Abstract
The number of anthracene-tetracene (AN-TN) doped p-terphenyl (p-TP) luminophors [(TN-AN/ p-TP) (D-A)] and thin films of polystyrene doped p-TP luminophors were prepared at different proportion by conventional technique called solid state reaction and spin coating technique respectively. Excitation energy transfer (EET) was studied by fluorimetry and cyclic voltammetry technique. The result showed that, TN-AN/ p-TP in crystalline state as well as in thin films exhibit outstanding green emission at 475–550 nm, peaking at 525 nm. Structural properties and thermal stability were studied by XRD, SEM and TGA-DSC. The HOMO and LUMO energy levels obtained by CV were in the range from 5.82–5.85 eV and 2.94–2.97 eV, respectively. The Eg calculated from the CV found 2.88 eV which are in close agreement with efficient energy transfer in prepared organoluminophors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
OLED’s paying more attention scientifically and commercially for the reason of their potential use in full color, high resolution and flat-panel displays [1,2,3,4,5]. To meet the same demands blue, green [6, 7] and red [8, 9] light emitting materials having high electroluminescence efficiencies, excellent thermal stability and good charge carrier abilities attracted more attention. The performance of red and green OLED’s is relatively good as compared to blue OLED’s. Because of their intrinsically wide band gap, the synthesis of highly efficient green light emitters showing good color purity remains a great challenge for the improvement of new OLED’s.
So, Polyaromatic Hydrocarbons (PAHs) have been studied extensively and developed green light emitting materials for OLEDs only because of their excellency in electroluminescence (EL) and photoluminescence (PL) properties.
These excellency in EL and PL is due to electronic excitation energy transfer in PAH crystals. Therefore, these materials are found be suitable for Donor-Acceptor (D-A) pairs in FRET and attracted more attention of new generation. The EET process is verified by fluorescence of donor measured with intentionally doped acceptor concentration. Various factors affect the energy transfer efficiency such as polymer, solvent, concentrations of acceptor and its morphology, etc. [10,11,12]. Out of them, thin film morphology played very important role for efficiency of energy transfer in holding D-A pair at close distance required for EET process [13].
Hence, in this study authors have developed efficient green light emitting materials by very simple doping technique and their doped thin films by using spin coating technique [14] at different concentrations of prepared AN-TN doped p-terphenyl crystalline materials. Anthracene and tetracene are very well known PAH materials used as dopant acting as excellent acceptor in electronic energy transfer processes [15, 24]. Whereas, p-terphenyl which acts as excellent organic semiconductor is used as a host material and also has capacity to solubilize guest impurity. Further, it is found that, p-terphenyl is more stable thermally [16,17,18].
Literature survey revealed that, no work is reported on tetracene and anthracene doped p-terphenyl emitting green light in crystal state and their energy transfer studies in thin films. Systematic quenching of p-terphenyl with instantaneous sensitization of AN-TN and their thin films were observed by fluorescence spectroscopy and electrochemical study by cyclic voltametry technique. Simultaneously structural and morphological study of crystalline materials have been done by using XRD and SEM. Studies on thermal stability have been done by TGA-DSC technique.
Experimental
Pure p-terphenyl, anthracene and tetracene of scintillation grade were brought from Merck-Schuchardt. Then the recrystallization and sublimation method were applied for it’s purification and further confirmed by fluorescence spectra.
Preparation of p-Terphenyl Luminophors and Doped Thin Films
The solid state reaction technique had been employed to prepare polycrystalline p-TP luminophors with different concentrations of AN and TN [19]. This was processed in silica crucible. The solid solution was heated at the temperature of the M.P. of p-TP (214 °C). Further, the melt obtained was slowly cooled to get finely grained powdered polycrystalline luminophors of p-TP. Finally this powder was subjected for characterization.
The polystyrene films doped by tricomponent mixed crystals were prepared by 1% polystyrene solution and spin coating the films on glass substrate [20, 21].
Characterization
The fluorescence spectra of guest doped p-TP were recorded by JOBIN YVON Fluorolog-3-11 spectrofluorimeter, at Indian Institute of Technology, Madras. The XRD spectra of doped and non-doped crystals were recorded with the Philips diffractometer (model PW-3710, Netherlands) with CrKα radiation (2.28) at Solapur University, Solapur. Also, (TGDTA-DSC) TA Inc. SDT-2790 with heating rate of 10 °C per minute under nitrogen atmosphere was used to perform thermogravimetric analysis at Solapur University, Solapur, while surface morphology of samples was studied by scanning electron microscope at SAIF, Indian Institute of Technology, Madras. Cyclic voltammogram was recorded at D.B.F. Dayanand Science College, Solapur.
Results and Discussion
Fluorescence Studies of p-Terphenyl Luminophors in Crystalline State
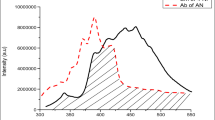
Figure 1 shows, fluorescence spectra of tetracene and anthracene doped p-terphenyl materials recorded at λmax = 290 nm [22]. The fluorescence spectra of doped p-terphenyl shows very negligible emission bands of p-terphenyl at 320–370 nm. The absence of p-terphenyl emission suggests an efficient transfer of excitation energy from p-terphenyl excitons to anthracene and tetracene. The fluorescence intensity of emission band of tetracene and anthracene doped p-terphenyl observed at 525 nm causes a green shift in emission wavelength. The presence of TN decreases the intensity of AN like emission. The observation led us to assume that, energy of excited AN is trapped by TN moiety before it is emitted radiatively. The possibility of two step energy transfer is confirmed on the basis of energy states of p-terphenyl, AN and TN. The first excited singlet state (S1) of p-terphenyl is higher than those of AN and TN while the first excited state (S1) of AN is lower than p-terphenyl but higher to that of TN. The TN singlet and p-terpenyl singlet reveal a wide gap by which the direct transfer of excitation energy to TN is not possible. However, it is transferred significantly to AN and then to TN. In the present work, we propose a two-step excitation energy transfer from p-terphenyl to the dopants.
Concentration effect is also observed. Higher concentrated solid solution (1 × 10−1 mol TN in 1 × 10−1 mol AN / p-TP) giving intense peak as compared to lower concentrated solid solution of TN.
Fluorescence Studies of p-Terphenyl Luminophors Doped with Polystyrene Thin Film
p-TP doped AN-TN mixed crystals showing green emission due to two step EET process from p-TP to TN via AN. These green light emitting luminophors for EL diodes require in thin film form. Polystyrene (PS) thin films doped with p-TP/AN-TN mixed crystals were prepared by spin coating technique in 1% polystyrene solution on glass substrate. p-TP fluoresces in the violet region at excitation wavelength 290 nm. Hence, doped films of p-TP luminophors, selectively excited at 290 nm.
The Fluorescence spectra of p-TP luminophors embedded in PS matrix is shown in Fig. 2. The careful examination of the spectra reveals, complete quenching of p-TP emission with two well separated emissive regions one in the range 385–440 nm and other in the range 475–575 nm. The earlier region corresponds to the AN like emission intensity with partial quenching while later is the TN monomer region. The observed TN monomer peak is very intense and broad, peaking at 525 nm as obtained for crystalline solid luminophors. Hence, present study provides materials suitable for use in EL devices.
X-Ray Diffraction Studies of p-Terphenyl Luminophors
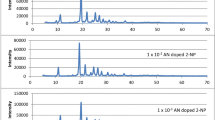
X-ray diffraction spectra of pure AN and TN doped p-TP is shown in Fig. 3. XRD profile of fine grained powder showed sharp peaks which specified crystallinity. The absence of any new peak in XRD spectra of doped luminophors confirms the homogeneity and formation of solid solution. Microstrain, dislocation density, grain size and stacking fault like structural parameters were calculated from XRD study shown in Table 1. Classical Sherrer formula has been used to estimate the average grain size of p-TP luminophors [23]. We found that, grain size increases with increase in concentration of AN and TN as a dopant into p-TP material. On doping of a pure crystal into host, it deforms by producing defects and imperfection into host lattice. This deformation changes microstrain marginally and dislocation density as expected [24]. The stacking fault observed to be improved with guest moiety in p-TP host material. These results supports the close packed structure of both host and guest molecules.
Surface Morphology of Tetracene and Anthracene Doped p-Terphenyl Luminophors
Fig. 4a indicate the SEM micrographs of pure p-TP and Fig. 4b indicate SEM micrographs of doped p-TP luminophors in crystal state. From SEM micrographs crystallite size observed for TN-AN doped p-TP is of 89.7 nm, required in optoelectronics
Thermal Studies of p-Terphenyl Luminophors
For optical applications, thermal stability of the material is essential. So, to study the alteration in thermal properties of p-TP doped AN and TN, thermogravimetric analysis has been done. Figures 5 and 6 show TGA and DSC thermographs of p-TP luminophors (1 × 10−1 mol AN-1 × 10−1 mol TN/ p-TP) under the nitrogen atmosphere within temperature range of 0–300 °C. From Fig. 5, it is observed that, the p-TP luminophors remains thermally stable up to 225 °C and after that the decomposition starts. The stage one from 250 °C to 275 °C, the stage two after 275 °C in which maximum weight loss is observed while decomposition of remaining compound indicate weight loss in third stage. The Fig. 6 show DSC curve which exhibits one endothermic peak at 215 °C respectively.
Electrical Properties of p-Terphenyl Luminophors
Electrochemical properties of AN and TN doped p-TP luminophors were studied by Cyclic Voltammetry (CV) in dichloromethane solution using ferrocene as an internal standard. The cyclic voltamogram is shown in Fig. 7. For the synthesized luminophors the HOMO and LUMO energy levels were observed in the range of 5.82–5.85 eV and 2.94–2.97 eV, respectively. The Eg calculated from the CV was 2.88 eV (Table 2) which found in close proximity with optical band gap.
Conclusion
Synthesis of highly fluorescent D-A based p-terphenyl luminophors and their thin films with green light emission were achieved. The XRD analysis indicated the formation of homogeneous solid solutions of the host and guest material. The SEM images evidenced crystals of ~ 89.7 nm of size. TGA-DSC and CV study revealed suitability of prepared luminophors used for optoelectronics.
References
Hosokawa C, Eida M, Matsuura M, Fukuoka K, Nakamura H, Kusumoto T (1997) Organic multi-color electroluminescence display with fine pixels. Synth Met 91:3–7
Forrest SR, Burrows PE, Shen Z, Gu G, Bulovic V, Thompson ME (1997) The stacked OLED (SOLEd): a new type of organic device for achieving high resolution full-color displays. Synth Met 91:9–13
Tullo AH (2001) Competition looms between LCDs and new organic light-emitting diode technology. Chem Eng News 79:49–54
Fuhrmann T, Salbeck J (2003) Organic materials for photonic devices. MRS Bull 28:354–359
Hung LS, Chen CH (2002) Recent progress of molecular organic electroluminescent matrials and devices. Mater Sci Eng R 39:143–222
Yao YS, Zhou QX, Wang XS, Wang Y, Zhang BW (2006) Fine tuning of the photophysical and electroluminescent properties of DCM-type dyes by changing the structure of the electron-donating group. J Mater Chem 16:3512
Jung BJ, Lee JI, Chu HY, Lee LM, Shim HK (2005) A new family of bis-DCM based dopants for red OLEDs. J Mater Chem 15:2470–2475
Tong Q-X, Lai S-L, Chan M-Y, Zhou Y-C, Kwong H-L, Lee C-S (2008) High efficiency nondoped green organic light emitting devices. Chem Phys Lett 455:79–82
Okumoto K, Kanno H, Hamaa Y, Takahashi H, Shibata K (2006) Green fluorescent organic light-emitting device with external quantum efficiency of nearly 10%. Appl Phys Lett 89:063504
Bhalla V, Vij V, Tejpal R, Singh G, Kumar M (2013) Solvent dependent competition between fluorescence resonance energy transfer and through bond energy transfer in rhodamine appended hexaphenylbenzene derivatives for sensing of Hg2+ ions. Dalton Trans 42:4456–4463
Yu JW, Kim JK, Kim DY, Kim C, Song NW, Kim D (2006) Prediction of efficient energy transfer in emissive polymer blends based on Forster radius and the excited state lifetime of acceptors. Curr Appl Phys 6(6):59–65
Lian H, Dai Y, Yang D, Cheng Z, Li C, Hou Z, Shang M, Lin J (2014) Morphology control, luminescence and energy transfer properties of NaCeF4and NaCeF4:Tb3+/Yb3+nanocrystals. Nanoscale 6:9703–9712
Gawrys P, Marszalek T, Bartnik E, Kucinska M, Ulanski J, Zagorska M (2011) Novel, low-cost, highly soluble n-type semiconductors: Tetraazaanthracene Tetraesters. Org Lett 13:6090–6093
Emslie AG, Bonner FT, Peck LG (1958) Flow of a viscous liquid on a rotating disk. J Appl Phys 29:858–862
Desai NK, Kolekar GB, Patil SR (2012) Preparation and characterization of anthracene doped p_terphenyl polycrystalline powders for scintillation application. International Journal of Luminescence and its application 2(I):38–40
Wang X, Lau KC, Li WK (2011) Doping Effects on Structural and Electronic Properties of Ladderanes and Ladder Polysilanes: A Density Functional Theory Investigation. J Phys Chem A 115:7656–7663
Shinohara H, Kotani M (1980) Singlet energy transfer in p-Terphenyl crystal doped with Tetracene. Bull Chem Soc Jpn 53:3171–3175
Mitsui M, Kawano Y (2013) Electronic energy transfer in tetracene-doped p-terphenyl nanoparticles: extraordinarily high fluorescence enhancement and quenching efficiency. Chem Phys 419:30–36
Mane KG, Nagore PB, Pujari SR (2017) Green light emitting tricomponent luminophors of 2-naphthol for construction of organic light emitting. JournalINX 3:38
Pujari SR, Bhosale PN, Rao PMR, Patil SR (2002) Structural and optical studies of perylene-doped polymer thin films. Indian J Pure Appl Phys 40:896
Pujari SR, Jadhav SA, Bhosale PN, Rao PMR, Patil SR (2002) Fluorescence studies of biphenyl doped by pyrene and perylene. Indian J Pure Appl Phys 40:115
Gharge MN, Bhattar SL, Kolekar GB, Patil SR (2008) Structural and photophysical aspects of perylene doped anthracene crystalline powders prepared by microwave heating. Indian J Chem A 47:1642
Cullity BD (1978) Elelments of X-ray Diffraction, 2nd Edn. Addision-Weslye Publshing Company, Inc., USA, p 102
Mane KG, Nagore PB, Pujari SR (2019) Synthesis, Photophysical, electrochemical and thermal study of biphenyl Luminophors: green light emitting materials. J fluor 29:177–183
Shaikh AM, Sharma BK, Chacko S, Kamble RM (2015) Synthesis, Photophysical, Electrochemical and Thermal Studies of Triarylamines based on benzo[g]quinoxalines. J Chem 127:1571
Mane KG, Nagore PB, Pujari SR (2018) Synthesis, Photophysical, electrochemical and thermal investigation of anthracene doped 2-Naphthol Luminophors and their thin films for optoelectronic devices. J Fluoresc 28:1023–1028
Acknowledgements
The authors are thankful to Sophisticated Analytical Instumentation Facility Centre, Indian Institute of Technology, Madras and to the Instrumentation Centre, Solapur University, Solapur, Maharashtra-India.
The authors also express special thanks to Dr. Sutrave and Mr. Sangam Gaikwad of Electronic Dept., D.B.F. Dayanand College of Science, Solapur, Maharashtra – India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mane, K.G., Nagore, P.B. & Pujari, S.R. Synthesis of Highly Fluorescent D-A Based p-Terphenyl Luminophors and their Thin Films for Optoelectronic Applications. J Fluoresc 29, 1001–1006 (2019). https://doi.org/10.1007/s10895-019-02413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02413-0