Abstract
Parkinson’s disease is a common neurodegenerative disease in the elderly, and mitochondrial defects underlie the pathogenesis of PD. Impairment of mitochondrial homeostasis results in reactive oxygen species formation, which in turn can potentiate the accumulation of dysfunctional mitochondria, forming a vicious cycle in the neuron. Mitochondrial fission/fusion and biogenesis play important roles in maintaining mitochondrial homeostasis. It has been reported that PGC-1α is a powerful transcription factor that is widely involved in the regulation of mitochondrial biogenesis, oxidative stress, and other processes. Therefore, we explored mitochondrial biogenesis, mitochondrial fission/fusion, and especially PGC-1α as the key point in the signaling mechanism of their interaction in rotenone-induced dopamine neurotoxicity. The results showed that mitochondrial number and mass were reduced significantly, accompanied by alterations in proteins known to regulate mitochondrial fission/fusion (MFN2, OPA1, Drp1, and Fis1) and mitochondrial biogenesis (PGC-1α and mtTFA). Further experiments proved that inhibition of mitochondrial fission or promotion of mitochondrial fusion has protective effects in rotenone-induced neurotoxicity and also promotes mitochondrial biogenesis. By establishing cell models of PGC-1α overexpression and reduced expression, we found that PGC-1α can regulate MFN2 and Drp1 protein expression and phosphorylation to influence mitochondrial fission/fusion. In summary, it can be concluded that PGC-1α-mediated cross talk between mitochondrial biogenesis and fission/fusion contributes to rotenone-induced dopaminergic neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD), resulting primarily from the death of dopaminergic (DA) neurons in the substantia nigra, is the second most common neurodegenerative disease. According to etiological research, both genetic factors and environmental toxins are responsible for PD [1, 2]. Pathogenesis research has revealed that mitochondrial dysfunction, oxidative stress, altered protein handling, and inflammatory changes are the main causes of cell death in the initiation and progression of PD [2, 3]. In particular, mitochondrial dysfunction has been thought to be the focus of the etiology of Parkinson’s disease [4].
Rotenone is a naturally occurring compound extracted from the roots of several plant species that has been extensively used as an insecticide [5]. It has been found that long-term chronic rotenone exposure can lead to symptoms of Parkinson’s disease. As a mitochondrial complex I inhibitor, rotenone has been extensively used to induce parkinsonian models, and it can reproduce most of the motor symptoms and histopathological features of PD. Moreover, obvious and selective DA neurodegeneration is also observed in neuron cells exposed to rotenone [3]. Our previous research [6] also showed that rotenone administration might disturb the distribution and metabolism of dopamine in PC12 cells, leading to ROS formation and cell death.
In recent decades, a great amount of evidence has accumulated focusing on the potential role of mitochondria in PD etiopathogenesis [7]. It is known that energy metabolism depends on continuous mitochondrial activity like fission, fusion, and remodeling of mitochondria in cells. Peroxisome proliferator-activated receptor gamma-coactivator 1-alpha (PGC-1α) is a transcriptional coactivator involved in energy metabolism through regulation of respiration and mitochondrial biogenesis. Low expression of PGC-1α can lead to a decrease in adenosine triphosphate (ATP) production and excessive ROS formation during stress response. PGC-1α, a strong stimulator of mitochondrial biogenesis, has been reported to be remarkably downregulated in PD patients, which implies a relationship between mitochondrial biogenesis and PD [8]. A recessive mutation in PTEN-induced putative kinase 1 (PINK1), DJ-1, or parkin is adequate to cause PD, and these genes interact with PGC-1α to regulate mitochondrial physiology [9]. Mitochondria are dynamic organelles incessantly remodeled by fission and fusion, which is very important to metabolic demand for bioenergetic adaptation. Mitochondrial fusion and fission play significant roles in maintaining mitochondrial function and morphology when cells experience stress [10]. Mitochondrial fission is essential for cells to acquire adequate numbers of mitochondria, and it is regulated by Drp1, Mid49, Mid51, and Mff. Mitochondrial fusion promotes complementation between differentially damaged mitochondria, and fusion is mediated by MFN1, MFN2, and OPA1 [11]. The latest research suggests that Drp1-dependent mitochondrial fragmentation plays a pivotal role in cellular dysfunction and mitochondria abnormalities in sporadic PD [12]. MFN2 is an outer mitochondrial membrane protein necessary for mitochondrial fusion. It has been confirmed that downregulation or loss of MFN2 expression can result in disturbance of mitochondrial energy dysmetabolism and mitochondrial fusion [13]. Recent studies have showed that PGC-1α participates in a pathway regulated by mitochondria through interaction with MFN2 in skeletal heart cells and muscle cells [14]. Accordingly, it has been speculated that PGC-1α/MFN2 may form a cascade pathway regulating mitochondrial energy metabolism that is impaired during IRI. However, whether there are interactions between mitochondrial fission/fusion and biogenesis remains unknown. Therefore, the role of mitochondrial biogenesis and mitochondrial fission/fusion in PD, especially the relationship between mitochondrial biogenesis and mitochondrial fission/fusion and their interaction, urgently needs further exploration.
Here, we evaluated how mitochondrial fission/fusion, biogenesis, and related genes contribute to mitochondrial quality control and their cross talk in a rotenone model of PD. In particular, we focused on PGC-1α as the axis of the regulatory mechanism in the interaction of mitochondrial biogenesis and mitochondrial fission/fusion in rotenone-induced dopamine neurotoxicity.
Materials and Methods
Materials
PC12 cells (adrenal gland; NGF-differentiated pheochromocytoma cells) were provided by Shanghai Cell Bank, Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. Rotenone, dimethylsulfoxide (DMSO), adenosine triphosphate (ATP), and mitochondrial fusion promoter M1 were purchased from Sigma (USA). Except for antibodies against mtTFA and ZNF746 (Santa Cruz, USA), antibodies against OPA1, MFN1, MFN2, Fis1, Drp1, and PGC-1α were purchased from Abcam (USA). Cell Counting kit-8 (CCK-8, DOJINDO, Japan), ReverTra Acea-Kit (Code No: FSK-100, Invitrogen, USA) and Liposome 2000 were used in this study.
Cell Culture and Treatment
Differentiated PC12 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) containing 10 % fetal bovine serum (HyClone) and 1 % (v/v) antibiotics (penicillin/streptomycin, Beyotime) under an atmosphere of 5 % CO2 at 37 °C. The culture medium was replenished every 2–3 days. Cells in the rotenone group were treated for 24 h with gradient concentrations (0.1, 0.5, 1.0, 1.5 μM) of rotenone dissolved in DMSO, and the control group was administered equal amounts of DMSO.
Cell Viability Assay
Viability of PC12 cells was tested with a CCK-8 kit as per the manufacturer’s instruction. Briefly, 1 × 104 cells/well were seeded into 96-well plates containing complete medium (100 μL) and were cultured in an incubator for 24 h. After the different treatments, each well was supplemented with 10 μL of CCK-8 solution and incubated at 37 °C for 90 min. The absorbance at 450 nm was assessed by a SpectraMax M2E microplate reader (Molecular Devices).
Confocal Laser Microscopy
The mitochondrial mass of PC12 cells was observed by using Mito-Tracker Green probe (Beyotime). Mito-Tracker Green, which is usually nonfluorescent in aqueous solution, becomes fluorescent upon its accumulation in the lipid environment of mitochondria. The fluorescent probe is insensitive to changes of membrane potential, redox status, or other mitochondrial conditions. To measure changes in mitochondrial mass, each group was treated with 50 nM Mito-Tracker Green at 37 °C for 40 min as described by the manufacturer. PC12 cells were observed under confocal laser microscopy (Leica TCS SP5) after two washes. The shape of mitochondria is usually either filamentous or fragmented. The filamentous mitochondria are structurally threadlike tubular, whereas the fragmented ones are shortened, spherical, or rod-like organelles. Mitochondria were classified into fragmented (<0.5 μm), intermediate (0.5–5 μm), and tubular (>5 μm) mitochondria. Cells showing 70 % intermediate or fragmented mitochondria were classified as fragmented cells. Several random fields (≥200 cells per sample) were selected for evaluation of mitochondrial mass.
RT-PCR
Total RNA was separated using TRIzol® Reagent (Invitrogen) as per the manufacturer’s directions. The quantity and purity of total RNA were determined with a NanoDrop ND-1000 spectrophotometer. The RNA samples were stored at −80 °C until use. All first-stand cDNA samples were prepared from total RNA (2 μg per 20 μL of reaction system) using a ReverTra Ace® PCR RT Kit (TOYOBO, Japan). Primers against each of the genes designed on Primer 5.0 are as follows: PGC-1α (S: GACCGTCCAAAGCATTCA; AS: GACTCATCCTTAGCCTCC), mtTFA (S: CGCCTGTCAGCCTTAT; AS: GACTCATCCTTAGCCTCC), OPA1 (S: AAGAGGCACTTCAAGGTCG; AS: GGGAGGAGGAAGAGCAGA), MFN2 (S: CGTCAAGAAGGATAAGCGACAC; AS: TCTGACCACTTCTTACCG), Fis1 (S: ATCCGTAGAGGCATCGTG; AS: TGTATTCGCC-GAACAGGA), Drp1 (S: TCTCCGAGTCCTTTATTG; AS: TGCTGTTGAAGTCGCAGGAG), and β-actin (S: TGGTGAAGCAGGCATCTGA; AS: TGCTGTTGAAGTCGCAGGAG). RT-PCR was carried out under the following conditions: 94 °C for 5 min; 35 cycles at 94 °C for 40 s, annealing for 30 s (mtTFA: 53.5 °C, PGC-1α: 55 °C, OPA1: 54.5 °C, MFN2: 56.5 °C, Fis1: 54 °C, Drp1: 53.5 °C, and β-actin: 55 °C), 72 °C for 45 s and then 72 °C for 5 min. The RT-PCR results were normalized with β-actin.
Mitochondrial Membrane Potential
Mitochondrial membrane potential (MMP) was determined using tetramethylrhodamine methyl (TMRM, Sigma), one commonly used probe for the analysis of MMP in intact cells. It has been reported that the whole-cell fluorescence of TMRM is highly sensitive to changes in MMP. PC12 cells were washed with PBS after different treatments. After that, the TMRM probe working solution was added to a final concentration of 20 nM, followed by incubation for 30 min. After rinsing three times with PBS, the PC12 cells were observed and photographed by confocal laser microscopy with the same excitation and emission wavelengths. Then, fluorescence intensity was computed.
Copy Number of Mitochondrial DNA Detected by Real-Time PCR
Mitochondrial DNA (mtDNA) was quantified by normalizing the mtco1 (mitochondrial-encoded gene) to the Ndufv1 (nuclear-encoded gene) using real-time PCR and the ΔΔCT method. The primers (F and R) were as follows: 5′-ACCCCCGCTATAACCCAATATCAGAC-3′ and 5′-TGGGT-GTCCGAAGAATCAAAATAG-3′ (mtco1); 5′-CGCCATGACTGGAGGTGAGGXAG-3′ and 5′-GGCCCCGTAAACCCGATGTCTTC-3′ (Ndufv1). Fluorescence of SYBR Green was detected by an iCycler iQ Real-time System (Bio-Rad). Totally, a 25-mL reaction system was used in containing 10 ng of total DNA, 0.2 mM F and R primers each, and SYBR Green mixture.
Western Blotting
PC12 cells were harvested after treatment, ice-lysed in RIPA lysis buffer (Beyotime), and centrifuged at 12,000g for 15 min. Protein content was detected using a BCA Protein Assay Kit (Beyotime). Sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed to separate the protein samples. Then, the proteins were transferred to polyvinylidene difluoride membranes, which were blocked in 5 % nonfat dry milk and incubated at 4 °C overnight with various primary antibodies. The primary antibodies were as follows: mouse anti-MFN2 (1:1000), mouse anti-OPA1 (1:500), mouse anti-Drp1 (1:1000), mouse anti-β-actin (1:1000), rabbit anti-Fis1 (1:500), rabbit anti-PGC-1α (1:500), and rabbit anti-mtTFA (1:500). After washing in TBST, the membranes were incubated with an anti-rabbit or anti-mouse IgG secondary antibody (1:2000) (Beyotime) for 1 h, followed by visualization by enhanced chemiluminescence with the ECL method. The individual band intensities of target proteins (MFN2, OPA1, Drp1, Fis1, PGC-1α, and mtTFA) were determined with ImageJ2x, and target proteins’ molecular masses were deduced from standard markers.
Immunofluorescence
PC12 cells were incubated on chamber slides for various periods. Then, after washing twice with PBS, the cells were fixed in 4 % paraformaldehyde for 30 min at room temperature. After washing three times with PBS, PC12 cells were blocked with 3 % BSA for 60 min and incubated at 4 °C overnight with various primary antibodies. The primary antibodies used in immunofluorescence (IF) analysis were as follows: rabbit anti-TOMM20 and mouse anti-p-Drp1 (both 1:1000). After washing three times with PBS, PC12 cells were cultured with secondary antibody 1:500 Alexa Fluor (Beyotime). After that, the cells were dyed with Hoechst at 37 °C in the dark for 5 min. Then, after three washes with PBS, mounting medium (Beyotime) was used to immobilize the coverslips. Fluorescence images were recorded by a Zeiss LSM500 confocal microscope and analyzed by the microscope and imaging software ZEN 2011.
Transmission Electron Microscopy
For transmission electron microscopy (TEM) experiments, PC12 cells were fixed with 2.5 % glutaraldehyde after washed with PBS. The cells were embedded and postfixed in 2 % osmium tetroxide, followed by staining with uranyl acetate and lead citrate. After that, PC12 cells were visualized by a Hitachi-7500 TEM device for detection of mitochondria as described, and the size of the mitochondria in the TEM images was computed as previously reported.
Transfection of Small Interfering RNA
PC12 cells (1 × 105/well) were seeded into 12-well plates containing 500 μL of complete medium and incubated for 24 h. Then, transfection with small interfering RNA (siRNA) was conducted using a Lipofectamine 2000 reagent as per the user’s guide. After 6 h, the medium was changed to normal culture medium. After another 48 h, the cells were harvested and sent for isolation of RNA and analysis of PGC-1α expression by real-time PCR. The sequences are as follows: 5-AAGACGGATTGCCCTCATTTG-3 (siPGC-1α) and 5-AACGUGACACGUUCG GAGAATT-3 (negative control).
Infection with Lentiviral Vectors
The lentiviral vector LV-PGC-1α-EGFP and the control vector LV-EGFP were constructed by Gikai Gene Company. PC12 cells were seeded into a six-pore board at a concentration of 1 × 106/pore. The medium was changed to 1 mL of complete culture medium containing lentivirus vector at a multiplicity of infection (MOI) of 10 and 5 μg/mL polybrene when the cell density reached 50 % confluence (usually after 2 days of subculture). PC12 cells infected with LV-PGC-1α-EGFP served as group lvPGC-1α; PC12 cells infected with LV-EGFP served as group lvCon. After 24 h of incubation with lentiviral vectors, the medium was changed to whole medium subsequently. The expression of GFP was detected by fluorescence microscopy after an extra 72-h culturing.
Statistical Analysis
Each experiment was performed at least three times. All data were reported as mean ± standard error. One-way analysis of variance (ANOVA) was used to compare the data between groups. Post hoc comparisons between groups were made using Tukey’s test. Statistical significance was set at a value of P < 0.05. All of the counting analyses were performed in a blinded manner.
Results
Rotenone Was Toxic to PC12 Cells and Induced a Decrease of MMP
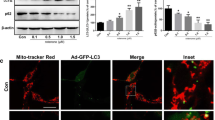
To confirm the toxic effect of rotenone, PC12 cells were treated for 24 h with rotenone dissolved in DMSO at gradient concentrations (0.1, 0.5, 1.0, 1.5 μM). The cell viability relative to the control group decreased significantly as the rotenone concentration increased (Fig. 1a). The level of tyrosine hydroxylase (TH, a key protein involved in tyrosine-to-dopamine conversion) also decreased in a dose-dependent manner (Fig. 1b). To further investigate the effect of rotenone on mitochondria, fluorescent intensity as an indicator of MMP was used and analyzed. The results showed that MMP did not change significantly in PC12 cells with low-dose rotenone treatment (0.1 μM). However, the fluorescent intensity decreased significantly in the other three higher dose groups in a dose-dependent way (Fig. 1c).
Rotenone-induced reduction of viability and MMP in PC12 cells. a Rotenone induced a significant dose-dependent decrease of cell viability. b A representative immunoblot and quantification of TH (59 kDa) in PC12 cells. The TH levels in group Rotenone decreased in a significant dose-dependent way. c The MMP did not change significantly in PC12 cells treated with a lower rotenone concentration. However, with increasing rotenone concentration, MMP decreased with obvious statistical significance compared with that in the control group. The data are representative of three independent experiments; the results are expressed as a percentage of the control, which was set at 100 %; the values are the means ± SEM; *P < 0.05; **P < 0.01 versus the control group
Rotenone Affected Mitochondrial Morphology and Induced Fragmented Mitochondria in PC12 Cells
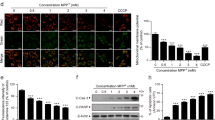
To confirm rotenone’s effects on mitochondrial quality in neuronal cells, the fluorescent intensity of Mito-Tracker Green was used and analyzed. As shown in Fig. 2a, mitochondria without rotenone treatment were filamentous and tubular or thread-like in appearance, often interconnecting to form a network. In comparison, the number of fragmented mitochondria forming small ring shapes was increased significantly by rotenone in a dose-dependent manner. In a further analysis, we found that a 24 h treatment with 0.5, 1.0, or 1.5 μM rotenone caused a reduction in mitochondrial mass (Fig. 2a). EM experiments revealed a decrease of mitochondrial length and area in rotenone-treated groups versus the control group (Fig. 2b), supporting the hypothesis that rotenone induces abnormal mitochondrial morphology in PC12 cells, leading to a decrease of mitochondrial mass.
Rotenone exposure disrupted mitochondrial mass and morphology in PC12 cells. a Obvious changes in mitochondrial mass were detected by confocal microscopy after PC12 cells were treated with rotenone (0.1∼1.5 μM) for 24 h; mitochondrial mass was analyzed by calculating the fluorescent intensity of Mito-Tracker Green, and it decreased in a dose-dependent manner in rotenone-treated groups. b Electron microscopy revealed obvious changes of mitochondrial morphology in the rotenone-treated groups. Mitochondrial area, mitochondrial length, and mitochondrial cristae were lower in the rotenone-treated groups than in the control group. The data are representative of three independent experiments. The results are expressed as a percentage of the control, which was set at 100 %. The values are the means ± SEM; *P < 0.05; **P < 0.01 versus the control group
Rotenone Impaired Mitochondrial Biogenesis in PC12 Cells
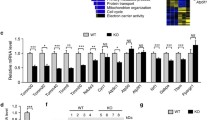
Impairment of mitochondrial biogenesis is another possible cause for the decreased number of mitochondria. To assess rotenone-induced mitochondrial biogenesis in PC12 cells, we analyzed the transcription and protein level of the PGC-1α gene and its target gene mtTFA, directly involved in mitochondrial biogenesis. As shown in Fig. 3a, b, messenger RNA (mRNA) transcript and protein levels of PGC-1α and mtTFA decreased dose-dependently in the rotenone-treated groups versus the control group. The results further proved that PGC-1α activity is altered and transcriptional deregulation of mtTFA occurs in PC12 cells after rotenone treatment. Furthermore, the rotenone treatment led to a significant dose-dependent decrease of mtDNA copy number in PC12 cells (Fig. 3c).
Rotenone impaired mitochondrial biogenesis in PC12 cells. a mRNA levels of PGC-1α and mtTFA, involved in mitochondrial biogenesis, as detected with RT-PCR were decreased in PC12 cells with rotenone treatment. b There was a dose-dependent decrease in protein levels of PGC-1α and mtTFA in the rotenone-treated groups. c MtDNA copy number as detected by quantitative real-time PCR decreased in a dose-dependent manner in the rotenone group. The results are expressed as a percentage of the control, which was set at 100 %. The values are the means ± SEM; *P < 0.05; **P < 0.01 versus the control group
Rotenone Promoted Mitochondrial Fission and Reduced Mitochondrial Fusion in PC12 Cells
To better understand how mitochondrial morphology is regulated in vivo, we analyzed proteins of mitochondrial fusion/fission. Various proteins responsible for the fission/fusion balance, including OPA1, MFN1/2, Drp1, and Fis1, were observed. The results showed that the mRNA and protein levels of Drp1 and Fis1, associated with mitochondrial fission, decreased significantly in a dose-dependent way in the rotenone-treated groups, while the level of p-Drp1 increased (Fig. 4a, b). The mRNA and protein levels of OPA1 and MFN2, connected with mitochondrial fusion, significantly decreased in a dose-dependent manner in the rotenone-treated groups (Fig. 4c, d). However, MFN1 was not detected in our model.
Expression of mitochondrial fusion/fission-related proteins. a Representative immunoblot and quantification of levels of proteins (Drp1 and Fis1) involved in mitochondrial fission revealed a dose-dependent decrease; however, the phosphorylation of Drp1 increased in rotenone-treated groups. b RT-PCR revealed a dose-dependent decrease of Drp1 and Fis1 in rotenone-treated groups. c Representative immunoblot and quantification of protein levels involved in mitochondrial fusion revealed a dose-dependent decrease of OPA1 and MFN2 in rotenone-treated groups. d Expression levels of OPA1 and MFN2 showed a dose-dependent decrease in rotenone-treated groups. The data are representative of three independent experiments. The results are expressed as a percentage of the control, which was set at 100 %. The values are the means ± SEM; *P < 0.05; **P < 0.01 versus the control group
Regulation of Mitochondrial Fission/Fusion Could Alter Rotenone-Induced Dopaminergic Neurotoxicity
To confirm the role of mitochondrial fission/fusion regulation in rotenone-induced dopaminergic neurotoxicity, M1 (a promoter of mitochondrial fusion) and Mdivi-1 (an inhibitor of mitochondrial fission, Md) were used to observe their effects on mitochondria in PC12 cells. PC12 cells in both group M1 + R and group Md + R showed much higher viability versus the rotenone group (Fig. 5a). The protein level of TH was much higher in group M1 + R and group Md + R versus the rotenone group (Fig. 5c). Moreover, M1 and Mdivi-1 pretreatment led to a significant decrease in the number of fragmented mitochondria and a significant increase of mtDNA copy number compared with the rotenone group (Fig. 5d, e).
Promoting fusion and inhibiting fission could reverse rotenone-induced neurotoxicity. a Cell viability analyzed using a SpectraMax M2E microplate reader. The viabilities of PC12 cells in group M1+R and group Md+R were both much higher compared with those of the rotenone group. b The concentration of Mdivi-1 (5 μM) used in our experiment was confirmed through a concentration gradient test. c Immunoblotting and quantification of protein levels of TH revealed an increase in group M1+R and group Md+R compared with the rotenone group. d Obvious changes in mitochondrial mass in PC12 cells as detected by confocal microscopy after different treatments. There were fewer fragmented mitochondria in group M1+R and group Md+R compared with the rotenone group. e M1 and Mdivi-1 pretreatment led to a significant increase of mtDNA copy number compared with the rotenone group. Furthermore, mtDNA copy number in group M1 and group Mdivi-1 was much higher than that in the control group. The results are expressed as a percentage of the control, which was set at 100 %. The values are the means ± SEM; *P < 0.05, **P < 0.01 versus the control group; #P < 0.05, ##P < 0.01 versus the rotenone group
Dependence of Rotenone-Induced Mitochondrial Fragmentation and mtDNA Injury on PGC-1α in PC12 Cells
Real-time PCR of RNA isolated from transfected cells confirmed that siRNA lead a 70 % decrease in PGC-1α mRNA levels compared with untransfected cells, while the mRNA levels of PGC-1α had no significant change transfected with negative control siRNA. Indeed, the viability of PC12 cells transfected with siRNA was lower than that of untransfected cells (Fig. 6a). In comparison, silencing of the PGC-1α gene led to a significant decrease of mitochondrial mass, a significant increase in the number of fragmented mitochondria, and a decrease of mtDNA copy number (Fig. 6b, c). By contrast, real-time PCR of RNA isolated from PC12 cells infected with LV-PGC-1α-EGFP confirmed the PGC-1α gene transfection with a 45 % increase in PGC-1α mRNA levels when compared to cells infected with LV-EGFP, and WB results further confirmed this conclusion (Fig. 6d). Meanwhile, no significant difference in any index was observed between groups lvPGC-1α and lvCon, excluding a negative effect of the lentiviral vectors. The viability of PC12 cells infected with LV-PGC-1α-EGFP did not change obviously versus cells infected with LV-EGFP. However, after being treated with rotenone, the cell viability of group lvPGC-1α increased significantly compared with that of group lvCon (Fig. 6e). Indeed, overexpression of the PGC-1α in PC12 cells led to a significant increase in mitochondrial mass, a significant decrease in the number of fragmented mitochondria and an increase in mtDNA copy number (Fig. 6f, g).
Mitochondrial fragmentation and mtDNA injury occurred through PGC-1α. a Cell viability was much lower in group siPGC-1α than in group siCon. b MtDNA copy number was significantly lower in group siPGC-1α than in group siCon. c Obvious changes in the mitochondrial mass of PC12 cells were detected by confocal microscopy after different treatments. There were more fragmented mitochondria in group siPGC-1α than in group siCon. d Overexpression of PGC-1α in PC12 cells using lentiviral vectors led to an increase in mRNA and protein levels of PGC-1α. e Cell viability in group lvPGC-1α + R was much higher than that in group rotenone. f Overexpression of PGC-1α gene led to a significant increase of mtDNA copy number in group lvPGC-1α and group lvPGC-1α + R. g Obvious changes in the mitochondrial mass of PC12 cells were detected by confocal microscopy after different treatments. There were fewer fragmented mitochondria in group lvPGC-1α + R compared with group rotenone. The data are representative of three independent experiments. The results are expressed as a percentage of the control, which was set at 100 %. The values are the means ± SEM; *P < 0.05, **P < 0.01 versus the siCon group; #P < 0.05 versus the rotenone group
PGC-1α Enhanced Mitochondrial Fusion and Inhibited Mitochondrial Fission in PC12 Cells
Western blot results showed that the protein level of MFN2 significantly decreased and the level of p-Drp1 increased significantly in siPGC-1α-transfected cells compared with untransfected cells (Fig. 7a). By contrast, overexpression of PGC-1α led to a significant increase in the protein level of MFN2 and a significant decrease in the protein level of p-Drp1 compared with cells infected with LV-EGFP (Fig. 7b). After treatment with rotenone, the same changes also occurred in PC12 cells. However, there were no obvious changes in the protein levels of OPA1, total Drp1, or Fis1 after siRNA transfection or lentiviral vector infection. Similarly, immunofluorescence results showed that the protein level of p-Drp1 in untransfected cells was much lower than that in cells with PGC-1α silencing and was much higher than that in cells with PGC-1α overexpression. Moreover, rotenone led to a further increase of p-Drp1 expression. Indeed, in normal PC12 cells without any treatment, p-Drp1 was mainly expressed in the cytoplasm, whereas in stressed PC12 cells exposed to rotenone, p-Drp1 was mainly translocated to the mitochondria (Fig. 7c). Silencing of PGC-1α could intensify the increase expression and translocation of p-Drp1. On the contrary, overexpression of PGC-1α could relieve the changes.
PGC-1α regulated the expression and phosphorylation of mitochondrial fission (Drp1) and mitochondrial fusion (MFN2) proteins. a Western blot results showed that the protein level of MFN2 in group siCon was higher than that in group siPGC-1α, and the level of p-Drp1 in group siCon was much lower than that in group siPGC-1α, while there were no obvious changes in the levels of OPA1 or Fis1 between group siCon and group siPGC-1α. b Western blot results showed that the protein level of MFN2 in group lvCon was lower than that in group lvPGC-1α, and the level of p-Drp1 in group lvCon was higher than that in group lvPGC-1α, while there were no obvious changes in the levels of OPA1 or Fis1 between group lvCon and group lvPGC-1α. c Immunofluorescence results showed that the protein level of p-Drp1 in the control group was much lower than that in group siPGC-1α and was much higher than that in group lvPGC-1α. The same changes also occurred after cells were treated with rotenone. Moreover, p-Drp1 was mainly translocated from cytoplasm to mitochondria in group rotenone, group siPGC-1α, and group siPGC-1α + R. The translocation was relieved in group lvPGC-1α + R. The data are representative of three independent experiments. The results are expressed as a percentage of the control, which was set at 100 %. The values are the means ± SEM; *P < 0.05, **P < 0.01 versus the lvCon group; #P < 0.05 versus the rotenone group
Discussion
PD is a common neurodegenerative disease occurring in the elderly. Recent data highlight the pivotal role of mitochondria in the neurodegeneration occurring in both familial and sporadic PD cases. In the present study, based on a rotenone-induced PD model we previously used [15–17], the status of mitochondrial dynamics and the possible underlying mechanism are explored.
A large number of fragmented mitochondria were observed evidenced by round, small, or dot-like dyeing patterns. The mitochondrial length, area, and cristae were also markedly reduced. Along with the observed mitochondrial fragmentation, disorganized structure of mitochondrial cristae, the reduction of MMP, mitochondrial ROS release, cell viability loss, and TH protein reduction were also observed in our rotenone-induced dopamine neurotoxicity. Rotenone could reduce mitochondrial number and mitochondrial mass significantly in PC12 cells. Therefore, the data shown in our study support the opinion that abnormal mitochondrial number and morphology contribute to rotenone-induced dopamine neurotoxicity. Normal mitochondrial number and mass is important for mitochondrial function. It can be speculated that excessive fragmented mitochondria lead to mitochondrial dysfunction.
Mitochondrial dysfunction is an early event in almost all neurodegenerative diseases, including PD [18]. In the past three decades, researchers have increasingly recognized that mitochondrial fusion, fission, biogenesis, transport, and degradation are all linked to the pathogenesis of PD [19]. Among these, mitochondrial fusion/fission defects would limit mitochondrial motility and lead to energy production reduction, excessive oxidative stress, and mtDNA deletion, finally resulting in cell death [18]. Mitochondrial fusion/fission events are regulated by several proteins: the pro-fission proteins Drp1 and Fis1 and pro-fusion proteins OPA1, MFN1 and MFN2. Of course, there are some other indirect proteins that interact with the proteins mentioned above. Therefore, a series of proteins directly related to mitochondrial fusion/fission was detected. The results showed that the mitochondrial fusion-related genes OPA1 and MFN2 decreased and MFN1 was not detected, while the mitochondrial fission-related gene p-Drp1 increased although other two genes, Drp1 and Fis1, declined. These results revealed that mitochondrial fusion/fission plays an important role in rotenone-induced dopamine neurotoxicity as measured by mitochondrial ROS production, MMP, and TH protein expression. To confirm the above conclusion, PC12 cells were pretreated with the fusion promoter M1 [20] or the fission inhibitor Mdivi-1 [21]. Promotion of mitochondrial fusion and inhibition of mitochondrial fission were found to correct the mitochondrial fragmentation, improve mitochondrial mass, and reduce mitochondrial impairment. Moreover, cell viability and TH expression were rescued significantly. Rotenone therefore disturbed the balance between mitochondrial fission and fusion and led to excessive mitochondrial fragmentation, breaking mitochondrial homeostasis. Regulating the dynamic balance of mitochondrial fission and fusion can have a protective effect on rotenone-induced dopamine neurotoxicity.
Recently, genes related to mitochondrial fusion or fission were found to play a significant role in PD. Drp1 and Fis1 are both key mediators of mitochondrial fission [22–24], and Fis1 helps Drp1 assemble into spirals or rings surrounding the mitochondrial outer membrane [25]. MFN1/2 facilitate mitochondrial outer membrane fusion [26, 27], and OPA1 is also a main mediator of maintenance of mtDNA and inner membrane in mammals [28]. Santos et al. found alterations of OPA1 and Drp1 in different cellular models of sporadic PD, and mitochondrial fragmentation can also be observed when Drp1 increased [12]. We found that in rotenone-induced dopamine neurotoxicity model, the increased phosphorylation levels of Drp1 could lead to an increase in mitochondrial fission. Recent data have shown that Drp1 plays a causal role in the impairing of mitochondrial fission in the pathogenesisof PD [29]. Indeed, an increase of Drp1 translocation to mitochondrial and the phosphorylation of Drp1 were detected in a cell model. Drp1 phosphorylation and translocation to mitochondria played an important role in rotenone-induced promotion of mitochondrial fission. Therefore, taking control of mitochondrial fission impairment mediated by Drp1 may be of extreme importance for inhibiting neurodegeneration. Consistent with a previous study, our results showed increases in mitochondrial fragmentation, the translocation of Drp1 to the mitochondria, and mitochondrial fission in cultured cells treated with Parkinsonism-inducing neurotoxins such as 6-hydroxydopamine and MPP+, thus causing the cell death of dopamine neurons [12]. Further, we demonstrated that PGC-1α, which has long been considered a nuclear protein participating in the regulation of transcription of nucleus-encoded mitochondrial genes [30], was reduced significantly in rotenone-induced dopamine neurotoxicity. During mitochondrial biogenesis, PGC-1α plays an important role in orchestrating the activation of a broad set of transcription factors and nuclear hormone receptors to promote the expression of nucleus-encoded mitochondrial genes [31]. Among the putative target genes, mtTFA, a transcription factor, was the focus of our experiments. The results showed that mtTFA was decreased markedly. mtTFA is supposed to have a role in regulating the expression of mitochondrial genes or in mtDNA maintenance [32]. Accordingly, consistent with our speculation, the mtDNA copy number was significantly decreased in PC12 cells treated with rotenone in our experiment. The protein expression of PGC-1α and mtTFA was lowered with rotenone treatment in PC12 cells, similar to the findings of a recent study [33]. The results further suggest that PGC-1α might participate in the pathogenesis of Parkinson’s disease. Recently, a lot of studies have found that PGC-1α is closely related to mitochondrial function, including induction of mitochondrial biogenesis, increasing the number and respiratory function of mitochondria, upregulating the antioxidant defense system, and even also playing an active effect on the mitochondria of DA [34, 35]. Therefore, in this study, we further used lentiviral vector infection to overexpress PGC-1α, and siRNA transfection to knock down PGC-1α in PC12 cells to confirm its role, pros and cons. Our research found that rotenone obviously decreased PC12 cell viability, mtDNA copy number, and mitochondrial mass while increasing mitochondrial fragmentation, but overexpression of PGC-1α significantly inhibited the death of PC12 cells, increasing both mtDNA copy number and mitochondrial mass. Conversely, PGC-1α knockdown cells were highly sensitive to the neurotoxin rotenone, with increased mitochondrial fragmentation and decreased cell viability and mtDNA copy number. These positive and negative results powerfully imply a mediator role of PGC-1α in the rotenone-induced PD model. Moreover, this work strengthens the role of the PGC-1α pathway in the regulation of mitochondrial biogenesis and metabolism and gives new insights into the regulatory mechanisms that govern mitochondrial genome expression, replication, and maintenance.
PGC-1α is a strong regulator of energy metabolism that regulates cellular responses to all kinds of metabolic stress including physical exertion, fasting, and cold temperature [36]. It has also been reported that dietary fucoxanthin increases the expression of mitochondrial biogenesis and fusion genes such as PGC-1α, MFN1, MFN2, and OPA1 (called the PGC-1α network) in mice’s white adipose tissues [14]. PGC-1α-mtTFA-mediated regulation of mitochondrial biogenesis has also been reported by many researchers [37]. Researches have proved that PGC-1α not only enhances the activity of mtTFA but also promotes its expression. Further experiments found that the expression of MFN2 went up to different degrees, and Drp1 decreases with overexpression of PGC-1α. Among these, the expression of p-Drp1 has the most significant change, and the expression levels of p-Drp1 was negatively correlated with the expression level of PGC-1α, suggesting that MFN2 and Drp1 may be downstream nuclear transcription factors of PGC-1α. Therefore, PGC-1α might mainly further regulate mitochondrial function via controlling the activity and expression of Drp1. Thus, it can be concluded that the positive regulation of PGC-1α-MFN2 and the negative regulation of PGC-1α-p-Drp1 impact the balance of mitochondrial fusion and fission. To our knowledge, this is the first evidence that PGC-1α regulates mitochondrial fusion/fission by targeting the expression of related genes in the rotenone-induced PD model. In addition, PGC-1α can promote oxidative phosphorylation and mitochondrial biogenesis through coordinating some gene expression programs. PGC-1α knockout mice display neurological abnormalities, such as exaggerated startle responses, dystonia, myoclonus, and clasping [9]. Activation of PGC-1α rescues the decrease of DA neurons in rotenone-induced primary neuron models, and altered PGC-1α activation and recessive mutations in parkin have also been linked to PD [33]. Guo et al. recently found that PGC-1α may protect diabetic nephropathy through the inhibition of Drp1-mediated mitochondrial dynamic remodeling and low-expression of PGC-1α leads to increased expression of p-Drp1 and increased mitochondrial fragmentation [38]. PGC-1α is a master promoter of oxidative metabolism and mitochondrial biogenesis by regulating nuclear respiratory factors such as ERRα and NRF1/2. It has been reported that ERRα can activate the transcriptional activity of the MFN2 promoter, and the effects are synergistic with those of PGC-1α [39]. Furthermore, NRF1/2 themselves are able to activate mtTFA which is crucial for the replication and transcription of mtDNA [40]. Now, combined with our results, it can be easily concluded that PGC-1α might participate in mitochondrial fusion and mitochondrial fission through regulating Drp1 and MFN2, being a key regulator of mitochondrial dynamics. Thus, PGC-1α is an important nuclear transcription factor. It regulates mitochondrial biogenesis, oxidative phosphorylation, mitochondrial transcription and replication, and antioxidant system. It can also regulate mitochondrial fission and fusion to maintain mitochondrial homeostasis [41]. At the same time, we found that M1 and Mdivi-1 could significantly improve mitochondrial DNA copy number, which indicated that mitochondrial fission and fusion might have a regulatory effect on mitochondrial biogenesis. Therefore, M1 and Mdivi-1 could modulate mitochondria dynamics by regulating mitochondrial fission/fusion and biogenesis. Combined with the regulatory effect of PGC-1α on mitochondrial fusion and fission, we inferred that mitochondrial fission/fusion and mitochondrial biogenesis regulated each other and influenced mitochondrial number and mitochondrial mass, which were very important for the balance between mitochondrial dynamics and mitochondrial homeostasis. Thus, there are cross talks between mitochondrial fission/fusion and biogenesis.
The dynamic balance between mitochondrial fission/fusion and biogenesis is important for maintenance of mitochondrial homeostasis and function. In addition, it has been reported that mitophagy is also involved in the regulation of mitochondrial homeostasis. The coordination between mitochondrial biogenesis and mitophagy plays a pivotal role in mitochondrial number homeostasis. Some studies have reported that a decline of mitophagy impairs mitochondrial biogenesis resulting in progressive mitochondrial accretion and, consequently, deterioration of cell function [42]. It is also has been reported that there are links between mitochondrial fission and mitophagy [43].
In summary, our research supplies a unique perspective that rotenone induces PD by reducing mitochondrial number and mitochondrial mass and that PGC-1α plays a mediating role in the process. There is cross talk between mitochondrial biogenesis and mitochondrial fission/fusion, which is regulated by PGC-1α (summarized in Fig. 8). Further work needs to explore the network as well as focus on the unbalanced statue of mitochondrial fission/fusion to explore the mechanism and find a new potential therapeutic approach.
The total frame diagram of the cross talk between mitochondrial biogenesis and fission/fusion regulated by PGC-1α in PC12 cells induced by rotenone. Rotenone induces the PD model by reducing mitochondrial number and mitochondrial mass, and PGC-1α plays a mediating role in the process. PGC-1α can not only regulate the expression of mtTFA to influence mitochondrial biogenesis but also has an impact on mitochondrial fission/fusion through regulating MFN2 and Drp1 protein expression and phosphorylation. At the same time, the regulation of mitochondrial fission/fusion can conversely influence mitochondrial biogenesis. Therefore, there is cross talk between mitochondrial biogenesis and mitochondrial fission/fusion
References
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39(6):889–909
Schapira AH, Jenner P (2011) Etiology and pathogenesis of Parkinson’s disease. Mov Disord 26(6):1049–1055. doi:10.1002/mds.23732
Xiong N, Long X, Xiong J, Jia M, Chen C, Huang J, Ghoorah D, Kong X et al (2012) Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson’s disease models. Crit Rev Toxicol 42(7):613–632. doi:10.3109/10408444.2012.680431
Hang L, Thundyil J, Lim KL (2015) Mitochondrial dysfunction and Parkinson disease: a Parkin-AMPK alliance in neuroprotection. Ann N Y Acad Sci 1350:37–47. doi:10.1111/nyas.12820
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3(12):1301–1306. doi:10.1038/81834
Sai Y, Wu Q, Le W, Ye F, Li Y, Dong Z (2008) Rotenone-induced PC12 cell toxicity is caused by oxidative stress resulting from altered dopamine metabolism. Toxicol In Vitro 22(6):1461–1468. doi:10.1016/j.tiv.2008.04.019
Arduino DM, Esteves AR, Cardoso SM (2011) Mitochondrial fusion/fission, transport and autophagy in Parkinson’s disease: when mitochondria get nasty. Park Dis 2011:767230. doi:10.4061/2011/767230
Pacelli C, De Rasmo D, Signorile A, Grattagliano I, di Tullio G, D’Orazio A, Nico B, Comi GP et al (2011) Mitochondrial defect and PGC-1alpha dysfunction in parkin-associated familial Parkinson’s disease. Biochim Biophys Acta 1812(8):1041–1053. doi:10.1016/j.bbadis.2010.12.022
Tsunemi T, La Spada AR (2012) PGC-1alpha at the intersection of bioenergetics regulation and neuron function: from Huntington’s disease to Parkinson’s disease and beyond. Prog Neurobiol 97(2):142–151. doi:10.1016/j.pneurobio.2011.10.004
Kowald A, Kirkwood TB (2011) Evolution of the mitochondrial fusion-fission cycle and its role in aging. Proc Natl Acad Sci U S A 108(25):10237–10242. doi:10.1073/pnas.1101604108
Youle RJ, van der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science 337(6098):1062–1065. doi:10.1126/science.1219855
Santos D, Esteves AR, Silva DF, Januario C, Cardoso SM (2015) The impact of mitochondrial fusion and fission modulation in sporadic Parkinson’s disease. Mol Neurobiol 52(1):573–586. doi:10.1007/s12035-014-8893-4
Martorell-Riera A, Segarra-Mondejar M, Munoz JP, Ginet V, Olloquequi J, Perez-Clausell J, Palacin M, Reina M et al (2014) Mfn2 downregulation in excitotoxicity causes mitochondrial dysfunction and delayed neuronal death. EMBO J 33(20):2388–2407. doi:10.15252/embj.201488327
Wu MT, Chou HN, Huang CJ (2014) Dietary fucoxanthin increases metabolic rate and upregulated mRNA expressions of the PGC-1alpha network, mitochondrial biogenesis and fusion genes in white adipose tissues of mice. Mar Drugs 12(2):964–982. doi:10.3390/md12020964
Sai Y, Chen J, Ye F, Zhao Y, Zou Z, Cao J, Dong Z (2013) Dopamine release suppression dependent on an increase of intracellular Ca(2+) contributed to rotenone-induced neurotoxicity in PC12 cells. J Toxicol Pathol 26(2):149–157. doi:10.1293/tox.26.149
Sai Y, Peng K, Ye F, Zhao X, Zhao Y, Zou Z, Cao J, Dong Z (2013) 14-3-3 Proteins in the regulation of rotenone-induced neurotoxicity might be via its isoform 14-3-3epsilon’s involvement in autophagy. Cell Mol Neurobiol 33(8):1109–1121. doi:10.1007/s10571-013-9977-9
Peng K, Tao Y, Zhang J, Wang J, Ye F, Dan G, Zhao Y, Cai Y et al (2016) Resveratrol regulates mitochondrial biogenesis and fission/fusion to attenuate rotenone-induced neurotoxicity. Oxidative Med Cell Longev 2016:6705621. doi:10.1155/2016/6705621
Knott AB, Bossy-Wetzel E (2008) Impairing the mitochondrial fission and fusion balance: a new mechanism of neurodegeneration. Ann N Y Acad Sci 1147:283–292. doi:10.1196/annals.1427.030
Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29(28):9090–9103. doi:10.1523/JNEUROSCI.1357-09.2009
Wang D, Wang J, Bonamy GM, Meeusen S, Brusch RG, Turk C, Yang P, Schultz PG (2012) A small molecule promotes mitochondrial fusion in mammalian cells. Angew Chem Int Ed Engl 51(37):9302–9305. doi:10.1002/anie.201204589
Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ et al (2014) Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J 28(1):316–326. doi:10.1096/fj.12-226225
Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM (1999) C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell 4(5):815–826
Smirnova E, Griparic L, Shurland DL, van der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12(8):2245–2256
Zhang Y, Chan DC (2007) Structural basis for recruitment of mitochondrial fission complexes by Fis1. Proc Natl Acad Sci U S A 104(47):18526–18530. doi:10.1073/pnas.0706441104
Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J (2005) Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol 170(7):1021–1027. doi:10.1083/jcb.200506078
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160(2):189–200. doi:10.1083/jcb.200211046
Ishihara N, Eura Y, Mihara K (2004) Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci 117(Pt 26):6535–6546. doi:10.1242/jcs.01565
Hudson G, Amati-Bonneau P, Blakely EL, Stewart JD, He L, Schaefer AM, Griffiths PG, Ahlqvist K et al (2008) Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain 131(Pt 2):329–337. doi:10.1093/brain/awm272
Huang S, Wang Y, Gan X, Fang D, Zhong C, Wu L, Hu G, Sosunov AA et al (2015) Drp1-mediated mitochondrial abnormalities link to synaptic injury in diabetes model. Diabetes 64(5):1728–1742. doi:10.2337/db14-0758
Scarpulla RC (2012) Nucleus-encoded regulators of mitochondrial function: integration of respiratory chain expression, nutrient sensing and metabolic stress. Biochim Biophys Acta 1819(9-10):1088–1097. doi:10.1016/j.bbagrm.2011.10.011
Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D et al (2011) ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med 17(9):1076–1085. doi:10.1038/nm.2439
Li M, Vascotto C, Xu S, Dai N, Qing Y, Zhong Z, Tell G, Wang D (2012) Human AP endonuclease/redox factor APE1/ref-1 modulates mitochondrial function after oxidative stress by regulating the transcriptional activity of NRF1. Free Radic Biol Med 53(2):237–248. doi:10.1016/j.freeradbiomed.2012.04.002
Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL et al (2011) PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell 144(5):689–702. doi:10.1016/j.cell.2011.02.010
Ciron C, Lengacher S, Dusonchet J, Aebischer P, Schneider BL (2012) Sustained expression of PGC-1alpha in the rat nigrostriatal system selectively impairs dopaminergic function. Hum Mol Genet 21(8):1861–1876. doi:10.1093/hmg/ddr618
Mudo G, Makela J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Malkia A et al (2012) Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci 69(7):1153–1165. doi:10.1007/s00018-011-0850-z
Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K et al (2006) Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4(11):e369. doi:10.1371/journal.pbio.0040369
Vaughan RA, Gannon NP, Barberena MA, Garcia-Smith R, Bisoffi M, Mermier CM, Conn CA, Trujillo KA (2014) Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes Metab 16(8):711–718. doi:10.1111/dom.12268
Guo K, Lu J, Huang Y, Wu M, Zhang L, Yu H, Zhang M, Bao Y et al (2015) Protective role of PGC-1alpha in diabetic nephropathy is associated with the inhibition of ROS through mitochondrial dynamic remodeling. PLoS One 10(4):e0125176. doi:10.1371/journal.pone.0125176
Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F et al (2005) Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol 567(Pt 1):349–358. doi:10.1113/jphysiol.2005.092031
Sun M, Huang C, Wang C, Zheng J, Zhang P, Xu Y, Chen H, Shen W (2013) Ginsenoside Rg3 improves cardiac mitochondrial population quality: mimetic exercise training. Biochem Biophys Res Commun 441(1):169–174. doi:10.1016/j.bbrc.2013.10.039
Valero T (2014) Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des 20(35):5507–5509
Palikaras K, Lionaki E, Tavernarakis N (2015) Coupling mitogenesis and mitophagy for longevity. Autophagy 11(8):1428–1430. doi:10.1080/15548627.2015.1061172
Zhou J, Li G, Zheng Y, Shen HM, Hu X, Ming QL, Huang C, Li P et al (2015) A novel autophagy/mitophagy inhibitor liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy 11(8):1259–1279. doi:10.1080/15548627.2015.1056970
Acknowledgments
This work was supported by grants from the NSFC (Natural Science Foundation of China) (81473006, 81273106) to Yan Sai and the Natural Science Foundation of Chongqing (cstc2012jjA10028) to Yan Sai.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Transparency Document
The transparency document associated with this article can be found in the online version.
Rights and permissions
About this article
Cite this article
Peng, K., Yang, L., Wang, J. et al. The Interaction of Mitochondrial Biogenesis and Fission/Fusion Mediated by PGC-1α Regulates Rotenone-Induced Dopaminergic Neurotoxicity. Mol Neurobiol 54, 3783–3797 (2017). https://doi.org/10.1007/s12035-016-9944-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9944-9