Abstract
For enhanced phytoextraction, mobilization of heavy metals (HMs) from the soil solid phase to soil pore water is an important process. A pot incubation experiment mimicking field conditions was conducted to investigate the performance of three soil additives in mobilizing HMs from contaminated paddy soil (Gleyi-Stagnic Anthrosol): the [S, S]-isomer of ethylenediamine disuccinate (EDDS) with application rates of 2.3, 4.3, and 11.8 mmol kg−1 of soil, ethylenediamine tetraacetate (EDTA; 1.4, 3.8, and 7.5 mmol kg−1), and elemental sulfur (100, 200, and 400 mmol kg−1). Temporal changes in soil pore water HM and dissolved organic carbon concentrations and pH were monitored for a period of 119 days. EDDS was the most effective additive in mobilizing soil Cu. However, EDDS was only effective during the first 24 to 52 days, and was readily biodegraded with a half-life of 4.1 to 8.7 days. The effectiveness of EDDS decreased at the highest application rate, most probably as a result of depletion of the readily desorbable Cu pool in soil. EDTA increased the concentrations of Cu, Pb, Zn, and Cd in the soil pore water, and remained effective during the whole incubation period due to its persistence. The highest rate of sulfur application led to a decrease in pH to around 4. This increased the pore water HM concentrations, especially those of Zn and Cd. Concentrations of HMs in the soil pore water can be regulated to a large extent by choosing the proper application rate of EDDS, EDTA, or sulfur. Hence, a preliminary work such as our pot experiment in combination with further plant experiments (not included in this study) will provide a good tool to evaluate the applicability of different soil additives for enhanced phytoextraction of a specific soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intensive human activities such as agriculture, traffic, mining, and industry have resulted in the accumulation of heavy metals (HMs) in soils in many areas of the world. For example, as much as 20 million hectares of land in China has been estimated to be contaminated by HMs (Chen, Zheng, Zhou, & Wang, 2004). Phytoextraction has been proposed as a cost-effective technology for the remediation of HM-contaminated soils (Cunningham & Ow, 1996; Marchiol, Sacco, Assolari, & Zerbi, 2004; Raskin, Smith, & Salt, 1997). The use of natural hyperaccumulating plants to clean up HM-contaminated soils has been intensively studied (Chardot, Massoura, Echevarria, Reeves, & Morel, 2005; Zhao, Lombi, & McGrath, 2003). However, hyperaccumulating plant species generally have a low biomass production, resulting in low HM removal rates from soil (Baker, 1981) and a relatively long phytoextraction duration before an acceptable clean-up level will be achieved (Japenga, Koopmans, Song, & Römkens, 2007; Koopmans, Römkens, Song, Temminghoff, & Japenga, 2007).
Alternatively, enhanced phytoextraction, i.e., the use of soil additives (e.g., sulfur or synthetic chelators) in combination with HM-tolerant plants, has been proposed as a promising option for the remediation of HM-contaminated soils (e.g., Blaylock et al., 1997; Cui, Dong, Li, & Wang, 2004; Grčman, Vodnic, Velikonja-Bolta, & Leštan, 2003; Huang, Chen, Berti, & Cunningham, 1997; Kayser et al., 2000). The application of soil additives increases the solubility of soil HMs and enhances plant uptake and accumulation of HMs in the above-ground plant parts, leading to higher HM removal rates from soil and thus a shorter phytoextraction duration. Elemental sulfur can induce soil acidification through its oxidation to sulphate and protons by sulfur-oxidizing bacteria (Jung, Jang, Sihn, Park, & Park, 2005; Moser & Olson, 1953; Slaton, Norman, & Gilmour, 2001). For example, the lowering of pH after sulfur application has been demonstrated to increase significantly the solubility and uptake of soil Zn and Cd by sunflower and maize (Kayser et al., 2000). Among the various synthetic chelators employed, ethylenediamine tetraacetate (EDTA) has been studied most intensively (e.g., Blaylock et al., 1997; Grčman, Velikonja-Bolta, Vodnic, & Leštan, 2001; Huang et al., 1997). EDTA has the ability to mobilize HMs from the soil solid phase through the formation of strong HM complexes in the soil pore water, leading to an increase in the availability of HMs for plant uptake. For example, EDTA application has been demonstrated to increase the uptake of soil Pb by Indian mustard 1,000 to 10,000 times compared with the control (Blaylock et al., 1997). However, due to the persistent nature of EDTA in the environment, its use can result in potential risks of leaching of the HMs to ground and surface waters (e.g., Chen, Li, & Shen, 2004; Sun, Zhao, Lombi, & McGrath, 2001). In addition, EDTA may have toxic effects on soil life (Grčman et al., 2001). These disadvantages limit the applicability of EDTA for enhanced phytoextraction in practice. As an alternative to EDTA, the use of ethylenediamine disuccinate (EDDS) has been proposed for enhanced phytoextraction (Grčman et al., 2003; Luo et al., 2005). The [S, S]-isomer of EDDS is readily biodegradable in soil (Bucheli-Witschel & Egli 2001; Schowanek et al., 1997) and no toxic effects of EDDS and the Cu-EDDS complex have been found on soil life (Kos & Leštan, 2004; Vandevivere, Saveyn, Verstraete, Feijtel, & Schowanek, 2001).
The key processes determining the phytoextraction rate of a HM-contaminated soil are:
-
1.
Mobilization of HMs from the soil solid phase to the bulk pore water after application of an additive
-
2.
Transport of HMs to the root surface
-
3.
Root uptake and translocation of HMs to the above-ground plant parts
In our study, we will mainly focus on the first process, i.e., comparison of the effects of different soil additives on the mobilization of soil HMs to the pore water. Knowledge gained from such studies is helpful in selecting the optimal soil additive as well as in determining the optimal application rate for enhanced phytoextraction of a specific HM-contaminated soil in the field. For a study such as ours, different approaches can be used, e.g., batch or column experiments. The batch approach has frequently been used to compare the effectiveness of different additives for mobilization of soil HMs and to determine kinetics of HM desorption (Hauser, Tandy, Schulin, & Nowack, 2005; Kim, Lee, & Ong, 2003; Papassiopi, Tambouris, & Kontopoulos, 1999). For batch experiments, however, usually a high solution to soil ratio is employed, varying from 50:1 to 2:1 (v/w), in combination with continuous shaking of the soil suspensions (e.g., Hauser et al., 2005; Tandy et al., 2004). These conditions are not fully representative of the field where little pore water comes into contact with much soil, yielding a low solution to soil ratio (Koopmans, McDowell, Chardon, Oenema, & Dolfing, 2002). Therefore, the partitioning behavior of HMs between the soil solid phase and solution in batch experiments and in the field may be rather different. In contrast, column experiments more closely approximate the conditions in the field. However, this approach has mostly been used to investigate the effects of different additives on the leaching behavior of soil HMs, while less or no attention was paid to HM partitioning inside the column (e.g., Grčman et al., 2003; Kos & Leštan 2003a, 2003b).
In this study, a pot incubation approach to mimic field conditions was used in combination with in situ porous suction tubes. This approach allowed us to study the partitioning of HMs between the soil solid phase and the pore water in the zone where uptake of HMs by plant roots potentially would occur. Few studies have been carried out on the HM-mobilizing effects by EDDS or comparison of the performance of different soil additives using a pot experiment approach. Meers, Ruttens, Hopgood, Samson, and Tack (2005) used a similar approach to study the HM-mobilizing effects of EDDS and EDTA. However, they only measured HM concentrations in the soil pore water, and other important parameters such as dissolved organic carbon (DOC) concentrations and pH were not recorded. The objectives of our study are:
-
1.
To determine temporal changes in HM and DOC concentrations and pH in the soil pore water after application of EDDS, EDTA, or sulfur at different rates
-
2.
To investigate the relationships among these parameters (HMs on the one hand and DOC and pH on the other) so as to determine the mechanisms controlling the solubility of HMs in our soil
-
3.
To contribute to an evaluation of the ability of EDDS, EDTA, and sulfur to increase the phytoextraction rate of HM-contaminated soils in the field while maintaining the potential risks of HM leaching at an acceptable level
Materials and methods
Soil
A silty clay loam soil (Gleyi-Stagnic Anthrosol) used in this incubation experiment was taken from the 0- to 15-cm layer of a paddy field adjacent to a Cu smelter in Zhejiang province located in the east of China. Soil was air-dried and subsequently passed through a 2-mm mesh sieve. Due to atmospheric deposition of alkaline metal-bearing dust and application of wastewater irrigation, the soil became heavily contaminated with Cu, Zn, Pb, and Cd. Total HM content in the soil (Table 1) was far above the Chinese environmental standards for agricultural soils (Cu: 100 mg kg−1, Zn: 250 mg kg−1, Pb: 300 mg kg−1, and Cd: 0.3 mg kg−1 at soil pH between 6.5 and 7.5).
Set-up of incubation experiment
The pot experiment was conducted in a growth chamber at 20 ± 1°C for an incubation period of 119 days. Cone-shaped pots with a top diameter of 14.0 cm, a bottom diameter of 9.5 cm, and a height of 14.0 cm were used. One Rhizon-Soil Moisture Sampler (Rhizon-SMS; Wageningen Research Products, Wageningen, The Netherlands) was installed at a 45° inclination in each pot. The Rhizon-SMS consists of a porous plastic suction tube with a diameter of 2.5 mm, an average pore diameter of 0.1 μm, and a length of 10 cm. This porous tube was capped with nylon at one end and attached to a polyethylene tube at the other. This polyethylene tube, with a length of 15 cm, was connected to a plastic syringe through which vacuum can be applied. In this study, the trisodium [S, S]-isomer of ethylenediamine disuccinate (Na3EDDS; Fluka, Buchs, Switzerland), disodium ethylenediamine tetraacetate (Na2EDTA), and fine powdered elemental sulfur were used as soil additives. For all soil additives, three application rates were used, and triplicate pots were installed at each rate. Before potting up, EDDS or EDTA solutions were thoroughly mixed with 1 kg of air-dried soil. In addition, a control treatment was prepared in which only demineralized water was applied. The actual EDDS and EDTA application rates were calculated from the DOC concentrations measured in the soil pore water on day 1 of the incubation experiment. For this purpose, DOC concentrations present in the control treatment were subtracted from the amounts of DOC measured in the soil pore water of the EDDS and EDTA treatments. Retention of EDDS and EDTA by the soil solid phase was assumed to be negligible at pore water pH > 7 (Güçlü & Apak, 2000; Jaworska, Schowanek, & Feijtel, 1999; Nowack, Lutzenkirchen, Behra, & Sigg, 1996; Vandevivere, Hammes, Verstraete, Feijtel, & Schowanek, 2001). The total of ten treatments are summarized in Table 2. Each pot was covered by a black film on the soil surface to avoid potential photodegradation of EDDS and EDTA (Metsärinne, Tuhkanen, & Aksela, 2001). Soil moisture content was adjusted to 80% of the water holding capacity (WHC) every 3 days during the incubation. The soil in each pot was kept aerobic by making a permanent small opening in the film covering the soil surface.
Sampling
Soil pore water samples of around 15 ml were extracted by Rhizon-SMS from each pot at day 1, 3, 10, 24, 31, 38, 45, 52, 59, 69, 89, 99, 109, and 119. The soil moisture content was adjusted to 80% of the WHC with demineralized water 12 h before each sampling. The pH values of the pore water samples were measured immediately after sampling. Soil pore water samples were acidified with concentrated HNO3 before HM analysis. An additional 2 ml of each pore water sample was stored at 4°C before determination of DOC.
Chemical analysis
Soil organic matter was analyzed by the modified Walkley-Black procedure (dichromate oxidation with external heating; Allison, 1965). Soil pH was measured in a suspension with a water to soil ratio of 2.5:1 (v/w). Soil carbonate content was determined by measuring the volume of CO2 emitted after adding hydrochloric acid into the soil. Soil particle size composition was determined using a Laser Diffraction Particle Size Analyzer (Beckman Coulter LS230). Soil moisture content was measured by drying the soil at 105°C. The WHC of our soil was determined by placing a cup filled with air-dried soil in a shallow pan with water to allow the soil to become saturated with water. After drainage of the excess of water out of the soil, the mass was recorded and the WHC was calculated. Total Cu, Zn, Pb, and Cd contents in soil were determined by a Flame Atomic Absorption Spectrometry (FAAS; Varian Spectrum FS220) after aqua regia digestion. Total HM contents in soil were expressed in mg kg−1 oven-dried soil. Concentrations of DOC in the soil pore water samples were determined as the difference between total carbon and inorganic carbon concentrations measured by a Shimadzu TOC analyzer (TOC500). Concentrations of Cu, Zn, Pb, and Cd in the soil pore water samples were determined by the FAAS as well.
Calculation of effectiveness of heavy metal mobilization
For evaluating the effectiveness of HM mobilization by the additives, the percentage of each HM in the soil pore water relative to the total amount in the pot soil can be calculated at each sampling step using the following equation:
where PHM refers to the percentage of each HM in the soil pore water relative to the total amount in the pot soil (%), HMpw represents HM concentration in the pore water (mg L−1), HMsoil represents the total HM content based on oven-dried weight (mg kg−1), V represents volume of soil pore water at 80% WHC (L), and M stands for the weight of the oven-dried soil in each pot (kg).
Calculation of half-life time of EDDS
For estimating the half-life time of EDDS in the different treatments of our incubation experiment, we used the first order degradation rate equation:
where C t refers to the concentration of soil pore water concentration of DOC in time (mg C l−1), C 0 is the initial DOC concentration (mg C l−1) in the pore water, k is the degradation constant (d−1), and t is time after application (d). The log-transformed form of Eq. 2 was used to fit the data of the DOC concentrations measured in the EDDS treatments:
For C 0, we used a fixed value, i.e., the DOC concentration measured on day 1. The DOC concentrations measured in the different treatments, which were used to derive Eq. 3, were corrected for DOC naturally present, as measured in the control treatment. For the EDDS-L, EDDS-M, and EDDS-H treatments (with L, M, and H indicating a low, medium or high application rate), the DOC concentrations from the first 24, 31, and 52 days were used respectively. At these sampling events, the EDDS concentrations decreased to levels of around 2% of the EDDS concentrations on day 1. The half-life time of EDDS was estimated according to:
where t 1/2 is the half-life time (d).
Results and discussion
EDDS and EDTA
Temporal changes in soil pore water Cu concentration
Figure 1 shows the temporal changes in the soil pore water Cu concentrations after application of EDDS and EDTA. For the EDDS-H and EDTA-H treatments, Cu concentrations at day 1 were clearly lower than those at day 3. This lag-phase may be due to slow kinetics of Cu desorption from the soil solid phase to the pore water (Meers et al., 2005). For both chelators, the Cu concentration was dependent on the application rate. However, EDDS was only effective for a relatively short period after the start of the pot incubation experiment, varying from 24 days for the EDDS-L treatment to 52 days for the EDDS-H treatment. Within this period, the Cu concentrations were 5 to 960 (EDDS-L) and 50 to 2,580 (EDDS-H) times higher than the Cu concentration in the control treatment (Fig. 1). After this period, Cu concentrations sharply decreased to the level observed in the control treatment. This is in agreement with results from Meers et al. (2005). In their study, the Cu concentration in the soil solution of an HM-contaminated soil sharply decreased 30 days after the application of 4 mmol EDDS kg−1. In contrast to EDDS, however, EDTA remained reasonably effective for all application rates within the whole incubation period. The Cu concentrations were 115 to 350 (EDTA-L) and 565 to 1,460 (EDTA-H) times higher than the Cu concentration observed in the control treatment (Fig. 1). Nevertheless, the Cu concentrations gradually decreased with time in all EDTA treatments. On day 119, the Cu concentration decreased 0.4 (EDTA-L) to 0.5 (EDTA-H) times compared with the Cu concentration on day 3 (Fig. 1).
Temporal changes in the soil pore water Cu concentration after (a) ethylenediamine disuccinate (EDDS), (b) ethylenediamine tetraacetate (EDTA), and (c) sulfur application. Error bars represent the standard deviation (n = 3). No replicate data are available for the EDTA-M and EDTA-H treatments, due to the malfunctioning of the Rhizon-SMS. L, M, and H indicate a low, medium or high application rate; CK refers to the control
In our study, EDDS and EDTA extracted 17 to 45% (EDDS) and 2 to 11% (EDTA) of the total Cu content from soil on day 3 (Table 3). In Table 3, we have chosen to present the data of day 3 instead of the data of day 1, because Cu concentrations on day 1 were clearly lower than those at day 3, probably due to slow Cu desorption kinetics (Fig. 1). However, the effectiveness of Cu extraction by these chelators was higher in the batch experiments of Tandy et al. (2004). In their study, Cu extraction by EDDS and EDTA within 1 day from three HM-contaminated soils with rather similar HM contents varied from 53 to 67% (EDDS) and from 29 to 84% (EDTA) of the total Cu content. These differences in Cu extraction effectiveness can be explained by the higher molar ratio between the amount of chelator applied and the sum of the total HM content in soil in the study of Tandy et al. (2004). For their soils, this ratio was 1 and 10 for both EDDS and EDTA. For our soil, it varied from 0.02 to 0.11 for EDDS and from 0.01 to 0.07 for EDTA, resulting in lower Cu extraction. In addition, one might extract more Cu using a batch approach than using a pot incubation experiment. This may be due to a better contact of the surface of suspended soil particles with the extracting solution facilitating an optimal transfer of Cu adsorbed at surface sites to the bulk solution during batch experiments.
Temporal changes in soil pore water DOC concentration
The mobilizing effect of EDDS on soil Cu was limited to a relatively short period after the start of the incubation experiment, as mentioned previously (Fig. 1). Figure 2 shows the temporal changes in the DOC concentration in the soil pore water after application of EDDS. For all EDDS treatments, DOC concentrations decreased sharply within 24 to 52 days to levels slightly above that observed in the control treatment. Apparently, the Cu-EDDS complex can be readily biodegraded, although our soil is severely contaminated with Cu and other HMs (Table 1). The sharp decrease in the DOC concentrations coincided with the sharp decrease in the Cu concentrations (Fig. 1). Since these trends in the DOC and Cu concentrations are clearly coupled, EDDS seemed to control the solubility of Cu. After biodegradation of EDDS, Cu re-adsorbs to the soil solid phase resulting in a decrease in the Cu concentration in the soil pore water. In contrast to Tandy, Ammann, Schulin, and Nowack (2006), however, we did not observe a lag-phase in the biodegradation of EDDS. Our soil may thus have been better acclimatized for the biodegradation of EDDS. Our sampling protocol did not contribute significantly to the decrease in the DOC concentrations in the EDDS treatments. Based on the volume of pore water sample (around 15 ml) taken at each sampling point during the incubation experiment, this contribution on day 119 was calculated to be <10% of the DOC concentrations measured on day 1, and is thus only of minor importance. For all EDTA treatments, the DOC concentrations in the soil pore water gradually decreased with time. On day 119, the DOC concentrations were 0.3 (EDTA-M) to 0.4 (EDTA-H) times lower than the DOC concentration observed on day 1. These trends in the DOC concentrations are in good agreement with those in the Cu concentrations (Fig. 1). Clearly, concentrations of DOC and Cu are coupled and EDTA seemed to control Cu solubility. The decrease in the DOC concentrations, however, can be largely explained by the calculated loss of DOC resulting from our sampling protocol. Despite the effects caused by this sampling artefact, the DOC concentrations in all EDTA treatments remained at elevated levels during the whole incubation period. This is in good agreement with the persistent nature of EDTA and its HM complexes in the environment (e.g., Chen, Li, Shen, 2004; Sun et al., 2001).
Temporal changes in the soil pore water DOC concentration after (a) EDDS and (b) EDTA application. Error bars represent the standard deviation (n = 3). No replicate data are available for the EDTA-M and EDTA-H treatments, due to the malfunctioning of the Rhizon-SMS. L, M, and H indicate either a low, medium or high application rate; CK refers to the control
In Table 4, the estimated degradation constants (k) and half-life times (t 1/2) are presented for the EDDS treatments. Biodegradation rates of EDDS clearly followed first order kinetics. The k and t 1/2 values were, however, dependent on the application rate, and varied from 0.17 day−1 and 4.1 day (EDDS-L) to 0.08 day−1 and 8.7 day (EDDS-H) respectively. This dependency of k and t1/2 on the EDDS application rate may be explained by Cu toxicity at increased levels of Cu mobilization, leading to a lower microbial activity and biodegradation rate (Meers et al., 2005). Instead of Cu toxicity, however, the biodegradation rate of EDDS may also have been limited by a fixed capacity of the micro-organisms in our soil for EDDS biodegradation, resulting in lower k and higher t1/2 values at higher application rates as well. The t 1/2 values for the EDDS treatments in our study are in good agreement with those found by Meers et al. (2005) and Tandy et al. (2006). In their studies, t 1/2 values varied from 3.8 to 7.5 days, depending on the initial EDDS concentration and the soil studied. Our t1/2 values were, however, higher than the t 1/2 value of 2.6 days reported by Schowanek et al. (1997) for EDDS biodegradation in a sewage sludge-amended soil. The application of sewage sludge may have caused the number of micro-organisms and the level of microbial activity in soil to increase, leading to a higher biodegradation rate (Tandy et al., 2006).
Interactions between soil pore water concentrations of Cu and DOC
During the effective period of EDDS, Cu concentrations were (much) higher than those in the EDTA treatments (Fig. 1). The effectiveness of Cu mobilization by EDDS and EDTA can be compared by calculating the ratio between the molar concentrations of Cu and the chelator in the soil pore water. Soil pore water concentrations of EDDS and EDTA were measured indirectly via DOC analysis. For calculating this ratio, the Cu and DOC concentrations in the EDDS and EDTA treatments were corrected for Cu and DOC present in the control treatment. In Fig. 3, the molar concentrations of Cu are plotted against those of EDDS for the soil pore water samples taken during the effective period of EDDS. For the EDDS-L and EDDS-M treatments, data points closely follow the 1:1 line. Since HMs form 1:1 complexes with EDDS (Vandevivere, Hammes, et al., 2001), the binding capacity of EDDS in these soil pore water samples was thus almost fully used by Cu. For the soil pore water samples of the EDDS-H treatment, however, some of the data points are clearly lying below the 1:1 line, and the Cu concentration seems to be moving toward a plateau with a further increase in the EDDS concentration. These data points, with an EDDS to Cu molar ratio varying between 1.5 and 3.0, represent the soil pore water samples taken on days 3 and 10 when relatively little biodegradation of EDDS had taken place (Fig. 2). Apparently, EDDS was not fully occupied by Cu in these soil pore water samples. This may be explained by depletion of the readily desorbable Cu pool in soil in the EDDS-H treatment, leading to a lower increase in the Cu concentration relative to the increase in the EDDS concentration. Hence, the effectiveness of EDDS to mobilize Cu was clearly lower at the highest application rate. In addition, mobilization of natural DOC from soil by EDDS in the EDDS-H treatment (Hauser et al., 2005) may have caused an overestimation of the EDDS concentrations, which also may explain why the data points of this treatment are lying below the 1:1 line. For enhanced phytoextraction of our soil, EDDS should thus not be applied at a rate higher than those of the EDDS-L and EDDS-M treatments (2.3 and 4.3 mmol kg−1). This result shows the advantage of conducting a preliminary experiment before starting with enhanced phytoextraction in the field. This allowed us to determine the optimal EDDS dose for Cu mobilization, thus both reducing the potential leaching risks of HMs and the high economic costs associated with the use of an unnecessarily high EDDS application rate.
Relationships between the molar concentrations of Cu and EDDS (a) for the effective period of EDDS and (b) for the whole incubation period of EDTA. Plotted data are individual data points from the EDDS-L (days 3 to 24), EDDS-M (days 3 to 31), and EDDS-H treatments (days 3 to 52; n = 41), and from all sampling events of all EDTA treatments (n = 65). No replicate data are available for the EDTA-M and EDTA-H treatments, due to the malfunctioning of the Rhizon-SMS. Data from day 1 are not included, because of disequilibrium in Cu mobilization (Fig. 1). L, M, and H indicate a low, medium or high application rate
For EDTA, the molar concentrations of Cu are plotted against those of EDTA for the soil pore water samples from the whole incubation period (Fig. 3). In contrast to EDDS, data points from all EDTA treatments clearly lie below the 1:1 line. Hence, only a small part of the EDTA binding capacity was used by Cu. Therefore, in our soil, EDDS is much more effective than EDTA at mobilizing Cu from the soil solid phase to the pore water. The higher effectiveness of Cu extraction by EDDS than by EDTA has been demonstrated before by Tandy et al. (2004) and Meers et al. (2005). The observed difference in Cu mobilization between EDDS and EDTA can be explained by the stability constants of the HM complexes for both chelators. The stability constants (log K) of Cu-EDDS and Cu-EDTA are nearly the same (Table 5). These values would suggest equal or better mobilization of Cu by EDTA. However, the log K value of Cu-EDDS is much higher than the log K values of the other HM-EDDS complexes, while the difference between the log K value of Cu-EDTA and the log K values of the other HM-EDTA complexes is much smaller (Table 5). Therefore, competition between Cu and other HMs, i.e., Zn, Pb, and Cd, for binding to EDTA will be stronger than for EDDS, resulting in a lower effectiveness of EDTA at mobilizing Cu. Our results have important consequences for selecting either EDDS or EDTA for enhanced phytoremediation of our HM-contaminated soil in the field. Since the effectiveness of EDDS to mobilize Cu from our soil is higher than that of EDTA, the use of EDDS for enhanced phytoextraction may ensure increased Cu uptake by plants, whereas the relatively short life span of EDDS would allow only for short-distance vertical transport in the soil profile, but would prevent long-term leaching risks of the Cu-EDDS complex to ground and surface waters (Meers et al., 2005). Nevertheless, careful management of the use of EDDS in the field is required, because if EDDS is applied in a period with rainfall or irrigation, a risk of Cu leaching does occur.
Temporal changes in soil pore water Pb, Zn, and Cd concentrations and interactions with DOC
Concentrations of Pb, Zn, and Cd in the soil pore water 3 and 119 days after the application of EDDS or EDTA are presented in Table 3. Similar to Cu (Fig. 1), the slow desorption kinetics of Pb, Zn, and Cd temporarily lowered the concentrations of these HMs in the soil pore water at day 1 (not shown). Concentrations of Pb and Cd in the soil pore water samples from the EDDS treatments on day 3 were 6 to 25 (Pb) and 2 to 3 times (Cd) higher than those in the control treatment (Table 3). The Zn concentrations in the EDDS-L and EDDS-M treatments on day 3 were 2 to 7 times higher respectively than the Zn concentration in the control treatment whereas the Zn concentration in the EDDS-H treatment increased 75 times (Table 3). Hence, soil Zn, Pb, and Cd were mobilized to the pore water to a much smaller extent than soil Cu, although these HMs were present at severely elevated levels in soil as well (Fig. 1; Tables 1, 3). The effects of EDDS on Pb, Zn, and Cd are limited to a relatively short period after the start of the incubation experiment, similar to the effect of EDDS on soil Cu (Fig. 1). The readily biodegradable nature of Zn-, Pb-, and Cd-EDDS complexes in our incubation experiment can explain this effect (Vandevivere, Saveyn, et al., 2001). In Fig. 4, the relationship between the sum of the molar concentrations of Cu and Zn and the molar concentration of EDDS is presented for the soil pore water samples taken during the effective period of EDDS. As previously discussed, the capacity of EDDS to bind HMs in the EDDS-L and EDDS-M treatments was almost fully used by Cu (Fig. 3). In the soil pore water samples taken from the EDDS-H treatment on days 3 and 10, Cu occupied a much smaller part of the EDDS binding capacity (Fig. 3). After including Zn, however, these data points now closely follow the 1:1 line as well. Apparently, the binding capacity of EDDS in the EDDS-H treatment is almost fully used by Cu and Zn. So EDDS first depletes the readily desorbable Cu pool in soil and the surplus of EDDS binding capacity is then used to extract the following available HM from soil with a high ability to form strong complexes with EDDS, i.e., Zn (Table 5). This is in agreement with trends observed by Meers et al. (2005) in the extraction of soil Cu and Zn by EDDS.
Relationships between the summed molar concentrations of (a) Cu and Zn and EDDS for the effective period of EDDS and (b) Cu, Zn, Pb, and Cd and EDTA for the whole incubation period. Plotted data are individual data points from the EDDS-L (days 3 to 24), EDDS-M (days 3 to 31), and EDDS-H treatments (days 3 to 52; n = 41) and from all sampling events of all EDTA treatments (n = 65). No replicate data are available for the EDTA-M and EDTA-H treatments, due to the malfunctioning of the Rhizon-SMS. Data from day 1 are not included, because of disequilibrium in Cu mobilization (Fig. 1). L, M, and H indicate a low, medium or high application rate
For EDTA, significant mobilization of soil Pb, Zn, and Cd was observed. Concentrations of these HMs in the soil pore water samples taken from the EDTA-L and EDTA-H treatments on day 3 were between 215 and 1,080 (Pb), 40 and 135 (Zn), and 35 and 130 (Cd) times higher than those in the control treatment (Table 3). For Cu, only a small part of the EDTA binding capacity was used (Fig. 3), as previously discussed. After including Pb, Zn, and Cd, however, the data points representing the soil pore water samples taken from all EDTA treatments for the whole incubation period all closely follow the 1:1 line now (Fig. 4). Apparently, the binding capacity of EDTA in all treatments is fully used by Cu, Pb, Zn, and Cd. These results may be explained by the relatively small differences between the stability constants for the different HM-EDTA complexes (Table 5). This causes strong competition among Cu, Pb, Zn, and Cd for binding to EDTA and thus increased concentrations in the soil pore water for all HMs, whereas EDDS is mainly effective at mobilizing soil Cu, as previously discussed. In contrast to EDDS, EDTA remained reasonably effective for Pb, Zn, and Cd during the whole incubation period, due to the low biodegradability and persistent nature of EDTA in soil.
Sulfur
Temporal changes in soil pore water Cu concentration and interaction with pH
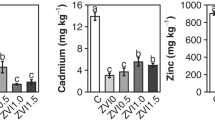
Sulfur application in the S-L and S-M treatments did not affect the pH and the Cu concentration in the soil pore water (Figs. 1, 5). This lack of effectiveness can be explained by the high proton buffering capacity of our soil, due to the large amount of carbonates initially present in soil (Table 1). At the end of the pot incubation experiment, still considerable amounts of carbonates remained in the soils of the S-L and S-M treatments (Table 6), explaining further why pH remained constant in these treatments. However, sulfur application in the S-H treatment led to a large decrease in the amounts of carbonates (Table 6) and a gradual decrease in pH with time to around 4 on day 119 (Fig. 5; Table 6). This caused a gradual but significant increase in the Cu concentration with time from day 24 onward (Fig. 1). On day 119, the Cu concentration in the S-H treatment was 645 times higher than the Cu concentration in the control treatment (Fig. 1). With a decrease in pH, the Cu concentration in soil solution increases, due to increased competition between Cu and protons for adsorption to the soil solid phase (Fest, Temminghoff, Griffioen, & Van Riemsdijk, 2005; Salam & Helmke, 1998; Weng, Temminghoff, & van Riemsdijk, 2001). Although pH decreased significantly, some carbonates were still present in the S-H treatment on day 119 (Table 6), due to slow dissolution kinetics. With time, pH may increase again after complete dissolution of the carbonates. For our soil, sulfur should be applied at a rate of around 400 mmol S kg−1 so as to decrease pH and to mobilize Cu to the soil pore water. The Cu concentrations in most of the EDDS and EDTA treatments were, however, higher than the Cu concentration in the S-H treatment (Fig. 1; Table 3). For our soil, EDDS and EDTA are more effective than sulfur in mobilizing soil Cu.
Temporal changes in soil pore water pH after (a) EDDS, (b) EDTA, and (c) sulfur applications. Error bars represent the standard deviation (n = 3). No replicate data are available for the EDTA-M and EDTA-H treatments, due to the malfunctioning of the Rhizon-SMS. L, M, and H indicate a low, medium or high application rate; CK refers to the control
Temporal changes in soil pore water Pb, Zn, and Cd concentrations and interactions with pH
Concentrations of Pb, Zn, and Cd in the soil pore water increased in the S-H treatment at the end of the incubation experiment (Table 3). Sulfur had the greatest effect on Zn; the Zn concentration on day 119 increased 200 times compared with the Zn concentration in the control treatment, whereas the Pb and Cd concentrations increased 40 and 45 times respectively (Table 3). The increase in these HM concentrations can be explained by the decrease in pH, as previously discussed (Fig. 5). However, solubilization of HM-carbonate precipitates (e.g., ZnCO3) under acidic conditions may have played a role as well (Madrid & Diazbarrientose, 1992). For the S-L and S-M treatments, however, the effects of sulfur application on mobilization of HMs were much smaller, due to the lack of a pH effect (Fig. 5). The effects of sulfur application on HM mobilization in the S-H treatment are in agreement with those observed in the studies of Cui et al. (2004) and Kayser et al. (2000). In these studies, sulfur application at a rate of 200 mmol kg−1 to HM-contaminated calcareous soils only increased the solubility of Cd and Zn, whereas no significant effects were found on soil Pb and Cu. This might be explained by the high buffering capacity of the soils used in these studies. The pH thus seemed to control the solubility of all Cu, Pb, Zn, and Cd in the soil pore water of the S-H treatment. In Fig. 6, the log concentrations of these HMs are presented as a function of pH. Good linear relationships were found between the log Cu, Pb, Zn, and Cd concentrations and pH. For our soil, sulfur application at a rate of 400 mmol S kg−1 was thus sufficient to cause significant mobilization of soil Zn to the pore water. Under the conditions of our incubation experiment, sulfur was more effective than EDDS and EDTA in mobilizing soil Zn and Cd. However, growth of plants that are not able to tolerate acidity may be severely inhibited at pH 4 (Fig. 5; Table 6), resulting in lower biomass production and thus a lower removal rate of HMs from the soil. Therefore, acid-tolerant plants such as maize (Salazar et al. 1997) should be used. In contrast to sulfur, pH in the soil pore water samples of the EDDS and EDTA treatments was only slightly affected (Fig. 5).
Conclusions
Ethylenediamine disuccinate (EDDS) was more effective than ethylenediamine tetraacetate (EDTA) at mobilizing soil Cu to the pore water, whereas it is less effective than EDTA at mobilizing soil Pb, Cd, and Zn. This is in accordance with the stability constants of HM-EDDS complexes.
Due to the short half-life (4.1 to 8.7 days) of EDDS, soluble HM concentrations decreased much quicker in the EDDS treatments than in the EDTA treatments, which indicated that EDDS will cause less risk of metal leaching than EDTA.
A high application rate of elemental sulfur caused a gradual decrease in soil pH and led to a sharp increase in the HM concentrations in the soil pore water.
For chemically induced phytoextraction, EDDS, EDTA, or sulfur application rates must be optimized to allow metal solubilization and subsequent plant uptake, and in the meantime to reduce potential metal leaching. Column study with plants is needed in future research.
References
Allison, L. E. (1965). Organic carbon. In: C. A. Black (Ed.), Methods of soil analysis. II. Agronomy Monograph 9 (pp. 1367–1378). Madison, WI: American Society of Agronomy.
Baker, A. J. M. (1981). Accumulators and excluders—Strategies in the response of plants on heavy metals. Journal of Plant Nutrition, 3, 677–686.
Blaylock, M. J., Slat, D. E., Dushenkov, S., Zakharova, O., Gussman, C., Kapulnik, Y., Ensley, B. D., & Raskin, I. (1997). Enhanced accumulation of Pb in India mustard by soil-applied chelating agents. Environmental Science & Technology, 31, 860–865.
Bucheli-Witschel, M., & Egli, T. (2001). Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiological Review, 25, 69–106.
Chardot, V., Massoura, S. T., Echevarria, G., Reeves, R. D., & Morel, J. L. (2005). Phytoextraction potential of the nickel hyperaccumulators Leptoplax emarginata and Bornmuellera tymphaea. International Journal of Phytoremediation, 7, 323–335.
Chen, H. M., Zheng, C. R., Zhou, D. M., & Wang, S. Q. (2004). Problems worthy of concern in soil environmental protection in China (In Chinese). Journal of Agro-Environmental Science, 2, 1244–1245.
Chen, Y. H., Li, X. D., & Shen, Z. G. (2004). Leaching and uptake of heavy metals by ten different species of plants during an EDTA-assisted phytoextraction process. Chemosphere, 57, 187–196.
Cui, Y. S., Dong, Y. T., Li, H. F., & Wang, Q. R. (2004). Effect of elemental sulfur on solubility of soil heavy metals and their uptake by maize. Environment International, 30, 323–328.
Cunningham, S. D., & Ow, D. W. (1996). Promises and prospects of phytoremediation. Plant Physiology, 110, 715–719.
Fest, E. P. M. J., Temminghoff, E. J. M., Griffioen, J., & Van Riemsdijk, W. H. (2005). Proton buffering and metal leaching in sandy soils. Environmental Science & Technology, 39, 7901–7908.
Grčman, H., Velikonja-Bolta, Š., Vodnic, D., & Leštan, D. (2001). EDTA enhanced heavy metal phytoextraction metal accumulation, leaching and toxicity. Plant and Soil, 235, 105–114.
Grčman, H., Vodnic, D., Velikonja-Bolta, Š., & Leštan, D. (2003). Ethylenediaminedissuccinate as a new chelate for environmentally safe enhanced lead phytoremediation. Journal of Environmental Quality, 32, 500–506.
Güçlü, K., & Apak, R. (2000). Modeling of copper(II), cadmium(II), and lead(II) adsorption on red mud from metal-EDTA mixture solutions. Journal of Colloid and Interface Science, 228, 238–252.
Hauser, L., Tandy, S., Schulin, R., & Nowack, B. (2005). Column extraction of heavy metals from soils using the biodegradable chelating agent EDDS. Environmental Science & Technology, 39, 6819–6824.
Huang, J. W. W., Chen, J. J., Berti, W. R., & Cunningham, S. D. (1997) Phytoremediation of lead-contaminated soils: Role of synthetic chelates in lead phytoextraction. Environmental Science & Technology, 31, 800–805.
Japenga, J., Koopmans, G. F., Song, J., & Römkens, P. F. A. M. (2007). A feasibility test to estimate the duration of phytoextraction of heavy metals from polluted soils. International Journal of Phytoremediation, 9, doi: 10.1080/15226510701232773.
Jaworska, J. S., Schowanek, D., & Feijtel, T. C. J. (1999). Environmental risk assessment for trisodium [S,S]-ethylene diamine disuccinate, a biodegradable chelator used in detergent applications. Chemosphere, 38, 3597–3625.
Jung, S. J., Jang, K. H., Sihn, E. H., Park, S. K., & Park, C. H. (2005) Characteristics of sulfur oxidation by a newly isolated Burkholderia spp. Journal of Microbiology and Biotechnology, 15, 716–721.
Kayser, A., Wenger, K., Keller, A., Attinger, W., Felix, H. R., Gupta, S. K., & Schulin, R. (2000). Enhancement of phytoextraction of Zn, Cd, and Cu from calcareous soil: The use of NTA and sulfur amendments. Environmental Science & Technology, 34, 1778–1783.
Kim, C., Lee, Y., & Ong, S. K. (2003). Factors affecting EDTA extraction of lead from lead-contaminated soils. Chemosphere, 51, 845–853.
Koopmans, G. F., McDowell, R. W., Chardon, W. J., Oenema, O., & Dolfing, J. (2002). Soil phosphorus quantity-intensity relationships to predict increased soil phosphorus loss to overland and subsurface flow. Chemosphere, 48, 679–687.
Koopmans, G. F., Römkens, P. F. A. M., Song, J., Temminghoff, E. J. M., Japenga, J. (2007). Predicting the phytoextraction duration of heavy metal contaminated soils. Water Air & Soil Pollution, doi: 10.1007/s11270-006-9307-7.
Kos, B., & Leštan, D. (2003a). Induced phytoextraction/soil washing of lead using biodegradable chelate and permeable barriers. Environmental Science & Technology, 37, 624–629.
Kos, B., & Leštan, D. (2003b). Influence of a biodegradable ([S, S]-EDDS) and nondegradable (EDTA) chelate and hydrogel modified soil water sorption capacity on Pb phytoextraction and leaching. Plant and Soil, 253, 403–411.
Kos, B., & Leštan, D. (2004). Chelator induced phytoextraction and in situ washing of Cu. Environmental Pollution, 132, 333–339.
Luo, C. L., Shen, Z. G., & Li, X. D. (2005). Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere, 59, 1–11.
Madrid, L., & Diaz-Barrientose, E. (1992). Influence of carbonate on the reaction of heavy-metals in soils. European Journal of Soil Science, 43, 709–721.
Marchiol, L., Sacco, P., Assolari, S., & Zerbi, G. (2004). Reclamation of polluted soil: Phytoremediation potential of crop-related Brassica species. Water, Air, & Soil Pollution, 158, 345–356.
Martell, A. E., Smith, R. M., & Motekaitis, R. J. (1989). NIST Critically Selected Stability Constants of Metal Complexes, Version 6.0. Gaithersburg, MD: National Institute of Standards and Technology.
Meers, E., Ruttens, A., Hopgood, M. J., Samson, D., & Tack, F. M. G. (2005). Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere, 58, 1011–1022.
Metsärinne, S., Tuhkanen, T., & Aksela, R. (2001). Photodegradation of ethylenediaminetetraacetic acid (EDTA) and ethylenediamine disuccinic acid (EDDS) within natural UV radiation range. Chemosphere, 45, 949–955.
Moser, U. S., & Olson, R. V. (1953). Sulfur oxidation in 4 soils as influenced by soil moisture tension and sulfur bacteria. Soil Science, 76, 251–257.
Nowack, B., Lutzenkirchen, T., Behra, P., & Sigg, L. (1996). Modeling the adsorption of metal-EDTA complexes onto oxides. Environmental Science & Technology, 30, 2397–2405.
Papassiopi, N., Tambouris, S., & Kontopoulos, A. (1999). Removal of heavy metals from calcareous contaminated soils by EDTA leaching. Water, Air, & Soil Pollution, 109, 1–15.
Raskin, I., Smith, R. D., & Salt, D. E. (1997). Phytoremediation of metals: Using plants to remove pollutants from the environment. Current Opinion in Biotechnology, 8, 221–226.
Salam, A. K., & Helmke, P. A. (1998). The pH dependence of free ionic activities and total dissolved concentrations of copper and cadmium in soil solution. Geoderma, 83, 281–291.
Salazar, F. S., Pandey, S., Narro, L., Perez, J. C., Ceballos, H., Parentoni, S. N., & Bahia, A. F. C. (1997). Diallel analysis of acid-soil tolerant and intolerant tropical maize populations. Crop Science, 37, 1457–1462.
Schowanek, D., Feijtel, T. C. J., Perkins, C. M., Hartman, F. A., Federle, T. W., & Larson, R. J. (1997). Biodegradation of [S,S], [R,R] and mixed stereoisomers of ethylene diamine disuccinic acid (EDDS), a transition metal chelator. Chemosphere, 34, 2375–2391.
Slaton, N. A., Norman, R. J., & Gilmour, J. T. (2001). Oxidation rates of commercial elemental sulfur products applied to an alkaline silt loam from Arkansas. Soil Science Society of America Journal, 65, 239–243.
Sun, B., Zhao, F. J., Lombi, E., & McGrath, S. P. (2001). Leaching of heavy metals from contaminated soils using EDTA. Environmental Pollution, 113, 111–120.
Tandy, S., Bossart, K., Mueller, R., Ritschel, J., Hauser, L., Schulin, R., & Nowack, B. (2004). Extraction of heavy metals from soils using biodegradable chelating agents. Environmental Science & Technology, 38, 937–944.
Tandy, S., Ammann, A., Schulin, R., & Nowack, B. (2006). Biodegradation and speciation of residual SS-ethylenediaminedisuccinic acid (EDDS) in soil solution left after soil washing. Environmental Pollution, 142, 191–199.
Vandevivere, P., Hammes, F., Verstraete, W., Feijtel, T. C. J., & Schowanek, D. (2001). Metal decontamination of soil, sediment, and sewage sludge by means of transition metal chelant [S,S]-EDDS. Journal of Environmental Engineering, 127, 802–811.
Vandevivere, P., Saveyn, H., Verstraete, W., Feijtel, T. C. J., & Schowanek, D. R. (2001). Biodegradation of metal-[S, S]-EDDS complexes. Environmental Science & Technology, 35, 1765–1770.
Weng, L. P., Temminghoff, E. J. M., & van Riemsdijk, W. H. (2001). Contribution of individual sorhents to the control of heavy metal activity in sandy soil. Environmental Science & Technology, 35, 4436–4443.
Zhao, F. J., Lombi, E., & McGrath, S. P. (2003). Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant and Soil, 249, 37–43.
Acknowledgements
This work was funded by the Natural Science Foundation of China and Jiangsu province (project no. 40301046 and BK2004166), Chinese Ministry of Science and Technology (project no. 2004CB720403 and 2002CB410809), and the Royal Dutch Academy of Sciences (contract no. 04-PSA-E-05). Furthermore, the authors are thankful to Walter Schenkeveld for his critical comments on a previous version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, G., Koopmans, G.F., Song, J. et al. Mobilization of heavy metals from contaminated paddy soil by EDDS, EDTA, and elemental sulfur. Environ Geochem Health 29, 221–235 (2007). https://doi.org/10.1007/s10653-006-9078-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-006-9078-5