Abstract
Traumatic brain injury (TBI) causes increased release of several mediators from injured and dead cells and elicits microglial activation. Activated microglia change their morphology, migrate to injury sites, and release tumor necrosis factor-alpha (TNF-α) and others. In this study we used a controlled fluid percussion injury model of TBI in the rat to determine whether early (4 h post-injury) or late (4 days post-injury) treatment with MLC 601, a Traditional Chinese Medicine, would affect microglial activation and improve recovery. MLC 601 was chosen for this study because its herbal component MLC 901 was beneficial in treating TBI in rats. Herein, rats with induced TBI were treated with MLC 601 (0.2–0.8 mg/kg) 1 h (early treatment) or 4 day post-injury (late treatment) and then injected once daily for consecutive 2 days. Acute neurological and motor deficits were assessed in all rats the day before and 4 days after early MLC 601 treatment. An immunofluorescence microscopy method was used to count the numbers of the cells colocalized with neuron- and apoptosis-specific markers, and the cells colocalized with microglia- and TNF-α-specific markers, in the contused brain regions 4 days post-injury. An immunohistochemistry method was used to evaluate both the number and the morphological transformation of microglia in the injured areas. It was found that early treatment with MLC 601 had better effects in reducing TBI-induced cerebral contusion than did the late therapy with MLC 601. Cerebral contusion caused by TBI was associated with neurological motor deficits, brain apoptosis, and activated microglia (e.g., microgliosis, amoeboid microglia, and microglial overexpression of TNF-α), which all were significantly attenuated by MLC 601 therapy. Our data suggest that MLC 601 is a promising agent for treatment of TBI in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is one of the leading causes of morbidity and mortality in young adults and children; it is a major global public health problem (Maas et al. 2008). Within minutes to months after the injury, the development of a cerebral contusion initiates ongoing neuronal degeneration (Morganti-Kossmann et al. 2007). Increased release of several mediators from injured and dead cells during TBI elicits microglial activation (Rivest 2009; Loane and Byrnes 2010). Activated microglia change morphology, migrate to injury sites, and release tumor necrosis factor-alpha (TNF-α) (Chio et al. 2010, 2013; Cheong et al. 2013), transforming growth factor-beta (TGF-β) (Lindholm et al. 1992; Rimaniol et al. 1995), and others.
MLC 601 (NeuroAid; Moleac Pte Ltd., Singapore), a Traditional Chinese Medicine, is currently used in both Asia and in the Middle East for stroke patients (Chen et al. 2009; Bavarsad Shahripour et al. 2011; Gan et al. 2008; Young et al. 2010). More recent evidence also provided updated references to several new trial papers on MLC 601 which include a neutral large clinical trial on stroke recovery (Chen et al. 2013a) with post hoc analyses showing possible efficacy in reducing recurrent vascular events (Chen et al. 2013b) and in patients with poorer prognostic factors (Navarro et al. 2014). In rodents with stroke, both MLC 601 and MLC 901 improved survival and brain damage (Heurteaux et al. 2010; Quintard et al. 2011). MLC 901 was also shown to induce neuroprotective and neurodegenerative benefits after TBI in rats (Quintard et al. 2014). However, to the best of our knowledge, it is unknown whether post-TBI administration of MLC 601 is beneficial in affecting the cerebral contusion, neurological motor deficits, and microglial activation that occurred during TBI in rodents.
Prior studies have shown that microgliosis, ameboid microglia, microglial expression of TNF-α caused by TBI have been collectively termed microglial activation (Chio et al. 2010, 2013; Cheong et al. 2013) . In the present study we used a controlled fluid percussion injury model of TBI in the rat to determine whether immediate treatment with MLC 601 would affect microglial activation and improve recovery. MLC was chosen for this study because its herbal component MLC 901 is beneficial in treating experimental TBI (Quintard et al. 2014).
Methods
Animals
Male Sprague–Dawley rats (260 ± 18 g) from the animal resource center of the Taiwan Department of National Science and Technology (TNOSAT) were used for all experiments. The TNOSAT’S policies on the care and use of laboratory animals were followed. The institutional review board of Chung Shan Medical University approved the experiments (protocol number: 100120701). All efforts were made to minimize animal suffering and reduce the number of rats used. The rats were housed under controlled laboratory conditions with a 12-h light/dark cycle, a temperature of 22 ± 2 °C, and a humidity of 60–70 % for at least 1 week before drug treatment or surgery. They had free access to a standard rodent diet and unlimited fresh drinking water.
Experimental Design
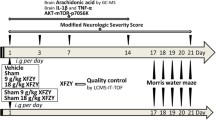
The rats were divided into three groups: (i) Sham group, (ii) TBI group+vehicle, and (iii) TBI group+MLC 601 (Moleac Pte Ltd, Singapore). The composition of the MLC 601 capsule was the following: 0.57 g of Radix astragali, 0.114 g of Radix Salvia miltiorrhizae, 0.114 g of Radix pas Paeoniae Rubra, 0.114 g of Rhizoma Chuanxiong, 0.114 g of Radix Angelicae Sinensis, 0.114 g of carthamus tinctorius L. (safflower), 0.114 g of Prunus persica (peach), 0.114 g of Radix polygalae, 0.114 g of Rhizoma Acori Tatarinowii, 0.095 g of Buthus martensü Karsch, 0.0665 of Hirudo (leech), 0.0665 g of Eupolyphaga seu Steleophaga, 0.0285 g of Calculus bovis artifactus (dried cattle gallstones), and 0.0285 g of Cornu (horn) saiga bovis arcticus and Cornu saiga tatarica. The drug was diluted in saline (used as a vehicle) at the concentration of 0.2 mg/ml, 0.4 mg/ml, or 0.8 mg/ml (stock solution) and incubated under agitation for 1 h at 37 °C. The solution was then strained using a 0.22-μm filter. Rats were intraperitoneally (i.p.) injected with a single dose of MLC 601 (0.2 mg/kg, 0.4 mg/kg, or 0.8 mg/kg) 1 h after TBI, and then with one injection per day for consecutive 2 days. In separate experiments, an i.p. dose of MLC (0.2–0.8 mg/kg) was injected at day 4 post-TBI once per day and consecutively for 2 days. The researchers who did the TBI surgery and measured the TBI were blinded to the treatment code.
Induction of TBI
TBI was induced using a fluid percussion injury device (Virginia Commonwealth University Biochemical Engineering, Richmond, VA, USA) (amplitude: 2.2 atm) on rats placed in a Kopf stereotaxic frame (McIntosh et al. 1987). In brief, rats were anesthetized using sodium pentobarbital (25 mg/kg, i.p.) (Sigma-Aldrich, St. Louis, MO, USA) and a mixture containing ketamine (4.4 mg/kg, intramuscularly [i.m.]) (Nankuang Pharmaceutical, Tainan, Taiwan), atropine (0.02633 mg/kg, [i.m.]) (Sintong Chemical, Taoyuan, Taiwan), and xylazine hydrochloride (6.77 mg/kg, [i.m.]) (Bayer AG, Leverkusen, Germany). Body temperature was maintained at 37 °C with a heating blanket during surgery and for the hour after surgery. Heart rate, blood pressure, and respiratory rate were monitored during the surgical procedure. Each injured and sham-injured rat was housed individually and closely evaluated for behavioral recovery immediately after TBI.
Cerebral Contusion Assay
The triphenyl tetrazolium chloride (TTC) staining procedures are used for determing cerebral ischemia extent caused by TBI (Chang et al. 2013). The TTC staining method described by Wang et al. (1997) was modified. In brief, at the indicated time after TBI, all the rats were deeply anesthetized and then intracardially perfused with saline. Their brain tissue was removed, immersed in cold saline for 5 min, and sliced into 2.0-mm thick sections with a tissue slicer. The brain slices were incubated in 2 % TTC dissolved in phosphate buffered saline (PBS) for 30 min at 37 °C and then transferred to a 5 % formaldehyde solution for fixation. The volume of the contusion, as revealed by negative TTC stains indicating dehydrogenase-deficient (or ischemic) tissue, was measured in each slice and summed using computerized planimetry (Image-Pro Plus 5.0; Media Cybernetics, Bethesda, MD, USA). The volume of the contusion was calculated as 2 mm (thickness of the slice) × (sum of the contusion area in all brain slices [mm2]). (Wang et al. 1997; Chang et al. 2013). Both cortical and hippocampal areas were affected in the injured side after TBI. The brain damaged volume for the TBI rats received vehicle solution was about 163 ± 18 mm3 (value is the mean ± S.D.).
Neurological Severity Scores
Acute neurological injury was assessed in all rats the day before and 4–21 days after surgery using a neurological severity score (NSS), a composite of the motor, sensory, and reflex test scores (Chen et al. 2008). One point was given for failure to perform a task. Thus, the higher the score (maximum: 14 points), the more severe the injury.
Spontaneous Forelimb Elevation (Cylinder) Test
Rats were placed in a glass cylinder (standard laboratory beaker) scaled to the size of the rat so that the cylinder diameter was roughly 4 cm greater than the length of the rat from nose to hindquarters, thus providing ample room for the rat to turn, but also minimizing horizontal exploration of the cylinder. Rats were observed for spontaneous rearings during a single 5-min observation session. The number of wall rearings using both left and right, right-only, or left-only forelimbs was recorded. A measurement was taken on the day before TBI surgery to control for pre-injury limb preference. The laterality score was computed as follows: (number of right-only − number of left-only)/(number of right-only + number of left-only + number of both together) (Schallert et al. 2000).
Foot-Fault Placing Test and Ladder-Climbing Task
All groups of rats were examined using the foot-fault placing test or ladder-climbing task before (0 day) and 1 and 21 days after the motor-training procedure. All rats were tested three times on each trial day.
A modified forelimb foot-fault placing test was used to examine forelimb function. The foot placing apparatus consisted of an elevated (10 cm) grid surface (10 × 110 cm2, with a square opening of 9 cm2 and a grid wire diameter of 1.0 mm) connected to platforms at each end (15 × 20 cm2). In each trial, the rat was encouraged by a noise or a prod to traverse the grid surface for 1 min. Occasionally, the rats inaccurately placed a forelimb, which fell through one of the openings in the grid. These mistakes were considered foot faults. The rate of contralateral forelimb foot faults made per meter in 1 min was calculated. Fewer errors made in this motor function indicated better hind-limb function (Ding et al. 2004).
To examine the coordination of their forelimbs and hind limbs, the rats were also tested using a ladder-climbing task that required motor skills (Ding et al. 2004). A single acrylic rod with crossbars at 3-cm intervals was suspended vertically from a platform 1 m above the base. In the present study, the number of rungs climbed in 60 s was recorded. The climbing velocity was then calculated as the number of rungs climbed per second (rungs/s). Higher scores in this motor function test indicated better limb coordination (Fujimoto et al. 2004).
Immunohistochemistry Assays
Serial 50-μm sections corresponding to coronal coordinates 0.8 mm to 5.3 mm posterior to the bregma were incubated in 2 mol/L of hydrogen chloride (HCl) for 30 min, rinsed in 0.1 mol/L boric acid (H3BO3) (pH 8.5) for 3 min at room temperature, and then incubated with primary antibodies in PBS containing 0.5 % normal bovine serum at 4 °C overnight, and the next day to secondary antibodies for 1 h at room temperature. The antibodies used were, sequentially, mouse monoclonal anti-NeuN (1:200) (Abcam Plc, Cambridge, UK), mouse monoclonal anti-Iba1 antibody (1:200) (Abcam), Alexa Fluor 568-conjugated donkey anti-mouse IgG antibody (1:400) (Invitrogen, Carlsbad, CA, USA), and DyLight 488-conjugated donkey-antibody IgG antibody (1:400) (Abcam). Coverslips were placed on the sections and fixed using fluorescent mounting medium (Dako, Carpinteria, CA, USA). The labeled cells were calculated in 5 coronal sections from each rat and expressed as the mean number of cells per section. For negative control sections, all the procedures were done without the primary antibody. Primary and secondary antibodies for multiple staining are listed in Table 1.
Images of the fluorescent immunohistochemistry for immune cells were captured at 100× magnification using a fluorescence microscope system (Axiovision; Zeiss Gmbh, Göttingen, Germany), and images from the bregma −0.8, −1.5, −2.5, −3.0, and −3.8 mm from each rat were evaluated. In each image, immune-positive cells showing staining with a cellular morphology and above background level were manually and exhaustively counted using Axiovision image analysis software (Zeiss). All cells were counted by an investigator (C.P.C.) blinded to the treatment status of each rat.
Statistical Analysis
Data are means ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) and then Tukey’s test was used to analyze differences between groups, except for the neurobehavioral scores, which were analyzed using nonparametric tests (Kruskal-Wallis, and then Dunn’s post hoc test). Significance was set at p < 0.05. SPSS 16.0 (SPSS, Chicago, IL, USA) was used for the statistical analysis.
Results
Acute Effect of TBI
The average intensity of the fluid pulse delivered to animals in the injured group was 2.25 ± 0.06 atm (mean ± S.E.M.). Immediately following this impact, all rats experienced a period of apnea (~25 s), hypertension (~140 mmHg for ~25 s), and tachycardia (~395 beats/min for ~125 min). Sham-injured animals showed no apnea, hypertension, or tachycardia. There was no difference between 2 treatment groups.
Early Administration of MLC 601 had Better Benefits in Treating TBI than did the Late MLC 601 Therapy
As demonstrated in Fig. 1a, early therapy of MLC 601 at a dose of 0.4 mg/kg or 0.8 mg/kg significantly attenuated TBI-induced cerebral contusion. In early injection procedure, MLC 601 was injected once daily 4 h post-injury and repeated for consecutive 2 days (so-called early therapy). However, when MLC 601 was injected once daily 4 days post-injury and repeated for consecutive 2 days (so-called late therapy), an i.p. of 0.8 mg/kg but not 0.4 mg/kg significantly attenuated TBI-induced cerebral contusion (Fig. 1b). In the following experiments early therapy with an i.p. dose of 0.4 mg/kg of MLC 601 was chosen for therapeutic verification.
Effects of early (a) or late (b) MLC 601 treatment on cerebral contusion volume, as evaluated by TTC staining, on 7 days post-injury. In a, animals were administered with an i.p. dose of 0.2 mg/kg, 0.4 mg/kg, or 0.8 mg/kg of MLC601 or vehicle solution once daily 1 h after TBI and for consecutive 2 days. In b, animals were injected with an i.p. dose of 0.2 mg/kg, 0.4 mg/kg, or 0.8 mg/kg of MLC601 or vehicle solution 4 days after TBI once daily and for consecutive 2 days. These groups of animals were killed for histological verification on day 7 post-TBI. Representative TTC-stained brain sections of a sham rat (A’), a TBI rats treated with vehicle (B’, TBI+vehicle), a TBI rat treated with 0.2 mg/kg MLC601 (C’, TBI+MLC601, 0.2 mg/kg), a TBI+MLC601 0.4 mg/kg (D’), and a TBI+MLC601 0.8 mg/kg (E’). Bar graphs demonstrating brain contusion volumes in vehicle-treated and MLC601-treaetd injured rats on days 7. Values are means ± S.E.M.; * P < 0.01 versus sham rats; + P < 0.05 versus vehicle-treated injured rats (n = 8 for each group). Middle panels showing representative tracings for different groups of rats. Arrow head indicates MLC injection or TTC stain was performed
Cortical Contusion and Neuronal Apoptosis were Lower in TBI Rat Treated with Early Administration of MLC 601
Four days after the rats had been subjected to TBI, TTC staining (Fig. 2) and TUNEL staining (Fig. 3), respectively, showed that the TBI rats treated with early administration of vehicle solution had significantly (p < 0.01) larger areas of brain contusion and more NeuN-TUNEL-positive cells in the core region of the contused cortex than did the sham group rats. Both the cerebral contusion and the neuronal apoptosis were significantly (p < 0.05) smaller in the TBI+MLC 601 group than in the TBI+vehicle group (Figs. 2 and 3).
Effects of early early MLC 601 therapy (an i.p. dose of 0.4 mg/kg of MLC 601 once daily 1 h after TBI and for consecutive 2 days) on cerebral contusion volume, as evaluated by TTC staining, on 4 days post-injury. a Representative TTC-stained brain sections of a sham rat, a vehicle-treated injured rat, and a MLC601-treated injured rat, showing dehydrogenase-deficient tissue (pale). Bar graphs demonstrating brain contused area (b) and contusion volumes (c) in sham group rats (●), TBI+vehicle group rats (○), and TBI+MLC601 group rats (▼). TBI+MLC group rat. MLC601-treated rats had a significant reduction in contusion volume relative to vehicle-treated rats on day 4. Values are means ± S.E.M.; * P < 0.01 versus sham rats; + P < 0.05 versus vehicle-treated injured rats (n = 8 for each group)
Effect of early MLC601 therapy (an i.p. dose of 0.4 mg/kg of MLC 601 once daily 1 h after TBI and for 2 consecutive 2 days) on neuronal apoptosis in the core contused cortex, as evaluated by TUNEL staining 4 day post-injury. Mean ± standard error values of the number of NeuN-TUNEL-positive cells in the core ischemic cortex. Sham (white column): rats given a sham traumatic brain injury (TBI) operation; TBI+vehicle (lined column): TBI rats treated with vehicle solution; TBI+MLC601 (crossed column); TBI rats treated with MLC601. The data were obtained 4 days after TBI or sham operation (n = 8 for each group). * P < 0.01 versus the sham group; + P < 0.05 versus TBI+vehicle group. Right panels depict representative NeuN-TUNEL double stainings for one sham rat, one TBI+vehicle rat, and one TBI+MLC601 rat
Effect of MLC 601 Treatment on Neurological and Motor Dysfunction in TBI Rats
TBI rats showed impaired performances on all tests (Fig. 4a: modified neurological severity score; b: cylinder test; c: foot-placing test; and d: ladder-climbing test). TBI rats treated with MLC 601 (0.4 mg/kg, i.p.) (TBI+MCL 601) performed significantly better on all the tests than did vehicle-treated rats between 4 and 21 days after TBI.
Effects of early MLC 601 therapy (an i.p. dose of 0.4 mg/kg of MLC once daily 1 h after TBI and for consecutive 2 days) on functional outcomes in rats with TBI, as evaluated by a modified neurological severity score (mNSS), b cylinder test, c foot placing test, and d ladder climbing test. Values are means ± S.E.M.; * P < 0.01 versus sham rats. + P < 0.05 versus vehicle-treated injured rats (n = 8 for each group). The higher the mNSS score (Chen et al. 2008), the laterality or spontaneous forelimb elevation (Schallert et al. 2000), or the foot placing error ratio (Ding et al. 2004; Fujimoto et al. 2004), the more severe the injury. In contrast, the lower the ladder climbing velocity, the more severe the injury (ding et al. 2004; Fujimoto et al. 2004)
Microgliosis and Ameboid Microglia were Significantly Lower in TBI+MCL 601 (0.4 mg/kg, i.p.) Group Rats
Four days after the rats had been subjected to TBI, immunohistochemical staining showed that the number of Iba1-positive cells around the contused cortex in the two TBI groups (Fig. 5, bottom) was significantly (p < 0.01) higher than the number in the sham group. Additionally, the microglia of the vehicle-treated injured rats were swollen and ameboid in shape (Fig. 5, top). Both microgliosis and swollen and ameboid-shaped microglia numbers were significantly lower in the TBI+MLC 601 group than in the vehicle-treated injured group rats (Fig. 5).
Effects of early MLC601 (an i.p. dose of 0.4 mg/kg of MLC once daily 4 h after TBI and for consecutive 2 days) therapy on cortical microglia morphology as well as the numbers of microglia in rats with TBI, as evaluated by immunohistochemical Iba1 staining 4 day post-injury. a A representative Iba1-stained brain section of a sham rat, a vehicle-treated injured rat, and a LC601-treated injured rat on day 4 post-injury. Scale bar is 50 μm. In vehicle-treated injured rats, microglia in the injured cortical tissue assume a swellon and an ameboid shape, which can be attenuated by MLC601 therapy (as indicated by the installs). b Bar graphs of mean densities of Iba1-positive cells in sham rats, vehicle-treated injured rats, and MLC601-treated injured rats in the cortical contusion margin 4 days after injury showing a significant decrease in the number of Iba1-postiive cells in the MLC601-treated injured group. Values are means ± S.E.M.; * P < 0.01 versus sham rats; + P < 0.05 versus vehicle-treated injured rats (n = 8 for each group)
TBI+MCL 601 Rats had Fewer Iba1+TNF-α-DAPI-Positive Cells than did TBI+Vehicle Rats
Four days after the rats had been subjected to TBI, triple-immunofluorescence staining showed that the number of colocalized Iba1+TNF-α+DAPI-positive cells (Fig. 6) around the contused cortex was significantly (p < 0.01) higher than in the Sham group. However, the number of colocalized Iba1+TNF-α-DAPI-positive cells (Fig. 6) around the contused cortex in the TBI+MLC 601 (0.4 mg/kg, i.p.) group rats was significantly (p < 0.05) lower than in the TBI+vehicle group (Fig. 6).
Effects of early MLC601 (an i.p. dose of 0.4 mg/kg of MLC once daily 4 h after TBI and for consecutive 2 days) therapy on microglial overexpression of TNF-α in the core contused cortex, as evaluated by Immunofluorescence assay 4 day post-injury. Mean ± S.E.M. of the number of Iba1-TNF-α-DAPI-positive cells in the core contused cortex. Sham (white column): rats given a sham traumatic brain injury (TBI) operation; TBI+vehicle (lined column): TBI rats treated with vehicle solution; TBI+MLC601 (crossed column): TBI rats treated with MLC601. The data were obtained 4 days after TBI or sham operation (n = 8 for each group). * P < 0.01 versus sham group; + P < 0.01 versus TBI+vehicle group. Right panels depict representative Iba1-TNF-α-DAPI triple stainings for one sham rat, one TBI+vehicle rat, and one TBI+MLC601 rat
Discussion
MLC 601 (NeuroAid I) contains 9 herbal and 5 animal components, whereas MLC 901 (NeuroAid II) bases on 9 herbal components (Heurteaux et al. 2010). In the present study, we observed that post-TBI treatment with MLC 601 significantly and dose-dependently attenuated TBI-induced cerebral contusion in rats. In addition, compared with those of the late treatment (4 days post-TBI), early treatment (1 h post-TBI) had significantly better beneficits in reducing TBI-induced cerebral contusion. The beneficial effects of MLC 601 were correlated with reduction in neurological and motor deficits, neuronal apoptosis, and microglial activation (in terms of microgliosis, morphological transformation of microglia, and microglial overexpression of tumor necrosis factor-alpha. Our results are consistent in part with those of Quintard et al. (2014). They reported that MLC 901 treatment (injected intraperitoneally at 2 h post-TBI and then administered in drinking water at a concentration of 10 mg/ml until sacrifice of the animals) induced neuroprotective and neuroregenerative beneficits after TBI in rats. It can be derived from these results that MLC 601 or MLC 901 has neuroprotective and neurorestorative effects which improve neurological and motor deficits in a rat model of TBI and a rationale for providing MLC 601 or MLC 901 therapy to improve functional recovery of patients with TBI.
Functional deficits are common neurological sequelae in patients with brain injuries and in animal models of TBI (Woodcock and Morganti-Kossmann 2013). The degree of sensorimotor dysfunction is an important indicator of the severity of the TBI (Fujimoto et al. 2004). A series of motor tasks—spontaneous forelimb elevation, a foot-fault placing test, and a ladder-climbing test—were used to assess motor functions. These motor tasks have been used in studies dealing with the effects of complex motor training on neuronal plasticity in normal animals (Black et al. 1990; Kleim et al. 1996; Isaacs et al. 1992) as well as on motor dysfunction in alcoholic, traumatic brain-injured and ischemic animals (Ding et al. 2001a, 2002a, b, 2004; Klintsova et al. 1998). We demonstrated that MLC 601 significantly improved neurological and motor outcomes assessed using a combination of neurobehavioral tests. The attenuation of neurological and motor dysfunction seen in the MLC 601-treated rats correlated with their histopathological findings. The TBI-MLC601 rats had smaller contusion volumes, fewer apoptotic neurons and less microgliosis, which led to the attenuation of brain injury and functional disorders.
It is believed that a post-TBI inflammatory response—activation of resident glial cells, microglia and astrocytes (Woodcock and Morganti-Kossmann 2013)—contributes to secondary injury in TBI and thus should be an important therapeutic target for reducing post-TBI contusion (Allan and Rothwell 2001; Morganti-Kossmann et al. 2002). We found that in response to TBI, activated microglia proliferated, became amoeboid-shaped, and released cytokines, which confirmed the findings of some other studies (Batchelor et al. 1999; Hanisch 2002).
Microglia are the resident macrophagy of the central nervous system (Kreutzberg 1996). Increased release of mediators from injured and dead cells during TBI elicit microglial activation (Rivest 2009; Loane and Byrnes 2010). Activated microglia change morphology, migrate to injury sites, and release reactive oxygen and nitrogen species, cytokines, and other factors. With respect to action, microglia is able to categorize as “classical” (or M1) or “alternatively activated” (or M2) subtype (Hernandez-Ontiveros et al. 2013). M1 subtype of microglia secretes high levels of TNF-α, IL-1, and low levels of IL-10. M2 subtype of microglia tend to attenuate inflammation, clear cellular debris, and produce very low levels of TNF-α, IL-1 and others, and high levels of IL-10, transforming growth factor-beta (TGF-β) and others. Activation of M 1 subtype of microglia can impair recovery and promote secondary (kreutzbery) death, while activation of M2 subtype of microglia can facilitate later tissue repair and functional recovery (Loane and Byrnes 2010). Activation of TNF receptor 1 (TNF-R1) causes pro-inflammatory and pro-apoptotic function of TNF-α, whereas activation of TNF-R2 tends to attenuate inflammation and to promote more pro-growth and survival pathways (McCoy and Tansey 2008; Knoblach et al. 1999; Iosif et al. 2008; Scherbel et al. 1997; Oshima et al. 2009). Base on the above statements, MLC601 therapy may improve outcomes of TBI by reducing overproduction of pro-inflammatory cytokines (via inhibiting both M1 subtype of microglia and TNF-R1). On the other hand, MLC601 therapy may facilitate later tissue repair and functional recovery by activating both M2 subtype of microglia and TNF-R2. The contention is supported in part by the many possible mechanisms of the beneficial effects of MLC601 or MLC901 against ischemia. MLC601 or MLC901 was found to stimulate BDNF expression (angiogenesis), to enhance neurogenesis, to promote cell proliferation, and to stimulate neurite outgrowth, the development of a dense axonal and dendritic network, and to activate KATP channels (Heurteaux et al. 2010; Quintard et al. 2011; Moha ou Maati et al. 2012). In addition, MLC601 may assist functional recovery after ischemic stroke by increasing cerebral blood flow (Bavarsad Shahripour et al. 2011). In addition, MLC 601 may assist functional recovery after ischemic stroke by increasing cerebral blood flow (Bavarsad Shahripour et al. 2011).
In fact, MLC601 contains 9 herbal and 5 animal components, while MLC901 bases on its 9 herbal components (Heurteaux et al. 2010). MLC901 shared with MLC601 the similar benefits in treating ischemic stroke (Quintard et al. 2011). It is likely that MLC901 may also possesses beneficial effect in treating TBI in rats. Of course, the requires further verification in futural studies. Of course, this requires further verification in futural studies.
Conclusion
In conclusion, NeuroAid (MLC 601) has been registered by the Sino food and Drug Administration in 2001 after being evaluated in clinical trials in China as a drug to facilitate recovery after stroke (Chen et al. 2009). It combines 9 herbal and 5 animal components. Our present study further provides first evidence showing that MLC601 is a promising agent for treatment of TBI in rats.
References
Allan SM, Rothwell NJ (2001) Cytokines and acute neurodegeneration. Nat Rev Neurosci 2:734–744
Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F et al (1999) Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci 19:1708–1716
Bavarsad Shahripour R, Shamsaei G, Pakdaman H, Majdinasab N, Nejad EM et al (2011) The effect of NeuroAiD™ (MLC601) on cerebral blood flow velocity in subjects’ post brain infarct in the middle cerebral artery territory. Eur J Intern Med 22:509–513
Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT (1990) Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A 87:5568–5572
Chang CP, Chio CC, Cheong CU, Chao CM, Cheng BC, Lin MT (2013) Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci 124:165–176
Chen SF, Hsu CW, Huang WH, Wang JY (2008) Post-injury baicalein improves histological and functional outcomes and reduces inflammatory cytokines after experimental traumatic brain injury. Br J Pharmacol 155:1279–1296
Chen C, Venketasubramanian N, Gan RN, Lambert C, Picard D et al (2009) Danqi Piantang Jiaonang (DJ), a traditional Chinese medicine, in poststroke recovery. Stroke 40:859–863
Chen CL, Venketasubramanian N, Lee CF, Wong KS, Bousser MG (2013a) Effects of MLC 601 on early vascular events in patients after stroke: the CHIMES study. Stroke 44:3580–3583
Chen CL, Young SH, Gan HH, Singh R, Lao AY et al (2013b) Chinese medicine neuroaid efficacy on stroke recovery: a double-blind, placebo-controlled, randomized study. Stroke 44:2093–2100
Cheong CU, Chang CP, Chao CM, Cheng BC, Yang CZ, Chio CC (2013) Etanercept attenuates traumatic brain injury in rats by reducing brain TNF- α contents and by stimulating newly formed neurogenesis. Mediat Inflamm 2013:620837
Chio CC, Lin JW, Chang MW, Wang CC, Kuo JR et al (2010) Therapeutic evaluation of etanercept in a model of traumatic brain injury. J Neurochem 115:921–929
Chio CC, Chang CH, Wang CC, Cheong CU, Chao CM et al (2013) Etanercept attenuates traumatic brain injury in rats by reducing early microglial expression of tumor necrosis factor-α. BMC Neurosci 14:33
Ding Y, Yao B, Lai Q, McAllister JP (2001) Impaired motor learning and diffuse axonal damage in motor and visual systems of the rat following traumatic brain injury. Neurol Res 23:193–202
Ding Y, Yao B, Zhou Y, Park H, McAllister JP 2nd, Diaz FG (2002a) Prereperfusion flushing of ischemic territory: a therapeutic study in which histological and behavioral assessments were used to measure ischemia-reperfusion injury in rats with stroke. J Neurosurg 96:310–319
Ding Y, Zhou Y, Lai Q, Li J, Park H, Diaz FG (2002b) Impaired motor activity and motor learning function in rat with middle cerebral artery occlusion. Behav Brain Res 132:29–36
Ding Y, Li J, Lai Q, Rafols JA, Luan X et al (2004) Motor balance and coordination training enhances functional outcome in rat with transient middle cerebral artery occlusion. Neuroscience 123:667–674
Fujimoto ST, Longhi L, Saatman KE, Conte V, Stocchetti N, McIntosh TK (2004) Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev 28:365–378
Gan R, Lambert C, Lianting J, Chan ES, Venketasubramanian N, Chen C, Chan BP, Samama MM, Bousser MG (2008) Danqi Piantan Jiaonang does not modify hemostasis, hematology, and biochemistry in normal subjects and stroke patients. Cerebrovasc Dis 25:450–456
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40:140–155
Hernandez-Ontiveros DG, Tajiri N, Acosta S, Giunta B, Tan J, Borlongan CV (2013) Microglia activation as a biomarker for traumatic brain injury. Front Neurol 4:30
Heurteaux C, Gandin C, Borsotto M, Widmann C, Brau F, Lhuillier M, Onteniente B, Lazdunski M (2010) Neuroprotective and neuroproliferative activities of NeuroAid (MLC601, MLC901), a Chinese medicine, in vitro and in vivo. Neuropharmacology 58:987–1001
Iosif RE, Ahlenius H, Ekdahl CT, Darsalia V, Thored P et al (2008) Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab 28:1574–1587
Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT (1992) Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab 12:110–119, Erratum in: J Cereb Blood Flow Metab 12:533
Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT (1996) Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci 16:4529–4535
Klintsova AY, Cowell RM, Swain RA, Napper RM, Goodlett CR, Greenough WT (1998) Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats. I. Behavioral results. Brain Res 800:48–61
Knoblach SM, Fan L, Faden AI (1999) Early neuronal expression of tumor necrosis factor-alpha after experimental brain injury contributes to neurological impairment. J Neuroimmunol 95:115–125
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318
Lindholm D, Castrén E, Kiefer R, Zafra F, Thoenen H (1992) Transforming growth factor-beta 1 in the rat brain: increase after injury and inhibition of astrocyte proliferation. J Cell Biol 117:395–400
Loane DJ, Byrnes KR (2010) Role of microglia in neurotrauma. Neurotherapeutics 2010(7):366–377
Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7:728–741
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation 5:45
McIntosh TK, Noble L, Andrews B, Faden AI (1987) Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent Nerv Syst Trauam 4:119–134
Moha Ou Maati H, Borsotto M, Chatelain F, Widmann C, Lazdunski M, Heurteaux C (2012) Activation of ATP-sensitive potassium channels as an element of the neuroprotective effects of the Traditional Chinese Medicine MLC901 against oxygen glucose deprivation. Neuropharmacology 63:692–700
Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T (2002) Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care 8:101–105
Morganti-Kossmann MC, Satgunaseeian L, Bye N, Kossmann T (2007) Modulation of immune response by head injury. Injury 38:1392–1400
Navarro JC, Gan HH, Lao AY, Baroque AC 2nd, Hiyadan JH et al (2014) Baseline characteristics and treatment responses of patients included from the phillipines in the CHIMES study. Abstract from session acute stroke: new treatment concepts. 23th European Stroke Conference Nice
Oshima T, Lee S, Sato A, Oda S, Hirasawa H, Yamashita T (2009) TNF-alpha contributes to axonal sprouting and functional recovery following traumatic brain injury. Brain Res 1290:102–110
Quintard H, Borsotto M, Veyssiere J, Gandin C, Labbal F et al (2011) MLC901, a traditional Chinese medicine protects the brain against global ischemia. Neuropharmacology 61:622–631
Quintard H, Lorivel T, Gandin C, Lazdunski M, Hevrteaux C (2014) MLC 901, a traditional Chinese medicine induces neuroprotective and neurodegenerative benefits after traumatic brain injury in rats. Neuroscience 277:72–86
Rimaniol AC, Lekieffre D, Serrano A, Masson A, Benavides J, Zavala F (1995) Biphasic transforming growth factor-beta production flanking the pro-inflammatory cytokine response in cerebral trauma. Neuroreport 7:133–136
Rivest S (2009) Regulation of innate response in the brain. Nat Reu Immunol 9:429–439
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39:777–787
Scherbel U, Raghupathi R, Nahamura M, Saatman K, McIntosh TK (1997) Evaluation of neurobehavioral deficits in brain-injured necrosis factor-deficient (TNF-1) mice after experimental brain injury. J Neurotrauma 14:781
Wang T, Lin SZ, Chiou AL, Williams LR, Hoffer BJ (1997) Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J Neurosci 17:4341–4348
Woodcock T, Morganti-Kossmann MC (2013) The role of markers of inflammation in traumatic brain injury. Front Neurol 4:18
Young SH, Zhao Y, Koh A, Singh R, Chan BP, Chang HM, Venketasubramanian N, Chen C (2010) CHIMES Investigators. Safety profile of MLC601 (Neuroaid) in acute ischemic stroke patients: a Singaporean substudy of the Chinese medicine neuroaid efficacy on stroke recovery study. Cerebrovasc Dis 30:1–6
Acknowledgments
This work was supported by research grant NSC 101-2314-B-218-001-MY3 from the Taiwan National Science and Technology Department.
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tsai, MC., Chang, CP., Peng, SW. et al. Therapeutic Efficacy of Neuro AiD™ (MLC 601), a Traditional Chinese Medicine, in Experimental Traumatic Brain Injury. J Neuroimmune Pharmacol 10, 45–54 (2015). https://doi.org/10.1007/s11481-014-9570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-014-9570-0