Abstract
A new class of 2-naphthol luminophors doped with varying amounts of pyrene were prepared by applying standard solid-state reaction method to sight new luminophors which emit at a higher wavelength. The optical and electrochemical properties of prepared luminophors were studied by fluorimetry and cyclic voltammetry technique, respectively. The fluorescence spectrum of doped 2-naphthol showed broad fluorescence band at maximum wavelength 450 nm for 1 × 10–1 mol pyrene per mole 2-naphthol. The shifted broad spectra confirmed the exciplex formation between 2-naphthol and pyrene in the excitation state. On the basis of electrochemical data, energy levels of HOMO and LUMO were obtained for the prepared luminophors. The observed values fit between 5.70–5.72 eV and 3.02–3.09 eV, respectively. Their thermal stability was studied by TGA–DSC analysis. The SEM images evidenced crystal size of ~ 115 nm. Thus, the overall study proposes that the prepared novel luminophors are useful in optoelectronics.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
1 Introduction
In present era, the fabrication of OLEDs [1,2,3,4,5], and devices such as light emitting diodes [6,7,8] attracted more attention by many researchers worldwide. The energy restructuring and transmission in polynuclear aromatic hydrocarbons turned important for researchers. The fluorescence phenomenon, such as quenching in fluorescence, excimer emission or exciplex emission and emission due to charge transfer, etc., has been applied to study the prepared organoluminophors [9].
The excimer and exciplex emission phenomena are popularly exhibited by polynuclear aromatic hydrocarbons (PAHs). The formation of exciplex turns feasible in pyrene and other PAHs, only when selection of host molecule is done properly [10]. Highly fluorescent pyrene functions as an effective donor by exciplex formation with biphenyl [11], while 2-naphthol is known as a good matrix in which homogeneous solid solution of pyrene shall be achieved. This article focuses mainly on the preparation of 2-naphthol luminophors with varying amounts of pyrene and the studies on photophysics of emissions observed from these luminophors. The weak violet fluorescence of 2-naphthol was replaced by an intense blue emission due to the exciplex formation with Pyrene. The synthesis of these blue-light-emitting lumiophors of 2-naphthol is more economic and could meet demands of fluorescent lamp technology.
2 Experimental
2-naphthol of AR grade and pyrene of scintillation grade were procured from Merck-Schuchardt. The recrystallization and sublimation methods were used for their purification and further the purity was confirmed by their fluorescence spectra.
2.1 Preparation of 2-naphthol doped luminophors
The solid-state reaction technique had been employed to prepare polycrystalline 2-naphthol luminohors with different concentrations of pyrene [12]. This was processed in sealed silica crucible. The solid solution was heated at the temperature of the M.P. of 2-NP (122 °C). Furthermore, the melt obtained was slowly cooled to get finely grained powdered polycrystalline luminophors of 2-NP containing pyrene. Finally, this powder was subjected for characterization.
2.2 Characterization
The fluorescence spectra of guest doped 2-naphthol were recorded by JOBIN YVON Fluorolog-3-11 spectrofluorimeter, at SAIF, Indian Institute of Technology, Madras. The XRD spectra of doped and non-doped crystals were recorded with the Philips diffractometer (model PW-3710, Netherlands) with Cr Kα radiation (2.28 Å). (TGDTA-DSC) TA Inc. SDT-2790 with heating rate of 10 °C per minute under nitrogen atmosphere was used to perform thermogravimetric analysis at Instrumentation centre of Solapur University, Solapur. The surface morphology of samples were studied by scanning electron microscope at SAIF, Indian institute of Technology, Madras.
3 Results and discussion
3.1 XRD analysis of doped 2-naphthol luminophors
Figure 1 shows the XRD spectra of undoped 2-naphthol and pyrene-doped 2-NP. The crystallinity of the doped 2-NP luminophors was identified by sharp peaks observed in XRD spectra. The additional peaks for pyrene were not seen in the XRD profile of the doped samples indicated the formation of homogeneous mixed crystals. The parameters like grain size, dislocation density and stacking fault were estimated by the usual methods and reported in Table1 [13]. On adding pyrene in 2-NP as a guest matrix, exhibited highest grain size at lower concentration, i.e. 1 × 10–3. The addition of guest impurities in the host produces defects, imperfections and deformations [14].
Figure 2 shows SEM images of doped 2-naphthol and pure exhibiting identical and finely detached crystallites of monoclinic form. It also provides crystals of ~ 115 nm size.
3.2 Photophysical properties
Monoclinic unit cells of 2-naphthol after excitation reveals fluorescence in the UV region [15]. Doping of pyrene in 2-naphthol showing emission with intense violet colour further be changed gradually to intense blue colour with increase in concentration of pyrene.
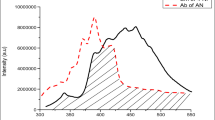
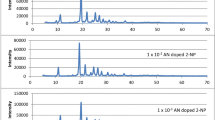
Figure 3a shows the emission spectra of 2-NP and absorption spectra of pyrene. The shaded area shows the overlapping region between the emission spectrum of 2-naphthol and absorption spectrum of pyrene. The Forster energy transfer distance, R0, estimated by the known method is 38 Å. This shows that the efficient excitation energy transfer (EET) from host 2-naphthol moiety to the guest pyrene moiety. Figure 3b reveals fluorescence spectra of varying amount of pyrene-doped 2-NP. 1 × 10–3, 1 × 10−2and 1 × 10–1 mol pyrene per mole 2-NP fluorescence spectra exhibits structured peaks at 356, 380 and 414 nm. This is attributed to the pyrene monomeric emission, as such emission being possible at low concentration [16].
However, at increased addition of pyrene in 2-naphthol mixed crystals, quenching of monomeric emission of pyrene is observed. At the same time broad emission observed in the range 430–540 nm. This red shifted emission band does not matches with well known crystalline pyrene excimer emission which is expected at 480 nm. The hetero dimeric species formed between 2-naphthol and pyrene. The lateral overlapping of π-molecular orbitals results in to formation of hetero-dimeric species in the excited state. The formation of exciplex between the guest and the host components is more probable due to the decrease in intermolecular distance between the components. However, at still higher concentration i.e. at 5 × 10–1 mol pyrene per mole 2-naphthol, this exciplex emission is quenched. This may be due to the concentration quenching phenomena.
The energy level diagram is shown in Fig. 4. The energy level diagram shows that the singlet level of pyrene (26802 cm−1) lie below the corresponding level of 2-naphthol (30303 cm−1). However strong vibrational coupling between excited singlet level of pyrene (Pst) and triplet level of 2-naphthol is possible. The transfer of excitation energy occurs from pyrene to 2-naphthol as shown in the figure and thus 2-naphthol triplets gets populated. At higher concentration due to lateral overlapping increases the possibility of formation of exciplex, i.e. hetero-dimeric species. The detailed mechanism of exciplex emission is depicted as below:
Mechanism
Presentation | Process |
|---|---|
\(P_{{{\text{S0}}}} \; + \;h\nu \; = \;P_{{{\text{S1}}}}\) | Pyrene excitation |
\(P_{{{\text{S1}}}} \; + \;N_{{{\text{S0}}}} \; = \;P_{{{\text{S0}}}} \; + \;N_{{{\text{T1}}}}\) | Singlet–triplet energy transfer (2-naphthol triplet formation) |
\(N_{{{\text{T1}}}} \; + \;P_{{{\text{S0}}}} \; = \;\left( {{\text{PN}}} \right)^{*}_{\text{Exciplex}}\) | Exciplex formation |
\(\left( {{\text{PN}}} \right)^{*}_{\text{Exciplex}} = \;P_{{{\text{S0}}}} \; + \;N_{{{\text{S0}}}} \; + \;h\nu_{{{\text{wx}}}}\) | Exciplex emission |
3.3 TGA–DSC analysis of doped 2-naphthol luminophors
The TGA–DSC analysis of prepared luminophors turns essential for its applications in optoelectronics. Therefore, thermogravimetric analysis was performed to scan the variation of thermal stability of pyrene doped with 2-naphthol as shown in Figs. 5 and 6 for 1 × 10–1 mol in the temperature range 0–300 °C. The 2-naphthol luminophors observed to be thermally stable at 125 °C and then start to decompose The first stage starts from 125 °C to 230 °C, the subsequent stage of 230 °C to 250 °C, suggesting a maximum loss in weight while whole decay in the third stage. Figure 5 shows a DSC curve presenting, respectively, two endothermic peaks at 105 °C and 120 °C.
3.4 Electrochemical properties
The cyclic voltammetry technique (CV) has been utilized to study the electrochemical properties of 2-NP luminophors doped with Py. The dichloromethane solution with ferrocene is used as an internal standard. In the obtained cyclic voltagramm, two quasi reversible waves were observed near − 1.0 and close to 1.15 at all curves (Fig. 7). From these obtained values, the energy levels of HOMO and LUMO were observed, respectively, in the range 5.70–5.72 eV and 3.02–3.09 eV. Furthermore, the values of Eg calculated were in the range 2.25–2.82 eV detected near the band gap [17, 18]. The electrochemical study further reveals that the prepared luminophors could meet the demands of the optoelectronic devices.
The details of analysis are given in Table 2.
4 Conclusions
The broad emission from the formed exciplex between pyrene-2-naphthol was observed for prepared novel pyrene-doped 2-naphthol luminophors. The XRD analysis indicated the formation of homogeneous solid solutions of the host and guest materials. The SEM images evidenced crystals of ~ 115 nm size. TGA–DSC and CV study revealed that the prepared 2-naphthol luminophors can be the suitable candidates for the optoelectronic devices. It is worthwhile to note that the intense exciplex emission is obtained from the prepared luminophors.
References
C.W. Tang, S.A. Van Slyke, Appl. Phys. Lett. 51, 913 (1987)
J.H. Burroughes, D.D.C. Bradley, A.R. Brown, R.N. Marks, K. Mackay, R.H. Friend, P.L. Burns, A.B. Homes, Nature 347, 539 (1990)
R.H. Friend, R.W. Gymer, A.B. Holmes, J.H. Burroughes, R.N. Marks, C. Taliani, D.D.C. Bradley, D.A. dos Santos, J.L. Brédas, M. Lögdlund, W.R. Salaneck, Nature 397, 121 (1999)
M.A. Baldo, M.E. Thompson, S.R. Forrest, Nature 403, 750 (2000)
K.-T. Wong, Y.-Y. Chien, R.-T. Chen, C.-F. Wang, Y.-T. Lin, H.-H. Chiang, P.-Y. Hsieh, C.-C. Wu, C.H. Chou, Y.O. Su, G.-H. Lee, S.-M. Peng, Ter(9,9-diarylfluorene)s. J. Am. Chem. Soc. 124, 11576 (2002)
A. Facchetti, M.-H. Yoon, C.L. Stern, H.E. Katz, J. MarksT, Angew. Chem. Int. Ed. 42, 3900 (2003)
L. Yang, A.-M. Ren, J.-K. Feng, X.-D. Liu, Y.-G. Ma, H.-X. Zhang, Theor. Inorg. Chem. 43, 5961 (2004)
Z. Ludmer, G. Berkovic, Chem. Phys. Lett. 100, 102 (1983)
W.-L. Jia, T. McCormick, Q.-D. Liu, H. Fukutani, M. Motala, R.-Y. Wang, Y. Tao, S. Wang, J. Mater. Chem. 22, 3344 (2004)
Kalyani N. Thejo, S. Dhoble, Renew. Sustain. Energy Rev. 44, 319 (2015)
S.R. Patil, S.B. Patwari, J. Lumin. 82, 115 (1999)
M.N. Gharge, S.L. Bhattar, G.B. Kolekar, S.R. Patil, Indian J. Chem. 47A, 1642 (2008)
S.A. Jadhav, S.R. Pujari, Indian J. Pure Appl. Phys. 38, 43 (2000)
K.G. Mane, P.B. Nagore, S.R. Pujari, IJRPET 3, 38 (2017)
S.R. Pujari, M.D. Kamble, P.N. Bhosale, P.M.R. Rao, S.R. Patil, Mater. Res. Bull. 37, 1641 (2000)
K.G. Mane, P.B. Nagore, S.R. Pujari, J. Fluoresc. 28, 1023 (2018)
A.M. Shaikh, B.K. Sharma, R.M. Kamble, J. Chem. Sci. 127, 1571 (2015)
K.G. Mane, P.B. Nagore, S.R. Pujari, J. Fluoresc. 29, 177 (2019)
Acknowledgements
The authors are thankful to Sophisticated Analytical Instrumentation Facility center (SAIF), Indian Institute of Technology, Madras and to the Instrumentation Centre, Solapur University, Solapur, Maharashtra, India. The authors also express special thanks to Dr. Sutrave and Mr. Sangam Gaikwad of Electronic Department of D.B.F. Dayanand College of Arts and Science, Solapur, Maharashtra, India
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mane, K.G., Nagore, P.B. & Pujari, S.R. Novel 2-naphthol luminophors for optoelectronics. Appl. Phys. A 125, 729 (2019). https://doi.org/10.1007/s00339-019-3024-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-3024-8