.4% at the seedling stage (SL) and the tillering stage (TL), respectively, compared to the CK1 (Fig. 1). At the booting stage (BT), the rates of increase were only 7.9–16.5% in the SD-Se treatment, while the application of Se at the tillering stage (TL-Se) significantly increased the concentration of DCB-extractable Fe by 30.6–34.5% (Fig. 1). At the mature stage, the concentration of DCB-extractable Fe had no significant difference among all of the treatments except for CK2 (Fig. 1). Similar results were observed in the acid soil. In addition, the lime application at the seeding stage (CK2) significantly decreased the concentration of DCB-extractable Fe by 22.5–39.3% during the whole growth period (Fig. 1).
Dynamics of cd sequestration in iron plaque
The amounts of DCB-extractable Cd on the root surface of rice grown in the neutral soil increased in parallel with the level of Cd in the soil (Fig. 2a, b, c). As the rice grew, the amounts of DCB-extractable Cd increased and reached their peak at the booting stage, then decreased at the mature stage (Fig. 2a, b, c). In the acid soil, the amounts of DCB-extractable Cd on the root surface were significantly higher than that in the neutral soil, and its peak occurred at the tillering and booting stages, then decreased at the mature stage (Fig. 2d, e, f).
The dynamics of DCB-extractable total Cd on the roots affected by Se application at different stages. Neutral soil contaminated with 0.33 (a), 0.62 (b), and 1.21 mg kg−1 (c) of Cd, and acid soil contaminated with 0.41 (d), 0.68 (e), and 1.28 mg kg−1 (f) of Cd; SL, TL, BT and MT mean seedlings, tillering, booting and mature stages of rice, respectively. SD-Se, TL-Se and BT-Se mean Se was applied at the seeding, tillering, and booting stages, respectively; CK1 and CK2 mean the blank control and lime treatment, respectively
In the neutral soil, the SD-Se treatment significantly increased the amounts of DCB-extractable Cd by 13.9–24.7% and 39.3–42.6% at the seedling and tillering stages, respectively, compared to the CK1; at the booting stage (BS), the amount of DCB-extractable Cd increased by only 5.0–10.1% in the SD-Se treatment, while it increased by 33.2–37.9% in the TL-Se treatment; at the mature stage, the DCB-extractable Cd did not differ significantly among the different treatments (Fig. 2a, b, c). Similar results were found in the acid soil, but the rates of increase in the TL-Se treatment were much higher, ranging from 28.7 to 100.0% at the booting stage; however, lime treatment significantly decreased the amounts of DCB-extractable Cd by 22.5–92.7% at the seedling and tillering stages (Fig. 2d, e, f).
Effects on the concentration and distribution of cd in different rice tissues
The concentrations of Cd were significantly different among the different tissues of rice that followed the order of root > shoot > brown rice > husk; the concentrations of Cd in the rice tissues from the acid soil were two–fold higher than that from the neutral soil (Table 3).
The application of Se decreased the concentration of Cd in the brown rice by 18.4–23.7% and 18.3–63.0% in the neutral and acid soils, respectively, compared to the CK1; among these treatments, the TL-Se treatment had the highest rate of reduction in both soils (Table 3). The Cd concentrations in the husk were significantly increased by the TL-Se and BT-Se treatments in the neutral soil; in other tissues, the Cd concentrations in both soils decreased after all of the treatments (Table 3). The allocation of Cd in the brown rice significantly decreased by 24.8 and 26.5% in the TL-Se and BT-Se treatments in the acid soil, respectively, as well as in the shoot; while the TL-Se and BT-Se treatments in the acid soil significantly increased the allocation of Cd in the root by 21.6 and 17.4%, respectively, as well as Cd allocation in the husk, the rates of increase were 41.4 and 38.7%, respectively (Table 3).
Effects on the accumulation of cd in the rice plant
The amounts of total Cd in the mature rice grown in the acid soil were two-fold higher than that grown in the neutral soil; in both soils, the amounts of total Cd increased with the increase of the Cd level in the soil (Fig. 3).
The accumulation of Cd in the mature rice affected by Se application at different stages in the neutral (a) and acid soils (b). Bars with the same letter(s) indicate no significant differences between different treatments at P < 0.05. SD-Se, TL-Se and BT-Se mean Se was applied at the seeding, tillering, and booting stages, respectively; CK1 and CK2 mean the blank control and lime treatment, respectively
The TL-Se treatment significantly decreased the total amounts of Cd uptake by 16.6–18.2% in three Cd levels of the neutral soil, while the reduction in the SD-Se and BT-Se treatments was only 6.0–11.5% and 5.7–15.5%, respectively, compared to that of CK1 (Fig. 3a). The inhibitory effects were enhanced in the acid soil. Compared with the CK1, the TL-Se treatment significantly decreased the total amounts of Cd uptake by 41.0–43.2% in the three Cd levels of the acid soil, and the reduction in the SD-Se and BT-Se treatments was 10.5–12.6% and 17.2–20.8%, respectively (Fig. 3b).
Effects on the transport of cd in the rice plant
In the neutral soil, the translocation factor (TFs) of Cd from the root to the brown rice was significantly decreased in the TL-Se and BT-Se treatments, within this transport process, the transport from the root to the shoot was decreased by 14.1 and 13.3%, and the transport from the husk to the brown rice was decreased by 34.2 and 33.4% in the TL-Se and BT-Se treatments, respectively, compared to that of the CK1 (Fig. 4a, b, c). Similar tendency was observed in the acid soil, while the reduction of TFs was higher than that in the neutral soil (Fig. 4d, e, f).
Box plots showing the translocation factors of Cd when Se was applied at different stages. The box plots show the medians (solid line) and means (short dash line). The bars with the same letter(s) indicate no significant differences between different treatments at P < 0.05 (n = 9). NS and AS mean the neutral and acid soil; SD-Se, TL-Se and BT-Se mean Se was applied at the seeding, tillering, and booting stages, respectively; CK1 and CK2 mean the blank control and lime treatment, respectively
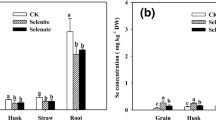
Effects on the accumulation of se in the brown rice
In the same application time of Se, the concentration of Se in the brown rice in the acid soil was approximately 2.50-fold higher than that in the neutral soil; The concentration of Se in the different treatments followed the order of BT-Se > TL-Se > SD-Se > CK1 in both soils (Fig. 5a, b). In addition, the concentration of Se in the brown rice obviously decreased as the level of Cd increased in both soils (Fig. 5a, b).
Changes of Se in the brown rice affected by the application of Se at different stages in the neutral (a) and acid soils (b). Bars with the same letter(s) indicate no significant differences between different treatments at P < 0.05. SD-Se, TL-Se and BT-Se mean Se was applied at the seeding, tillering, and booting stages, respectively; CK1 and CK2 mean the blank control and lime treatment, respectively
Cd accumulation in brown rice in field trials
In both sites of the field trial, the concentrations of Cd in the brown rice without the application of Se exceeded the Chinese food safety standard of 0.20 mg kg−1 (GB 2762–2017) (National Health and Family Planning Commission of People’s Republic of China 2017), and the levels of Cd in the brown rice in site 1 were much higher than those in site 2 (Fig. 6). The application of 0.50 mg kg−1 Se (0.25 g Na2SeO3 m−2) at the tillering stage, significantly decreased the concentration of Cd in the brown rice by 35.1% (P < 0.01) in site 1 and decreased by 27.1% in site 2 compared with the corresponding control (CK1) (Fig. 6).
Discussion
Current results showed that the amounts of iron plaque increased quickly at the early to middle growth stages, and then decreased at the mature stage (see Fig. 1). A similar result was observed in the study of Wang et al. (2013). This might be attributed to the change in radial oxygen loss (ROL) involved in the oxidation process of ferrous ion in the rhizosphere (Wang et al. 2013; Yang et al. 2014). Interestingly, although the concentration of total iron in the neutral soil was higher than the acid soil (see Table 1), the amounts of iron plaque on the rice roots grown in the neutral soil were significantly lower than that in the acid soil in this study (Fig. 1). This could be due to the higher concentration of ferrous and water-extractable iron in the acid soil than that in the neutral soil (see Table 1). Thus, the iron readily moved to the rhizosphere in the acid soil to form iron plaque. The mobility of the iron was mainly associated with the soil pH. As the soil pH decreased, more iron ions would be desorbed from the soil colloids (Mendelssohn et al. 1995).
Previous studies had reported that Se can inhibit the uptake and accumulation of Cd in rice (Liao et al. 2016; Wan et al. 2016), which was consistent with the present results that 5.7–15.5% and 10.5–43.2% of total Cd were reduced in the neutral and acid soils (see Fig. 3). In this study, enhancing the formation of iron plaque on the root surface was considered to be one of the main underlying mechanisms (see Fig. 1). Generally, plant will suffer physiological toxicity under the stress of heavy metals or nanoparticles (Song et al. 2014; Rastogi et al. 2017), e.g., Cd accumulation in plant will induce the production of O2•−, •OH, and H2O2 (Lin et al. 2012; Xu et al. 2016). Application of some special substances can alleviate those stresses, such as Se fertilizer and salicylic acid which involved in eliminating the reactive oxygen species (ROS) and alleviating the oxidative stress induced by toxic metal (Lin et al. 2012; Song et al. 2014). The results of oxidative parameters from the seedling stage in this study also showed the similar results (see Fig. S2). Those ROS in the rhizosphere are beneficial to the oxidation of ferrous ion, and might enhance the formation of iron plaque. Iron plaque on the root surface was mainly composed of iron oxyhydroxide that had a large surface area of more than 200 m2 g−1, thus providing reactive substrates to bind metals (Hansel et al. 2001). Therefore, increasing the Cd sequestration in the iron plaque is an important reason contributing to the reduction of Cd accumulation in the rice plant caused by Se. However, this inhibitory effect depended on the application time of the Se. The application of Se at the early stage, such as seeding stage, slightly reduced the brown rice Cd (see Table 3), since the iron plaque mainly formed at the tillering and booting stages of rice (see Fig. 1). The application of Se at the tillering stage significantly increased the amounts of iron plaque at the booting stage (see Fig. 1) and had the greatest reduction on the amount of Cd in the rice (see Fig. 3). At the mature stage of rice, the amounts of iron plaque dropped to a low and similar level in all of the treatments (see Fig. 1). Therefore, controversial results of the effects of Se on the iron plaque were observed in the previous studies that only focused on the mature stage (Chang et al. 2013; Hu et al. 2014). In addition, soil pH is also an important factor affecting the uptake of Cd by rice by adjusting the phytoavailability of Cd (Ding et al. 2013), and the phytoavailability of Cd decrease with increasing soil pH, thus lime treatment significantly decreased the Cd accumulation in rice plant (see Fig. 3). However, the increased soil pH decreased the formation of iron plaque and Cd sequestration on root surface (see Figs. 1 and 2), which might attribute to the immobilization of iron in the soil induced by lime (Barman et al. 2014). Therefore, the decreased amount of iron plaque is considered a potential reason accounting for that the lower reduction of Cd accumulation in rice in lime treatment than the TL-Se treatment. In this study, the acid soil had a much higher phytoavailability of Cd (see Table 1), since the increasing rate of Cd in the iron plaque in the acid soil was much more than that in the neutral soil as Se was applied (see Fig. 2), the suppressive effect on Cd accumulation in rice was greater in the acid soil. In addition, Fan et al. (2010) previously reported that sulfur can also inhibit Cd accumulation in rice, but the effect of exogenous sulfur was not taken into consideration in this study due to the relatively low addition of 3CdSO4·8H2O (0.09–0.26 mg kg−1 S), with respect to soil S content (see Table 1).
Se application increased the allocation of Cd in the root and husk (see Table 3), and decreased the transport of Cd from the root to brown rice, in which the transports from the root to the shoot and from the husk to the brown rice were inhibited (see Fig. 4). Se can decrease the mobility of Cd in the root cell by increasing the proportion of Cd in the cell wall (Pang et al. 2014). Alternatively, Se is a component of glutathione (GSH) involved in the synthesis of phytochelatins (PCs) (Al-Lahham et al. 1999). Thus, Se addition increased the synthesis of PCs in the rice tissues (Kumar et al. 2016). The mobility of Cd in the rice tissues could be restricted by the chelation of PCs. The restriction on Cd transport was also affected by the application time of the Se (see Fig. 4). At the early growth stage of rice, the root was too small to adsorb enough Se. Since paddy clay minerals prove to be highly efficient at adsorbing Se (Johnsson 1991), the photoavailability of Se which applied at the early stage was weakened in the later growth stages. When Se was applied at the tillering and booting stages, more Se would be adsorbed by the rice roots. Thus, the restriction on Cd transport in the rice tissues was greater (see Fig. 4). Although some previous studies reported that Se phytoavailability decreased with decreasing soil pH within a range of 5–7 (Eich-Greatorex et al. 2007; Gisselnielsen et al. 1984), Se in brown rice accumulated to higher levels in the acid soil than the neutral soil in this study (see Fig. 5). This could be due to two possible reasons, on one hand, the acid soil has a much higher component of sand than the neutral soil (see Table 1), which was beneficial to enhance the phytoavailability of Se in the soil (Fernandes et al. 2014); on the other hand, Se concentrations in DCB extracts in the acid soil were much higher than that in the neutral soil (see Fig. S1), which is considered to act as a pool to the uptake of Se by rice during the whole growth stage (Huang et al. 2015). Se accumulation in the brown rice was also inhibited by the level of Cd in the soils (see Fig. 5), revealing a significant antagonistic effect between Se and Cd that agrees with the results of our previous study (Huang et al. 2017). Therefore, we considered that tillering application of Se was more applicable in the soils with low level of Se and slight to moderate contamination of Cd, while its effects may also depend on climates, season variation, and other properties of soil. The results from field trials also demonstrated that tillering application of Se can significantly reduce Cd accumulation in brown rice, but the Cd level still exceeded the food limit (see Fig. 6). Therefore, other feasible technologies such as immobilization, should be applied to Cd-contaminated field together with tillering application of Se to further reduce brown rice Cd.
Conclusion
The pot experiment showed that the tillering to booting stage is the key period for the formation of iron plaque on root surface, and the tillering stage is the optimal time for Se application to reduce Cd accumulation in brown rice. The enhancement of the formation of iron plaque and Cd sequestration on the root surface at the booting stage induced by tillering application of Se, was considered as an important reason. In addition, the restriction on Cd transport from the root to brown rice was another important factor controlling the accumulation of Cd in brown rice. Field experiments in the following year also demonstrated that tillering application of Se had significant reductions of Cd in brown rice.
References
Al-Lahham A, Rohde V, Heim P, Leuchter R, Veeck J, Wunderlich C, Wolf K, Zimmermann M (1999) Biosynthesis of phytochelatins in the fission yeast. Phytochelatin synthesis: A second role for the glutathione synthetase gene of Schizosaccharomyces pombe. Yeast 15:385–396
Alexander PD, Alloway BJ, Dourado AM (2006) Genotypic variations in the accumulation of cd, cu, Pb and Zn exhibited by six commonly grown vegetables. Environ Pollut 144:736–745
Arao T, Maejima Y, Baba K (2009) Uptake of aromatic arsenicals from soil contaminated with diphenylarsinic acid by rice. Environ Sci Technol 43:1097–1101
Baba H, Tsuneyama K, Yazaki M, Nagata K, Minamisaka T, Tsuda T, Nomoto K, Hayashi S, Miwa S, Nakajima T, Nakanishi Y, Aoshima K, Imura J (2013) The liver in itai-itai disease (chronic cadmium poisoning): pathological features and metallothionein expression. Modern Pathol 26:1228–1234
Barman M, Shukla LM, Datta SP, Rattan RK (2014) Effect of applied lime and boron on the availability of nutrients in an acid soil. J Plant Nutr 37:357–373
Bolan NS, Adriano DC, Mani PA, Duraisamy A (2003) Immobilization and phytoavailability of cadmium in variable charge soils. II. Effect of lime addition. Plant Soil 251:187–198
Chang H, Zhou XB, Wang WH, Zhou YX, Dai WC, Zhang CM, Yu SH (2013) Effects of selenium application in soil on formation of iron plaque outside roots and cadmium uptake by rice plants. Adv Mater Res 750-752:1573–1576
Cheng H, Wang MY, Wong MH, Ye ZH (2014) Does radial oxygen loss and iron plaque formation on roots alter cd and Pb uptake and distribution in rice plant tissues? Plant Soil 375:137–148
Ding CF, Zhang TL, Wang XX, Zhou F, Yang YR, Huang GF (2013) Prediction model for cadmium transfer from soil to carrot (Daucus carota L.) and its application to derive soil thresholds for food safety. J Agr Food Chem 61:10273–10282
Eich-Greatorex S, Sogn TA, Ogaard AF, Aasen I (2007) Plant availability of inorganic and organic selenium fertiliser as influenced by soil organic matter content and pH. Nutr Cycl Agroecosys 79:221–231
Fan JL, Hu ZY, Ziadi N, Xia X, Wu CYH (2010) Excessive sulfur supply reduces cadmium accumulation in brown rice (Oryza sativa L.). Environ Pollut 158:409–415
Feng RW, Wei CY, Tu SX, Ding YZ, Song ZG (2013) A dual role of se on cd toxicity: evidences from the uptake of cd and some essential elements and the growth responses in paddy rice. Biol Trace Elem Res 151:113–121
Fernandes KFM, Berton RS, Coscione AR (2014) Selenium biofortification of rice and radish: effect of soil texture and efficiency of two extractants. Plant Soil Environ 60:105–110
Gisselnielsen G, Gupta UC, Lamand M, Westermarck T (1984) Selenium in soils and plants and its importance in livestock and human-nutrition. Adv Agron 37:397–460
Hansel CM, Fendorf S, Sutton S, Newville M (2001) Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants. Environ Sci Technol 35:3863–3868
Hu Y, Norton GJ, Duan GL, Huang YC, Liu YX (2014) Effect of selenium fertilization on the accumulation of cadmium and lead in rice plants. Plant Soil 384:131–140
Huang GX, Ding CF, Guo FY, Li XG, Zhang TL, Wang XX (2017) Underlying mechanisms and effects of hydrated lime and selenium application on cadmium uptake by rice (Oryza sativa L.) seedlings. Environ Sci Pollut R 24:18926–18935
Huang QQ, Wang Q, Luo Z, Yu Y, Jiang RF, Li HF (2015) Effects of root iron plaque on selenite and selenate dynamics in rhizosphere and uptake by rice (Oryza sativa). Plant Soil 388:255–266
Johnsson L (1991) Selenium uptake by plants as a function of soil type, organic-matter content and pH. Plant Soil 133:57–64
Khan MIR, Nazir F, Asgher M, Per TS, Khan NA (2015) Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18
Kumar A, Dixit G, Singh AP, Dwivedi S, Srivastava S, Mishra K, Tripathi RD (2016) Selenate mitigates arsenite toxicity in rice (Oryza sativa L.) by reducing arsenic uptake and ameliorates amino acid content and thiol metabolism. Ecotox Environ Safe 133:350–359
Liao GJ, Xu Y, Chen C, Wu QH, Feng RW, Guo JK, Wang RG, Ding YZ, Sun Y, Xu YM, Xia W, Fan ZL, Mo LY (2016) Root application of selenite can simultaneously reduce arsenic and cadmium accumulation and maintain grain yields, but show negative effects on the grain quality of paddy rice. J Environ Manag 183:733–741
Lin L, Zhou WH, Dai HX, Cao FB, Zhang GP, Wu FB (2012) Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J Hazard Mater 235:343–351
Liu W, Yang YS, Li PJ, Zhou QX, Xie LJ, Han YP (2009) Risk assessment of cadmium-contaminated soil on plant DNA damage using RAPD and physiological indices. J Hazard Mater 161:878–883
Liu WX, Feng X, Shang S, Zhang G, Wu FB (2015) Selenium reduces cadmium accumulation and alleviates cadmium-induced quality degradation in tobacco. Plant Soil Environ 61:444–450
Lovley DR, Phillips EJP (1987) Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microb 53:1536–1540
Meharg AA, Norton G, Deacon C, Williams P, Adomako EE, Price A, Zhu YG, Li G, Zhao FJ, McGrath S, Villada A, Sommella A, De Silva PMCS, Brammer H, Dasgupta T, Islam MR (2013) Variation in rice cadmium related to human exposure. Environ Sci Technol 47:5613–5618
Mendelssohn IA, Kleiss BA, Wakeley JS (1995) Factors controlling the formation of oxidized root channels - a review. Wetlands 15:37–46
MEP, MLR (2014) National survey of soil pollution. China Environ Protection Ind
Moulis JM, Thevenod F (2010) New perspectives in cadmium toxicity: an introduction. Biometals 23:763–768
National Health and Family Planning Commission of People’s Republic of China (2017) National standard for food safety (GB2762–2017). Chinese Standard Press, Beijing
Nishita H, Haug RM (1974) Water and ammonium acetate-extractable Zn, Mn, cu, Cr, co, and Fe in heated soils. Soil Sci 118:421–424
Oono Y, Yazawa T, Kawahara Y, Kanamori H, Kobayashi F, Sasaki H, Mori S, Wu JZ, Handa H, Itoh T, Matsumoto T (2014) Genome-wide transcriptome analysis reveals that cadmium stress signaling controls the expression of genes in drought stress signal pathways in rice. PLoS One 9:e96946
Otte ML, Rozema J, Koster L, Haarsma MS, Broekman RA (1989) Iron plaque on roots of aster-Tripolium L. - interaction with zinc uptake. New Phytol 111:309–317
Pang XC, Wang H, Wu ZY, Wang S, Liu CY, He GX, Ge Y (2014) Alleviation by selenium of cadmium toxicity to rice and its mechanisms. Journal of Agro-Environment Science 33:1679–1685 (in Chinese)
Rastogi A, Zivcak M, Sytar O, Kalaji HM, He XL, Mbarki S, Brestic M (2017) Impact of metal and metal oxide nanoparticles on plant: a critical review. Front Chem 5:78
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Skroder H, Hawkesworth S, Kippler M, El Arifeen S, Wagatsuma Y, Moore SE, Vahter M (2015) Kidney function and blood pressure in preschool-aged children exposed to cadmium and arsenic - potential alleviation by selenium. Environ Res 140:205–213
Song WY, Yang HC, Shao HB, Zheng AZ, Brestic M (2014) The alleviative effects of salicylic acid on the activities of catalase and superoxide dismutase in malting barley (Hordeum uhulgare L.) seedling leaves stressed by heavy metals. Clean-Soil Air Water 42:88–97
Taylor GJ, Crowder AA (1983) Use of the DCB technique for extraction of hydrous iron-oxides from roots of wetland plants. Am J Bot 70:1254–1257
Uraguchi S, Kamiya T, Sakamoto T, Kasai K, Sato Y, Nagamura Y, Yoshida A, Kyozuka J, Ishikawa S, Fujiwara T (2011) Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. P Natl Acad Sci USA 108:20959–20964
Wan YN, Yu Y, Wang Q, Qiao YH, Li HF (2016) Cadmium uptake dynamics and translocation in rice seedling: influence of different forms of selenium. Ecotox Environ Safe 133:127–134
Wang X, Liang CH, Yin Y (2015) Distribution and transformation of cadmium formations amended with serpentine and lime in contaminated meadow soil. J Soils Sediments 15:1531–1537
Wang X, Yao HX, Wong MH, Ye ZH (2013) Dynamic changes in radial oxygen loss and iron plaque formation and their effects on cd and as accumulation in rice (Oryza sativa L.). Environ Geochem Hlth 35:779–788
Wu C, Ye ZH, Li H, Wu SC, Deng D, Zhu YG, Wong MH (2012) Do radial oxygen loss and external aeration affect iron plaque formation and arsenic accumulation and speciation in rice? J Exp Bot 63:2961–2970
Xiao M, Yang WJ, Zhang Z, Lv X, Chi DZ (2014) Cadmium accumulation in soil and risk prediction in the Qaidam Basin. J Plant Nutr Fert 20:1271–1279
Xu Z, Fang Y, Chen Y, Yang WJ, Ma N, Pei F, Kimatu BM, Hu QH, Qiu WF (2016) Protective effects of se-containing protein hydrolysates from se-enriched rice against Pb2+-induced cytotoxicity in PC12 and RAW264.7 cells. Food Chem 202:396–403
Yamaguchi N, Ohkura T, Takahashi Y, Maejima Y, Arao T (2014) Arsenic distribution and speciation near rice roots influenced by iron plaques and redox conditions of the soil matrix. Environ Sci Technol 48:1549–1556
Yang JX, Tam NFY, Ye ZH (2014) Root porosity, radial oxygen loss and iron plaque on roots of wetland plants in relation to zinc tolerance and accumulation. Plant Soil 374:815–828
Zhu YG, Pilon-Smits EAH, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14:436–442
Acknowledgements
This work was jointly sponsored by the National Key Technology Research and Development Program of China (2015BAD05B04), Agricultural Synergy Innovation Alliance Program of Jiangxi Province, and Agro-Environmental Protection program of Jiangxi province.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo
Electronic supplementary material
ESM 1
(DOCX 119 kb)
Rights and permissions
About this article
Cite this article
Huang, G., Ding, C., Guo, F. et al. The optimum Se application time for reducing Cd uptake by rice (Oryza sativa L.) and its mechanism. Plant Soil 431, 231–243 (2018). https://doi.org/10.1007/s11104-018-3768-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3768-5