Abstract
The skeleton is a living organ that undergoes constant changes, including bone formation and resorption. It is affected by various diseases, such as osteoporosis, osteopenia, and osteomalacia. Nowadays, several methods are applied to protect bone health, including the use of hormonal and non-hormonal medications and supplements. However, certain drugs like glucocorticoids, thiazolidinediones, heparin, anticonvulsants, chemotherapy, and proton pump inhibitors can endanger bone health and cause bone loss. New studies are exploring the use of supplements, such as conjugated linoleic acid (CLA) and glucosamine, with fewer side effects during treatment. Various mechanisms have been proposed for the effects of CLA and glucosamine on bone structure, both direct and indirect. One mechanism that deserves special attention is the regulatory effect of RANKL/RANK/OPG on bone turnover. The RANKL/RANK/OPG pathway is considered a motive for osteoclast maturation and bone resorption. The cytokine system, consisting of the receptor activator of the nuclear factor (NF)-kB ligand (RANKL), its receptor RANK, and its decoy receptor, osteoprotegerin (OPG), plays a vital role in bone turnover. Over the past few years, researchers have observed the impact of CLA and glucosamine on the RANKL/RANK/OPG mechanism of bone turnover. However, no comprehensive study has been published on these supplements and their mechanism. To address this gap in knowledge, we have critically reviewed their potential effects. This review aims to assist in developing efficient treatment strategies and focusing future studies on these supplements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The skeleton is a living organ that is in a permanent state of turnover, including bone resorption and formation [1]. The adult human skeleton comprises approximately 206 bones [2]. Bones are responsible for body form and protecting vital organs, such as the brain and heart. Additionally, there are stem cells in the bone marrow that are remarkably important for hematopoiesis [3, 4]. As the world population ages and life expectancy increases, the number of people who suffer from bone discomfort also increases. According to reports, more than 200 million individuals worldwide struggle with osteoporosis. The International Osteoporosis Foundation indicates that one in three women and one in five men experience bone discomfort in their lifetime [5]. Bone mass density is a value used to measure bone health and strength, which naturally decreases due to aging or pathologically due to some diseases. Losing about 0.5-1% of bone mass each year after achieving peak bone mass is a natural occurrence. Recent investigations indicate that men and women are at risk of bone loss in almost equal amounts, but the difference is that women usually suffer from severe bone loss during postmenopause and afterward, while bone resorption in men occurs slowly over time [6]. Alongside genetic factors, physical activity, and a healthy food regime affect bone health. Various materials are needed for a healthy bone structure, such as calcium, magnesium, and phosphate [7], as well as protein and fat [8]. Nowadays, various methods are used to protect bone health, including drugs, surgeries, and physical therapies [9]. Bisphosphonates [10], Denosumab, Calcitonin [11], and Estrogen antagonists [12] are some of the most common medications used to prevent bone loss. Nutrient factors like vitamin D, vitamin K, and calcium [13], whether obtained through diet or supplements, can also be useful for bone amplification.
On the other hand, several medications can put bone health at risk and lead to bone loss. These medications include glucocorticoids, thiazolidinediones, heparin, anticonvulsants, chemotherapies, and proton pump inhibitors [14].
Osteoblasts [15], osteoclasts [16], osteocytes [15], and bone lining cells are the fundamental cells of bones. In a healthy body, the balance between osteoblasts (responsible for bone formation) and osteoclasts (responsible for bone resorption) keeps the skeleton healthy [17]. Recent investigations have been focusing on strategies to enhance bone formation [18,19,20,21]. In the middle of 1990s, it was revealed that osteoclast maturation depends on three markers belonging to the TNF superfamily. Receptor activator of NF-kB (RANK) is a receptor on the surface of preosteoclasts. Its binding with receptor activator of NF-kB ligand (RANKL) marks the onset of preosteoclast changes. Osteoprotegerin (OPG) participates as a decoy receptor for RANKL and prevents RANKL/RANK interaction. In conclusion, a higher OPG/RANKL ratio is a symptom of lower bone resorption [22].
The RANKL/RANK/OPG pathway is a crucial cytokine system involved in the regulation of bone turnover, and its significance in bone health has been extensively documented in recent papers [22, 23]. This pathway plays a pivotal role in osteoclast maturation and bone resorption, making it a fundamental mechanism to investigate in the context of bone health [22, 24]. The RANKL/RANK/OPG system stands as one of the most significant discoveries in bone biology in the past decade, and its disruption leads to or causes numerous bone diseases. Various factors, including cytokines, hormones, and growth factors, regulate the RANKL/RANK/OPG pathway [23]. In conclusion, a higher OPG/RANKL ratio is a symptom of lower bone resorption, and the elimination of RANKL and RANK in animal studies shows a major effect in inhibiting bone mass loss and osteoporosis [22, 23].
Conjugated linoleic acid (CLA) is a type of polyunsaturated fatty acid that contains two double bonds between carbons in conjugated and is made by an isomerization pathway [25]. There are two major forms of CLA: cis-9 trans-11 CLA and trans-10 cis-12 CLA [26]. Many advantages have been suggested for CLA, including anti-carcinoma effects, anti-diabetes effects, and a reduced risk of atherosclerosis [27]. Athletes have used CLA specifically for its effect of reducing body fat mass and boosting lean body mass to maximize exercise outcomes [28]. Furthermore, some examinations have shown that CLA’s anti-inflammatory effects could be beneficial in reducing post-exercise inflammation in athletes [29].
Glucosamine is an essential precursor for glycoproteins and glycosaminoglycans. It is a glucose-derived amino sugar that can be naturally produced in the human body [30]. Apart from its natural role in cartilage and synovial fluid, glucosamine could have anti-inflammatory effects as a supplement [31]. Additionally, glucosamine has been used to repair joint injuries and alleviate skeletal pain in athletes. Exercise-induced upregulation in athletes’ cartilage metabolism increases the need for glucosamine as well [32].
CLA and glucosamine are under investigation in new studies as potential therapeutic approaches with fewer side effects.
The selection of conjugated linoleic acid (CLA) and glucosamine supplements was based on their potential benefits for bone health, as supported by existing research [32, 33]. CLA has been shown to have positive effects on bone structure in various in vitro and in vivo studies. It has been found to increase bone mass by reducing bone resorption and increasing bone mineralization [6]. Glucosamine, on the other hand, has shown promise in supporting healthy bone growth and reducing the weakening of bones. Early studies suggest that glucosamine supplements may help prevent the progression of osteoporosis post-menopause [34]. The direct and indirect effects of CLA and glucosamine on bone structure have been linked to a number of different mechanisms. One particular mechanism that demands special focus is the regulatory impact of RANKL/RANK/OPG on bone turnover. This mechanism is common in bone turnover effects of both CLA and glucosamine [35, 36]. The RANKL/RANK/OPG pathway is considered a motive for osteoclast maturation and bone resorption [24]. However, no in-depth research on these supplements, particularly the RANKL/RANK/OPG pathway, has been released. We have critically examined their potential effects to fill in this knowledge gap, guide future research on these supplements, and aid in the development of effective treatment plans.
Two common sports supplements: conjugated linoleic Acid/Glucosamine and bone health
Conjugated linoleic acid
CLA is an isoform of linoleic acid with a conjugated double bond. The term “conjugated” refers to molecules having a single bond separating two double or triple bonds. Conjugated linoleic acids are 18-carbon fatty acids (C18:2 n-6), mostly having 2 double bonds in a conjugated form at positions (9, 11) or (10, 12) [37]. The amount of CLA humans receive from dietary sources is about 10% of the suggested CLA per day. The content of CLA intake from dietary sources in the USA is about 104–151 mg in women and 176–212 mg in men [38]. Based on animal studies, at least 3 g/d of CLA is recommended to achieve its health benefits [39]. Almost 4/5 of the existing CLA in dietary sources is c9t11-CLA. Most CLA supplements contain the same amount of CLA isomers [40]. Synthetic forms of CLA are usually plant-based and typically contain equal amounts of cis 9, trans 11, and trans 10, cis 12 [41].
Dietary sources of CLA, including meats, eggs, and dairy, contain cis-9, trans-11, and trans-10, cis-12 in a 3:1 ratio [40]. Animal age, diet, and species are some of the factors influencing the amount of CLA in their tissues or products, such as milk or eggs [42]. For example, feeding animals with oilseeds and fish oil could result in more CLA production in their tissues [43].
Multiple microorganisms have the ability to perform CLA biohydrogenation [44]. In fact, CLAs are intermediates formed during the production of polyunsaturated fatty acids such as linoleic acid and linolenic acid [43]. Several investigations have shown that CLA is the product of a species of Lactobacillus acidophilus. These microorganisms produce CLA from linoleic acid under microaerobic conditions [44]. Hydroxy fatty acids like 10-hydroxy-12-octadecenoic acid are intermediates in the CLA production pathway [45]. Butyrivibrio fibrisolvens is another ruminal microorganism participating in CLA production in animal tissues [46]. Additionally, Clostridium sporogenes, Bifidobacterium breve, Propionibacterium acnes, Lactobacillus Reuteri, and Propionibacterium freudenreichii are named as bacterial agents of CLA production [47].
In a mice study, it was reported that CLA could prevent chemically-induced skin neoplasia, leading to comprehensive studies about CLA’s health benefits [48]. Based on studies, several effects have been suggested for CLA intake. It has been shown that CLA intake could lower the risk of type 2 diabetes, cancer, and atherosclerosis, and it might be helpful for weight loss [44]. It seems that the anti-carcinogenic and metabolism-regulation effects of CLA are influenced by modification in PPAR α and γ [49]. It has been reported that t10c12 CLA appears to suppress lipid uptake by blocking lipoprotein lipase and stearoyl-CoA desaturase activities. Additionally, it has been shown to regulate lipid metabolism in hepatic cells in in-vitro studies [50]. Furthermore, several studies suggest that CLA has advantages for diabetic people, as it reduces insulin resistance and increases GLUT4 expression [51]. Regarding CLA’s anti-atherosclerosis effects, various mechanisms have been suggested, including reducing macrophage LDL uptake, monocyte adhesion, and monocyte migration, all leading to the improvement of the damaged environment and regulating atherosclerotic mediators [52]. However, several investigations have achieved some undesirable effects of CLA consumption, such as oxidative stress, insulin resistance, and gastrointestinal irritation [27]. These contradictory results indicate the importance of more studies about CLA consumption, especially CLA supplementation.
CLA’s effect on bone metabolism is one of the most ambiguous effects of CLA on the human body. The limited information available suggests that CLA affects the skeleton mainly by reducing prostaglandins, which could augment bone resorption and formation based on their concentration in bones, and increasing calcium absorption [53]. Several animal and in vitro studies have shown that CLA supplementation leads to a lower risk of bone fractures by different mechanisms (Table 1) [41].
Glucosamine
Investigations are examining new alternative strategies to control the degeneration of cartilage in bone-related diseases, including osteoarthritis [54,55,56]. Glucosamine (2-amino-2-deoxy-β-d-glucopyranose), as a possible treatment agent, is a glucose-based endogenous amino-monosaccharide primarily expressed in cartilage and connective tissues [57]. Its active form, D-glucosamine, results from an interaction between fructose 6-phosphate and glutamine through the hexosamine biosynthesis pathway [58]. Glucosamine appears to participate in cartilage and synovial fluid as part of glycosaminoglycan, proteoglycan, and hyaluronic acid structures [59]. Moreover, some evidence indicates that glucosamine has anti-inflammatory, antiviral, and antioxidative effects [30]. Various studies suggest that glucosamine could inhibit inflammation by suppressing the Nf-Kb pathway [58]. New strategies of dietary herbal supplements focus on the regulation of inflammation and immune regulation as target mechanisms [60,61,62,63].
In addition to endogenous production, glucosamine supplements are used as adjuvant therapy for several health issues, including osteoarthritis [64]. Since there are few food sources of glucosamine, most supplements are obtained from shrimp shells, chitosan, and other sea crustaceans [65]. Glucosamine supplements are reportedly safe and have no serious side effects, but they might cause some general side effects, such as gastric pain and diarrhea. Additionally, allergic reactions might occur due to the sea-crustacean-based ingredients [66].
Based on different studies, oral consumption of glucosamine with 26% bioavailability is the most common way of taking glucosamine. Glucosamine sulfate and glucosamine hydrochloride are the most popular types of glucosamine supplements [30]. Valid evidence shows that glucosamine sulfate has the highest bioavailability among all forms of glucosamine supplements [67].
CLA and bone health
Various investigations have examined the effects of CLA on bone health, both in vivo and in vitro. Since most of the studies on CLA’s skeletal effects are animal studies, their results partly depend on the type of animal being examined. Investigations have shown that CLA consumption might increase bone mineral density in chicks and mice, but no significant changes were observed in pigs and rats [68].
A number of mechanisms have been proposed for CLA’s effects on bone structure, both direct and indirect (see Fig. 1). Consuming dietary CLA can increase bone formation by regulating prostaglandin E2 in young chicks [36]. In human osteoblast-like cells, PGE2 is reduced with the consumption of trans-10, cis-12 CLA, but no effects were observed from cis-9, trans-11. PGE2 has been described as a modulator for bone formation and resorption [41]. The reduction in PGE2 might result from arachidonic acid restriction [49]. Additionally, an in vitro study has suggested CLA’s potential to upregulate alkaline phosphatase activities [69]. Alkaline phosphatase is an enzyme that plays a major role in bone mineralization through the hydrolysis of phosphate-containing strata, synthesizing orthophosphate, and enhancing calcium uptake [70]. Despite its benefits, CLA isomers could have some unwanted effects on the skeleton. Some experiments revealed that the 9 cis, 11 trans isomer of CLA resulted in the growth of the number and size of mineralized bone nodules [71].
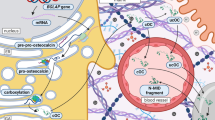
An illustration of the RANKL-RANK-OPG pathway. Numerous cell types, including osteoblasts and bone marrow stromal cells, produce RANKL as a membrane-bound or secreted ligand. When RANKL binds to the corresponding RANK receptor on the surface of these cells, a number of intracellular processes are triggered, resulting in the maturation and differentiation of precursor osteoclasts into activated osteoclasts. Possible blocking RANKL expression by CLA and glucosamine leads to diminished levels of the RANKL/RANK combination, which in turn prevents the maturation of the precursor of osteoclast to activated osteoclast. The RANKL/RANK signaling pathway plays a crucial role in osteoclast formation, activation, and survival. When RANKL binds to its receptor RANK on the surface of osteoclast precursor cells, it triggers the fusion and differentiation of these cells into mature osteoclasts. However, blocking the interaction between RANKL and RANK can inhibit osteoclastogenesis and prevent the formation of mature osteoclasts. This can be achieved by using specific inhibitors or agents including CLA and glucosamine that target RANKL expression or interfere with the RANKL-RANK interaction. By disrupting this pathway, the maturation of osteoclast precursors into activated osteoclasts can be effectively prevented, thereby potentially reducing bone resorption and maintaining bone health. The soluble RANKL decoy receptor, OPG, which shares structural similarities with RANK, can inhibit the activity of RANKL. By blocking RANKL’s ability to associate with RANK, OPG prevents all downstream molecular processes that would otherwise result in osteoclast differentiation and bone resorption. CLA and glucosamine can regulate bone remodeling and osteoclast activation by increasing OPG expression and decreasing RANKL in osteoblasts. Therefore, by increasing OPG expression and decreasing RANKL, CLA and glucosamine can affect bone resorption by decreasing mature osteoclast formation and activity. Abbreviations: RANKL, receptor activator of nuclear factor (NF)-kB ligand; OPG, osteoprotegrin; CLA, conjugated linoleic acid
It seems that feeding animals with CLA-rich food like butter leads to an increase in bone synthesis [72]. Another experiment has indicated that simultaneous use of CLA and calcium may possibly protect the bone against postmenopausal bone loss [73]. Co-supplementation of CLA and calcium modulated bone markers and caused a slowing down in bone resorption [73]. A closer look indicated that CLA supplementation could augment bone mineral resources in animals with higher calcium in their diet [68]. Some investigations suggest that regulating PPAR γ leads to an enhancement in osteoblasts’ number and bone formation after a bone fracture. As CLA could modulate PPAR, CLA is suggested for bone treatment [49].
Glucosamine and bone health
Glucosamine supplementation has widespread use in bone-related disorders, not only as an over-the-counter dietary supplement (in the US) but also as a slow-acting medication (in Europe), particularly in osteoarthritis (OA) [65]. Several investigations have indicated that 1500 mg/day of glucosamine sulfate improved OA symptoms in many cases. However, glucosamine hydrochloride has not shown the same impact [74]. Moreover, the anti-inflammatory effect of glucosamine sulfate is comparable to NSAIDs such as ibuprofen, with better toleration in patients [74]. Limited trials that have been done on glucosamine’s effects in osteoporosis support the idea of its anti-osteoporotic effects as well [75]. It is indicated that the mechanism of glucosamine supplements is basically the same as the endogenous form of it [66]. In vitro and in vivo investigations have suggested different mechanisms of action for glucosamine in bone health, including regulating glycosaminoglycans and type II collagen expression, reducing inflammatory agents, down-regulating catabolic enzymes, and interfering with the NF-kB pathway (Table 1) [76].
As mentioned above, there seem to be potential effects of CLA and glucosamine supplements on bone health. Although various inflammatory mechanisms may be involved in the effective role of these supplements, it seems that they can exert their protective effects by regulating the RANKL/RANK/OPG pathway.
**RANKL/RANK/OPG Pathway**
The discovery of the RANKL/RANK/OPG pathway was the result of long and accurate investigations aimed at finding the mechanisms of osteoclast maturation and bone resorption [77]. The cytokine system formed by receptor activator of nuclear factor (NF)-kB ligand (RANKL), its receptor RANK, and its decoy receptor Osteoprotegerin (OPG) has a vital role in bone turnover [78]. Additionally, this pathway has a role in the alteration of breast glands through pregnancy and progesterone-positive breast cancer [79]. It has been suggested that the RANKL/RANK/OPG system is an effective factor in autoimmune diseases such as rheumatoid arthritis and might have interactions with the immune system as well [80].
RANKL (receptor activator of NF-kB ligand) is a transmembrane protein expressed by osteoblasts, tumor cells, and immune cells. As a ligand, RANKL starts a chain reaction in immature osteoclasts when it binds to its receptor, RANK, a TNF receptor superfamily member expressed on the osteoclasts’ membrane. The RANKL-RANK connection starts a cascade of reactions in osteoclasts that result in osteoclast maturation [81].
Osteoprotegerin (OPG), another member of the TNF-receptor superfamily, which, unlike RANK, is not bonded to the cell membrane surface, acts as a decoy receptor for RANKL. OPG, produced by osteoblast lineage cells and several other cells in the bone marrow, binds with RANKL to prevent RANKL/RANK connection and following reactions. As a result, the number of mature osteoclasts naturally decreases, and bone resorption is controlled [82]. Based on former findings, when some mediator decreases RANKL expression level, most of the time, OPG expression gets somewhat limited or even decreases as well [22]. Additionally, OPG expression is affected by cytokines, hormones, growth factors, and Wnt/β-catenin [22].
In most bone diseases, many agents like hormones, cytokines, and growth factors play their roles by making changes in OPG levels, RANKL levels, and disrupting their balance [83]. An enhancement in the RANKL/OPG ratio is observable in metastatic bones. This condition could put bone integrity in danger, lead to some unwanted changes, and also keep the risk of tumor returns at high levels [84]. Bone discomforts, especially osteoporosis, usually have a significant relation to individuals’ genetics. Several studies reported that single nucleotide polymorphisms (SNPs) located near TNFRSF11B, TNFSF11, and TNFRSF11A are in charge of encoding OPG, RANKL, and RANK, respectively [85].
Although RANKL, RANK, and OPG have the main roles in this pathway, multiple cytokines, prostaglandins, and hormones participate in it, such as IL-1, IL-6, vitamin D, and PTH [86]. Additionally, external factors could have a significant role in the regulation of RANKL, OPG, and even RANK to prevent bone loss or accelerate it. Agents like Zinc [87], probiotics [24], and exercise have shown to play a role in regulation [23].
**CLA and RANKL/RANK/OPG**
An examination was conducted in ovariectomized mice to clarify the effect of conjugated linoleic acid on postmenopausal bone conditions. This 24-week study compared the effects of safflower oil and CLA on bone inflammation, osteoblast genesis, and osteoclast genesis. The results indicated that using CLA could prevent RANKL expression, increase OPG expression, and regulate anti-inflammatory and pro-inflammatory cytokines [88].
To display the effect of various CLA isomers on age-related bone resorption, a study was conducted on female mice. Sixty mice were split into four groups equally, and each group was fed corn oil, corn oil plus c9t11-CLA, corn oil plus t10c12-CLA, or corn oil plus t10c12-CLA plus c9t11-CLA. After six months, the results of the examinations demonstrated that sRANKL significantly decreased in groups fed t10c12-CLA and CLA-mix compared to other groups. Mice fed with t10c12-CLA showed higher bone mass density associated with a reduction in RANKL, TNF-a, and IL-6 compared to c9t11-CLA [36].
Another in vitro study investigated the effect of CLA on human CD14 + monocytes. This study showed that both c9t11 and t10c12 isomers of CLA decreased osteoclast proliferation and activity, but c9t11-CLA showed stronger results. Both of the isomers demonstrated decreasing effects on RANKL levels [89]. An experiment on murine RAW264.7 cells showed that CLA isomers prevented RANKL-induced osteoclastogenesis depending on their doses. CLA might reduce RANKL-induced TNF-a, and it could inhibit osteoclast-specific genes and osteoclast-specific transcription factors [90].
Glucosamine and RANKL/RANK/OPG
The results of a clinical trial indicated that OPG expression was unaffected by glucosamine sulfate, either alone or in combination with vitamin D3. Surprisingly, glucosamine enhanced RANKL levels both alone and in the presence of vitamin D3. All samples were derived from OA patients [35]. However, in the investigation by Yong Sun et al., glucosamine seemed to affect OPG and RANKL expression. In this study, conducted on Knee Osteoarthritis (KOA) patients, both the intervention and control groups took Etoricoxib as a basic medication, while the intervention group also received glucosamine. The reported outcomes indicated that OPG levels increased in both groups, particularly in the intervention group, and RANKL down-regulation was specifically observable in the glucosamine group [91].
Another study, performed on collagenase-induced osteoarthritis (CIOA) mice, demonstrated that glucosamine hydrochloride might reduce RANKL concentration. The outcome of this study shows that the amount of soluble RANKL in CIOA mice, after treatment with glucosamine hydrochloride, was significantly lower than in the non-treated group; however, it still was much higher than in healthy cases [92]. Igarashi et al.‘s study on newborn mouse osteoblasts (Mc3T3-E1) concluded that glucosamine diminished RANKL expression. Based on the results of this study, glucosamine at a higher dosage (1mM) suppressed RANKL mRNA more effectively than at a lower dose (0.1mM) [93].
Conclusion
Bone health may be impacted by CLA and glucosamine supplements. Alongside direct anti-inflammatory mechanisms, they appear to influence the RANKL/RANK/OPG pathway to display their protective effects. More research is recommended to determine the impact of CLA and glucosamine supplements on bone health, with a focus on the RANKL/RANK/OPG mechanism. Despite recent reports suggesting that using CLA could reduce RANKL expression, increase OPG expression, and regulate anti-inflammatory and pro-inflammatory cytokines, further investigation is necessary.
Data Availability
Not applicable.
References
Szulc P (2018) Bone turnover: Biology and assessment tools. Best Pract Res Clin Endocrinol Metab 32(5):725–738. https://doi.org/10.1016/j.beem.2018.05.003
Iwaniec UT, Turner RT (2016) Influence of body weight on bone mass, architecture and turnover. J Endocrinol 230(3):R115–130. https://doi.org/10.1530/joe-16-0089
Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS (2008) The cell biology of bone metabolism. J Clin Pathol 61(5):577–587. https://doi.org/10.1136/jcp.2007.048868
Albdeery A, Alzamily A (2022) Evaluation of the effect of injections of both platelet-rich plasma and hyaluronic acid in patients with early knee osteoarthritis via the concentration of interleukin-1β in serum. J Biomed Biochem 1(3):39–49. https://doi.org/10.57238/jbb.2022.20103
Sözen T, Özışık L, Başaran NÇ (2017) An overview and management of osteoporosis. Eur J Rheumatol 4(1):46
Banu J, Bhattacharya A, Rahman M, O’Shea M, Fernandes G (2006) Effects of conjugated linoleic acid and exercise on bone mass in young male Balb/C mice. Lipids Health Dis 5(1):7
Martinez de Victoria E (2016) Calcium, essential for health. Nutr Hosp 33(Suppl 4):341. https://doi.org/10.20960/nh.341
Corwin RL (2003) Effects of dietary fats on bone health in advanced age. Prostaglandins, leukotrienes, and essential fatty acids 68. 6379–386. https://doi.org/10.1016/s0952-3278(03)00062-0
Zhang B, Xie Y, Ni Z, Chen L (2020) Effects and Mechanisms of Exogenous Electromagnetic Field on Bone cells: a review. Bioelectromagnetics. https://doi.org/10.1002/bem.22258
Pizones J, Plotkin H, Parra-Garcia JI, Alvarez P, Gutierrez P, Bueno A, Fernandez-Arroyo A (2005) Bone healing in children with osteogenesis imperfecta treated with bisphosphonates. J Pediatr Orthop 25(3):332–335
Ubios A, Furno GJ, Guglielmotti M (1991) Effect of calcitonin on alveolar wound healing. J oral Pathol Med 20(7):322–324
Grasser WA, Pan LC, Thompson DD, Paralkar VM (1997) Common mechanism for the estrogen agonist and antagonist activities of droloxifene. J Cell Biochem 65(2):159–171. https://doi.org/10.1002/(sici)1097-4644(199705)65:2>159::aid-jcb3<3.0.co;2-t
Fischer V, Haffner-Luntzer M, Amling M, Ignatius A (2018) Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur Cells Mater 35:365–385. https://doi.org/10.22203/eCM.v035a25
Panday K, Gona A, Humphrey MB (2014) Medication-induced osteoporosis: screening and treatment strategies. Therapeutic Adv Musculoskelet Disease 6(5):185–202
Komori T (2016) Cell death in chondrocytes, osteoblasts, and Osteocytes. Int J Mol Sci 17(12). https://doi.org/10.3390/ijms17122045
Ono T, Nakashima T (2018) Recent advances in osteoclast biology. Histochem Cell Biol 149(4):325–341. https://doi.org/10.1007/s00418-018-1636-2
Ohlsson C, Sjogren K (2015) Effects of the gut microbiota on bone mass. Trends Endocrinol Metab 26(2):69–74. https://doi.org/10.1016/j.tem.2014.11.004
Bolamperti S, Villa I, Rubinacci A (2022) Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res 10(1):48
Kim JH, Liu X, Wang J, Chen X, Zhang H, Kim SH, Cui J, Li R, Zhang W, Kong Y (2013) Wnt signaling in bone formation and its therapeutic potential for bone diseases. Therapeutic Adv Musculoskelet Disease 5(1):13–31
Yu Y, Wang L, Ni S, Li D, Liu J, Chu HY, Zhang N, Sun M, Li N, Ren Q (2022) Targeting loop3 of sclerostin preserves its cardiovascular protective action and promotes bone formation. Nat Commun 13(1):4241
Wang L, Yu Y, Ni S, Li D, Liu J, Xie D, Chu HY, Ren Q, Zhong C, Zhang N (2022) Therapeutic aptamer targeting sclerostin loop3 for promoting bone formation without increasing cardiovascular risk in osteogenesis imperfecta mice. Theranostics 12(13):5645
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473(2):139–146. https://doi.org/10.1016/j.abb.2008.03.018
Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S (2020) RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. BioMed research international 2020
Amin N, Boccardi V, Taghizadeh M, Jafarnejad S (2020) Probiotics and bone disorders: the role of RANKL/RANK/OPG pathway. Aging Clin Exp Res 32(3):363–371
Guo Q, Li T, Qu Y, Liang M, Ha Y, Zhang Y, Wang Q (2022) New research development on trans fatty acids in food: Biological effects, analytical methods, formation mechanism, and mitigating measures. Progress in lipid research:101199
Ing SW, Belury MA (2011) Impact of conjugated linoleic acid on bone physiology: proposed mechanism involving inhibition of adipogenesis. Nutr Rev 69(3):123–131. https://doi.org/10.1111/j.1753-4887.2011.00376.x
Benjamin S, Prakasan P, Sreedharan S, Wright AD, Spener F (2015) Pros and cons of CLA consumption: an insight from clinical evidences. Nutr Metabolism 12:4. https://doi.org/10.1186/1743-7075-12-4
Terasawa N, Okamoto K, Nakada K, Masuda K (2017) Effect of conjugated linoleic acid intake on endurance Exercise Performance and Anti-fatigue in Student athletes. J Oleo Sci 66(7):723–733. https://doi.org/10.5650/jos.ess17053
Baghi AN, Mazani M, Nemati A, Amani M, Alamolhoda S, Mogadam RA (2016) Anti-inflammatory effects of conjugated linoleic acid on young athletic males. JPMA The Journal of the Pakistan Medical Association 66(3):280–284
Kirkham S, Samarasinghe R (2009) Glucosamine. J Orthop Surg 17(1):72–76
Kantor ED, Lampe JW, Navarro SL, Song X, Milne GL, White E (2014) Associations between glucosamine and chondroitin supplement use and biomarkers of systemic inflammation. J Altern Complement Med 20(6):479–485
Nagaoka I, Tsuruta A, Yoshimura M (2019) Chondroprotective action of glucosamine, a chitosan monomer, on the joint health of athletes. Int J Biol Macromol 132:795–800. https://doi.org/10.1016/j.ijbiomac.2019.03.234
Kerksick CM, Wilborn CD, Roberts MD, Smith-Ryan A, Kleiner SM, Jäger R, Collins R, Cooke M, Davis JN, Galvan E (2018) ISSN exercise & sports nutrition review update: research & recommendations. J Int Soc Sports Nutr 15(1):38
Jiang Z, Li Z, Zhang W, Yang Y, Han B, Liu W, Peng Y (2018) Dietary natural N-Acetyl-d-glucosamine prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. Molecules 23(9):2302
Tat SK, Pelletier JP, Vergés J, Lajeunesse D, Montell E, Fahmi H, Lavigne M, Martel-Pelletier J (2007) Chondroitin and glucosamine sulfate in combination decrease the pro-resorptive properties of human osteoarthritis subchondral bone osteoblasts: a basic science study. Arthritis Res Therapy 9(6):R117. https://doi.org/10.1186/ar2325
Rahman MM, Halade GV, Williams PJ, Fernandes G (2011) t10c12-CLA maintains higher bone mineral density during aging by modulating osteoclastogenesis and bone marrow adiposity. J Cell Physiol 226(9):2406–2414
Lehnen TE, da Silva MR, Camacho A, Marcadenti A, Lehnen AM (2015) A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. J Int Soc Sports Nutr 12:36. https://doi.org/10.1186/s12970-015-0097-4
Kim Y, Kim J, Whang KY, Park Y (2016) Impact of conjugated linoleic acid (CLA) on skeletal muscle metabolism. Lipids 51(2):159–178. https://doi.org/10.1007/s11745-015-4115-8
Gangidi RR, Lokesh BR (2014) Conjugated linoleic acid (CLA) formation in edible oils by photoisomerization: a review. J Food Sci 79(5):R781–785. https://doi.org/10.1111/1750-3841.12449
Koronowicz AA, Banks P (2018) Antitumor Properties of CLA-Enriched Food Products. Nutr Cancer 70(4):529–545. https://doi.org/10.1080/01635581.2018.1460684
Roy BD, Antolic A (2009) Conjugated linoleic acid (CLA) and bone health: a review. Curr Top Nutraceutical Res 7(1):27
Dhiman TR, Nam SH, Ure AL (2005) Factors affecting conjugated linoleic acid content in milk and meat. Crit Rev Food Sci Nutr 45(6):463–482. https://doi.org/10.1080/10408390591034463
Kramer JK, Cruz-Hernandez C, Deng Z, Zhou J, Jahreis G, Dugan ME (2004) Analysis of conjugated linoleic acid and trans 18: 1 isomers in synthetic and animal products. Am J Clin Nutr 79(6):1137S–1145S
Kishino S, Ogawa J, Omura Y, Matsumura K, Shimizu S (2002) Conjugated linoleic acid production from linoleic acid by lactic acid bacteria. J Am Oil Chem Soc 79(2):159–163
Ogawa J, Matsumura K, Kishino S, Omura Y, Shimizu S (2001) Conjugated linoleic acid accumulation via 10-hydroxy-12-octadecaenoic acid during microaerobic transformation of linoleic acid by Lactobacillus acidophilus. Appl Environ Microbiol 67(3):1246–1252
Gorissen L, Leroy F, De Vuyst L, De Smet S, Raes K (2015) Bacterial production of conjugated linoleic and linolenic acid in foods: a technological challenge. Crit Rev Food Sci Nutr 55(11):1561–1574. https://doi.org/10.1080/10408398.2012.706243
Yang B, Gao H, Stanton C, Ross RP, Zhang H, Chen YQ, Chen H, Chen W (2017) Bacterial conjugated linoleic acid production and their applications. Prog Lipid Res 68:26–36. https://doi.org/10.1016/j.plipres.2017.09.002
Belury MA, Nickel KP, Bird CE, Wu Y (1996) Dietary conjugated linoleic acid modulation of phorbol ester skin tumor promotion
Shan Z, Luo ZP, Shen X, Chen L (2017) Promotion of fracture healing by conjugated linoleic acid in rats. J Orthop Surg 25(2):2309499017718910. https://doi.org/10.1177/2309499017718910
Pariza MW, Park Y, Cook ME (2001) The biologically active isomers of conjugated linoleic acid. Prog Lipid Res 40(4):283–298. https://doi.org/10.1016/s0163-7827(01)00008-x
Cho K, Song Y, Kwon D (2016) Conjugated linoleic acid supplementation enhances insulin sensitivity and peroxisome proliferator-activated receptor gamma and glucose transporter type 4 protein expression in the skeletal muscles of rats during endurance exercise. Iran J Basic Med Sci 19(1):20–27
Bruen R, Fitzsimons S, Belton O (2017) Atheroprotective effects of conjugated linoleic acid. Br J Clin Pharmacol 83(1):46–53. https://doi.org/10.1111/bcp.12948
Brownbill RA, Petrosian M, Ilich JZ (2005) Association between dietary conjugated linoleic acid and bone mineral density in postmenopausal women. J Am Coll Nutr 24(3):177–181
Roseti L, Desando G, Cavallo C, Petretta M, Grigolo B (2019) Articular cartilage regeneration in osteoarthritis. Cells 8(11):1305
Liu H, Xiang X, Huang J, Zhu B, Wang L, Tang Y, Du F, Li L, Yan F, Ma L (2021) Ultrasound augmenting injectable chemotaxis hydrogel for articular cartilage repair in osteoarthritis. Chin Chem Lett 32(5):1759–1764
Li L, Xu H, Qu L, Nisar M, Nisar MF, Liu X, Xu K (2023) Water extracts of Polygonum Multiflorum Thunb. And its active component emodin relieves osteoarthritis by regulating cholesterol metabolism and suppressing chondrocyte inflammation. Acupunct Herb Med 3(2):96–106
Dahmer S, Schiller R (2008) Glucosamine. Am Family Phys 78(4):471–476
Dalirfardouei R, Karimi G, Jamialahmadi K (2016) Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sci 152:21–29
Matheson AJ, Perry CM (2003) Glucosamine Drugs & Aging 20(14):1041–1060
Sun S-J, Deng P, Peng C-E, Ji H-Y, Mao L-F, Peng L-Z (2022) Extraction, structure and immunoregulatory activity of low molecular weight polysaccharide from Dendrobium officinale. Polymers 14(14):2899
Wu Q, Gao Z-J, Yu X, Wang P (2022) Dietary regulation in health and disease. Signal Transduct Target Therapy 7(1):252
Huang K, Huang J, Zhao J, Gu Z, Wu J (2022) Natural lotus root-based scaffolds for bone regeneration. Chin Chem Lett 33(4):1941–1945
Epsley S, Tadros S, Farid A, Kargilis D, Mehta S, Rajapakse CS (2021) The effect of inflammation on bone. Front Physiol 11:1695
Reginster J-Y, Neuprez A, Lecart M-P, Sarlet N, Bruyere O (2012) Role of glucosamine in the treatment for osteoarthritis. Rheumatol Int 32(10):2959–2967
Kang HE, Kim SJ, Yeo E-j, Hong J, Rajgopal A, Hu C, Murray MA, Dang J, Park E (2022) Pharmacokinetic comparison of Chitosan-Derived and Biofermentation-Derived glucosamine in Nutritional supplement for Bone Health. Nutrients 14(15):3213
Williams C, Ampat G (2020) Glucosamine Sulfate
Meulyzer M, Vachon P, Beaudry F, Vinardell T, Richard H, Beauchamp G, Laverty S (2008) Comparison of pharmacokinetics of glucosamine and synovial fluid levels following administration of glucosamine sulphate or glucosamine hydrochloride. Osteoarthr Cartil 16(9):973–979. https://doi.org/10.1016/j.joca.2008.01.006
Ing SW, Belury MA (2011) Impact of conjugated linoleic acid on bone physiology: proposed mechanism involving inhibition of adipogenesis. Nutr Rev 69(3):123–131
Cusack S, Jewell C, Cashman K (2005) The effect of conjugated linoleic acid on the viability and metabolism of human osteoblast-like cells. Prostaglandins Leukot Essent Fatty Acids 72(1):29–39
Piattelli A, Scarano A, Corigliano M, Piattelli M (1996) Effects of alkaline phosphatase on bone healing around plasma-sprayed titanium implants: a pilot study in rabbits. Biomaterials 17(14):1443–1449. https://doi.org/10.1016/0142-9612(96)87288-7
Platt I, Rao LG, El-Sohemy A (2007) Isomer-specific Effects of conjugated linoleic acid on mineralized bone nodule formation from human Osteoblast-LikeCells. Experimental Biology and Medicine 232(2):246–252
Watkins BA, Shen C-L, McMurtry JP, Xu H, Bain SD, Allen KG, Seifert MF (1997) Dietary lipids modulate bone prostaglandin E2 production, insulin-like growth factor-I concentration and formation rate in chicks. J Nutr 127(6):1084–1091
Park Y, Kim J, Scrimgeour AG, Condlin ML, Kim D, Park Y (2013) Conjugated linoleic acid and calcium co-supplementation improves bone health in ovariectomised mice. Food Chem 140(1–2):280–288
Reginster JY, Neuprez A, Lecart MP, Sarlet N, Bruyere O (2012) Role of glucosamine in the treatment for osteoarthritis. Rheumatol Int 32(10):2959–2967. https://doi.org/10.1007/s00296-012-2416-2
Lv C, Wang L, Zhu X, Lin W, Chen X, Huang Z, Huang L, Yang S (2018) Glucosamine promotes osteoblast proliferation by modulating autophagy via the mammalian target of rapamycin pathway. Biomed Pharmacotherapy = Biomedecine Pharmacotherapie 99:271–277. https://doi.org/10.1016/j.biopha.2018.01.066
Henrotin Y, Chevalier X, Herrero-Beaumont G, McAlindon T, Mobasheri A, Pavelka K, Schön C, Weinans H, Biesalski H (2013) Physiological effects of oral glucosamine on joint health: current status and consensus on future research priorities. BMC Res Notes 6:115. https://doi.org/10.1186/1756-0500-6-115
Khosla S (2001) Minireview: the opg/rankl/rank system. Endocrinology 142(12):5050–5055
Hofbauer L, Kuhne C, Viereck V (2004) The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact 4(3):268
Wunderle M, Ruebner M, Haberle L, Schwenke E, Hack CC, Bayer CM, Koch MC, Schwitulla J, Schulz-Wendtland R, Kozieradzki I, Lux MP, Beckmann MW, Jud SM, Penninger JM, Schneider MO, Fasching PA (2020) RANKL and OPG and their influence on breast volume changes during pregnancy in healthy women. Sci Rep 10(1):5171. https://doi.org/10.1038/s41598-020-62070-3
Liu W, Zhang X (2015) Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues. Mol Med Rep 11(5):3212–3218
Kovács B, Vajda E, Nagy EE (2019) Regulatory Effects and interactions of the wnt and OPG-RANKL-RANK signaling at the bone-cartilage interface in Osteoarthritis. Int J Mol Sci 20(18). https://doi.org/10.3390/ijms20184653
Udagawa N, Koide M, Nakamura M, Nakamichi Y, Yamashita T, Uehara S, Kobayashi Y, Furuya Y, Yasuda H, Fukuda C, Tsuda E (2021) Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab 39(1):19–26. https://doi.org/10.1007/s00774-020-01162-6
Kostenuik PJ (2005) Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol 5(6):618–625
Dougall WC (2012) Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res 18(2):326–335
Tu P, Duan P, Zhang R-S, Xu D-B, Wang Y, Wu H-P, Liu Y-H, Si L (2015) Polymorphisms in genes in the RANKL/RANK/OPG pathway are associated with bone mineral density at different skeletal sites in post-menopausal women. Osteoporos Int 26(1):179–185
Hsu Y-H, Niu T, Terwedow HA, Xu X, Feng Y, Li Z, Brain JD, Rosen CJ, Laird N, Xu X (2006) Variation in genes involved in the RANKL/RANK/OPG bone remodeling pathway are associated with bone mineral density at different skeletal sites in men. Hum Genet 118(5):568–577
Amin N, Clark CC, Taghizadeh M, Djafarnejad S (2020) Zinc supplements and bone health: the role of the RANKL-RANK axis as a therapeutic target. J Trace Elem Med Biol 57:126417
Rahman MM, Fernandes G, Williams P (2014) Conjugated linoleic acid prevents ovariectomy-induced bone loss in mice by modulating both osteoclastogenesis and osteoblastogenesis. Lipids 49(3):211–224. https://doi.org/10.1007/s11745-013-3872-5
Platt I, El-Sohemy A (2009) Effects of 9cis,11trans and 10trans,12cis CLA on osteoclast formation and activity from human CD14 + monocytes. Lipids Health Dis 8:15. https://doi.org/10.1186/1476-511x-8-15
Rahman MM, Bhattacharya A, Fernandes G (2006) Conjugated linoleic acid inhibits osteoclast differentiation of RAW264.7 cells by modulating RANKL signaling. J Lipid Res 47(8):1739–1748. https://doi.org/10.1194/jlr.M600151-JLR200
Sun Y, Wang C, Gong C (2020) Repairing effects of glucosamine sulfate in combination with etoricoxib on articular cartilages of patients with knee osteoarthritis. J Orthop Surg Res 15(1):150. https://doi.org/10.1186/s13018-020-01648-z
Ivanovska N, Dimitrova P (2011) Bone resorption and remodeling in murine collagenase-induced osteoarthritis after administration of glucosamine. Arthritis Res Therapy 13(2):1–13
Ali Abd Z, Jabbar N (2023) Circulating microrna-22 as a biomarker related to oxidative stress in hyperthyroid women patient. J Biomed Biochem 2(3):28–37. https://doi.org/10.57238/jbb.2023.7019.1039
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Shaymaa J. Abdulrahman, Abduladheem Turki Jalil, Mohanad Ali Abdulhadi, Dumooa Falah, Muna S. Merza, Abbas F. Almulla, Ahmed Ali and Ronak Taher Ali all contributed significantly to the development and completion of this research paper. Shaymaa J. Abdulrahman, Abduladheem Turki Jalil and Mohanad Ali Abdulhadi were responsible for conceptualizing the study and designing the research framework. Ronak Taher Ali, Dumooa Falah, and Muna S. Merza conducted the literature review, gathering relevant information on bone health, osteoporosis, and the impact of various drugs and supplements. Abbas F. Almulla and Ahmed Ali were involved in analyzing and interpreting the data related to the regulatory effect of RANKL/RANK/OPG on bone turnover concerning the use of CLA and glucosamine supplements. All authors participated in the critical review of the manuscript, providing valuable insights, and revising the content to ensure the accuracy and clarity of the information presented. Shaymaa J. Abdulrahman, Abduladheem Turki Jalil, Mohanad Ali Abdulhadi, Dumooa Falah, Muna S. Merza, Abbas F. Almulla, Ahmed Ali and Ronak Taher Ali contributed equally in the preparation of the final version of the paper, approving it for submission, and agreeing to be accountable for all aspects of the work. Their collaborative efforts have been instrumental in addressing the knowledge gap regarding the potential effects of CLA and glucosamine on the RANKL/RANK/OPG mechanism on bone turnover, thus paving the way for the development of more effective treatment strategies and guiding future studies on these supplements and their mechanisms in bone health.
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdulrahman, S., Abdulhadi, M.A., Turki Jalil, A. et al. Conjugated linoleic acid and glucosamine supplements may prevent bone loss in aging by regulating the RANKL/RANK/OPG pathway. Mol Biol Rep 50, 10579–10588 (2023). https://doi.org/10.1007/s11033-023-08839-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08839-x