Abstract
A series of novel luminophors of 2-naphthol by doping anthracene were prepared using conventional solid state reaction technique. The photophysical, electrochemical and thermal properties were studied by Fluorescence spectroscopy, XRD, SEM, TGA-DSC and by Cyclic Voltammetry techniques. The thin films were characterized by Fluorescence spectroscopy. XRD study of fine grained powders exhibited sharp peaks which specify crystallinity and homogeneity of the doped luminophors. The fluorescence spectra of doped 2-naphthol exhibited emission of anthracene at 413 nm i.e. blue emission with instantaneous fluorescence quenching of 2-NP due to excitation energy transfer (EET). Electrochemical data specify that the HOMO and LUMO energy levels of the synthesized luminophors are in the range of 5.55–5.71 eV and 3.03–3.24 eV, respectively. TGA-DSC study confirmed thermal stability of prepared luminophors. Hence, overall study proposes that these luminophors seems applicable to be used as n-type materials for Optoelectronic devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays organic semiconductors have attracted more attention for their application in optoelectronic devices such as organic solar cells, organic light emitting diodes (OLEDs), organic photovoltaic devices, etc. [1,2,3]. Furthermore, demand from optoelectronic industries for these materials encouraged researchers to go for it. As compared to the metals doped in inorganic materials, organic doped materials find greater applications in LEDs with respect to effortless synthesis, reasonable, lightweight property, flexibility, etc. and embrace a productive field. These crucial facts encouraged their applications in solid-state reactions for lightening. Hence, these mesmerizing properties attracted various researchers and added more to existing literature which throws light on purely organic materials based luminophors and their use in optoelectronics [4,5,6,7,8,9]. But many of the methods used by researchers in organic synthesis are very tedious, time consuming and need expensive catalysts [10]. Vapour phase method, crystal growth method from solution, melt growth method and solid state growth method by heating have also been employed for the same purpose [11, 12]. Amongst which, doping technique turns efficient method named as conventional solid state reaction technique to get the emission of fluorescent materials due to its simple processing, informal heating and proficient output with great purity materials [13]. In conventional solid state reaction technique, selection of host and guest materials play a dynamic role. Intermolecular energy transfer in between host and guest is considered because of intramolecular interactions [14, 15]. In present article, monoclinic host material has been chosen and purposely doped with blue light emitting guest as an effective donor of excitation energy with the D-type (diffusion spectrum) crystal lattice which trap the excitation energy of host and attain alteration in the fluorescence spectrum of the host material [16,17,18,19]. Hence, in this study, 2-naphthol is preferred as a host material which solubilize anthracene as a guest material and transfer electronic excitation energy to the guest molecules via EET process, because of two benzene rings of 2-naphthol which are not co-planar in ground state but oriented in the plane in an electronically excited state by absorbing UV radiations. Aim of current article is to improve emission of 2-naphthol by integrating selected dopant with different concentrations and to make it applicable for optoelectronic devices.
Experimental
Pure 2-naphthol and anthracene of scintillation grade were procured from Merck-Schuchardt. The same were recrystallized and purified by the sublimation method. The similarity in the fluorescence spectra at different excited wavelength in the UV region confirmed the purity of these substances.
Preparation of Doped 2-Naphthol Luminophors
The conventional solid-state reaction technique was employed to prepare polycrystalline luminophors of 2-NP containing AN [20, 21]. Different concentrations of AN doped 2-NP solid solutions were formed by mixing appropriate amount of these two in silica crucible with heating at the temperature just above the melting point of 2-NP(122 °C). Then the obtained melt was cooled slowly to get polycrystalline luminophors of AN doped 2-NP with fine crushed powder. Finally, the fine powder of doped 2-NP was used to study the photophysical, thermal and electrochemical properties.
Characterization Techniques
The fluorescence spectra of doped 2-naphthol was recorded by JOBIN YVON Fluorolog-3-11 spectrofluorimeter, at IIT Madras. The XRD analysis of doped and undoped crystals was carried out by Philips Diffractometer (PW-3710 model, Holland) with CrKα radiation (2.28 Ǻ). (TGDTA-DSC) TA Inc. SDT- 2790 with heating rate 10 °C per minute under the nitrogen atmosphere was employed to carry out Thermogravimetric analysis, while FEI Quanta FEG 200 -Scanning Electron Microscope was used to study surface morphology of samples.
Results and Discussion
Structural Studies of Doped 2-Naphthol Luminophors by XRD
X-ray diffraction spectra of pure and anthracene doped 2-naphthol is shown in Fig. 1. XRD profile of fine grained powder exhibited sharp peaks which specify crystallinity and homogeneity of the doped luminophors. No additional peak for AN was observed in the spectrum of 2-NP luminophors. This reflection specify that, the mixed crystals of two components are homogenous. Along with this, the structural parameters like microstrain, dislocation density, grain size and stacking fault were also calculated and tabulated in (Table 1). Classical Sherrer formula has been used to estimate the average grain size of 2-naphthol luminophors [22]. The addition of AN as a guest material into 2-NP showed highest grain size for 1 × 10−2 concentration. On doping of a pure crystal into host, it deforms by producing defects and imperfection into host lattice. Hence, this deformation changes microstrain marginally and dislocation density as per the expectation [23]. It has been calculated and tabulated in (Table 1). Also Stacking fault observed to be improved with guest anthracene in 2-naphthol host material. This result concluded to consider close packed structure of both host and guest molecules. SEM micrographs (Fig. 2) of doped and undoped 2-naphthol exhibits well separated, identical crystallites of monoclinic form. Also, SEM micrographs noted average crystallite size of 133 nm which is the range desired in optoelectronic devices.
Photophysical Properties
2-naphthol which is monoclinic unit cell when excited at 330 nm radiation exhibits fluorescence in the UV region [24]. Anthracene is well identified for it's emission like naphthalene matrix by EET process.
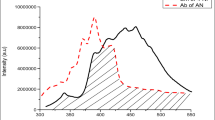
The fluorescence spectrum of 2-NP and AN doped 2-NP are shown in Fig. 3. It has been observed that, fluorescence spectra of anthracene doped 2-naphthol is structured and appears to be blue shifted viz. 413 nm which differ completely from fluorescence spectrum of 2-naphthol. The absence of 2-naphthol emission bands clearly specify that the excitation energy of 2-naphthol exciton is trapped by anthracene due to excitation energy transfer (EET) with immediate fluorescence quenching of 2-NP. Hence, for 1 × 10−3 and 1 × 10−2 anthracene doped 2-naphthol, the peak appeared at 412 nm and 413 nm. As the concentration of anthracene increased, peak shift towards longer wavelength with increase in intensity of peak.
Also the fluorescence study of thin films of anthracene doped 2-naphthol by employing spin coating technique was carried out as shown in Fig. 3c and result listed in Table 2. The results fulfilled our expectations.
Thermal Properties of Doped 2-Naphthol Luminophors
To exhibit optical applications, materials need to be thermally stable. To examine alteration in thermal properties of 2-naphthol doped anthracene, thermogravimetric analysis has been done. Figures 4 and 5 show TGA and DSC thermographs of 2-naphthol doped anthracene under the nitrogen atmosphere within temperature range of 0–300 °C. From Fig. 4, it is observed that, the anthracene doped 2-naphthol remains thermally stable up to 125 °C and after that the decomposition starts. The complete process of decomposition proceeds in three stages. The stage one from 125 °C to 230 °C, the stage two from 230 °C to 250 °C in which maximum weight loss is observed while decomposition of remaining compound indicate weight loss in third stage. The Fig. 5 show DSC curve which exhibits one endothermic peak at 120 °C.
From thermogram it is clear that doped 2-naphthol is suitable for device fabrication and to facilitate an enhanced lifetime of the devices.
Electrochemical Properties
Electrochemical properties of AN doped 2-NP luminophors were studied by Cyclic Voltammetry technique (CV) in dichloromethane solution using ferrocene as an internal standard.On anodic sweep, two quasi-reversible waves were observed near-0.2 and near 0.4 in the first and third curve which may be due to two electron process in anthracene impurity doped in 2-naphthol (Fig. 6). For the synthesized luminophors the HOMO and LUMO energy levels were observed in the range of 5.55–5.71 eV and 3.03–3.24 eV respectively. The Eg calculated from the CV were in the range of 2.25–2.82 eV which found in close proximity with optical band gap [25, 26]. Thus, the prepared luminophors are good applicants as n-type materials used in optoelectronic devices.
The observed parameters are tabulated in Table 3.
Conclusion
The structural, emission, electrochemical and thermal properties of the synthesized luminophors are significantly influenced by the concentration of guest material with emission at blue region. The electrochemical and thermal study of the synthesized luminophors confirmed their use as n-type material for Optoelectronic devices.
References
Pron A et al (2011)Triarylamine substituted Arylene Bisimides as solution Processable organic semiconductors for field effect transistors. Effect of substituent position on their spectroscopic, electrochemical, structural, and electrical transport properties. J Phys Chem C 115:15008–15017
Bujak P, Kulszewicz-Bajer I, Zagorska M, Maurel V, Wielgus I, Pron A (2013) Polymers for electronics and spintronics. Chem Soc Rev 42:8895–8999
Lincker F, Heinrich B, De Bettignies R, Rannou P, Pecaut J, GreB B, Donnio RD (2011) Fluorenone core donor–acceptor–donor π-conjugated molecules end-capped with dendritic oligo(thiophene)s: synthesis, liquid crystalline behaviour, and photovoltaic applications. J Mater Chem 21:5238
Friend RH, Gymer RW, Holmes AB, Burroughes JH, Marks RN, Taliani C, Bradley DDC, Santos DAD, Bredas JL, Logdlund M, Salaneck WR (1999) Study on the Optoelectronic Properties of UV Luminescent Polymer: ZnO Nanoparticles Dispersed PANI. Nature 397:121–128
Kim DY, Cho HN, Kim CY (2000) Prog. Synthesis and optical properties of poly[(4-(benzoxazole-2-yl)phenyl)methyl methacrylate] with 1,8-naphthalimide end group. Polym Sci 25:1089
Shinohara H, Kotani M (1980) Singlet energy transfer of p-terphenyl doped anthracene. Bulletin of the Chemical Society of Japan 53:3171
Kohler A, Wilson JS, Friend RH (2002) Fluorescence and phosphorescence in organic materials. Adv Eng Mater 4:453–459
Hung LS, Chen CH (2002) Anthracene derivative for a non-doped blue-emitting organic electroluminescence device with both excellent color purity and high efficiency. Mat Sci Eng R 39:143
Ping Q, Akiyama T, Abe K, Shigenari T (1995) Fluorescence spectra and ifetime of 2-naphthol in H2O- and D2O-ice(Ih) single crystal. Solid State Commun 95(3):177–180
Schab-Balcerzak E et al (2015) New core-substituted with electron-donating group 1,8-naphthalimides towards optoelectronic applications. J Lumin 166:22–39
Wang H, Yang Z, Xie Z, Wang H, Wang B, Ma Y (2014) The thermodynamic characteristics of organic crystal growth by physical vapor transport: towards high-quality and color-tunable crystal preparation. Cryst Eng Comm 16:4539–4545
Ravi G, Srinivasan K, Anbukumar S, Ramasamy P (1994) Growth and characterization of sulphate mixed L-arginine phosphate and ammonium dihydrogen phosphate/potassium dihydrogen phosphate mixed crystals. J Cryst Growth 137:598–604
Desai NK, Kolekar GB, Patil SR (2012) Preparation and characterization of anthracene doped p-terphenyl polycrystalline powders for scintillation application. Int J Lumin Appl 2(I):38–40
Desai NK, Mahajan PG, Kumbhar AS, Kolekar GB, Patil SR (2016) Nanoporous p-terphenyl polystyrene films containing perylene; fabrication, characterization and remarkable fluorescence resonance energy transfer based blue emitting properties. J Mater Sci Mater Electron 27:1118
Desai RR, Lakhminarayana D, Patel PB, Panchal CJ (2002) Electrical and optical properties of indium sesquitelluride (In2Te3) thin films. J Mater Sci 41:2019
Patil SR, Patwari SB (1999) Red shift in fluorescence of naphthalene doped by anthracene and perylene. J Lumin 82:115–119
Desai NK, Gupta MK, Kolekar GB, Patil SR (2013) Fluorescence enhancement effect in pyrene and perylene doped nanoporous polystyrene films: mechanistic and morphology. Phys Status Solidi A 210:2121
Mitsui M, Kawano Y (2013) Electronic energy transfer in tetracene-doped p-terphenyl nanoparticles: extraordinarily high fluorescence enhancement and quenching. Chem Phys 419:30
Masuko M, Ohuchi S, Sode K, Ohtani H, Shimadzu A (2000) Fluorescence resonance energy transfer from pyrene to perylene labels for nucleic acid hybridization assays under homogeneous solution conditions. Nucl Acids Res 28:1
Wang X, Lau KC, Li WK (2011) Doping effects on structural and electronic properties of ladderanes and ladder polysilanes: a density functional theory investigation. J Phys Chem A 115:7656
Pujari SR, Bhosale PN, Rao PMR, Patil SR (2002) Sensitized monomer fluorescence and excitation energy transfer in perylene-doped phenanthrene in crystalline and in polymer matrix. Mater Res Bull 37:439–448
Gharge MN, Bhattar SL, Kolekar GB, Patil SR (2008) Structural and photophysical aspects of perylene- doped anthracene crystalline powders prepared by microwave heating. Indian J Chem Sect A 47:1642–1648
Kalita PK, Sarma BK, Das HL (2000) Structural characterization of vacuum evaporated ZnSe thin films. Bull Mater Sci 23(4):313–317
Marciniak B, Rozycka-Sokolowska E, Pavlyuk V (2003) 2-Naphthalenol. Acta Cryst E59:o52
Shaikh AM, Sharma BK, Kamble RM (2015) Synthesis, Photophysical, electrochemical and thermal studies of Triarylamines based on benzo[g]quinoxalines. J Chem Sci 127(9):1571–1579
Sharma BK, Dixit S, Chacko S, Kamble RM, Agarwal N (2017) Synthesis and studies of Imidazoanthraquinone derivatives for applications in organic electronics. Eur J Org Chem :4389
Acknowledgements
The authors express sincere thanks to IIT Madras, SAIF and Instrumentation Centre, Solapur University, Solapur, Maharashtra-India and D.B.F. Dayanand College of Science, Solapur, Maharashtra -India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mane, K.G., Nagore, P.B. & Pujari, S.R. Synthesis, Photophysical, Electrochemical and Thermal Investigation of Anthracene Doped 2-Naphthol Luminophors and their Thin Films for Optoelectronic Devices. J Fluoresc 28, 1023–1028 (2018). https://doi.org/10.1007/s10895-018-2265-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2265-9