Abstract
Cognitive decline associated with type 2 diabetes mellitus (T2DM) is a risk factor to impair human health. Although light-intensity exercise prevents hippocampal memory dysfunction in pre-symptomatic T2DM animals by altering hippocampal lactate transport and neurotrophic factors, the effects of light-intensity exercise in an advanced stage of T2DM animals remain unclear. Here, ob/ob mice, an animal model of T2DM, were subjected to light-intensity exercise (5.0 m/min) for 30 min/day, five days/week for four weeks. The effects of light-intensity exercise on hippocampal complications, mRNA expressions of monocarboxylate transporter (MCT), and miRNA levels were assessed. The light-intensity exercise improved hippocampal memory retention in ob/ob mice. Downregulated hippocampal Mct2 mRNA levels in T2DM were improved with light-intensity exercise. Hippocampal mRNA levels of Mct1 and Mct4 were unchanged within groups. Based on miRNA sequencing, sedentary ob/ob mice exhibited that 71 miRNAs were upregulated, and 77 miRNAs were downregulated in the hippocampus. In addition, the exercise significantly increased 24 miRNAs and decreased 4 miRNAs in the T2DM hippocampus. The exercise reversed T2DM-induced alterations of hippocampal 9 miRNAs, including miR-200a-3p. Our findings imply that miR-200a-3p/Mct2 in the hippocampus would be a possible clinical target for treating T2DM-induced memory dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since physical exercise positively affects peripheral organs and the brain, exercise is widely employed as a therapeutic strategy for metabolic syndrome and mental disorders (van der Heijden et al. 2013). Exercise therapy for type 2 diabetes (T2DM) is a representative case of that and focuses on treating insulin resistance and glycemic control (Praet and van Loon 2009; Colberg et al. 2010). On the other hand, T2DM induces several complications in peripheral organs and the brain, particularly in the hippocampus, like cognitive decline (Cukierman et al. 2005; Whitmer 2007; McCrimmon et al. 2012); however, an exercise strategy for such hippocampal complications remains unestablished. Thus, developing a therapeutic strategy for the hippocampus in T2DM is needed to maintain human health.
T2DM-induced hippocampal complications with learning and memory function can be caused by several factors, such as angiogenesis, inflammation, oxidative stress, and insulin resistance (Whitmer 2007; Sickmann and Waagepetersen 2015; Stranahan 2015; De Sousa et al. 2020a; Sousa et al. 2020b). We have recently reported the downregulation of lactate transport through monocarboxylate transporter 2 (MCT2) into neurons as a potential etiologic brain mechanism (Shima et al. 2017, 2018; Jesmin et al. 2022). Lactate is served to neurons through blood circulation and astrocytes via glycolysis and/or glycogenolysis (Pierre and Pellerin 2005; Pellerin et al. 2007; Benarroch 2010; Pellerin and Magistretti 2012). Glycogen stored in astrocytes is an essential source of lactate. Glycogen-derived lactate is released into the extracellular fluid from astrocytes via MCT1 and MCT4, and is taken up by neurons via MCT2 (Pierre and Pellerin 2005). The lactate is used as an energy substrate for neuronal activity and as a neuro-modulator to enhance neuronal plasticity, such as neurogenesis (Tsacopoulos and Magistretti 1996; Benarroch 2010; Suzuki et al. 2011; Descalzi et al. 2019; Carrard et al. 2021). Previous studies have shown that learning and memory dysfunction are caused by inhibition of lactate supply and/or downregulated MCT2 expression and function in the hippocampus (Newman et al. 2011; Suzuki et al. 2011; Duran et al. 2013; Descalzi et al. 2019; Korol et al. 2019; Netzahualcoyotzi and Pellerin 2020; Zhou et al. 2021; Chen et al. 2021). We have already found that the T2DM animal exhibits lower MCT2 expressions in their hippocampi than those in counterpart control animals (Shima et al. 2017, 2018; Jesmin et al. 2022); thus, diminished utilization of lactate via MCT2 is likely an essential factor in hippocampal complications with cognitive decline in T2DM.
It is expected that the beneficial effects of exercise on memory function in T2DM (De Sousa et al. 2021). A previous study showed that an exercise regimen at a moderate-intensity (approximately 60% \(\mathrm{\dot{V}O_{2max}}\)) for four weeks improves hippocampal memory dysfunction and MCT2 expression in the advanced stage of T2DM rodents (Shima et al. 2017). Thus, restoring hippocampal MCT2 expression would be necessary for the exercise-induced improvement of memory dysfunction in the advanced stage of T2DM. Even light-intensity exercise (< 45% \(\mathrm{\dot{V}O_{2max}}\)) enhances neuronal activity in the hippocampus (Soya et al. 2007; Suwabe et al. 2018) and hippocampus-based memory function in healthy animals with modulating hippocampal neurogenesis (Okamoto et al. 2012, 2015; Inoue et al. 2014, 2015). Our recent study has reported that light-intensity and moderate-intensity exercise regimens can similarly improve hippocampal complications in pre-symptomatic diabetic rodents with a restoration of hippocampal MCT2 expression (Jesmin et al. 2022). Since lactate utilization via MCT2 contributes to hippocampal neurogenesis (Lev-Vachnish et al. 2019), light-intensity exercise could be a beneficial therapeutic approach for maintaining hippocampal function in healthy but also diabetic subjects. However, it is unclear whether light-intensity exercise improves hippocampal function in an advanced stage of T2DM.
Here, we tested the effects of 4 weeks of light-intensity exercise, a published exercise model (Yook et al. 2019), on hippocampal memory dysfunction focused on lactate transporter in the hippocampus of ob/ob mice, an animal model of T2DM. We subsequently determined the hippocampal miRNA profile changes using miR-Seq to gain insight into the modulation of hippocampal lactate transporter expression.
Materials and methods
Animals
Eight-week-old male C57BL/6 mice and ob/ob mice (a T2DM mouse model) obtained from SLC Inc. (Shizuoka, Japan) were housed in temperature-controlled rooms (21–23℃) under a 12-hour light/dark cycle (lights on 8:00–20:00). The mice were given a standard pellet diet (Rodent Diet CE-2, CLEA Japan Inc., Tokyo, Japan) and water ad libitum. The experiments were pre-approved (approval No. 21-001) and conducted following the Gunma University Animal Care and Experimentation Committee.
Exercise training
Ob/ob mice were divided into exercise groups and non-exercise (sedentary) groups after one week of acclimatization. The mice in the exercise group were subjected to running habituation on a forced exercise wheel bed at 3.0–5.0 m/min for 30 min/day, five days/week (a total of five sessions over six days), one week, and then subjected to run at 5.0 m/min on the same equipment for 30 min/day, five days/week, three weeks. This exercise program is recognized as light-intensity below the ventilatory threshold of ob/ob mice (Yook et al. 2019). All sessions were performed during a light period (8:00–10:00). C57BL/6 mice were set only sedentary group.
Memory performance test
All mice were subjected to the Morris water maze for five days and the fourth week’s exercise period. The Morris water maze test was conducted in a circular pool (100 cm in diameter and 30 cm in depth) with an invisible platform (10 cm in diameter) located in the center of one quadrant. The experimental room had some extra-maze cues. All four start-points were used during the learning sessions in different orders. Mice were given a maximum of 60 s to explore and get to the platform. If the mice failed to find the platform within 60 s, they were hand-guided to it. After reaching the platform, rats were allowed to remain there for 10 s. During the learning sessions, escape latency (s), swim length (cm), and speed (cm/s) were recorded by a video tracking system (O’hara & Co., Ltd., Japan). One day after the last learning session, the platform was removed from the pool, and mice were allowed a 60 s probe trial to look for it in the pool. The crossing times where the platform had been placed during learning sessions were measured using the same system.
Tissue preparation
Two days after the probe trial, mice were anesthetized with isoflurane (30% isoflurane in propylene glycol; Dainippon Sumitomo Pharma Co., Osaka, Japan), and the blood samples were obtained from mice by cardiac puncture. And then, the hippocampus was collected in RNAlater™ Stabilization Solution (Invitrogen™). The hippocampus samples were stored at -20℃ for subsequent biochemical analysis.
Blood glucose and HbA1C assays
Blood glucose and HbA1C levels were measured by a glucometer (FreeStyle Libre, Abbot, Japan) and an A1CNow+ kit (Finggal Link Co., Ltd., Japan), respectively.
Real-time PCR
Total RNA was extracted from the hippocampus tissue using Trizol (Qiagen Inc., Valencia, CA, USA) and RNeasy (Qiagen Inc., Valencia, CA, USA) according to the protocol provided. Then, we did DNase I treatment and quantification, and then 1000 ng of RNA was reverse transcribed to cDNA with GeneAce cDNA Synthesis Kit (Nippon Gene, Japan). After that, we measured the mRNA expression levels of target genes using five ng of cDNA, primers for each target gene, and PowerTrack™ SYBR™ Green Master Mix in StepOne Plus Real-Time PCR 96-well system (Thermo Fisher Scientific Inc., USA). The sequences of primers were as Table 1. The relative levels of each mRNA were calculated by the ΔΔCT method and normalized by β-actin mRNA levels.
miRNA isolation and next generation sequencing of miRNA
Hippocampal miRNAs were isolated using miRNeasy Micro Kit (Qiagen, Inc., Valencia, CA, USA). The libraries for miRNA sequencing were prepared using 100 ng of RNA, QIAseq miRNA Library Kit, and QIAseq miRNA NGS 48 Index IL (Qiagen Inc., Valencia, CA, USA), and the library quality was subsequently assessed by measuring library size in base pair using Agilent High Sensitivity DNA Kit (Agilent Technologies, Santa Clara, CA) and Agilent Bioanalyzer (Agilent Technologies). Each miRNA molecule was tagged with a UMI while building the library. The libraries were subjected to single-end sequencing of 86-bp reads on NextSeq 500 with NextSeq 500/550 High Output Kit v2.5 (Illumina Inc., San Diego, CA, USA); the average and range of total read numbers were 11,404,458 and 10,509,339−12,190,862, respectively. The sequenced data were then subjected to calibration, adapter trimming, identification insert sequencing and UMI sequencing, alignment to the mouse-specific miRbase mature database and GRCm38 sequence, and read counting using “Primary Quantification” on the GeneGlobe (https://geneglobe.qiagen.com/jp/). miRDB (http://www.mirdb.org) and TargetScan (http://www.targetscan.org/mmu_80/) were used to explore the target mRNAs.
Statistical analysis
Data in hippocampal memory performance and mRNA are expressed as mean ± standard error (SEM) and were analyzed using Prism version 9 (MDF, Tokyo, Japan). Group comparisons were performed using two-way ANOVA (only for escape latency, swim length, and speed during the learning sessions) or one-way ANOVA with Tukey’s post hoc tests. Statistical significance was set at p < 0.05. Log2 fold changes in each miRNA and statistical significance were analyzed by the TCC-iDEGES-edgeR pipeline on R (https://www.R-project.org/, version 4.0.3) package TCC (version 1.30.0) (Sun et al. 2013) and edgeR (version 3.32.1).
Results
Physiological and biochemical variables
Both sedentary and exercise groups of ob/ob mice exhibited increased body weight and the ratio of fat to body weight compared to C57BL/6 mice (Table 2; all p < 0.0001). The fat to body weight ratio in exercised ob/ob mice was significantly higher than that in sedentary ob/ob mice (Table 2; p = 0.0088). Although HbA1C levels were significantly higher in both groups of ob/ob mice compared to C57BL/6 mice (Table 2; ob/ob-sedentary: p < 0.0001, ob/ob-exercised: p = 0.0006), only sedentary mice showed higher levels of blood glucose than C57BL/6 mice (Table 2; p = 0.0306), but exercised ob/ob mice did not (Table 2; p = 0.8235).
Hippocampal learning and memory function
The ob/ob mice exhibited longer escape latency than C57BL/6 mice (Fig. 1A; effects of group: F(2, 228) = 26.52, p < 0.0001; effects of time: F(3, 228) = 2.62, p = 0.0516; interaction: F(6, 228) = 2.09, p = 0.0549); there is no effect of exercise on the escape latency in ob/ob mice (p > 0.05). The longer swim distance (Fig. 1B; effects of group: F(2, 228) = 12.05, p < 0.0001; effects of time: F(3, 228) = 10.40, p < 0.0001; interaction: F(6, 228) = 0.90, p = 0.4983) and the faster speed of swimming (Fig. 1C; effects of group: F(2, 228) = 26.52, p < 0.0001; effects of time: F(3, 228) = 2.62, p = 0.0516; interaction: F(6, 228) = 2.09, p = 0.0549) were shown in C57BL/6 mice compared to both sedentary and exercised ob/ob mice. The times of crossing the target platform during the probe test in C57BL/6 mice were significantly greater than that in sedentary ob/ob mice (Fig. 1D; F(2, 12) = 3.91, p = 0.0493; sedentary C57BL/6 mice vs. sedentary ob/ob mice: p = 0.0416), but not than that in exercised ob/ob mice (sedentary C57BL/6 mice vs. exercised ob/ob mice: p = 0.2431).
Effect of light-intensity exercise on memory function. Escape latency (A), swim length (B), and speed (C) during the learning session in mice (mean ± SEM). White circles: sedentary C57BL/6 mice, white triangles: sedentary ob/ob mice, and black triangles: exercised ob/ob mice. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. sedentary C57BL/6 mice. D Effect of exercise on the probe trial, showing the crossing times where the platform had been placed. White bars: sedentary C57BL/6 mice, dark gray bars: sedentary ob/ob mice, and light gray bars: exercised ob/ob mice. *p < 0.05. Data are expressed as mean ± SEM, n = 5 mice for each group. E Representative swimming paths during the probe trial. Red circles mean the target platform area
Quantification of mRNA in the hippocampus
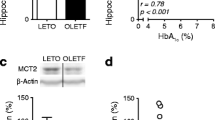
Mct2 mRNA levels had significant downregulation in the hippocampus of ob/ob mice compared to control mice, and light-intensity exercise improved hippocampal mRNA levels of Mct2 in ob/ob mice (Fig. 2A; F(2, 12) = 6.29, p = 0.0135; sedentary C57BL/6 mice vs. sedentary ob/ob mice: p = 0.0334, sedentary ob/ob mice vs. exercised ob/ob mice: p = 0.0186). Hippocampal mRNA levels of Mct1 and Mct4 were unchanged with group (Fig. 2B-C; Mct 1, F(2, 12) = 1.84, p = 0.2017; Mct 4, F(2, 12) = 1.43, p = 0.2777).
Effect of light-intensity exercise on mRNA levels of Mct2 (A), Mct1 (B), and Mct4 (C) in the hippocampus. The group of sedentary C57BL/6 mice was normalized as 100%. White bars: sedentary C57BL/6 mice, dark gray bars: sedentary ob/ob mice, and light gray bars: exercised ob/ob mice. *p < 0.05. Data are expressed as mean ± SEM, n = 5 mice for each group
Alteration of hippocampal miRNA expressions with T2DM and exercise
The total reads of hippocampal miRNA in sedentary and exercised ob/ob mice were significantly lower than that in sedentary C57BL/6 mice (Fig. 3A; all p < 0.05). Sedentary ob/ob mice exhibited upregulated 71 miRNAs and downregulated 77 miRNAs in the hippocampus compared to sedentary C57BL/6 mice (Fig. 3B). The exercise significantly upregulated 24 miRNAs and downregulated 4 miRNAs in ob/ob mice (Fig. 3C). In addition, the exercise reversed T2DM-induced alterations of 9 miRNAs, such as miR-871-5p, miR-200a-3p, miR-200b-3p, miR-322-3p, miR-344d-1-5p, miR-351-5p, miR-429-3p, miR-542-3p and miR-3474 in the hippocampus (Fig. 3D). A miRNA, miR-200a-3p, is predicted as a modulator for MCT2 (Slc16a7) on miRDB (http://www.mirdb.org) and TargetScan (http://www.targetscan.org/mmu_80/).
Total reads of miRNA by miR-seq in the hippocampus for each group (A). White bars: sedentary C57BL/6 mice, dark gray bars: sedentary ob/ob mice, and light gray bars: exercised ob/ob mice. *p < 0.05. Data are expressed as mean ± SEM, n = 3 mice for each group. Significantly modulated miRNA in the hippocampus with T2DM (B) or light-intensity exercise in T2DM (C). D Venn diagrams show the overlap of the modulated genes between T2DM and exercise in T2DM.
Discussion
The current study investigated the effects of light-intensity exercise for four weeks on cognitive dysfunction in T2DM mice. Here, the investigation has shown that light-intensity exercise improves memory dysfunction by restoring hippocampal Mct2 mRNA levels. Furthermore, light-intensity exercise significantly reversed levels of 9 miRNAs, including miR-200a-3p, a modulator for MCT2, in the hippocampus of T2DM mice.
Four weeks of the present light-intensity exercise improved hippocampal cognitive decline in T2DM mice (Fig. 1D). Light-intensity exercise has beneficial effects on the hippocampus in pre-symptomatic diabetic animals (Jesmin et al. 2022), and our findings indicate the possible efficacy of light-intensity exercise for hippocampal function in advanced T2DM. In the current study, we investigated only the effects of light-intensity exercise; we could not compare it with the exercise at different intensities. Recently, it has been reported that the beneficial effects of high-intensity interval training (HIT) on T2DM subjects, such as glycemic control and aerobic capacity (Little et al. 2011; Hwang et al. 2019). HIT can also improve hippocampal dysfunction in T2DM (Li et al. 2021); thus, further investigations are needed to consider the benefits of low-intensity exercise on T2DM compared with HIT.
Hippocampal Mct2 mRNA levels were restored with an exercise-induced improvement of hippocampal memory function in T2DM mice (Fig. 2A). The hippocampal mRNA levels of Mct1 and Mct4 were unchanged with the present light-intensity exercise (Fig. 2B and C). MCT2 plays a role in the uptake of lactate into neurons (Bliss et al. 2004; Pierre and Pellerin 2005; Aveseh et al. 2014). Previous studies reported that downregulated hippocampal MCT2 expression induces hippocampus-based memory dysfunction and neuroplasticity dysregulation (Suzuki et al. 2011; Descalzi et al. 2019; Netzahualcoyotzi and Pellerin 2020; Chen et al. 2021). Therefore, based on our findings, there is a possibility that restoring hippocampal MCT2-mediated lactate uptake might partially cause a light-intensity exercise-induced improvement of memory dysfunction in T2DM mice. However, we know little about whether the lactate transported via MCT2 in the exercised hippocampus is used as an energy substrate for a neuronal activity or a neuro-modulator to enhance neuronal plasticity (Tsacopoulos and Magistretti 1996; Benarroch 2010; Suzuki et al. 2011). Further studies are needed in this regard.
Since the data of miR-Seq in the current study, the expression of miR-200a-3p, a regulator for MCT2, was reversed with light-intensity exercise in T2DM mice (Fig. 3B-D, and Supplementary Tables 1 and 2). Previous studies reported that upregulation of Mct2 (Slc16a7) mRNA levels was observed with increased miR-200a-3p expression (Yao et al. 2019). In addition, miR-200a-3p is predicted as a modulator for MCT2 (Slc16a7) on miRDB (http://www.mirdb.org) and TargetScan (http://www.targetscan.org/mmu_80/); it led us to speculate that the alteration of miR-200a-3p levels may be related to Mct2 modulation and memory performance in T2DM mice. Further pharmacological studies with miRNA interference are needed in this regard. We also found the hippocampal expressions of some miRNAs in exercised T2DM mice (Fig. 3D). For example, it is known that miR-200b-3p and miR-429-3p contributes to suppressing apoptosis (Li et al. 2020; Zheng et al. 2021), and miR-542-3p contributes to suppressing inflammation (Cai et al. 2021). Based on these previous reports, there is a possibility that exercise-induced restorations of these miRNAs also relate to the improvement of T2DM-induced memory dysfunction; further studies should be addressed in this regard. On the other hand, upregulation of miR-351-5p induces hippocampal neuronal death and oxidative stress (Zheng et al. 2019; Woo et al. 2021); at least, the alteration of miR-351-5p expression might not be related to memory function in T2DM mice.
Although HbA1C levels were unchanged with four weeks of light-intensity exercise in T2DM mice, only sedentary T2DM mice showed significantly higher blood glucose levels than C57BL/6 mice. Since daily walking improves blood glucose levels in diabetic patients (Duvivier et al. 2017; Hayashi et al. 2018; Jesmin et al. 2021), even light-intensity exercise in the current study could contribute to treating blood glucose levels in T2DM mice (Table 2). Although there is a possibility that light-intensity exercise treats lipid metabolism (Grace et al. 2017; Jesmin et al. 2020), fat mass in T2DM mice could not be improved with 4 weeks of light-intensity exercise in the current study (Table 2). These findings postulate that glycometabolism in T2DM has a higher sensitivity to light-intensity exercise than lipid metabolism.
The current study has some limitations. First, we still know very little about why and how lactate uptake through hippocampal neuronal MCT2 interacts with memory dysfunction with T2DM; thus, further studies should address this. Second, we could not identify hippocampal protein levels of lactate transporters. Third, we limited our focus on hippocampal lactate transporter in the present study. We did not measure the hippocampal molecules involved in T2DM-induced memory dysfunction, such as angiogenesis, inflammation, oxidative stress, and insulin resistance (Whitmer 2007; Sickmann and Waagepetersen 2015; Stranahan 2015; De Sousa et al. 2020a; Sousa et al. 2020b). Future studies are warranted in this regard. Finally, it is impossible to ultimately reveal the effects of exercise on T2DM-induced memory dysfunction based on the current results; further mechanism-based studies are needed.
In conclusion, our current findings show for the first time that four weeks of light-intensity exercise treat T2DM-induced memory dysfunction with a restoration of hippocampal Mct2 gene levels. Since light-intensity exercise reversed miR-200a-3p in the hippocampus of T2DM mice, modulation of miR-200a-3p/Mct2 in the hippocampus with exercise would partly contribute to treating memory dysfunction with T2DM. Our findings could facilitate the development of a new clinical target for treating T2DM-induced memory dysfunction.
Data availability
The datasets in the current study are available from the corresponding author on reasonable request.
References
Aveseh M, Nikooie R, Sheibani V, Esmaeili-Mahani S (2014) Endurance training increases brain lactate uptake during hypoglycemia by up regulation of brain lactate transporters. Mol Cell Endocrinol 394:29–36. https://doi.org/10.1016/j.mce.2014.06.019
Benarroch EE (2010) Glycogen metabolism: Metabolic coupling between astrocytes and neurons. Neurology 74:919–923. https://doi.org/10.1212/WNL.0b013e3181d3e44b
Bliss TM, Ip M, Cheng E et al (2004) Dual-gene, dual-cell type therapy against an excitotoxic insult by bolstering neuroenergetics. J Neurosci 24:6202–6208. https://doi.org/10.1523/JNEUROSCI.0805-04.2004
Cai G, Cai G, Zhou H et al (2021) Mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction. Stem Cell Res Ther 12:1–12. https://doi.org/10.1186/s13287-020-02030-w
Carrard A, Cassé F, Carron C et al (2021) Role of adult hippocampal neurogenesis in the antidepressant actions of lactate. Mol Psychiatry 26:6723–6735. https://doi.org/10.1038/s41380-021-01122-0
Chen W, Sun X, Zhan L et al (2021) Conditional knockout of Pdha1 in mouse hippocampus impairs cognitive function: The possible involvement of lactate. Front Neurosci 15:1–11. https://doi.org/10.3389/fnins.2021.767560
Colberg SR, Sigal RJ, Fernhall B et al (2010) Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 33:e147–e167. https://doi.org/10.2337/dc10-9990
Cukierman T, Gerstein HC, Williamson JD (2005) Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia 48:2460–2469. https://doi.org/10.1007/s00125-005-0023-4
De Sousa RAL, Harmer AR, Freitas DA et al (2020) An update on potential links between type 2 diabetes mellitus and Alzheimer’s disease. Mol Biol Rep 47:6347–6356. https://doi.org/10.1007/s11033-020-05693-z
De Sousa RAL, Improta-Caria AC, de Jesus-Silva FM et al (2020) High-intensity resistance training induces changes in cognitive function, but not in locomotor activity or anxious behavior in rats induced to type 2 diabetes. Physiol Behav 223:112998. https://doi.org/10.1016/j.physbeh.2020.112998
De Sousa RAL, Improta-Caria AC, Cassilhas RC (2021) Effects of physical exercise on memory in type 2 diabetes: a brief review. Metab Brain Dis 36:1559–1563. https://doi.org/10.1007/s11011-021-00752-1
Descalzi G, Gao V, Steinman MQ et al (2019) Lactate from astrocytes fuels learning-induced mRNA translation in excitatory and inhibitory neurons. Commun Biol 2:247. https://doi.org/10.1038/s42003-019-0495-2
Duran J, Saez I, Gruart A et al (2013) Impairment in long-term memory formation and learning-dependent synaptic plasticity in mice lacking glycogen synthase in the brain. J Cereb Blood Flow Metab 33:550–556. https://doi.org/10.1038/jcbfm.2012.200
Duvivier BMFM, Schaper NC, Hesselink MKC et al (2017) Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia 60:490–498. https://doi.org/10.1007/s00125-016-4161-7
Grace MS, Dempsey PC, Sethi P et al (2017) Breaking up prolonged sitting alters the postprandial plasma lipidomic profile of adults with type 2 diabetes. J Clin Endocrinol Metab 102:1991–1999. https://doi.org/10.1210/jc.2016-3926
Hayashi A, Oguchi H, Kozawa Y et al (2018) Daily walking is effective for the management of pregnant women with gestational diabetes mellitus. J Obstet Gynaecol Res 44:1731–1738. https://doi.org/10.1111/jog.13698
Hwang C-L, Lim J, Yoo J et al (2019) Effect of all-extremity high-intensity interval training vs. moderate-intensity continuous training on aerobic fitness in middle-aged and older adults with type 2 diabetes: A randomized controlled trial. Exp Gerontol 116:46–53. https://doi.org/10.1016/j.exger.2018.12.013
Inoue K, Hanaoka Y, Nishijima T et al (2014) Long-term mild exercise training enhances hippocampus-dependent memory in rats. Int J Sports Med 36:280–285. https://doi.org/10.1055/s-0034-1390465
Inoue K, Okamoto M, Shibato J et al (2015) Long-term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS ONE 10:e0128720. https://doi.org/10.1371/journal.pone.0128720
Jesmin S, Sohael F, Rahman MA et al (2020) Short-term lifestyle intervention program through daily walking improves circulatory low HDL level in rural Bangladeshi women. J Phys Fit Sport Med 9:181–190. https://doi.org/10.7600/jpfsm.9.181
Jesmin S, Shima T, Yamaguchi N et al (2021) Daily walking habit reduces fasting blood sugar level in newly diagnosed diabetic participants in rural Bangladesh. J Phys Fit Sport Med 10:85–97. https://doi.org/10.7600/jpfsm.10.85
Jesmin S, Shima T, Soya M et al (2022) Long-term light and moderate exercise intervention similarly prevent both hippocampal and glycemic dysfunction in presymptomatic type 2 diabetic rats. Am J Physiol Metab 322:E219–E230. https://doi.org/10.1152/ajpendo.00326.2021
Korol DL, Gardner RS, Tunur T, Gold PE (2019) Involvement of lactate transport in two object recognition tasks that require either the hippocampus or striatum. Behav Neurosci 133:176–187. https://doi.org/10.1037/bne0000304
Lev-Vachnish Y, Cadury S, Rotter-Maskowitz A et al (2019) L-lactate promotes adult hippocampal neurogenesis. Front Neurosci 13:403. https://doi.org/10.3389/fnins.2019.00403
Li C, Niu J, Zhou B et al (2020) Dexmedetomidine attenuates cisplatin-induced cognitive impairment by modulating miR-429-3p expression in rats. 3 Biotech 10:244. https://doi.org/10.1007/s13205-020-02217-1
Li X, He Q, Zhao N et al (2021) High intensity interval training ameliorates cognitive impairment in T2DM mice possibly by improving PI3K/Akt/mTOR Signaling-regulated autophagy in the hippocampus. Brain Res 1773:147703. https://doi.org/10.1016/j.brainres.2021.147703
Little JP, Gillen JB, Percival ME et al (2011) Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol 111:1554–1560. https://doi.org/10.1152/japplphysiol.00921.2011
McCrimmon RJ, Ryan CM, Frier BM (2012) Diabetes and cognitive dysfunction. Lancet 379:2291–2299. https://doi.org/10.1016/S0140-6736(12)60360-2
Netzahualcoyotzi C, Pellerin L (2020) Neuronal and astroglial monocarboxylate transporters play key but distinct roles in hippocampus-dependent learning and memory formation. Prog Neurobiol 194:101888. https://doi.org/10.1016/j.pneurobio.2020.101888
Newman LA, Korol DL, Gold PE (2011) Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS ONE 6:e28427. https://doi.org/10.1371/journal.pone.0028427
Okamoto M, Hojo Y, Inoue K et al (2012) Mild exercise increases dihydrotestosterone in hippocampus providing evidence for androgenic mediation of neurogenesis. Proc Natl Acad Sci 109:13100–13105. https://doi.org/10.1073/pnas.1210023109
Okamoto M, Yamamura Y, Liu Y et al (2015) Hormetic effects by exercise on hippocampal neurogenesis with glucocorticoid signaling. Brain Plast (Amsterdam Netherlands) 1:149–158. https://doi.org/10.3233/BPL-150012
Pellerin L, Bouzier-Sore A-K, Aubert A et al (2007) Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55:1251–1262. https://doi.org/10.1002/glia.20528
Pellerin L, Magistretti PJ (2012) Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32:1152–1166
Pierre K, Pellerin L (2005) Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem 94:1–14. https://doi.org/10.1111/j.1471-4159.2005.03168.x
Praet SFE, van Loon LJC (2009) Exercise therapy in type 2 diabetes. Acta Diabetol 46:263–278. https://doi.org/10.1007/s00592-009-0129-0
Shima T, Matsui T, Jesmin S et al (2017) Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia 60:597–606. https://doi.org/10.1007/s00125-016-4164-4
Shima T, Jesmin S, Matsui T et al (2018) Differential effects of type 2 diabetes on brain glycometabolism in rats: focus on glycogen and monocarboxylate transporter 2. J Physiol Sci 68:69–75. https://doi.org/10.1007/s12576-016-0508-6
Sickmann HM, Waagepetersen HS (2015) Effects of diabetes on brain metabolism - is brain glycogen a significant player? Metab Brain Dis 30:335–343. https://doi.org/10.1007/s11011-014-9546-z
Soya H, Nakamura T, Deocaris CC et al (2007) BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun 358:961–967. https://doi.org/10.1016/j.bbrc.2007.04.173
Stranahan AM (2015) Models and mechanisms for hippocampal dysfunction in obesity and diabetes. Neuroscience 309:125–139. https://doi.org/10.1016/j.neuroscience.2015.04.045
Sun J, Nishiyama T, Shimizu K, Kadota K (2013) TCC: an R package for comparing tag count data with robust normalization strategies. BMC Bioinformatics 14:219. https://doi.org/10.1186/1471-2105-14-219
Suwabe K, Byun K, Hyodo K et al (2018) Rapid stimulation of human dentate gyrus function with acute mild exercise. Proc Natl Acad Sci 115:10487–10492. https://doi.org/10.1073/pnas.1805668115
Suzuki A, Stern SA, Bozdagi O et al (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144:810–823. https://doi.org/10.1016/j.cell.2011.02.018
Tsacopoulos M, Magistretti P (1996) Metabolic coupling between glia and neurons. J Neurosci 16:877–885. https://doi.org/10.1523/JNEUROSCI.16-03-00877.1996
van der Heijden MMP, van Dooren FEP, Pop VJM, Pouwer F (2013) Effects of exercise training on quality of life, symptoms of depression, symptoms of anxiety and emotional well-being in type 2 diabetes mellitus: a systematic review. Diabetologia 56:1210–1225. https://doi.org/10.1007/s00125-013-2871-7
Whitmer R (2007) Type 2 diabetes and risk of cognitive impairment and dementia. Curr Neurol Neurosci Rep 7:373–380. https://doi.org/10.1007/s11910-007-0058-7
Woo H-N, Park S, Kim HL et al (2021) miR-351-5p/Miro2 axis contributes to hippocampal neural progenitor cell death via unbalanced mitochondrial fission. Mol Ther - Nucleic Acids 23:643–656. https://doi.org/10.1016/j.omtn.2020.12.014
Yao J, Cheng Y, Zhang D et al (2019) Identification of key genes, MicroRNAs and potentially regulated pathways in alcoholic hepatitis by integrative analysis. Gene 720:144035. https://doi.org/10.1016/j.gene.2019.144035
Yook JS, Rakwal R, Shibato J et al (2019) Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc Natl Acad Sci USA 201815197. https://doi.org/10.1073/pnas.1815197116
Zheng L, Han X, Hu Y et al (2019) Dioscin ameliorates intestinal ischemia/reperfusion injury via adjusting miR-351-5p/MAPK13-mediated inflammation and apoptosis. Pharmacol Res 139:431–439. https://doi.org/10.1016/j.phrs.2018.11.040
Zheng C, Lu T, Fan Z (2021) miR-200b-3p alleviates TNF-α-induced apoptosis and inflammation of intestinal epithelial cells and ulcerative colitis progression in rats via negatively regulating KHDRBS1. Cytotechnology 73:727–743. https://doi.org/10.1007/s10616-021-00490-3
Zhou Z, Okamoto K, Onodera J et al (2021) Astrocytic cAMP modulates memory via synaptic plasticity. Proc Natl Acad Sci 118:1–10. https://doi.org/10.1073/pnas.2016584118
Acknowledgements
We thank the Laboratory for Analytical Instruments, Education and Research Support Center, Gunma University Graduate School of Medicine. We also thank Dr. Yuichi Uosaki, Mr. Yohei Morishita, Ms. Yoko Yokoyama, Ms. Hiroko Matsuda, and Ms. Saori Umezawa for their helpful technical assistance.
Funding
This work was supported by the Japan Society for the Promotion of Science (Grant-in-Aid for Early-Career Scientists, No. 20K19565; 22K17711); and the Fostering Health Professionals for Changing Needs of Cancer; and the Promotion Plan for the Platform of Human Resource Development for Cancer, New Paradigms – Establishing Center for Fostering Medical Researchers of the Future Programs [Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan]; and Gunma University Initiative for Advanced Research (GIAR). This work was the result of using research equipment shared in the MEXT Project for promoting public utilization of advanced research infrastructure (Program for supporting the introduction of the new sharing system) Grant Number JPMXS0420600120.
Author information
Authors and Affiliations
Contributions
Takeru Shima: Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Writing - original draft. Reika Kawabata-Iwakawa: Formal analysis, Funding acquisition, Investigation, Writing - review & editing. Hayate Onishi: Investigation, Writing - review & editing. Subrina Jesmin: Investigation, Supervision, Writing - review & editing. Tomonori Yoshikawa: Investigation, Writing - review & editing.
Corresponding author
Ethics declarations
Ethics approval
The experiments were pre-approved (approval No. 21-001) and conducted following the Gunma University Animal Care and Experimentation Committee.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(PDF 296 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shima, T., Kawabata-Iwakawa, R., Onishi, H. et al. Light-intensity exercise improves memory dysfunction with the restoration of hippocampal MCT2 and miRNAs in type 2 diabetic mice. Metab Brain Dis 38, 245–254 (2023). https://doi.org/10.1007/s11011-022-01117-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01117-y