Abstract
The number of anthracene (AN) and anthracene-tetracene (AN-TN) doped 4-nitrophenol (4-NP) luminophors (AN/4-NP and AN-TN/4-NP) were prepared at different proportion by conventional technique called solid state reaction. Excitation energy transfer was observed by fluorescence spectroscopy and cyclic voltammetry. 4-NP acts as an outstanding light emitting matrix. The effect of the donor emission was perceived by changing the dopant concentration; this showed that AN/4-NP and TN-AN/4-NP exhibit fluorescence emission ranging from blue to green i.e. in the range from 400 to 525 nm and 535–550 nm, respectively. Structural properties and thermal stability was studied by XRD, SEM and TGA-DSC techniques. The study reveals that the prepared materials show excellent properties which can meet the demands of optoelectronic devices as well as for light emitting devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The huge usage of doped organoluminophors motivated their study in organic molecular solids. Nowadays white organic light emitting materials (OLEDs) formed by doped materials have drawn more attention due to their various applications. In recent years many organoluminophors being synthesized and investigated for their properties to meet the demands of phosphors for optoelectronic devices.
The transfer of excitation energy in doped organic aggregates has been widely examined for over half century because of its central role as organic semiconductor materials. One more reason to study the energy transfer in doped organic matrix is sensitized acceptor emissions. This methodology has been applied in numerous studies of doped molecular crystals and thin films [1,2,3,4]. The intramolecular charge transfer (ICT) in excited states or excimers or exciplex production [5] are responsible for this type of energy transfer in doped organic matrix.
In last few years, a variety of doped organic semiconducting materials are being synthesized using different donor-acceptor materials such as tetracene doped p-terphenyl, biphenyl doped pyrene [6], pyrene and perylene doped fluorine [7], tetracene and anthracene doped biphenyl [8], anthracene doped 2-naphthol [9], tetracene and anthracene doped 2-naphthol [10], tetracene and anthracene doped p-terphenyl [11], etc.
Considering the scope of this research, the authors synthesized blue and green light emitting materials by using organic materials viz. AN, TN and 4-NP and the characteristic properties were studied.
Experimental
4-nitrophenol of AR grade, anthracene and tetracene of scintillation grade were procured from Merck-Schuchardt. The purchased host and guest materials were further recrystallized before use. The purity of these materials were further confirmed by fluorescence spectra.
Preparation of 4-Nitrophenol Doped Luminophors
The solid state reaction technique had been employed to prepare polycrystalline 4-NP luminophors with varying concentrations of AN and TN [12]. Silica crucibles were used for the preparation of doped materials. The solid solution was heated at the temperature corresponding to the M.P. of host 4-NP i.e. at 114 °C. The melt obtained was slowly cooled to get finely grained polycrystalline luminophors of 4-NP. This powder was finally crushed to fine powder and subjected to the characterization.
Characterization
The fluorescence spectra of pure components as well as guest doped 4-NP were recorded by JOBIN YVON Fluorolog-3-11 spectrofluorimeter, at SAIF, Indian Institute of Technology, Madras. The XRD spectra of doped and un-doped crystals were recorded with the Philips diffractometer (model PW-3710, Netherlands) with CrKα radiation (2.28). (TGDTA-DSC) TA Inc. SDT-2790 with heating rate of 10 °C per minute under nitrogen atmosphere was used to perform thermogravimetric analysis at Solapur University, Solapur, while surface morphology of samples was studied by SEM at SAIF, Indian Institute of Technology, Madras.
Results and Discussion
Selection of Guest Materials
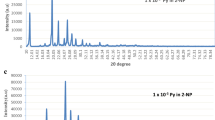
The initial studies on anthracene, tetracene and 4-nitrophenol had been performed to choose first and second guest (impurity) in 4-nitrophenol host to prepare bicomponent and tricomponent mixed crystals. Figure 1 shows overlapping region between absorption spectra of AN and emission spectra of 4-NP while Fig. 2 indicates overlapping region between absorption spectra of TN and emission spectra of 4-NP. Shaded area shown in the figure was used to determine Förster energy transfer distance (R0) values. Calculated values of R0 are tabulated in Table 1. The values of R0 are in the range of 37–49 Å indicating the possibility of EET between host and guest [13]. This study further supports to use AN as first and TN as second guest in 4-NP host matrix.
Photophysical Studies of 4-Nitrophenol Luminophors
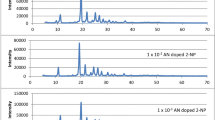
Figures 3 and 4 shows the fluorescence spectra of AN doped 4-NP luminophors and AN-TN doped 4-NP luminophors at excitation wavelength 301 nm. Fluorescence spectrum of anthracene doped 4-nitrophenol shows partial quenching of host fluorescence with appearance of additional emission bands in the wavelength region 335–380 nm. The monomeric peaks of anthracene is seen in the region 400–525 nm. The appeared anthracene emission specifies the transfer of excitation energy of 4-NP to AN either by exciton or by EET process. The quenching of host fluorescence and appearnce of guest fluorescence is identified by these processes.
Figure 4 shows monomeric peaks of the tetracene in the wavelength region 480–590 nm [14]. The most intense peak maxima seen at 539 nm. These monomeric emission arises due to the excitation energy transfer from anthracene to tetracene. This bathochromic shift is seen as a result of two step EET from host 4-NP to tetracene via anthracene.
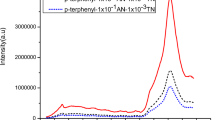
The comparison between fluorescence spectra of bicomponent luminophors containing 1 × 10−1 mol AN per mole 4-NP and tricomponent luminophors containing 1 × 10−1 mol AN and 1 × 10−1 mol TN per mole 4-NP was shown in Fig. 5. The spectra reveals the fluorescence emission of anthracene doped 4-NP seen in the blue region while tricomponent luminophors emits in the green region peaking at 539 nm.
Structural Studies of Bicomponent and Tricomponent Luminophors of4-Nitrophenol
Figure 6 shows the XRD spectra of pure 4-NP, AN, TN and doped 4-NP luminophors. The crystallinity of the doped 4-NP luminophors is identified by sharp peaks observed in XRD spectra. Absence of XRD peaks corresponding to AN and TN confirms the homogeneity of the doped samples. The prepared luminophors are highly crystalline. The calculated parameters viz., grain size, microstrain, dislocation density and stacking fault are shown in Table 2 These parameters are calculated by using FWHM value of intense band in XRD spectra. The grain size varies as the dopant concentration changes in the host. The matrix exhibited highest grain size at lower concentration i.e. 1 × 10−3.The addition of guest impurities in the host produces defects, imperfections and deformations which affect on other parameters such as microstrain, dislocation density and stacking fault [15].
The SEM images of pure and doped 4-NP luminophors are shown in Fig. 7. The micrographs exhibits identical and finely detached crystallites. It also provides crystallite size which was found as ∼95 nm in bicomponent and ~102 nm in tricomponent luminophors. These study reveals that the prepared luminophors in the present investigation can be used in optoelectronics.
Surface Morphology of Anthracene and Tetracene Doped 4-Nitrophenol Luminophors
Thermal Studies of 4-Nitrophenol Luminophors
For optical applications, thermal stability of the material is essential. Hence, to study the alteration in thermal properties of AN-TN doped 4-NP, thermogravimetric analysis has been done. Figures 8 and 9 shows TGA and DSC thermographs of 4-NP luminophors (1 × 10−1TN-1 × 10−1AN mole in 4-NP) under the nitrogen atmosphere within temperature range of 0–300 °C. From Fig.8, it is observed that the 4-nitrophenol luminophors remains thermally stable up to 225 °C and after that the decomposition starts. The stage one from 195 °C to 225 °C, the stage two from 225 °C to 275 °C in which maximum weight loss is observed while decomposition of remaining compound indicate weight loss in third stage. Figure 9 show DSC curve which exhibits one endothermic peak at 115 °C [16].
Electrical Properties 4-Nitrophenol Luminophors
Electrochemical properties of AN doped 4-NP and TN-AN doped 4-NP luminophors were studied by Cyclic Voltammetry (CV) in dichloromethane solution using ferrocene as an internal standard. On anodic sweep, two quasi-reversible waves were observed near −1.25 and near 1.2 in the first and third curve which may be due to two electron process in anthracene impurity doped in 4-NP and near −1.0 and 1.1 for TN-AN in 4-NP as observed in Figs. 10 and 11, respectively. For the prepared luminophors energy levels of HOMO and LUMO were detected in the range of 5.38–5.85 eV and 2.78–3.08 eV, respectively for both components. The Eg calculated from the CV shown in Table 3 were in range of 2.37–3.07 eV which found in close proximity with optical band gap [18]. This CV data shows that the prepared luminophors may be serve as a good candidates as n-type materials used in optoelectronic devices.
Conclusion
The novel luminophors of 4-NP emitting in the blue and green region are prepared. The two step EET process is observed from host to second guest in 4-NP solid matrix. The XRD analysis indicated the formation of homogeneous solid solutions of the host and guest material. The SEM images evidenced crystals of ∼95 nm and ∼105 nm size for AN doped 4-NP and AN-TN doped 4-NP luminophors repsectively. TGA-DSC and CV study revealed suitability of 4-nitrophenol luminophors in the optoelectronic devices.
References
Powell RC, Kepler RG (1969) Evidence for long-range Exciton-impurity interaction in Tetracene-doped anthracene crystals. Phys Rev Lett 22:636
Davies MJ, Jones AC, Williams JO, Munn RW (1983) Phonon-assisted energy transfer in the mixed molecular crystal p-terphenyl/tetracene. J Phys Chem 87:541
Huppert D, Rojansky D (1985) Picosecond study of electronic energy transfer in tetracene-doped anthracene crystals. Chem Phys Lett 114:149
Fujino T, Fujima T, Tahara T (2005) Femtosecond fluorescence dynamics imaging using a fluorescence up-conversion microscope. J Phys Chem B 109:15327
Gierschner J, Egelhaaf H-J, Oelkrug D, Müllen K (1998) Electronic deactivation and energy transfer in doped oligophenylenevinylene nanoparticles. J Fluoresc 8:37
Jadhav SA, Pujari SR, Patil SR (2000) Photophysics of exciplex emission of crystalline biphenyl doped by pyrene. Ind J of pure & Appl Phys 38:43
Pujari SR, Jadhav SA, Patil SR (2001) Tuning of wavelength of exciplex emission of pyrene and perylene in fluorene matrix. Ind J of Chem 40A:933
Mane KG, Nagore PB, Pujari SR (2019) Synthesis, Photophysical, electrochemical and thermal study of biphenyl Luminophors: green light emitting materials. J fluoresce 29:177
Mane KG, Nagore PB, Pujari SR (2018) Synthesis, Photophysical, electrochemical and thermal investigation of anthracene doped 2-Naphthol Luminophors and their thin films for optoelectronic devices. J Fluoresc 28:1023
Mane KG, Nagore PB, Pujari SR Synthesis of highly fluorescent D-A based p-TerphenylLuminophorsand their thin films for optoelectronic applications. J Fluore. https://doi.org/10.1007/s10895-019-02413-0
Mane KG, Nagore PB, Pujari SR (2017) Green light emitting tricomponentluminophors of 2-naphthol for construction of organic light emitting. International Journal of Research Publications in Engineering and Technology 8:38
Leung LM, Lo WT, So SK et al (2000) A high-efficiency blue emitter for small molecule-based organic light-emitting diode. J Am Chem Soc 122:5640
Baldo MA, Soos ZG, Forrests R (2001) Local order in amorphous organic molecular thin films. Chem Phys Lett 347:297
Yoshikawa H, Sasaki K, Masuhara H (2000) Picosecond near-field microspectroscopic study of a single anthracene microcrystal in evaporated anthracene−Tetracene film: inhomogeneous inner structure and growth mechanism. J Phys Chem B 104:3429
Sathyamoorthy R & Dheepa (2007) “Structural characterization of thermally evaporated Bi2Te3 thin films”, J Phys Chem Sol, 68: 111
Kalbende PP, Tasare MV and Zade AB (2013) Preparation, characterization, and thermal degradation studies of p-nitrophenol-based copolymer, J. of Chemistry, Article ID 846327, 9 pages
Wu C, Djurovich P. I. and Thompson M. E. (2009) “Study of energy transfer and triplet Exciton diffusion in hole-transporting host materials”, Adv Func Mater 19: 3157
Chiu KY, Su TX, Li JH, Lin T-H, Liou GS, Cheng S-H (2005) Novel trends of electrochemical oxidation of amino-substituted triphenylamine derivatives. J Electroanal Chem 575:95
Acknowledgements
The authors are thankful to Sophisticated Analytical Instrumentation Facility center, Indian Institute of Technology, Madras and to the Instrumentation Centre, Solapur University, Solapur, Maharashtra-India.
The authors also express special thanks to Dr. D. S. Sutrave and Mr. Sangam Gaikwad of Electronic Department of D.B.F. Dayanand College of Arts and Science, Solapur, Maharashtra – India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mane, K.G., Nagore, P.B. & Pujari, S.R. Synthesis of Novel Blue and Green Light Emitting 4-Nitrophenol Luminophors for Optoelectronics. J Fluoresc 29, 1371–1380 (2019). https://doi.org/10.1007/s10895-019-02447-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02447-4