Abstract
Cholestasis is a bile flow reduction that is induced following Bile Duct Ligation (BDL). Cholestasis impairs memory and induces apoptosis. Apoptosis consists of two pathways: intrinsic and extrinsic. The intrinsic pathway is modulated by BCL-2 (B cell lymphoma-2) family proteins. BCL-2 (a pro-survival BCL-2 protein) has anti-apoptotic effect, while BAD (BCL-2-associated death) and BAX (BCL-2-associated X), the other members of BCL-2 family have pro-apoptotic effect. Furthermore, TFAM (mitochondrial transcriptional factor A) is involved in transcription and maintenance of mitochondrial DNA and PGC-1α (peroxisome proliferator-activated receptor γ coactivator-1α) is a master regulator of mitochondrial biogenesis. On the other hand, NeuroAid is a Traditional Chinese Medicine with neuroprotective and anti-apoptosis effects. In this study, we evaluated the effect of cholestasis on spatial memory and expression of BCL-2, BAD, BAX, TFAM, and PGC-1α in the hippocampus of rats. Additionally, we assessed the effect of NeuroAid on cholestasis-induced cognitive and genetic alterations. Cholestasis was induced by BDL surgery and NeuroAid was injected intraperitoneal at the dose of 0.4 mg/kg. Furthermore, spatial memory was evaluated using Morris Water Maze (MWM) apparatus. The results showed cholestasis impaired spatial memory, increased the expression of BAD and BAX, decreased the expression of TFAM and PGC-1α, and did not alter the expression of BCL-2. Also, NeuroAid decreased the expression of BAD and BAX and increased the expression of TFAM, PGC-1α, and BCL-2. In conclusion, cholestasis impaired spatial memory and increased the expression of pro-apoptotic genes. Also, cholestasis decreased the expression of TFAM and PGC-1α. Interestingly, NeuroAid restored the effects of cholestasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bile formation is a special function of liver that is crucial for liver health [1]. Bile Duct Ligation (BDL) disrupts bile secretion and induces liver damage [2]. BDL also induces hepatic fibrosis, necrosis, and apoptosis [3, 4]. Furthermore, BDL induces cholestasis [2, 5]. Cholestasis reduces the bile flow and induces liver damage [6,7,8]. Cholestasis also impairs learning and memory in different cognitive tasks [9, 10]. It has been revealed that cholestasis disrupts memory retrieval in one-trial step-down memory task in mice [11]. Cholestasis also impairs spatial recognition memory in Y-maze task [12]. Furthermore, mild BDL can impair learning in Y-maze task in rats [13]. Interestingly, human studies have shown that biliary cirrhosis and liver diseases can induce cognitive impairments and dementia [14, 15]. It should be noted that molecular mechanisms of cholestasis involved in cognitive impairments are largely unknown. However, it has been suggested that BDL-induced hyperammonia may be a main factor for cognitive and neurological impairments [16, 17]. In addition, disruptions in neurotransmitter systems including glutamatergic, cholinergic, dopaminergic, opioidergic, GABAergic, and serotonergic have been observed following BDL or liver diseases [18,19,20]. Further, as mentioned before, BDL plays an important role in the induction of necrosis and apoptosis [21,22,23].
Apoptosis is a form of programmed cell death. The inappropriate form of apoptosis (too little or too much) induces neurodegenerative and autoimmune diseases, and various types of cancer [24]. Apoptosis occurs via two pathways: the extrinsic and the intrinsic [25]. The intrinsic pathway (also called mitochondria-dependent) is modulated by BCL-2 (B cell lymphoma-2) family of proteins [26, 27]. BCL-2 proteins integrate both death and survival signals, and based on the composition of typical BH domains are divided into anti-apoptotic and pro-apoptotic groups [28, 29]. The integrity of the Outer Mitochondrial Membrane (OMM) is regulated by anti-apoptotic BCL-2 proteins including BCL-2 (a pro-survival BCL-2 protein) [30, 31]. Furthermore, pro-apoptotic BCL-2 proteins such as BAD (BCL-2-associated death) and BAX (BCL-2-associated X) activate caspases and promote cell death via releasing mitochondrial Inter-Membrane Space (IMS) proteins [32, 33].
TFAM (mitochondrial transcriptional factor A) has a critical role in transcription and maintenance of mitochondrial DNA (mtDNA) [34, 35]. TFAM also protects mtDNA from free radicals [36]. Abnormal function of mtDNA can lead to severe disorders. For example, decrease in functional mtDNA abundance may induce Parkinson’s disease, Alzheimer’s disease (AD), and Amyotrophic lateral sclerosis (ALS) [37, 38]. On the other hand, PGC-1α (peroxisome proliferator-activated receptor γ coactivator-1α) is a master regulator of mitochondrial biogenesis and regulates energy metabolism and antioxidant pathways [39, 40]. PGC-1α has a critical role in the pathogenesis of various diseases such as AD [41].
The hippocampus has high expression of BCL-2 proteins family including BAX and BAD [42]. It has been revealed that stress upregulates the expression of BAX and BAD [42]. Stress damages the neurons probably via induction of pro-apoptotic factors, while upregulation of anti-apoptotic BCL-2 proteins may be a compensatory response [43, 44]. Furthermore, the hippocampus has high expression of PGC-1α and TFAM [40]. The expression of PGC-1α and TFAM is downregulated following dementia and AD in memory-related brain regions especially hippocampus [45].
NeuroAid (as a Traditional Chinese Medicine) has anti-apoptotic and neuroprotective effects [46, 47]. NeuroAid (MLC901) has nine herbal components per each capsule including 0.80 g Radix astragali, 0.16 g Radix salvia miltiorrhizae, 0.16 g Radix paeoniae rubra, 0.16 g Rhizoma chuanxiong, 0.16 g Radix angelicae sinensis, 0.16 g Car-thamus tinctorius, 0.16 g Prunus persica, 0.16 g Radix polygalae, and 0.16 g Rhizoma acori tatarinowii [48]. NeuroAid inhibits necrosis and apoptosis of neurons in ischemic rats [49]. Also, NeuroAid plays an important role in the repair of neurovascular unit after ischemic stroke [50]. Recent pharmacological studies have shown that NeuroAid protects the brain from ischemic injury, decreases functional deficits in animal models of stroke, and prevents neural death in in-vitro models of excitotoxicity [51]. NeuroAid also induces synaptogenesis, promotes cell proliferation, and stimulates the development of dense axonal and dendritic networks [51]. In addition, BDNF (brain-derived neurotrophic factor) and VEGF (vascular endothelial growth factor), the key mediators of adaptive remodeling of surviving neurons and neural networks [52] are upregulated following NeuroAid treatment [51, 53].
According to the mentioned findings, the goal of this research is to investigate the effect of NeuroAid on cholestasis-induced spatial memory impairment with respect to the expression of BAX, BCL-2, BAD, PGC-1α, and TFAM in the hippocampus of male Wistar rats.
Material and Method
Animals
In the present study, male Wistar rats (220-240 g, 11–12 weeks old) bred at Iranian National Center for Addiction Studies (INCAS), Tehran, Iran were used. Rats were kept in the lab with a 12/12 h light-dark cycle and standard temperature (22 ± 2 °C). Rats were placed in Plexiglas cages in groups of 4 and free access to food and water was provided except during the experiments. Each experimental group consisted of eight rats. Furthermore, the behavioral experiments were done only during the light phase. All the experiments were done in accordance with the guidelines of NIH (NIH Guide for the Care and Use of Laboratory Animals) and the guidelines of Institute for Cognitive Science Studies (ICSS), Tehran, Iran.
Bile Duct Ligation (BDL) Surgery
Rats were anesthetized by intraperitoneal injection of ketamine hydrochloride (50 mg/kg) plus xylazine (5 mg/kg). During surgery, the common bile duct was located and ligated using 4 − 0 silk at two points anterior to the pancreas and posterior to the hilum of the liver. One ligation was made just above the duodenum; the second ligation was made approximately 2 mm above the first ligation and then transected at the midpoint between the two ligatures [11, 54]. Sham-ligation surgery was performed by locating and manipulating the common bile duct. Sterile 0.9% NaCl solution (1 mL/rat) was injected intraperitoneal immediately after the surgery. All surgeries were performed using aseptic technique. After the operation, each rat was placed in a cage by itself to prevent wound dehiscence and 4 h later was transferred to its original cage [11, 55].
Drug
NeuroAid (MLC901) (Moleac, Singapore) including 9 herbal components per each capsule: 0.80g Radix astragali, 0.16g Radix salvia miltiorrhizae, 0.16g Radix paeoniae rubra, 0.16g Rhizoma chuanxiong, 0.16g Radix angelicae sinensis, 0.16g Car-thamus tinctorius, 0.16g Prunus persica, 0.16 g Radix polygalae, and 0.16g Rhizoma acori tatarinowii [48] was injected intraperitoneal at the dose of 0.4 mg/kg.
Experimental Groups
This study consisted of four experimental groups including: Sham of BDL with saline (1 mL/kg), Sham of BDL with NeuroAid (0.4 mg/kg), BDL with saline (1 mL/kg), and BDL with NeuroAid (0.4 mg/kg). NeuroAid (Moleac, Singapore) was diluted in saline (used as a vehicle) at concentration of 0.4 mg/mL (stock solution) and incubated under agitation for 1 h at 37 °C. The solution was strained using a 0.22-µm filter. One day after BDL surgery, NeuroAid (0.4 mg/kg) was injected intraperitoneal; and then, was injected every other day up to 28 days [46]. After 28 days, spatial memory was assessed. 28-day period was selected because BDL induces a strong fibrotic response after 21 to 28 days [56]. Additionally, many studies have used a 28-day period to evaluate the effect of BDL on cognitive and non-cognitive functions [10, 57,58,59]. We designed sham of BDL with saline group to assess the effect of both injection stress and surgery stress on memory performance.
Morris Water Maze
Morris Water Maze (MWM) is a valid test to assess spatial learning and memory [60, 61]. MWM is a circular black tank (150 cm in diameter and 60 cm depth) filled with water to a depth of 30 cm. The water temperature in the tank was about 22° C. The visual signs were placed on the walls around the tank. The maze was divided into four quadrants and each quadrant had a starting location: north (N), south (S), west (W), and east (E). The hidden platform (10 cm in diameter) was submerged 1 cm beneath the surface of the water in the center of the target quadrant (the north-west quadrant) [62]. All rats had to find the hidden platform by referring to the visual cues. The test began after a 4-week treatment. One day before the start of learning trials, the rats were familiarized with the apparatus. The learning session consisted of eight trials with four different starting positions. The hidden platform was in the north-west quadrant (the target quadrant). A camera was located above the tank and attached to a computer. The performance of each rat was recorded by a smart video tracking software (BorjSanatAzma). During learning trials, the escape latency, the traveled distance, and the mean velocity of each rat to find the hidden platform were measured. After finding the platform, each rat was allowed to remain on the platform for 20s (to memorize visual cues). In learning trials, more time spent or longer distance traveled means poor spatial learning. 24h after learning trials, the probe test was performed. In the probe test rats were placed in the tank for 1 min, while the hidden platform was removed. The escape latency, the traveled distance, and the mean velocity of the rats only in the target quadrant were measured. In probe trial, more time spent or longer distance traveled in the target quadrant means better spatial memory. After the probe test, we performed visible test with the visible platform to ensure that motor functions, visuo–motor abilities, or motivation of the rats to escape water or anything else did not influence the results.
Statistical Analysis for Spatial Memory
Statistical analyses were performed using SPSS software (V. 26). Given the normality of distribution and the homogeneity of data variance, the results were statistically evaluated using two-way ANOVA. Further analyses for individual “between-group” comparisons were done using post hoc Tukey test. In all comparisons, P < 0.05 was considered as statistical significance.
Hippocampus Sample Preparation
After performing the tests, rats were placed in a special chamber and killed with CO2 gas. The rat’s brain was removed. After a brief wash with normal saline, the brain was placed inside a coronal section matrix suitable for the brain of rat weighing 200 to 250 g; and then, the hippocampus was separated and placed into the nitrogen tank. (Note: all stages of tissue extraction should not last longer than 2 min). After 24 h, fifty milligrams of hippocampus samples were diluted in 10 ng phosphate-buffered saline (PBS) (PH = 7.4) and homogenized by a homogenizer. The samples were centrifuged at 2000–3000 RPM for 20 min and the supernatants were collected carefully for measuring the expression level of BCL-2, BAX, BAD, PGC-1α, and TFAM genes. All samples were kept at −80 °C [63, 64].
Real-time PCR
In order to measure BCL-2, BAX, BAD, PGC-1α, and TFAM genes’ mRNA expression, complementary DNA (cDNA) was prepared from the whole cellular RNA. The total RNA was extracted using BioFACT™ Total RNA Prep Kit. The RNA was quantified by Picodrop Microliter Spectrometer. cDNA was prepared using BioFACT™ OneStep RT-PCR Kit according to the manufacturer’s method in a final volume of 40 µl. Finally, the cDNA was stored at −20 °C [35].
In order to normalize target gene expression, GAPDH was used as the housekeeping gene. The primers used for the real-time PCR were BCL-2, BAX, BAD, PGC-1α, and TFAM.
After preparing hippocampus samples, the extracted RNA was purified and the high quality RNAs were selected and kept at − 80 °C until using for cDNA synthesis. Up to 1 µg RNA was converted to cDNA using Quantitect reverse transcription kit (Qiagen). The primers for Real-time PCR were designed and underwent an extensive search using BLAST tool. The characteristics of the primers used in this study have been summarized in Table 1. Real-time PCR was carried out using the following cycling conditions: 95 °C for 10 min, and 40 cycles at 95 °C for 15 s, and 60 °C for 1 min. Each complete amplification stage was followed by a dissociation stage: at 95 °C for 15 s, 60 °C for 30 s, then temperature was ramped up from 60 to 95 °C (at the rate of 0.03 °C/s). Melting curve analysis was performed according to the dissociation stage data and reactions.
Genetic Data Analysis
Quantitative analysis was performed by the measurement of Ct values during the exponential phase of amplification. Relative quantity of genes was determined using comparative Ct method and ΔCt was calculated as the difference between the Ct values of the target gene and the Ct value of GAPDH gene. The results were analyzed using this formula: Gene dosage ratio = 2−ΔΔCt. Statistical significance of difference was calculated using t-test.
Results
BDL Impaired Spatial Memory and NeuroAid Restored this Effect
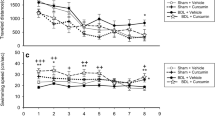
Trials- The results of two-way ANOVA showed that the effect of BDL surgery, drug, and the interaction effect for traveled distance, escape latency, and mean velocity in both Sec. Introduction (the average data of trials 1 to 4) & 2 (the average data of trials 5 to 8) was not significant (Fig. 1). Thus, our data showed that spatial learning was not altered following BDL surgery or NeuroAid administration.
Shows the effects of bile duct ligation surgery and NeuroAid injection on traveled distance (a), escape latency (b), and swimming speed (c) in the Morris Water Maze apparatus in two sections (Sec. Introduction: average data of trials 1 to 4, Sec. Material and Method: average data of trials 5 to 8), showing spatial learning performance. Two-way ANOVA and post hoc Tukey’s were used to analyze data (n = 8)
Probe- The results of two-way ANOVA for escape latency showed that the effect of BDL surgery (F1, 28 = 4.32, P < 0.05), drug (F1, 28 = 12.90, P < 0.001), and the interaction effect (F1, 28 = 4.86, P < 0.05) was significant. The results of two-way ANOVA for traveled distance showed that the effect of drug (F1, 28 = 11.70, P < 0.01), and the interaction effect (F1, 28 = 8.90, P < 0.01) was significant, while the effect of BDL surgery (F1, 28 = 1.70, P > 0.05) was not significant. The results of post hoc Tukey’s showed that there is a significant difference between sham + saline and BDL + saline groups for traveled distance (P < 0.001) and escape latency (P < 0.001). The BDL rats could not distinguish the target quadrant. Therefore, the results showed that BDL decreased the traveled distance and the escape latency in the target quadrant, meaning impaired spatial memory consolidation. Also, post hoc Tukey’s showed that there is a significant difference between BDL + saline and BDL-NeuroAid groups for traveled distance (P < 0.001) and escape latency (P < 0.001). Administration of NeuroAid in BDL group increased the traveled distance and the escape latency in the target quadrant, meaning that NeuroAid restored the impairment effect of cholestasis on spatial memory consolidation. Furthermore, the results of two-way ANOVA for the mean velocity were not significant (Fig. 2). Also, the results of the visible tests were not significant, meaning that motor functions, visuo–motor abilities, or motivation of the rats to escape water or anything else did not influence the results (data not shown).
Shows the effects of bile duct ligation surgery and NeuroAid injection on traveled distance (a), escape latency (b) and swimming speed (c) in the target quadrant of the Morris Water Maze apparatus, showing spatial memory consolidation. ***P < 0.001 different from sham + saline group, and ###P < 0.001 different from the BDL + saline group. Two-way ANOVA and post hoc Tukey’s were used to analyze data (n = 8)
Real Time PCR Analysis Shows Increased Expression of BAD and BAX in BDL Rats
BAX- The results of two-way ANOVA showed that the effect of BDL surgery (F1, 28 = 149.75, P < 0.001), drug (F1, 28 = 231.25, P < 0.001), and the interaction effect (F1, 28 = 146.20, P < 0.001) was significant. The results of post hoc Tukey’s showed that the expression of BAX (P < 0.001) was increased in the BDL group, while NeuroAid reversed this effect (P < 0.001).
BAD- The results of two-way ANOVA showed that the effect of BDL surgery (F1, 28 = 85.54, P < 0.001), drug (F1, 28 = 144.39, P < 0.001), and the interaction effect (F1, 28 = 86.19, P < 0.001) was significant. The results of post hoc Tukey’s showed that the expression of BAD (P < 0.001) was increased in the BDL group, while NeuroAid reversed this effect (P < 0.001).
BCL-2- The results of two-way ANOVA showed that the effect of BDL surgery (F1, 28 = 752.24, P < 0.001), drug (F1, 28 = 898.33, P < 0.001), and the interaction effect (F1, 28 = 1139.92 P < 0.001) was significant. The results of post hoc Tukey’s showed that the expression of BCL-2 (P < 0.001) was increased in the BDL-NeuroAid group.
PGC-1α- The results of two-way ANOVA showed that the effect of BDL surgery (F1, 28 = 633.65, P < 0.001), drug (F1, 28 = 782.39, P < 0.001), and the interaction effect (F1, 28 = 733.40, P < 0.001) was significant. The results of post hoc Tukey’s showed that the expression of PGC-1α (P < 0.05) was decreased in the BDL group, while NeuroAid reversed this effect (P < 0.001).
TFAM- The results of two-way ANOVA showed that the effect of BDL surgery (F1, 28 = 3.73, P > 0.05), and the interaction effect (F1, 28 = 2.12, P > 0.05) was not significant, while the effect of drug (F1, 28 = 21.76, P < 0.001) was significant. The results of post hoc Tukey’s showed that the expression of TFAM (P < 0.05) was decreased in the BDL group, while NeuroAid reversed this effect (P < 0.05) (Fig. 3).
Shows the results of Real-time PCR analyses for the expression of BAX, BCL-2, BAD, PGC-1α, and TFAM. Sham-NeuroAid and BDL groups were compared with the sham group. P < 0.001*P < 0.05 and ***P < 0.001 different from the sham-saline group, #P < 0.05 and ###P < 0.001 different from the BDL-saline group. Two-way ANOVA and post hoc Tukey’s were used to analyze data (n = 8)
Discussion
Impairment Effect of BDL on Spatial Memory
Our results showed that spatial learning was not altered following BDL surgery or NeuroAid administration (Fig. 1 a and b). Furthermore, our data showed that BDL decreased the traveled distance and the escape latency in the target quadrant in the probe test meaning the impairment of spatial memory consolidation (Fig. 2a and b). Previous studies have shown the impairment effect of BDL on learning and memory. BDL impairs spatial performance in MWM apparatus and impairs learning in passive avoidance task [65]. It has been revealed that BDL impairs spatial memory consolidation in MWM apparatus [66]. Furthermore, BDL disrupts learning and memory retrieval in step-through passive avoidance task in rats [67]. BDL is a chronic liver injury that impairs learning and memory. It has been reported that acute or chronic liver injury impairs cognitive functions [68, 69]. Note that, the molecular mechanism of BDL involved in cognitive impairments is still unknown. In this research, BDL impaired spatial memory in MWM apparatus. It has been suggested that hyperammonia induced by the liver disease is one of the main factors responsible for cognitive impairments following BDL [16, 17]. Furthermore, disruptions in many neurotransmitter systems have been observed in liver diseases [18,19,20]. Previous study has shown that cholestasis-induced glutamatergic disruption impairs memory formation in different brain regions especially the dorsal region of the hippocampus [11]. Previous reports have also suggested that cholestasis changes nitric oxide (NO) level, increases oxidative stress, disrupts calcium homeostasis, and induces cell death; all these mechanisms are involved in memory impairment [70,71,72,73]. Additionally, it seems that disruption in the release of corticotrophin-releasing hormone and homeostasis of manganese following cholestasis has a critical role in the induction of cognitive impairments [74,75,76].
NeuroAid Restored the Impairment Effect of BDL on Spatial Memory
The results showed that NeuroAid restored the impairment effect of BDL on spatial memory (Fig. 2). Previous studies have shown the neuroprotective effects of NeuroAid on memory function. For example, NeuroAid restores fear memory impairment induced by sleep deprivation [77]. NeuroAid also improves cognitive dysfunctions in AD patients [78]. Furthermore, NeuroAid reduces tau phosphorylation [79]. NeuroAid improves performance in novel object recognition, MWM apparatus, and passive avoidance learning task [48]. Interestingly, NeuroAid attenuates hippocampal oxidative stress induced by ROS (reactive oxygen species) accumulation [80]. Also, NeuroAid has neuro-restorative effect that stimulates brain neuro-repair processes including neuroplasticity and neurogenesis [53]. It’s important to note that the beneficial effects of NeuroAid may be related to multi-target effects [77]. For example, NeuroAid acts as an activator of KATP channels [81]. Furthermore, NeuroAid activates the serine/threonine kinase Akt (protein kinase B) pathway in global ischemia model [82]. NeuroAid increases hippocampal neurogenesis via promoting proliferation, neural differentiation, and survival of young neurons [48]. On the other hand, BDNF (brain-derived neurotrophic factor) and VEGF (vascular endothelial growth factor) are critically involved in neuronal repair [83]. BDNF and VEGF are also involved in learning and memory processing [84, 85]. Interestingly, NeuroAid stimulates the expression of BDNF in brain tissue after focal and global ischemia [51]. Furthermore, NeuroAid enhances the expression of VEGF in the hippocampus and cortex of TBI (traumatic brain injury) rats [53]. All these findings show that NeuroAid induces peripheral and central neuroprotective effects in the body, promotes endogenous neural repair processes in the brain, and reverses the impairment effect of BDL on memory.
Increase in BAD and BAX Expression and Decrease in TFAM and PGC-1α Expression Following BDL
The results showed that the expression of BAD and BAX was increased following BDL (Fig. 3). As mentioned, BCL-2 family proteins have a crucial role in modulating apoptosis [86]. BAD (BCL-2-associated death) and BAX (BCL-2-associated X) are pro-apoptotic proteins that activate caspases via releasing IMS proteins [32]. As the data showed, BDL increased the expression of these genes. BDL accumulates P53 (a gene involved in regulating cell cycle) in the nucleus; while the overexpression of P53 increases the expression of BAX and induces apoptosis [87]. Furthermore, maternal obstructive cholestasis during pregnancy (OCP) increases the expression of BAX via increase in apoptosis in the OCP placentas [88]. Our previous study has shown that BDL increases BAX expression in the striatum of rats [89]. In another study, a dramatic increase in BAX expression was observed following BDL [90]. BDL also translocates the cytoplasmic BAX to the mitochondria and induces apoptosis via releasing Cytochrome c (Cyt c) into the cytoplasm [91]. Thus, increase in the expression of BAX and BAD may be related to BDL-induced apoptosis. Additionally, BDL-induced P53 overexpression may lead to the increase in BAX expression and apoptosis.
On the other hand, the results showed that the expression of TFAM and PGC-1α was decreased following BDL (Fig. 3). Previous research has revealed that TFAM downregulation following BDL reduces mtDNA copy number in rats [92]. It has been also revealed that long-term cholestasis induces a significant decrease in TFAM level [93]. Furthermore, long-term cholestasis decreases PGC-1α expression via induction of mitochondrial oxidative stress [94]. In this study, the expression of TFAM and PGC-1α was decreased following BDL. TFAM has a main role in mtDNA maintenance and stabilization [95]. Also, it protects mtDNA from ROS [96]. Note that, activation of TFAM is modulated by PGC-1α in response to various conditions such as oxidative stress, liver injuries, and BDL [97, 98]. As mentioned before, PGC-1α is a master regulator of mitochondrial biogenesis and involved in energy homeostasis [99, 100]. Also, PGC-1α has an important role in oxidative stress [101]. We suggest that decrease in TFAM level may be related to BDL-induced oxidative stress. TFAM is crucial to protect mtDNA from ROS and if the level of ROS gets too high, the function of TFAM may be suppressed. Furthermore, the failure of mitochondrial biogenesis following cholestasis may downregulate the expression of TFAM. On the other hand, it can be suggested that failure of mitochondrial biogenesis following TFAM downregulation [92] reduces PGC-1α [102]. Also, decrease of mitochondrial biogenesis and increase of oxidative stress following BDL may suppress the expression of PGC-1α [94].
NeuroAid Reversed the Effects of BDL on Genes Expression
Our data showed that NeuroAid reversed all the effects of BDL on the expression of genes (Fig. 3). NeuroAid has neuroprotective properties in rodent models of focal and global ischemia [103]. Previous reports have demonstrated that NeuroAid prevents the death of threatened neuronal tissues [49]. Interestingly, NeuroAid decreases the activity of apoptotic pathways via reducing BAX activity in the CA1 pyramidal neurons [49]. NeuroAid also decreases BAX expression in the hippocampus [77]. In addition, NeuroAid restores BDL-induced BAX upregulation in the striatum of rats [104]. In this research, NeuroAid decreased the expression of pro-apoptotic genes (BAX and BAD). The limitation of the present study is the lack of immunohistochemical studies to show the exact location of apoptosis within the hippocampus and the exact location of protection. However, we refer to an interesting study. Previous study has reported that NeuroAid decreases the number of injured neurons in the ipsilateral hippocampal CA3 substructure, dentate gyrus, and thalamus following TBI in rats [53]. Furthermore, TBI-induced necrotic and apoptotic neuronal death is significantly reduced after treatment with NeuroAid [53]. GFAP (glial fibrillary acidic protein) is the cell-specific intermediate filament in astrocytes and its upregulation is considered as a definite feature of activated astrocytes [105]. It has been shown that NeuroAid can strongly reduce GFAP expression in both injured cortex and dentate gyrus neurons of TBI rats [53]. Therefore, it seems that NeuroAid induces a neuroprotective effect in both mature neurons and the dentate gyrus (and its subgranular cell layer). Thus, we can suggest that the neuroprotective effect of NeuroAid may also occur in the other important regions of the brain. Additionally, this mentioned study has reported that at 4 h following TBI, the expression of MAP2 (microtubule-associated protein 2) in the CA3 region and dentate gyrus is decreased [53]. MAP2 is expressed in the soma and dendrites of neuronal cells and is critical for microtubule stability and neuroplasticity. Changes in the expression of MAP2 can induce neuronal degeneration following brain injury [106]. Interestingly, NeuroAid prevents MAP2 changes in TBI rats [53]. It has been revealed that NeuroAid can also increase the number of axons of newly generated cells to the CA3 region [53]. We suggest that the decrease in BAX and BAD expression may be related to the repressive effect of NeuroAid on apoptosis pathways. In addition, NeuroAid via reducing susceptibility of mitochondrial pathways may decrease the expression of BAX and BAD. On the other hand, the expression of TFAM and PGC-1α was increased following NeuroAid treatment. NeuroAid by decrease in ROS accumulation attenuates BDL-induced oxidative stress. Thus, we can suggest that NeuroAid increases the expression of TFAM via reducing ROS. In addition, it seems that NeuroAid enhances mitochondrial biogenesis. Thus, NeuroAid may increase the expression of TFAM via attenuating mitochondrial biogenesis failure. Furthermore, NeuroAid via decrease of oxidative stress and improvement of mitochondrial biogenesis upregulates PGC-1α. It can be also suggested that activation of PGC-1α plays an important role in the modulation of TFAM level.
Conclusions
In conclusion, the results of the present study showed that BDL impaired spatial memory in rats. Furthermore, NeuroAid restored BDL-induced spatial memory impairment. In addition, BDL increased the expression of pro-apoptotic genes (BAD and BAX) and decreased the expression of TFAM and PGC-1α. While, NeuroAid reversed all the effects of BDL on genes expression. Note that, our data only shows mRNA and genetic changes but not changes in protein expression. Often, changes in genetic and mRNA levels do not lead to changes in protein levels. Thus, conducting studies using other methods such as western blotting is necessary to complete our conclusions.
Data Availability
Data will be made available on the reasonable request.
References
Boyer JL (2013) Bile formation and secretion. Compr Physiol 3(3):1035–1078. doi:https://doi.org/10.1002/cphy.c120027
Wang K (2015) Molecular mechanisms of hepatic apoptosis regulated by nuclear factors. Cell Signal 27(4):729–738. doi:https://doi.org/10.1016/j.cellsig.2014.11.038
Aktas C, Kanter M, Erboga M, Mete R, Oran M (2014) Melatonin attenuates oxidative stress, liver damage and hepatocyte apoptosis after bile-duct ligation in rats. Toxicol Ind Health 30(9):835–844. doi:https://doi.org/10.1177/0748233712464811
Motteleb AE, Ibrahim DM, Elshazly I SM (2017) Sildenafil protects against bile duct ligation induced hepatic fibrosis in rats: Potential role for silent information regulator 1 (SIRT1). Toxicol Appl Pharmacol 335:64–71. doi:https://doi.org/10.1016/j.taap.2017.09.021
Yokota S, Ono Y, Nakao T, Zhang P, Michalopoulos GK, Khan Z (2018) Partial bile duct ligation in the mouse: a controlled model of localized obstructive cholestasis. J Vis Exp. https://doi.org/10.3791/56930
Lleo A, Marzorati S, Anaya JM, Gershwin ME (2017) Primary biliary cholangitis: a comprehensive overview. Hepatol Int 11(6):485–499. doi:https://doi.org/10.1007/s12072-017-9830-1
Sirpal S, Chandok N (2017) Primary sclerosing cholangitis: diagnostic and management challenges. Clin Exp Gastroenterol 10:265–273. doi:https://doi.org/10.2147/CEG.S105872
Santamaria E, Rodriguez-Ortigosa CM, Uriarte I, Latasa MU, Urtasun R, Alvarez-Sola G, Barcena-Varela M, Colyn L, Arcelus S, Jimenez M, Deutschmann K, Peleteiro-Vigil A, Gomez-Cambronero J, Milkiewicz M, Milkiewicz P, Sangro B, Keitel V, Monte MJ, Marin JJG, Fernandez-Barrena MG, Avila MA, Berasain C (2018) The epidermal growth factor receptor ligand amphiregulin protects from cholestatic liver injury and regulates bile acids synthesis. Hepatology. https://doi.org/10.1002/hep.30348
Hosseini N, Nasehi M, Radahmadi M, Zarrindast MR (2013) Effects of CA1 glutamatergic systems upon memory impairments in cholestatic rats. Behav Brain Res 256:636–645. doi:https://doi.org/10.1016/j.bbr.2013.08.018
Dastgheib M, Dehpour AR, Heidari M, Moezi L (2015) The effects of intra-dorsal hippocampus infusion of pregnenolone sulfate on memory function and hippocampal BDNF mRNA expression of biliary cirrhosis-induced memory impairment in rats. Neuroscience 306:1–9. doi:https://doi.org/10.1016/j.neuroscience.2015.08.018
Zarrindast MR, Hoseindoost S, Nasehi M (2012) Possible interaction between opioidergic and cholinergic systems of CA1 in cholestasis-induced amnesia in mice. Behav Brain Res 228(1):116–124. doi:https://doi.org/10.1016/j.bbr.2011.11.039
Javadi-Paydar M, Ghiassy B, Ebadian S, Rahimi N, Norouzi A, Dehpour AR (2013) Nitric oxide mediates the beneficial effect of chronic naltrexone on cholestasis-induced memory impairment in male rats. Behav Pharmacol 24(3):195–206. doi:https://doi.org/10.1097/FBP.0b013e3283618a8c
Gimenez-Garzo C, Salhi D, Urios A, Ruiz-Sauri A, Carda C, Montoliu C, Felipo V (2015) Rats with mild bile duct ligation show hepatic encephalopathy with cognitive and motor impairment in the absence of cirrhosis: effects of alcohol ingestion. Neurochem Res 40(2):230–240. doi:https://doi.org/10.1007/s11064-014-1330-2
Newton JL, Hollingsworth KG, Taylor R, El-Sharkawy AM, Khan ZU, Pearce R, Sutcliffe K, Okonkwo O, Davidson A, Burt J, Blamire AM, Jones D (2008) Cognitive impairment in primary biliary cirrhosis: symptom impact and potential etiology. Hepatology 48(2):541–549. doi:https://doi.org/10.1002/hep.22371
Yilmaz Y, Ozdogan O (2009) Liver disease as a risk factor for cognitive decline and dementia: an under-recognized issue. Hepatology 49(2):698. https://doi.org/10.1002/hep.22752
Pantiga C, Rodrigo LR, Cuesta M, Lopez L, Arias JL (2003) Cognitive deficits in patients with hepatic cirrhosis and in liver transplant recipients. J Neuropsychiatry Clin Neurosci 15(1):84–89. doi:https://doi.org/10.1176/jnp.15.1.84
Weissenborn K, Heidenreich S, Giewekemeyer K, Ruckert N, Hecker H (2003) Memory function in early hepatic encephalopathy. J Hepatol 39(3):320–325
Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM (2010) Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br J Pharmacol 159(4):950–957. doi:https://doi.org/10.1111/j.1476-5381.2009.00589.x
Palomero-Gallagher N, Bidmon HJ, Cremer M, Schleicher A, Kircheis G, Reifenberger G, Kostopoulos G, Haussinger D, Zilles K (2009) Neurotransmitter receptor imbalances in motor cortex and basal ganglia in hepatic encephalopathy. Cell Physiol Biochem 24(3–4):291–306. doi:https://doi.org/10.1159/000233254
Butterworth RF (1996) The neurobiology of hepatic encephalopathy. Semin Liver Dis 16(3):235–244. doi:https://doi.org/10.1055/s-2007-1007236
Shipovskaya AA, Dudanova OP (2018) Intrahepatic cholestasis in nonalcoholic fatty liver disease. Ter Arkh 90(2):69–74. doi:https://doi.org/10.26442/terarkh201890269-74
Wang K (2014) Molecular mechanisms of liver injury: apoptosis or necrosis. Exp Toxicol Pathol 66(8):351–356. doi:https://doi.org/10.1016/j.etp.2014.04.004
Malhi H, Gores GJ, Lemasters JJ (2006) Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 43(2 Suppl 1):S31-44. https://doi.org/10.1002/hep.21062
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. doi:https://doi.org/10.1080/01926230701320337
Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G (2016) Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging 8(4):603–619. doi:https://doi.org/10.18632/aging.100934
Giam M, Huang DC, Bouillet P (2008) BH3-only proteins and their roles in programmed cell death. Oncogene 27(Suppl 1):S128–S136. https://doi.org/10.1038/onc.2009.50
Zhang L, Zhou Y, Chen K, Shi P, Li Y, Deng M, Jiang Z, Wang X, Li P, Xu B (2017) The pan-Bcl2 Inhibitor AT101 Activates the Intrinsic Apoptotic Pathway and Causes DNA Damage in Acute Myeloid Leukemia Stem-Like Cells. Target Oncol 12(5):677–687. doi:https://doi.org/10.1007/s11523-017-0509-2
Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ (2017) From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov 16(4):273–284. doi:https://doi.org/10.1038/nrd.2016.253
Yap JL, Chen L, Lanning ME, Fletcher S (2017) Expanding the Cancer Arsenal with Targeted Therapies: Disarmament of the Antiapoptotic Bcl-2 Proteins by Small Molecules. J Med Chem 60(3):821–838. doi:https://doi.org/10.1021/acs.jmedchem.5b01888
Shoshan-Barmatz V, Mizrachi D, Keinan N (2013) Oligomerization of the mitochondrial protein VDAC1: from structure to function and cancer therapy. Prog Mol Biol Transl Sci 117:303–334. doi:https://doi.org/10.1016/B978-0-12-386931-9.00011-8
Wang T, Yang Z, Zhang Y, Zhang X, Wang L, Zhang S, Jia L (2018) Caspase cleavage of Mcl-1 impairs its anti-apoptotic activity and proteasomal degradation in non-small lung cancer cells. Apoptosis 23(1):54–64. doi:https://doi.org/10.1007/s10495-017-1436-5
Vogtle FN, Burkhart JM, Rao S, Gerbeth C, Hinrichs J, Martinou JC, Chacinska A, Sickmann A, Zahedi RP, Meisinger C (2012) Intermembrane space proteome of yeast mitochondria. Mol Cell Proteomics 11(12):1840–1852. doi:https://doi.org/10.1074/mcp.M112.021105
Han X, Cong H (2017) Enterovirus 71 induces apoptosis by directly modulating the conformational activation of pro-apoptotic protein Bax. J Gen Virol 98(3):422–434. doi:https://doi.org/10.1099/jgv.0.000705
Zhang R, Wang J (2018) HuR stabilizes TFAM mRNA in an ATM/p38-dependent manner in ionizing irradiated cancer cells. Cancer Sci 109(8):2446–2457. doi:https://doi.org/10.1111/cas.13657
Malboosi N, Nasehi M, Hashemi M, Vaseghi S, Zarrindast MR (2020) The neuroprotective effect of NeuroAid on morphine-induced amnesia with respect to the expression of TFAM, PGC-1alpha, DeltafosB and CART genes in the hippocampus of male Wistar rats. Gene 742:144601. doi:https://doi.org/10.1016/j.gene.2020.144601
Kunkel GH, Kunkel CJ, Ozuna H, Miralda I, Tyagi SC (2019) TFAM overexpression reduces pathological cardiac remodeling. Mol Cell Biochem 454(1–2):139–152. doi:https://doi.org/10.1007/s11010-018-3459-9
Coskun P, Wyrembak J, Schriner SE, Chen HW, Marciniack C, Laferla F, Wallace DC (2012) A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim Biophys Acta 1820(5):553–564. doi:https://doi.org/10.1016/j.bbagen.2011.08.008
Chaturvedi RK, Flint Beal M (2013) Mitochondrial diseases of the brain. Free Radic Biol Med 63:1–29. doi:https://doi.org/10.1016/j.freeradbiomed.2013.03.018
Villena JA (2015) New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J 282(4):647–672. doi:https://doi.org/10.1111/febs.13175
Pedros I, Petrov D, Allgaier M, Sureda F, Barroso E, Beas-Zarate C, Auladell C, Pallas M, Vazquez-Carrera M, Casadesus G, Folch J, Camins A (2014) Early alterations in energy metabolism in the hippocampus of APPswe/PS1dE9 mouse model of Alzheimer’s disease. Biochim Biophys Acta 1842(9):1556–1566. doi:https://doi.org/10.1016/j.bbadis.2014.05.025
Onyango IG, Khan SM, Bennett JP Jr (2017) Mitochondria in the pathophysiology of Alzheimer’s and Parkinson’s diseases. Front Biosci (Landmark Ed) 22:854–872
Reisi P, Eidelkhani N, Rafiee L, Kazemi M, Radahmadi M, Alaei H (2017) Effects of doxepin on gene expressions of Bcl-2 family, TNF-alpha, MAP kinase 14, and Akt1 in the hippocampus of rats exposed to stress. Res Pharm Sci 12(1):15–20. doi:https://doi.org/10.4103/1735-5362.199042
Murthy SR, Thouennon E, Li WS, Cheng Y, Bhupatkar J, Cawley NX, Lane M, Merchenthaler I, Loh YP (2013) Carboxypeptidase E protects hippocampal neurons during stress in male mice by up-regulating prosurvival BCL2 protein expression. Endocrinology 154(9):3284–3293. doi:https://doi.org/10.1210/en.2013-1118
Moretti M, Budni J, Dos Santos DB, Antunes A, Daufenbach JF, Manosso LM, Farina M, Rodrigues AL (2013) Protective effects of ascorbic acid on behavior and oxidative status of restraint-stressed mice. J Mol Neurosci 49(1):68–79. doi:https://doi.org/10.1007/s12031-012-9892-4
Manczak M, Kandimalla R, Yin X, Reddy PH (2018) Hippocampal mutant APP and amyloid beta-induced cognitive decline, dendritic spine loss, defective autophagy, mitophagy and mitochondrial abnormalities in a mouse model of Alzheimer’s disease. Hum Mol Genet 27(8):1332–1342. doi:https://doi.org/10.1093/hmg/ddy042
Tsai MC, Chang CP, Peng SW, Jhuang KS, Fang YH, Lin MT, Tsao TC (2015) Therapeutic efficacy of Neuro AiD (MLC 601), a traditional Chinese medicine, in experimental traumatic brain injury. J Neuroimmune Pharmacol 10(1):45–54. doi:https://doi.org/10.1007/s11481-014-9570-0
Chan HY, Stanton LW (2016) A pharmacogenomic profile of human neural progenitors undergoing differentiation in the presence of the traditional Chinese medicine NeuroAiD. Pharmacogenomics J 16(5):461–471. doi:https://doi.org/10.1038/tpj.2016.21
Lorivel T, Gandin C, Veyssiere J, Lazdunski M, Heurteaux C (2015) Positive effects of the traditional Chinese medicine MLC901 in cognitive tasks. J Neurosci Res 93(11):1648–1663. doi:https://doi.org/10.1002/jnr.23591
Quintard H, Borsotto M, Veyssiere J, Gandin C, Labbal F, Widmann C, Lazdunski M, Heurteaux C (2011) MLC901, a traditional Chinese medicine protects the brain against global ischemia. Neuropharmacology 61(4):622–631. doi:https://doi.org/10.1016/j.neuropharm.2011.05.003
Gandin C, Widmann C, Lazdunski M, Heurteaux C (2016) MLC901 Favors Angiogenesis and Associated Recovery after Ischemic Stroke in Mice. Cerebrovasc Dis 42(1–2):139–154. doi:https://doi.org/10.1159/000444810
Heurteaux C, Gandin C, Borsotto M, Widmann C, Brau F, Lhuillier M, Onteniente B, Lazdunski M (2010) Neuroprotective and neuroproliferative activities of NeuroAid (MLC601, MLC901), a Chinese medicine, in vitro and in vivo. Neuropharmacology 58(7):987–1001. doi:https://doi.org/10.1016/j.neuropharm.2010.01.001
Chopp M, Li Y, Zhang ZG (2009) Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke 40(3 Suppl):S143–S145. doi:https://doi.org/10.1161/STROKEAHA.108.533141
Quintard H, Lorivel T, Gandin C, Lazdunski M, Heurteaux C (2014) MLC901, a Traditional Chinese Medicine induces neuroprotective and neuroregenerative benefits after traumatic brain injury in rats. Neuroscience 277:72–86. doi:https://doi.org/10.1016/j.neuroscience.2014.06.047
Bergasa NV, Alling DW, Vergalla J, Jones EA (1994) Cholestasis in the male rat is associated with naloxone-reversible antinociception. J Hepatol 20(1):85–90
Rastegar H, Homayoun H, Afifi M, Rezayat M, Dehpour AR (2002) Modulation of cholestasis-induced antinociception by CCK receptor agonists and antagonists. Eur Neuropsychopharmacol 12(2):111–118
Tag CG, Sauer-Lehnen S, Weiskirchen S, Borkham-Kamphorst E, Tolba RH, Tacke F, Weiskirchen R (2015) Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp. https://doi.org/10.3791/52438
Villar-Lorenzo A, Rada P, Rey E, Maranon P, Arroba AI, Santamaria B, Saiz J, Ruperez FJ, Barbas C, Garcia-Monzon C, Valverde AM, Gonzalez-Rodriguez A (2019) Insulin receptor substrate 2 (IRS2) deficiency delays liver fibrosis associated with cholestatic injury. Dis Model Mech. https://doi.org/10.1242/dmm.038810
Vartak N, Damle-Vartak A, Richter B, Dirsch O, Dahmen U, Hammad S, Hengstler JG (2016) Cholestasis-induced adaptive remodeling of interlobular bile ducts. Hepatology 63(3):951–964. doi:https://doi.org/10.1002/hep.28373
Mohammadian Z, Eidi A, Mortazavi P, Tavangar MM, Asghari A (2015) Effects of folic acid on dyslipidemia and serum homocysteine in a rat model of cholestasis and hepatic fibrosis. Pol J Pathol 66(1):49–56. doi:https://doi.org/10.5114/pjp.2015.51153
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848–858. doi:https://doi.org/10.1038/nprot.2006.116
Vaseghi S, Babapour V, Nasehi M, Zarrindast MR (2019) Synergistic but not additive effect between ACPA and lithium in the dorsal hippocampal region on spatial learning and memory in rats: Isobolographic analyses. Chem Biol Interact 315:108895. doi:https://doi.org/10.1016/j.cbi.2019.108895
Vaseghi S, Babapour V, Nasehi M, Zarrindast MR (2018) The role of CA1 CB1 receptors on lithium-induced spatial memory impairment in rats. EXCLI J 17:916–934. doi:https://doi.org/10.17179/excli2018-1511
Nadimi H, Djazayery A, Javanbakht MH, Dehpour A, Ghaedi E, Derakhshanian H, Mohammadi H, Mousavi SN, Djalali M (2020) Effect of vitamin D supplementation on CREB-TrkB-BDNF pathway in the hippocampus of diabetic rats. Iran J Basic Med Sci 23(1):117–123. doi:https://doi.org/10.22038/IJBMS.2019.38170.9068
Kordestani-Moghadam P, Nasehi M, Khodagholi F, Vaseghi S, Zarrindast MR, Khani M (2020) The fluctuations of metabotropic glutamate receptor subtype 5 (mGluR5) in the amygdala in fear conditioning model of male Wistar rats following sleep deprivation, reverse circadian and napping. Brain Res 1734:146739. doi:https://doi.org/10.1016/j.brainres.2020.146739
Aghaei I, Shabani M, Doustar N, Nazeri M, Dehpour A (2014) Peroxisome proliferator-activated receptor-gamma activation attenuates motor and cognition impairments induced by bile duct ligation in a rat model of hepatic cirrhosis. Pharmacol Biochem Behav 120:133–139. doi:https://doi.org/10.1016/j.pbb.2014.03.002
Swain MG (2001) Cytokines and neuroendocrine dysregulation in obstructive cholestasis: pathophysiological implications. J Hepatol 35(3):416–418
Hosseini N, Alaei H, Zarrindast MR, Nasehi M, Radahmadi M (2014) Cholestasis progression effects on long-term memory in bile duct ligation rats. Adv Biomed Res 3:215. doi:https://doi.org/10.4103/2277-9175.143263
Cauli O, Lopez-Larrubia P, Rodrigues TB, Cerdan S, Felipo V (2007) Magnetic resonance analysis of the effects of acute ammonia intoxication on rat brain. Role of NMDA receptors. J Neurochem 103(4):1334–1343. doi:https://doi.org/10.1111/j.1471-4159.2007.04878.x
Jover R, Rodrigo R, Felipo V, Insausti R, Saez-Valero J, Garcia-Ayllon MS, Suarez I, Candela A, Compan A, Esteban A, Cauli O, Auso E, Rodriguez E, Gutierrez A, Girona E, Erceg S, Berbel P, Perez-Mateo M (2006) Brain edema and inflammatory activation in bile duct ligated rats with diet-induced hyperammonemia: A model of hepatic encephalopathy in cirrhosis. Hepatology 43(6):1257–1266. doi:https://doi.org/10.1002/hep.21180
Keller JN, Guo Q, Holtsberg FW, Bruce-Keller AJ, Mattson MP (1998) Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. J Neurosci 18(12):4439–4450
Stewart SM, Campbell RA, McCallon D, Waller DA, Andrews WS (1992) Cognitive patterns in school-age children with end-stage liver disease. J Dev Behav Pediatr 13(5):331–338
Huang LT, Chen CC, Sheen JM, Chen YJ, Hsieh CS, Tain YL (2010) The interaction between high ammonia diet and bile duct ligation in developing rats: assessment by spatial memory and asymmetric dimethylarginine. Int J Dev Neurosci 28(2):169–174. doi:https://doi.org/10.1016/j.ijdevneu.2009.11.006
Nasehi M, Piri M, Abbolhasani K, Zarrindast MR (2013) Involvement of opioidergic and nitrergic systems in memory acquisition and exploratory behaviors in cholestatic mice. Behav Pharmacol 24(3):180–194. doi:https://doi.org/10.1097/FBP.0b013e3283618aab
Forton DM, Patel N, Prince M, Oatridge A, Hamilton G, Goldblatt J, Allsop JM, Hajnal JV, Thomas HC, Bassendine M, Jones DE, Taylor-Robinson SD (2004) Fatigue and primary biliary cirrhosis: association of globus pallidus magnetisation transfer ratio measurements with fatigue severity and blood manganese levels. Gut 53(4):587–592
Burak KW, Le T, Swain MG (2002) Increased sensitivity to the locomotor-activating effects of corticotropin-releasing hormone in cholestatic rats. Gastroenterology 122(3):681–688
Bearn J, Allain T, Coskeran P, Munro N, Butler J, McGregor A, Wessely S (1995) Neuroendocrine responses to d-fenfluramine and insulin-induced hypoglycemia in chronic fatigue syndrome. Biol Psychiatry 37(4):245–252. doi:https://doi.org/10.1016/0006-3223(94)00121-I
Nasehi M, Mohammadi A, Ebrahimi-Ghiri M, Hashemi M, Zarrindast MR (2019) MLC901 during sleep deprivation rescues fear memory disruption in rats. Naunyn Schmiedebergs Arch Pharmacol. doi:https://doi.org/10.1007/s00210-018-01612-z
Chen CLH, Sharma PR, Tan BY, Low C, Venketasubramanian N (2019) The Alzheimer’s disease THErapy with NEuroaid (ATHENE) study protocol: Assessing the safety and efficacy of Neuroaid II (MLC901) in patients with mild-to-moderate Alzheimer’s disease stable on cholinesterase inhibitors or memantine-A randomized, double-blind, placebo-controlled trial. Alzheimers Dement (N Y) 5:38–45. doi:https://doi.org/10.1016/j.trci.2018.12.001
Lee WT, Hsian CCL, Lim YA (2017) The effects of MLC901 on tau phosphorylation. Neuroreport 28(16):1043–1048. doi:https://doi.org/10.1097/WNR.0000000000000884
Friberg H, Wieloch T, Castilho RF (2002) Mitochondrial oxidative stress after global brain ischemia in rats. Neurosci Lett 334(2):111–114
Moha Ou Maati H, Borsotto M, Chatelain F, Widmann C, Lazdunski M, Heurteaux C (2012) Activation of ATP-sensitive potassium channels as an element of the neuroprotective effects of the Traditional Chinese Medicine MLC901 against oxygen glucose deprivation. Neuropharmacology 63(4):692–700. doi:https://doi.org/10.1016/j.neuropharm.2012.05.035
Franke TF, Kaplan DR, Cantley LC (1997) PI3K: downstream AKTion blocks apoptosis. Cell 88(4):435–437
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25(4):581–611. doi:https://doi.org/10.1210/er.2003-0027
Bekinschtein P, Cammarota M, Medina JH (2014) BDNF and memory processing. Neuropharmacology 76:677–683. https://doi.org/10.1016/j.neuropharm.2013.04.024
Maass A, Duzel S, Brigadski T, Goerke M, Becke A, Sobieray U, Neumann K, Lovden M, Lindenberger U, Backman L, Braun-Dullaeus R, Ahrens D, Heinze HJ, Muller NG, Lessmann V, Sendtner M, Duzel E (2016) Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage 131:142–154. doi:https://doi.org/10.1016/j.neuroimage.2015.10.084
Lomonosova E, Chinnadurai G (2008) BH3-only proteins in apoptosis and beyond: an overview. Oncogene 27(Suppl 1):S2–S19. https://doi.org/10.1038/onc.2009.39
Xiang H, Kinoshita Y, Knudson CM, Korsmeyer SJ, Schwartzkroin PA, Morrison RS (1998) Bax involvement in p53-mediated neuronal cell death. J Neurosci 18(4):1363–1373
Perez MJ, Macias RI, Marin JJ (2006) Maternal cholestasis induces placental oxidative stress and apoptosis. Protective effect of ursodeoxycholic acid. Placenta 27(1):34–41. doi:https://doi.org/10.1016/j.placenta.2004.10.020
Nasehi M, Torabinejad S, Hashemi M, Vaseghi S, Zarrindast MR (2020) Effect of cholestasis and NeuroAid treatment on the expression of Bax, Bcl-2, Pgc-1alpha and Tfam genes involved in apoptosis and mitochondrial biogenesis in the striatum of male rats. Metab Brain Dis 35(1):183–192. doi:https://doi.org/10.1007/s11011-019-00508-y
Shi SH, Jiang L, Xie HY, Xu J, Zhu YF, Zheng SS (2015) The effect of secondary cholestasis on the CD68-positive and CD163-positive macrophage population, cellular proliferation, and apoptosis in rat testis. J Reprod Immunol 110:36–47. doi:https://doi.org/10.1016/j.jri.2015.03.008
Oh SH, Yun KJ, Nan JX, Sohn DH, Lee BH (2003) Changes in expression and immunolocalization of protein associated with toxic bile salts-induced apoptosis in rat hepatocytes. Arch Toxicol 77(2):110–115. doi:https://doi.org/10.1007/s00204-002-0415-x
Tiao MM, Lin TK, Liou CW, Wang PW, Chen JB, Kuo FY, Huang CC, Chou YM, Chuang JH (2009) Early transcriptional deregulation of hepatic mitochondrial biogenesis and its consequent effects on murine cholestatic liver injury. Apoptosis 14(7):890–899. doi:https://doi.org/10.1007/s10495-009-0357-3
Arduini A, Serviddio G, Escobar J, Tormos AM, Bellanti F, Vina J, Monsalve M, Sastre J (2011) Mitochondrial biogenesis fails in secondary biliary cirrhosis in rats leading to mitochondrial DNA depletion and deletions. Am J Physiol Gastrointest Liver Physiol 301(1):G119–G127. doi:https://doi.org/10.1152/ajpgi.00253.2010
Serviddio G, Pereda J, Pallardo FV, Carretero J, Borras C, Cutrin J, Vendemiale G, Poli G, Vina J, Sastre J (2004) Ursodeoxycholic acid protects against secondary biliary cirrhosis in rats by preventing mitochondrial oxidative stress. Hepatology 39(3):711–720. doi:https://doi.org/10.1002/hep.20101
Kang D, Kim SH, Hamasaki N (2007) Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion 7(1–2):39–44. doi:https://doi.org/10.1016/j.mito.2006.11.017
Theilen NT, Kunkel GH, Tyagi SC (2017) The Role of Exercise and TFAM in Preventing Skeletal Muscle Atrophy. J Cell Physiol 232(9):2348–2358. doi:https://doi.org/10.1002/jcp.25737
Nisoli E, Clementi E, Moncada S, Carruba MO (2004) Mitochondrial biogenesis as a cellular signaling framework. Biochem Pharmacol 67(1):1–15
Tiao MM, Lin TK, Chen JB, Liou CW, Wang PW, Huang CC, Chou YM, Huang YH, Chuang JH (2011) Dexamethasone decreases cholestatic liver injury via inhibition of intrinsic pathway with simultaneous enhancement of mitochondrial biogenesis. Steroids 76(7):660–666. doi:https://doi.org/10.1016/j.steroids.2011.03.002
Wang P, Guo X, Zong W, Li Y, Liu G, Lv Y, Zhu Y, He S (2019) PGC-1alpha/SNAI1 axis regulates tumor growth and metastasis by targeting miR-128b in gastric cancer. J Cell Physiol. doi:https://doi.org/10.1002/jcp.28193
Puigserver P, Spiegelman BM (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24(1):78–90. doi:https://doi.org/10.1210/er.2002-0012
Peng K, Yang L, Wang J, Ye F, Dan G, Zhao Y, Cai Y, Cui Z, Ao L, Liu J, Zou Z, Sai Y, Cao J (2017) The Interaction of Mitochondrial Biogenesis and Fission/Fusion Mediated by PGC-1alpha Regulates Rotenone-Induced Dopaminergic Neurotoxicity. Mol Neurobiol 54(5):3783–3797. doi:https://doi.org/10.1007/s12035-016-9944-9
de Andrade DC, de Carvalho SN, Pinheiro D, Thole AA, Moura AS, de Carvalho L, Cortez EA (2015) Bone marrow mononuclear cell transplantation improves mitochondrial bioenergetics in the liver of cholestatic rats. Exp Cell Res 336(1):15–22. doi:https://doi.org/10.1016/j.yexcr.2015.05.002
Heurteaux C, Widmann C, Moha ou Maati H, Quintard H, Gandin C, Borsotto M, Veyssiere J, Onteniente B, Lazdunski M (2013) NeuroAiD: properties for neuroprotection and neurorepair. Cerebrovasc Dis 35(Suppl 1):1–7. doi:https://doi.org/10.1159/000346228
Nasehi M, Torabinejad S, Hashemi M, Vaseghi S, Zarrindast MR (2019) Effect of cholestasis and NeuroAid treatment on the expression of Bax, Bcl-2, Pgc-1alpha and Tfam genes involved in apoptosis and mitochondrial biogenesis in the striatum of male rats. Metab Brain Dis. doi:https://doi.org/10.1007/s11011-019-00508-y
Kernie SG, Erwin TM, Parada LF (2001) Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res 66(3):317–326. doi:https://doi.org/10.1002/jnr.10013
Johnson GV, Jope RS (1992) The role of microtubule-associated protein 2 (MAP-2) in neuronal growth, plasticity, and degeneration. J Neurosci Res 33(4):505–512. doi:https://doi.org/10.1002/jnr.490330402
Acknowledgements
Special thanks to Iranian National Center for Addiction Studies (INCAS), Tehran, Iran, for providing laboratory tools and rats.
Funding
There is no providing financial support to this project.
Author information
Authors and Affiliations
Contributions
P. Molaei conducted the experiments. S. Vaseghi and M. Hashemi wrote the manuscript and managed the literature search. M. Entezari analyzed data. M. Nasehi designed the study. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Molaei, P., Vaseghi, S., Entezari, M. et al. The Effect of NeuroAid (MLC901) on Cholestasis-Induced Spatial Memory Impairment with Respect to the Expression of BAX, BCL-2, BAD, PGC-1α and TFAM Genes in the Hippocampus of Male Wistar Rats. Neurochem Res 46, 2154–2166 (2021). https://doi.org/10.1007/s11064-021-03353-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03353-7