Abstract
Summary
Tocotrienols have shown bone-protective effect in animals. This study showed that a 12-week tocotrienol supplementation decreased concentrations of bone resorption biomarker and bone remodeling regulators via suppressing oxidative stress in postmenopausal osteopenic women.
Introduction
Tocotrienols (TT) have been shown to benefit bone health in ovariectomized animals, a model of postmenopausal women. The purpose of this study was to evaluate the effect of 12-week TT supplementation on bone markers (serum bone-specific alkaline phosphatase (BALP), urine N-terminal telopeptide (NTX), serum soluble receptor activator of nuclear factor-kappaB ligand (sRANKL), and serum osteoprotegerin (OPG)), urine calcium, and an oxidative stress biomarker (8-hydroxy-2′-deoxyguanosine (8-OHdG)) in postmenopausal women with osteopenia.
Methods
Eighty-nine postmenopausal osteopenic women (59.7 ± 6.8 year, BMI 28.7 ± 5.7 kg/m2) were randomly assigned to three groups: (1) placebo (430 mg olive oil/day), (2) low TT (430 mg TT/day, 70% purity), and (3) high TT (860 mg TT/day, 70% purity). TT, an extract from annatto seed with 70% purity, consisted of 90% delta-TT and 10% gamma-TT. Overnight fasting blood and urine samples were collected at baseline, 6, and 12 weeks for biomarker analyses. Eighty-seven subjects completed the 12-week study.
Results
Relative to the placebo group, there were marginal decreases in serum BALP level in the TT-supplemented groups over the 12-week study period. Significant decreases in urine NTX levels, serum sRANKL, sRANKL/OPG ratio, and urine 8-OHdG concentrations and a significant increase in BALP/NTX ratio due to TT supplementation were observed. TT supplementation did not affect serum OPG concentrations or urine calcium levels throughout the study period. There were no significant differences in NTX level, BALP/NTX ratio, sRANKL level, and sRANKL/OPG ratio between low TT and high TT groups.
Conclusions
Twelve-week annatto-extracted TT supplementation decreased bone resorption and improved bone turnover rate via suppressing bone remodeling regulators in postmenopausal women with osteopenia. Such osteoprotective TT’s effects may be, in part, mediated by an inhibition of oxidative stress.
Trial registration
ClinicalTrials.gov identifier: NCT02058420. Title: Tocotrienols and bone health of postmenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing body of evidence suggests a detrimental effect of excessive oxidative stress on bone metabolism (bone formation < bone resorption) [1], microstructural deterioration, and bone loss [1], significantly increasing bone fragility and susceptibility to fracture [2]. Half of postmenopausal women will experience a bone fracture due to osteoporosis [2]. According to the World Health Organization, it is estimated that about 22 million women have low bone mass (called osteopenia) in the USA and 8 million women have severe bone loss (called osteoporosis) [3]. Although the usage of antiosteoporotic therapies (e.g., hormone therapy, bisphosphonates, parathyroid hormone) has been widely accepted, their inherent side effects after long-term use have raised some concerns [4]. Thus, identifying alternative natural compounds to mitigate the progression of bone deterioration and bone loss in postmenopausal women has become a high public health priority.
Tocotrienols (TTs), a subgroup of vitamin E with four isomers—alpha, beta, gamma, and delta, have gained much attention on management of chronic diseases including osteoporosis in the past decade [5] due to their strong antioxidant and anti-inflammation properties [6]. TTs protect osteoblasts from lipid peroxidation [6] and suppress osteoclast differentiation [7], osteoclast maturation [7], and bone resorption activity [7] by inhibiting the activation of nuclear factor κB (NF-kB) and extracellular signal-regulated kinase (ERK) [7].
The rodent model of ovariectomy (OVX)-induced bone loss and microstructure deterioration has been widely used to mimic estrogen deficiency-induced bone loss in postmenopausal women with low bone mass [9]. Previous animal studies have shown the bone-protective effects of TTs, in the forms of palm-oil-derived TT mixture [10], pure TTs (γ-TT in Deng et al. [11], δ-TT in Abdul-Majeed et al. [12]), or TT-rich fraction Aktifanus et al. [13]) in the OVX animals, due to TT’s anti-inflammatory properties. Gamma- or delta-TT supplementation to OVX animals had similar osteoprotective effects shown as increased bone formation, decreased bone resorption, and improved bone strength [11, 12].
These evidence from in vitro and animal studies provide the rationale for the translation of the findings to postmenopausal women [10,11,12,13]. However, no study has ever been conducted to demonstrate the antioxidant role of TT in postmenopausal women with low bone mass. This study was designed to examine the effects of dietary TT (consisting of 90% δ-TT and 10% γ-TT extracted from annatto seeds) supplementation on bone turnover biomarkers, bone modeling regulators, and urine calcium in postmenopausal osteopenic women. In addition, we also evaluated the biomarker for oxidative stress-mediated DNA damage, 8-hydroxy-2′-deoxyquanosine (8-OHdG), in urine of study participants. Our hypotheses were (1) 12 weeks of dietary TT supplementation favors bone remodeling in postmenopausal women with osteopenia as shown in an increase in bone formation, a decrease in bone resorption, and a decrease in osteoclastic activity; and (2) the changes in bone markers are associated with reduced oxidative stress. These hypotheses are based on the premise that TT would protect osteoblasts and osteoclasts from excessive oxidative stress-induced damage, resulting in osteoprotective benefit to postmenopausal osteopenic women who are at increased fracture risk.
Methods
Study design
This was a 12-week double-blinded, placebo-controlled, randomized trial to investigate the effects of TT on relevant primary and secondary outcomes in postmenopausal osteopenic women. The primary outcome measure included urine N-terminal telopeptide (NTX, a bone resorption marker), while the secondary outcome measures included serum bone-specific alkaline phosphatase (BALP), serum soluble receptor activator of nuclear factor-kappaB ligand (sRANKL), serum osteoprotegerin (OPG), urine calcium (Ca), and urine 8-OHdG.
In this study, we used tocotrienols extracted from annatto seeds. Each TT capsule contains 90% δ-TT and 10% γ-TT with a 70% purity. The tocopherol-free TT sample precludes the potential tocopherol-mediated attenuation of the TT effect [14]. The δ-TT and γ-TT contained in the capsule are the most active TT isomers for bone health with their antioxidant and mevalonate-suppressive activities [15]. The selection of 600 mg TT daily was based on the following: (i) previous animal studies showing the osteoprotective impacts of TT at 60 mg/kg body weight on the OVX animals [12] and (ii) dose conversion from rat to human based on body surface area [16]. Two dosages of TT at 300 and 600 mg daily were used to evaluate their effects on bone biomarkers. Since there is no study evaluating dietary TT supplement on bone turnover markers, a 12-week intervention period was chosen based on previous studies using dietary supplements on postmenopausal women for bone turnover marker assessment [17, 18].
Participants
The complete study protocol has been published previously, and only a brief description is provided here [19]. Postmenopausal women were recruited through study flyers, local media, community centers, and clinics. Of 326 potential participant candidates, 89 were recruited after screening based on the inclusion and exclusion criteria. The included participants had to be ≥ 45-year postmenopausal women with no menses for at least 1 year; bone mineral density (BMD) T-score greater than − 2.5 at measured site (spine and/or hip); normal function of thyroid, liver, and kidney; serum 25-hydroxy vitamin D ≥ 20 ng/mL; and no bisphosphonate use for at least 12 months before the start date of study. Exclusion criteria included (1) having history of, or evidence for, metabolic bone disease including recent fracture (other than low BMD); (2) having history of cancer within the last 5 years, except for treated superficial basal or squamous cell carcinoma of the skin; (3) having history or evidence of endocrine disease or malabsorption syndrome that would be a contraindication to the investigation of TT absorption; (4) having HbA1c of ≥ 7% in the last 3 months; (5) having alcohol intake > 1 drink/day; (6) having use of non-steroidal anti-inflammatory drugs on a regular basis; (7) taking medication that is known to affect bone metabolism; (8) taking statin or other drugs for cholesterol control ≤ 3 months before the start date of study; (9) taking hormone/hormone-like therapy ≤ 6 months before the start date of study; (10) taking anticoagulants that may interact with TT; (11) having cognitive impairment, depression, or other medical/eating disorders; (12) smoking > 10 cigarettes/day; or (13) unwillingness to accept randomization. Written informed consent was obtained from all the participants before enrollment into the study. This study was approved by Texas Tech University Health Sciences Center Institutional Review Board, and it was registered at ClinicalTrials.gov as NCT02058420.
Randomization, blinding, and intervention
Participants were randomly assigned in a 1:1:1 ratio in block size of 3 and 6 to one of three treatments: placebo, low TT, and high TT groups with matched body mass index (BMI) (≥ 30 or < 30 kg/m2) and age (≥ 50 or < 50 years) for 12 weeks. Both the study participants and investigators were blinded to treatment allocation.
The participants in the placebo group received two 430 mg olive oil soft gels per day (one in the morning and another in the evening). The low TT participants received one 430 mg olive oil soft gel in the morning and one 430 mg TT soft gel from Delta Gold® Tocotrienol 70% (containing 300 mg TT) in the evening. Delta Gold® Tocotrienol 70% is an extract of annatto containing 90% δ-tocotrienols and 10% γ-tocotrienols with 70% purity. The high TT participants received two 430 mg TT soft gels per day (containing 600 mg TT) with one in the morning and another in the evening. Placebo (olive oil soft gels) and intervention (TT soft gels, registered with FDA Investigational New Drug (IND) number 120761) were provided by American River Nutrition, Inc., Hadley, MA. The olive oil soft gels and TT soft gels were indistinguishable in taste, smell, and appearance. During the intervention, all participants were provided with a 500 mg elemental calcium as Oster Shell and 400 IU vitamin D as cholecalciferol supplement daily (GlaxoSmithKline, PA).
Data collection
Medical history, food intake, physical activity level, height, and body composition were recorded at the time of enrollment. For screening purpose, both BMD (dual-energy X-ray absorptiometry, Norland Excel X-Ray Bone Densitometer) and laboratory blood chemistry parameters were measured prior to the baseline data collection. At baseline, 6, and 12 weeks, body composition including body weight, fat mass, fat-free mass, and bone mass was determined through bioimpedance measurement (SC-331S Body Composition Analyzer, Tanita Corporation of America, Inc., Arlington Height, IL, USA). Overnight fasting blood and urine samples were collected for outcome measures. In addition, at baseline and 12 weeks, food intake and physical activity level were monitored for any deviation from baseline macro- and micro-nutrient intake as well as routine physical activity level throughout 12-week study period.
Compliance and adverse event monitoring
At 6- and 12-week visits, participants were asked to return any remaining soft gels. Compliance of placebo and TT groups was determined as the number of soft gels ingested divided by the number a participant should have ingested throughout the study period. Adverse effects, if any, were self-reported by the participants. In addition, the activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in serum were determined at 6 and 12 weeks to monitor liver function. All adverse events, whether observed by investigators or voluntarily disclosed by subjects, were recorded on the adverse event form throughout the study.
Measurement of bone markers, urine calcium, and 8-OHdG
Serum BALP concentrations were measured using Metra™ BALP immunoassay (Quidel Corporation, San Diego, CA), intra-assay and inter-assay CVs < 5.0%. Urine NTX concentrations were measured using an ELISA kit (Alere, Providence, RI), intra-assay and inter-assay CVs < 3.0%. Urine creatinine concentrations were measured using MicroVue™ Creatinine Assay Kit (Quidel Corporation). The final NTX was normalized by urinary creatinine. Serum sRANKL concentrations were measured using sRANKL (total) human ELISA (BioVendor, LLC, Asheville, NC), intra-assay and inter-assay < 12%. Serum OPG concentrations were measured using a MicroVue OPG kit (Quidel Corporation), intra-assay and inter-assay < 5%. Urine Ca levels were analyzed by a certified diagnostic laboratory (Quest Diagnostic Laboratory, Dallas, TX, USA). Urine 8-OHdG levels were measured by OxiSelect™ Oxidative DNA Damage ELISA Kit (Cell Biolabs, Inc., San Diego, CA, USA), and the intra-assay and inter-assay CVs were 9.38 and 11.99%, respectively.
Statistical analysis
The minimum sample size for assessing urine NTX was calculated using the reported urine NTX level [20] as the baseline value for placebo and intervention groups. Compared with the placebo group with no anticipated changes throughout the study period, the low TT and high TT groups were expected to decrease by 15 and 20% from the baseline, respectively. We also assumed that the adjusted covariates explained about 20% of the variability in NTX, i.e., a Pearson correlation coefficient of 0.45 (R2 = 0.20) between NTX and the adjusted covariates. With an estimated attrition rate of 15% over 12 weeks of intervention, a minimal sample size of 78 participants was needed.
The outcome measures and other cofactors were summarized using appropriate summary measures as per the type and distribution of the variable according to each intervention level. The baseline cofactors were compared among the three groups using one-way ANOVA or Fisher’s exact test/chi-squared test as appropriate.
To assess the effect of intervention on NTX at 12 weeks, repeated measures ANCOVA was conducted to adjust the baseline NTX and other significant cofactors, including participant age, BMI, baseline calcium intake, and exercise frequency. A post hoc comparison among the three intervention groups was made, if there was a significant effect of interaction. The assumptions of the ANCOVA model were assessed, and appropriate transformations were made, if needed. An intention-to-treat analysis was carried out for efficacious end point. The results were summarized using mean differences and their 95% confidence intervals. The same analysis approach used for NTX was also employed for BALP, sRANKL, OPG, Ca, and 8-OHdG.
For the secondary analysis, Spearman correlation between change in NTX and change in the secondary biomarker (BALP, sRANKL, OPG, Ca, and 8-OHdG) was computed. All the statistical analyses were carried out using SAS 9.3 (Cary, NC).
Results
Study participants
Figure 1 is a flow chart showing the recruitment and group assignment of study participants. A total of 89 subjects were recruited, and 87 subjects completed the study (attrition rate 2%). Based on the results of pill count, all groups have a high average compliance rate (> 90%) at 12 weeks. Neither serum ALT nor AST activity was affected throughout the study. No adverse event due to study treatment was reported by the participants. In addition, there was no difference in body composition, self-reported physical activity level, or food intake (micro- and macro-nutrients) among three groups at baseline and 12 weeks. Readers are advised to anticipate a follow-up report detailing the impacts of tocotrienols on safety, body composition, physical activity, and food intake report.
Table 1 describes the baseline characteristics of study participants. At the baseline, there were no significant differences in age, BMI, BMD, dietary calcium intake, serum 25-hydroxy vitamin D and thyroid-stimulating hormone, race, education, lifestyle profiles, general health, and outcome measures among three groups, except for serum HbA1c, exercise frequency, alcohol consumption, urine NTX, and urine 8-OHdG. In terms of outcome measures, at the baseline, the placebo group had the lowest urine NTX level and the low TT group had the highest urine 8-OHdG concentration among three groups.
Bone biomarkers
We have employed a mixed regression model to investigate the effect of treatment on bone marker while adjusting for participant age, BMI, calcium intake, and exercise frequency at baseline level. The results of mixed regression model are described in Fig. 2 (a (BALP), b (NTX), c (BALP/NTX)). There was marginal and significant difference in the change of serum BALP in the TT-treated groups over the 12-week study period (Fig. 2a). Relative to the placebo group, the low TT group had a weekly decrease of 0.207 U/L in BALP (p = 0.026), while the high TT group had a weekly decrease of 0.230 U/L in BALP (p = 0.013). Participant’ age (p = 0.772), BMI (p = 0.082), and calcium intake (p = 0.672) seemed to be not associated with serum BALP level. Each additional session of excise per week was also not associated with serum absolute BALP level (p = 0.097).
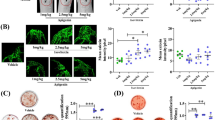
Effect of tocotrieniol supplementation on bone markers, serum BALP (a), urine NTX (b), BALP/NTX ratio (c), serum sRANKL (d), serum OPG (e), and sRANKL/OPG ratio (f), in postmenopausal women with osteopenia. Data are presented as means of groups. BALP bone-specific alkaline phosphatase, NTX N-terminal telopeptide.  Placebo;
Placebo;  low TT;
low TT;  high TT
high TT
There was highly significant difference in the change of urine NTX (bone resorption marker) over the study period (Fig. 2b). Compared to the placebo group, the low TT group had a weekly decrease of 0.048 nM BCE/mM Crt of urine NTX (p < 0.001), while the high TT group had a weekly decrease of 0.062 nM BCE/mM Crt in urine NTX (p < 0.001). Compared to participants with BMI ≥ 30 kg/m2, urine NTX level was 0.195 nM BCE/mM Crt greater for those with BMI < 30 kg/m2 (p = 0.001). Participant’s age (p = 0.290), calcium level (p = 0.450), and exercise frequency (p = 0.720) were not associated with urine absolute NTX level.
Compared with the placebo group, the low TT group and high TT group had a weekly increase of 5.06 and 5.50% in the BALP/NTX ratio, respectively (both at p < 0.001) (Fig. 2c). Comparing with participants with BMI ≥ 30 kg/m2, the BALP/NTX ratio for those with BMI < 30 kg/m2 was 16.5% lower (p = 0.043). Participant’s age, baseline calcium level, and baseline exercise frequency were not associated with the BALP/NTX ratio (p = 0.146, 0.961, and 0.705, respectively).
Table 2 assesses the total within-subject change in BALP, NTX, and the BALP/NTX ratio from baseline to 12 weeks. Kruskal test showed that there was no difference among the three treatments in relative % change in BALP. There was a significant decline in NTX after 12 weeks of low TT and high TT compared to placebo (p < 0.001). Moreover, there was a significant increase in BAP/NTX after 12 weeks of low TT and high TT compared to placebo (p < 0.001). There was no significant difference between the low TT and the high TT groups in relative % change of NTX (p = 0.632) and BALP/NTX (p = 0.865).
Figure 2 also illustrates the effects of TT supplementation on bone remodeling regulators including sRANKL (Fig. 2d), OPG (Fig. 2e), and sRANKL/OPG (Fig. 2f). Relative to the placebo group, (i) the low TT group and the high TT group had a weekly decrease of 2.0 and 2.2% in serum sRANKL throughout the study period, respectively (p = 0.006 for low TT group, p = 0.002 for high TT group), and (ii) there was no difference in serum sRANKL levels between the low TT and the high TT groups (p = 0.765) (Fig. 2d). Neither age (p = 0.714), BMI (p = 0.256), baseline calcium intake (p = 0.093), nor baseline exercise frequency (p = 0.659) of participant was associated with serum sRANKL level. Regarding serum OPG, (i) the high TT group had a weekly increase of 1.1 pmol/L comparing with the placebo group, (ii) there was no significant difference in serum OPG between the placebo and the low TT groups, and (iii) there was no significant difference in OPG between the low TT and high TT groups (Fig. 2e). Participant’s age (p = 0.424), BMI (p = 0.277), calcium intake (p = 0.779), and exercise frequency (p = 0.861) were not associated with serum OPG level. Furthermore, compared to the placebo group, the low TT group had a weekly decrease of 2.6% in the sRANKL/OPG ratio (p = 0.001), while the high TT group had a weekly decrease of 3.6% in the sRANKL/OPG ratio (p < 0.001) throughout the study period (Fig. 2f). There was no significant difference in the sRANKL/OPG ratio between the low TT group and the high TT group (p = 0.235). Age (p = 0.940), BMI (p = 0.690), calcium intake (p = 0.053), and exercise frequency (p = 0.237) were not associated with the sRANKL/OPG ratio.
In the study, we further determined the proportion of subjects in whom the decline in NTX at 12 weeks exceeded the least significant change (LSC) using a LSC formula described by Naylor et al. [21]. We found that (1) for the placebo group, none of the subjects was a LSC responder, and (2) for both the low TT and the high TT groups, there were four LSC responders, which corresponded to approximately 15% by LSC.
Urine calcium levels
No significant difference was found in the change of urine Ca/Crt over the 12-week period among the placebo, low, and high TT groups (p = 0.806) (Fig. 3). Participant’s age (p = 0.484), calcium intake (p = 0.187), and exercise frequency (p = 0.369) were not associated with urine Ca/Crt level; however, participants with BMI < 30 kg/m2 had significantly higher Ca/Crt than those with BMI ≥ 30 kg/m2 (p = 0.002).
Urinary 8-OHdG levels
Figure 4 illustrates the urine 8-OHdG levels at 0, 6, and 12 weeks. Using a similar approach of mixed regression model, we found that relative to the placebo group, the low TT group and the high TT groups had weekly decreases of 11.2% (p < 0.001) and 8.0% (p < 0.001), respectively, in urine 8-OHdG. The participant’s age (p = 0.907), BMI (p = 0.895), calcium level (p = 0.126), and exercise frequency (p = 0.335) were not associated with urine 8-OHdG. There was a statistically significant correlation between 8-OHdG and NTX (p = 0.342, p < 0.001).
Discussion
The present study is the first to evaluate dietary TT supplementation on bone biomarkers along with a possible mechanism in postmenopausal women with osteopenia. We observed a marginal decreasing trend of serum BALP, an indicator of bone formation, in the TT-supplemented groups. Lower values of BALP were observed at 6 weeks and sustained at 12 weeks, although the trend was marginal (0.05 < p < 0.10). The observation of suppressed bone formation due to TT treatment corroborates with Muhammad et al. [13] using palm oil-derived TT mixture (60 mg/kg body weight) for 8 weeks in Wistar OVX rats, a model of postmenopausal women. Intriguingly, the lower values of NTX, an indicator of bone resorption, were also observed at 6 and 12 weeks in both TT-supplemented groups (low TT and high TT), in a time-dependent and dose-dependent pattern. This finding was consistent with previous animal studies that TT supplementation, in the forms of pure γ-TT [11], palm TT [10], or a mixture of TT and tocopherols [22], suppressed bone resorption activity in OVX animals. The ability of TT to suppress bone resorption biomarker demonstrated in this study corroborates with TT’s effect on osteoclastic activity suggested in in vitro study [8]. Brook et al. [8] reported TT (α-, δ-, and γ-TT) treatments suppressed osteoclast formation in pre-osteoclasts. Besides individual bone biomarkers, the ratio of bone formation to resorption (BALP/NTX) has been also used as an indicator of the state of bone turnover to evaluate the effects of dietary supplement on bone metabolism [23, 24]. In this study, the TT-mediated increases in BALP/NTX ratio at 6-week (35.70% for low TT group and 48.28% for high TT group) and 12-week (112.82% for low TT group and 98.75% for high TT group) time points are mainly due to a slightly decreased bone formation (BALP) and significantly suppressed bone resorption (NTX), suggesting that TT supplementation favored bone remodeling of postmenopausal osteopenic women.
In addition to estrogen deficiency-induced bone loss [25], centralized fat deposition (obesity) [26] and loss of muscle mass/strength (sarcopenia) [27] also have significant negative impacts on musculoskeletal health in postmenopausal women. Fu et al. reported that central body fat mass has a negative relationship with total and regional BMD in postmenopausal women [28]. Although the data of fat mass of study participants was not available in this study, we evaluated the potential impact of BMI on bone turnover biomarkers. We found that BMI at ≥ 30 kg/m2 contributed to a greater urine NTX level without affecting serum BALP level, resulting in a lower BALP/NTX ratio. Our findings are consistent with the Fu’s cross-sectional findings that fat mass was negatively associated with BMD, a status of bone health in postmenopausal women [28].
The OPG/RANK/RANKL pathway plays a central role in bone modeling by coupling osteoblasts and osteoclasts [29,30,31,32,33,34]. RANK is expressed on precursors of osteoblasts, and it is a member of tumor necrosis factor (TNF) receptor family [29]. RANKL, a ligand for RANK, is expressed on the cell surface of both osteoblasts and stromal cells. Once RANK binds to its ligand (RANKL), it initiates the differentiation and maturation of osteoclasts through NF-κB pathways [30], resulting in bone resorption. OPG, a glycoprotein, is also expressed by osteoblasts, and it is a member of TNF receptor superfamily [31]. OPG is a decoy receptor for RANKL. Once OPG binds to RANKL, it blocks the interaction of RANKL and RANK [32], resulting in downregulation of osteoclast formation and bone resorption [33]. Thus, a greater RANKL/OPG ratio expressed by osteoblasts is commonly used as an indicator of an increased rate of osteoclastogenesis with a result of increased bone resorption. The loss of estrogen in postmenopausal women has major effects on bone remodeling, resulting in imbalance between bone resorption (osteoclasts) and bone formation (osteoblasts) activities [34]. An ample body of evidence suggests that the impacts of estrogen on bone are mediated through OPG/RANK/RANKL pathway [35, 36]. Nabipour et al. [37] reported that the circulating levels of OPG and the RANKL/OPG ratio were significantly correlated with age, and age, BMI, and RANKL appeared to be independently associated with femoral neck BMD of postmenopausal women.
Dietary bioactive compounds have been considered as alternatives in mitigating the progression of bone loss in OVX animals and postmenopausal women. One of molecular mechanisms underlying osteoprotective effect is to lower the RANKL/OPG ratio [32, 33]. In this study, the placebo group had the highest RANKL and RANKL/OPG ratio among three groups, suggesting a higher rate of bone resorption. Such observation in the placebo group was consistent with findings of higher urine NTX levels of placebo participants. When we expressed the respective percentage change of RANKL/OPG ratio at 6 weeks relative to the baseline, there were a 35.78% increase, a 6.81% decrease, and a 12.8% decrease in the placebo, low TT, and high TT, respectively (p < 0.001). Similar to the changes at 6 weeks, there were a 21.06% increase in the placebo group, a 12.47% decrease in the low TT group, and a 23.78% decrease in the high TT group (p = 0.031) at 12 weeks compared to baseline (0 week). The changes in %RANKL/OPG ratio further confirm the benefit of dietary TT supplementation on bone remodeling through a suppression of osteoclastic activity (as shown in decreased urine NTX and serum sRANKL levels), resulting in a reduced bone resorption. Our clinical findings on suppression of RANKL by TT supplementation were supported by Ha’s cellular study [7]. In co-culture of bone marrow cells and osteoblasts, Ha et al. [7] have demonstrated α-TT-suppressed RANKL induced the differentiation of osteoclasts from precursors, probably through inhibiting NF-κB and ERK activation, and reduced bone-resorbing activity of mature osteoclasts without affecting their survival.
Osteoporosis is attributed, in part, to excessive oxidative stress caused by reactive oxygen species (ROS) and low-grade chronic inflammation [1]. Excess ROS increases osteoclastogenesis and bone resorption and degradation [1, 38] but induces osteoblast and osteocyte apoptosis, resulting in suppression of bone formation [39]. Cervellati et al. recently [40] reported an independent and positive association between urine 8-OHdG (oxidative stress biomarker) and RANKL/OPG ratio in postmenopausal women with osteopenia but not in those with normal bone or osteoporosis, further implicating oxidative stress in the imbalance of bone homeostasis that precedes development of postmenopausal women. In this study, both low TT and high TT significantly suppressed urine 8-OHdG by 37.8 and 33.5%, respectively (p < 0.001), at 6 weeks. Such a suppression sustained at week 12 (a 48.5% decrease in the low TT group and a 30.9% decrease in the high TT group, p < 0.001). We also noted that the placebo group had a 121.3 and 105.5% increase at weeks 6 and 12 compared to week 0. Our urine 8-OHdG observation provides a possible mechanism on how TT supplementation is beneficial to bone remodeling in postmenopausal women due to TT’s antioxidant and anti-inflammatory properties. Tocotrienols have been shown to increase antioxidant capacity and decrease oxidative stress/inflammation in bone cells [6, 8, 41, 42] and in OVX animals [10]. The impacts of δ-TT on inflammatory biomarkers have been studied in hypercholesterolemic subjects [43, 44]. Authors reported a dose-dependent reduction in inflammation (i.e., resistin, IL-1δ, IL-12, TNF-α, IFN-δ, IL-6) induced by daily intake of 125–750 mg Delta Gold TT (the same study supplement used in our study) in hypercholesterolemic subject [43, 44]. Recently, Qureshi et al. further demonstrated that consumption of Delta Gold TT plus American Heart Association Step-1 diet significantly reduced serum inflammatory cytokines (TNF-α, IL-2, IL-4, IL-6, IL-8, IL-10) and lipids (total cholesterol, LDL-cholesterol, and triglycerides) in hypercholesterolemic subjects [44].
There are limitations in the present study: (1) since this is a short-term 12-week study, we did not assess the changes in BMD of study participants pre- and post-intervention, and (2) the study agent TT (90% δ-TT and 10% γ-TT) has a 70% purity, and we do not know if the remaining 30% (mainly plant essential oils) would also play a role in bone health. Future study is suggested to assess the effects of long-term TT supplementation on change of BMD using DXA, a gold standard clinical parameter. Other advanced imaging technology, such as peripheral quantitative computed tomography (pQCT), may be employed to evaluate the changes in bone microstructure to further evaluate the clinical impact of dietary TT on bone health. Furthermore, examination of potential interactions (additive, synergistic, or counter effect) of TT and other dietary bioactive compounds may be warranted in long-term clinical trials.
Conclusions
This study showed that supplementation of TTs (mainly delta-TT) suppressed bone resorption and RANKL and increased BALP/NTX ratio. These TT’s osteoprotective effects are, in part, mediated through a suppression of oxidative stress and the OPG/RANK/RANKL pathway in postmenopausal women with osteopenia.
References
Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y (2009) Oxidative stress in bone remodelling and disease. Trends Mol Med 15(10):468–477. https://doi.org/10.1016/j.molmed.2009.08.004
NIH (2001) Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 285(6):785–795
National Osteoporosis Foundation. American’s bone health: the state of osteoporosis and low bone mass in our nation. Washington, DC: National Osteoporosis Foundation, 2002;1–55
Rizzoli R, Reginster JY (2011) Adverse drug reactions to osteoporosis treatments. Expert Rev Clin Pharmacol 4(5):593–604. https://doi.org/10.1586/ecp.11.42
Shahidi F, de Camargo AC (2016) Tocopherols and tocotrienols in common and emerging dietary sources: occurrence, applications, and health benefits. Int J Mol Sci 17(10):E1745
Nizar AM, Nazrun AS, Norazlina M, Norliza M, Ima Nirwana S (2011) Low dose of tocotrienols protects osteoblasts against oxidative stress. Clin Ter 162(6):533–538
Ha H, Lee JH, Kim HN, Lee ZH (2011) Alpha-tocotrienol inhibits osteoclastic bone resorption by suppressing RANKL expression and signaling and bone resorbing activity. Biochem Biophys Res Commun 406(4):546–551. https://doi.org/10.1016/j.bbrc.2011.02.085
Brooks R, Kalia P, Ireland DC, Beeton C, Rushton N (2011) Direct inhibition of osteoclast formation and activity by the vitamin E isomer gamma-tocotrienol. Int J Vitam Nutr Res 81(6):358–367. https://doi.org/10.1024/0300-9831/a000087
Gürkan L, Ekeland A, Gautvik KM, Langeland N, Rønningen H, Solheim LF (1986) Bone changes after castration in rats. A model for osteoporosis. Acta Orthop Scand 57(1):67–70. https://doi.org/10.3109/17453678608993219
Soelaiman IN, Ming W, Abu Bakar R, Hashnan NA, Mohd Ali H, Mohamed N, Muhammad N, Shuid AN (2012) Palm tocotrienol supplementation enhanced bone formation in oestrogen-deficient rats. Int J Endocrinol 2012:532862
Deng L, Ding Y, Peng Y, Wu Y, Fan J, Li W, Yang R, Yang M, Fu Q (2014) γ-Tocotrienol protects against ovariectomy-induced bone loss via mevalonate pathway as HMG-CoA reductase inhibitor. Bone 67:200–207. https://doi.org/10.1016/j.bone.2014.07.006
Abdul-Majeed S, Mohamed N, Soelaiman IN (2012) Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid Based Complement Alternat Med 2012:960742
Aktifanus AT, Shuid AN, Rashid NHA, Ling TH, Loong CY, Saat NM, Muhammad N, Mohamed N, Soelaiman IN (2012) Comparison of the effects of tocotrienol and estrogen on the bone markers and dynamic changes in postmenopausal osteoporosis rat model. Asian J Anim Vet Adv 7:225–234
Shibata A, Nakagawa K, Sookwong P, Tsuduki T, Asai A, Miyazawa T (2010) α-Tocopherol attenuates the cytotoxic effect of δ-tocotrienol in human colorectal adenocarcinoma cells. Biochem Biophys Res Commun 397(2):214–219. https://doi.org/10.1016/j.bbrc.2010.05.087
Shen CL, Klein A, Chin KY, Mo H, Tsai P, Yang RS, Chyu MC, Ima-Nirwana S (2017) Tocotrienols for bone health: a translational approach. Ann N Y Acad Sci 1401:150–165.
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22(3):659–661. https://doi.org/10.1096/fj.07-9574LSF
Kruger MC, Ha PC, Todd JM, Kuhn-Sherlock B, Schollum LM, Ma J, Qin G, Lau E (2012) High-calcium, vitamin D fortified milk is effective in improving bone turnover markers and vitamin D status in healthy postmenopausal Chinese women. Eur J Clin Nutr 66(7):856–861. https://doi.org/10.1038/ejcn.2012.54
Li Z, Karp H, Zerlin A, Lee TY, Carpenter C, Heber D (2010) Absorption of silicon from artesian aquifer water and its impact on bone health in postmenopausal women: a 12 week pilot study. Nutr J 9(1):44. https://doi.org/10.1186/1475-2891-9-44
Shen CL, Mo H, Yang S, Wang S, Felton CK, Tomison MD, Soelaiman IN (2016) Safety and efficacy of tocotrienol supplementation for bone health in postmenopausal women: protocol for a dose-response double-blinded placebo-controlled randomised trial. BMJ Open 6(12):e012572. https://doi.org/10.1136/bmjopen-2016-012572
Iwamoto J, Takada T (2015) Effect of minodronate on the speed of sound of the calcaneus in postmenopausal women with an increased risk of fractures: a clinical practice-based observational study. J Chin Med Assoc 78(10):591–596. https://doi.org/10.1016/j.jcma.2015.06.010
Naylor KE, Jacques RM, Peel NF, Gossiel F, Eastell R (2016) Response of bone turnover markers to raloxifene treatment in postmenopausal women with osteopenia. Osteoporos Int 27(8):2585–2592. https://doi.org/10.1007/s00198-016-3573-z
Norazlina M, Ima-Nirwana S, Gapor MT, Khalid BA (2000) Palm vitamin E is comparable to alpha-tocopherol in maintaining bone mineral density in ovariectomised female rats. Exp Clin Endocrinol Diabetes 108(04):305–310. https://doi.org/10.1055/s-2000-7758
Zittermann A, Geppert J, Baier S, Zehn N, Gouni-Berthold I, Berthold HK, Reinsberg J, Stehle P (2004) Short-term effects of high soy supplementation on sex hormones, bone markers, and lipid parameters in young female adults. Eur J Nutr 43(2):100–108. https://doi.org/10.1007/s00394-004-0447-5
Shen CL, Chyu MC, Yeh JK, Zhang Y, Pence BC, Felton CK, Brismée JM, Arjmandi BH, Doctolero S, Wang JS (2012) Effect of green tea and Tai Chi on bone health in postmenopausal osteopenic women: a 6-month randomized placebo-controlled trial. Osteoporos Int 23(5):1541–1552. https://doi.org/10.1007/s00198-011-1731-x
Clarke BL, Khosla S (2010) Physiology of bone loss. Radiol Clin N Am 48(3):483–495. https://doi.org/10.1016/j.rcl.2010.02.014
Tchernof A, Poehlman ET, Després JP (2000) Body fat distribution, the menopause transition, and hormone replacement therapy. Diabetes Metab 26(1):12–20
Maltais ML, Desroches J, Dionne IJ (2009) Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact 9(4):186–197
Fu X, Ma X, Lu H, He W, Wang Z, Zhu S (2011) Associations of fat mass and fat distribution with bone mineral density in pre- and postmenopausal Chinese women. Osteoporos Int 22(1):113–119. https://doi.org/10.1007/s00198-010-1210-9
Nelson CA, Warren JT, Wang MWH, Teitelbaum SL, Fremont DH (2012) RANKL employs distinct binding modes to engage RANK and the osteoprotegerin decoy receptor. Structure 20(11):1971–1982. https://doi.org/10.1016/j.str.2012.08.030
Wada T, Nakashima T, Hiroshi N, Penninger JM (2006) RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 12(1):17–25. https://doi.org/10.1016/j.molmed.2005.11.007
Weichhaus M, Segaran P, Renaud A, Geerts D, Connelly L (2014) Osteoprotegerin expression in triple-negative breast cancer cells promotes metastasis. Cancer Med 3(5):1112–1125. https://doi.org/10.1002/cam4.277
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473(2):139–146. https://doi.org/10.1016/j.abb.2008.03.018
Boyce BF, Xing L (2007) Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 9 Suppl 1:S1. Review
Raisz LG (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115(12):3318–3325. https://doi.org/10.1172/JCI27071
Kearns AE, Khosla S, Kostenuik PJ (2008) Receptor activator of nuclear factor кB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocrine Rev 29(2):155–928. https://doi.org/10.1210/er.2007-0014
Vega D, Maalouf NM, Sakhaee K (2007) The role of receptor activator of nuclear factor-휅B (RANK)/RANK ligand/osteoprotegerin: clinical implications. J Clin Endocrinol Metab 92(12):4514–4521. https://doi.org/10.1210/jc.2007-0646
Nabipour I, Larijani B, Vahdat K, Assadi M, Jafari SM, Ahmadi E, Movahed A, Moradhaseli F, Sanjdideh Z, Obeidi N, Amiri Z (2009) Relationships among serum receptor of nuclear factor-kappaB ligand, osteoprotegerin, high-sensitivity C-reactive protein, and bone mineral density in postmenopausal women: osteoimmunity versus osteoinflammatory. Menopause 16(5):950–955. https://doi.org/10.1097/gme.0b013e3181a181b8
Garrett JR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 85(3):632–639. https://doi.org/10.1172/JCI114485
Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ (2004) Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun 314(1):197–207. https://doi.org/10.1016/j.bbrc.2003.12.073
Cervellati C, Romani A, Cremonini E, Bergamini CM, Fila E, Squerzanti M, Greco P, Massari L, Bonaccorsi G (2016) Higher urinary levels of 8-hydroxy-2′-deoxyguanosine are associated with a worse RANKL/OPG ratio in postmenopausal women with osteopenia. Oxidative Med Cell Longev 2016:6038798
Wang Y, Park NY, Jang Y, Ma A, Jiang Q (2015) Vitamin E γ-tocotrienol inhibits cytokine-stimulated NF-κB activation by induction of anti-inflammatory A20 via stress adaptive response due to modulation of sphingolipids. J Immunol 195(1):126–133. https://doi.org/10.4049/jimmunol.1403149
Abd Manan N, Mohamed N, Shuid AN (2012) Effects of low-dose versus high-dose γ-tocotrienol on the bone cells exposed to the hydrogen peroxide-induced oxidative stress and apoptosis. Evid Based Complement Alternat Med 2012:680834
Qureshi AA, Khan DA, Mahjabeen W, Trias AM, Silswal N, Qureshi N (2015) Impact of δ-Tocotrienol on inflammatory biomarkers and oxidative stress in hypercholesterolemic subjects. J Clin Exp Cardiolog 6(4):1000367
Qureshi AA, Khan DA, Mahjabeen W, Qureshi N (2015) Dose-dependent modulation of lipid parameters, cytokines and RNA by δ-tocotrienol in hypercholesterolemic subjects restricted to AHA Step-1 diet. Br J Med Medical Res 6(4):351–366. https://doi.org/10.9734/BJMMR/2015/13820
Acknowledgements
This study was supported by American River Nutrition, Inc., Hadley, MA.
Funding
This study is funded by American River Nutrition, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Shen, CL., Yang, S., Tomison, M.D. et al. Tocotrienol supplementation suppressed bone resorption and oxidative stress in postmenopausal osteopenic women: a 12-week randomized double-blinded placebo-controlled trial. Osteoporos Int 29, 881–891 (2018). https://doi.org/10.1007/s00198-017-4356-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4356-x






 Placebo;
Placebo;  low TT;
low TT;  high TT
high TT
 Placebo;
Placebo;  low TT;
low TT;  high TT
high TT