Abstract
Novel luminophors of anthracene (AN) and tetracene (TN) doped biphenyl were prepared using Conventional Solid State reaction technique. Fluorescence spectroscopy, XRD, SEM, TGA-DSC and Cyclic Voltammetry techniques have been employed for photophysical, electrochemical and thermal study. The X-ray diffraction study revealed the formation of homogeneous biphenyl solid solutions with the added guests AN and TN. Fluorescent biphenyl absorbing short wave UV radiation and emitting at long wave UV radiation has been used as a solid matrix. From the fluorescence spectra it is seen that the added guests shifts the UV fluorescence of biphenyl emitting in green region of visible spectrum at 532 nm. SEM images of the prepared luminophors showed the crystallites of average size 140 nm which makes them suitable candidates for their use in Optoelectronic devices. HOMO and LUMO energy levels of the synthesized luminophors from electrochemical data observed in 5.50–5.64 eV and 3.09–3.13 eV with band gap 2.37–2.55 eV, respectively. TGA-DSC study revealed the thermal stability of prepared luminophors.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently organic semiconductors got significant attention of researchers because of their ultimate electro-optical properties, technological industrial uses. Many of Organic conjugated molecules have been synthesized for their application as organic light emitting diodes (OLEDs), organic field effect transistors (OFETs) [1] and dye-sensitized solar cells [2]. Electroluminescent multifunctional materials consists of donor-acceptor (D-A) molecules which have substantial importance for OLEDs in recent time [3]. These molecules have been found to show fascinating charge-transporting properties in electroluminescent devices [4]. These properties found to be achieved by doping technique known as Conventional Solid State reaction technique [5,6,7]. The conjugated molecules like anthracene, tetracene, pyrene, perylene, p-terphenyl, etc., have been used as organic scintillators. But the potential of anthracene and tetracene to interact with host molecule is well known [8, 9]. The role of scintillators is to produce light in fluorescent tubes and to transform UV light into visible light [10, 11]. Hence there has been much interest in synthesis, photo-physical, electrochemical and thermal investigation of Organo-luminophors with its utility in solid state electronics emitting in preferred colors.
Mixed crystals of biphenyl doped anthracene in which the fluorescence of biphenyl emission get quenched and shows new anthracene like emission in the spectral region of 400–450 nm. Tetracene exihibits very weak fluorescence in solid state. However, it forms the series of solid solution with anthracene to yield green emission [12, 13]. The present work reports, synthesis of tricomponent luminophors of biphenyl doped by anthracene and tetracene with enhancement in fluorescence emission from blue to green light with their photo-physical, electrochemical and thermal properties by selective excitation of host biphenyl.
Experimental
Biphenyl, anthracene and tetracene of scintillation grade were purchased from Merck-Schuchardt and used as received. Biphenyl was used as a host matrix while AN and TN were used as a guests. The series of solid solutions of biphenyl were prepared by conventional solid state reaction method. Different concentrations of AN and TN doped biphenyl solid solutions were prepared by using appropriate amount and mixing all in silica crucible with heating at the melting point of biphenyl. The melt thus obtained was cooled to get polycrystalline luminophors of AN and TN doped biphenyl. The fine powder of doped biphenyl luminophors were then subjected to photophysical, thermal and electrochemical characterization.
Characterization Techniques
JOBIN YVON Fluorolog-3-11spectrofluorimeter, at IIT Madras has been used to record the fluorescence emission of doped biphenyl. For structural analysis of the doped and undoped crystals, X-ray analysis was carried out by Philips Diffractometer (PW-3710 model, Holland) with CrKα radiation (2.28 Ǻ). Thermogravimetric analysis has been done by (TGDTA-DSC) TA Inc. SDT- 2790 with heating rate 10 °C per minute under the nitrogen atmosphere. FEI Quanta FEG 200 -Scanning Electron Microscope was employed to study surface morphology of samples.
Results and Discussion
Structural Studies of Doped Biphenyl Luminophors
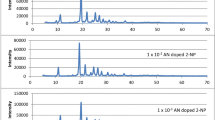
X-ray diffraction spectra of pure AN and TN doped biphenyl is shown in Figs. 1, 2 and 3. XRD profile of fine grained powder showed sharp peaks which specified crystallinity. The absence of any new peak in XRD spectra of doped luminophors confirms the homogeneity and formation of solid solution [14]. Microstrain, dislocation density, grain size and stacking fault like structural parameters were calculated from XRD study shown in Tables 1 and 2. Classical Sherrer formula has been used to estimate the average grain size of biphenyl luminophors [15]. We found that, grain size increases with increase in concentration of AN and TN as a dopant into biphenyl material. On doping of a pure crystal into host, it deforms by producing defects and imperfection into host lattice. This deformation changes microstrain marginally and dislocation density as per the hope [16]. The stacking fault observed to be improved with guest moiety in biphenyl host material. These results supports the close packed structure of both host and guest molecules. SEM micrographs of doped and undoped biphenyl showed well separated identical crystallites of monoclinic form. SEM micrographs noted average crystallite size at 140 nm which is in the range desired in Optoelectronic devices.
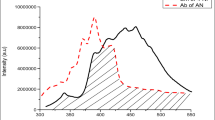
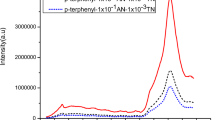
AN doped biphenyl gives anthracene like emission. The anthracence like emission rules out the possibility of direct excitation of AN. The presence of TN decreases the intensity of AN like emission and new emission appears in the region 480–575 nm. In comparison of the luminophor containing 10−3 mol AN and 10−3 mol TN per mole biphenyl, the emission intensity of the AN like emission decreases in the luminophor containing 10−3 mol AN and 10−2 mol TN per mole biphenyl. The observation led us to assume that, energy of excited AN is trapped by TN moiety before it is emitted radiatively. The possibility of two step energy transfer is confirmed on the basis of energy states of biphenyl, AN and TN. The first excited singlet state (S1) of biphenyl is higher than those of AN and TN while the first excited state (S1) of AN is lower than biphenyl but higher to that of TN. The TN singlet and biphenyl singlet reveal a wide gap by which the direct transfer of excitation energy to TN is not possible. However, it is transferred significantly to AN and then to TN. In the present work we proposed a two step excitation energy transfer from biphenyl to the dopants.
Thermal Properties
The thermal stability of doped materials is important for their applications in optics. To examine thermal properties of biphenyl luminophors, thermogravimetric analysis has been done. Figure 4 shows TGA of doped biphenyl luminophors under the nitrogen atmosphere within temperature range of 0–300 °C. From Fig. 4, it is observed that, the anthracene doped biphenyl remains thermally stable up to 120 °C and after that decomposition starts. The complete process of decomposition proceeds in three stages. The stage one from 120 °C to 185 °C, the second stage from 185 °C to 250 °C in which maximum weight loss is observed while decomposition of remaining compound indicate weight loss in third stage. From this thermal study it is clear that, doped biphenyl luminophors are thermally stable and makes them suitable for device fabrication and lifetime enhancement of the device.
Electrochemical Properties
Electrochemical properties of AN and TN doped biphenyl luminophors were studied by Cyclic Voltammetry (CV) in dichloromethane solution using ferrocene as an internal standard. The cyclic voltamogram is shown in Fig. 5. For the synthesized luminophors the HOMO and LUMO energy levels were observed in the range of 5.50–5.64 eV and 3.09–3.13 eV, respectively. The Eg calculated from the CV were in the range 2.37–2.55 eV which found in close proximity with optical band gap [17]. On anodic sweep, two quasi-reversible waves were observed near −0.3 and near 0.7 in the first and second curve which may be due to two electron transfer processes in AN and TN doped biphenyl luminophors. This data shows the prepared luminophors can be used as a n-type materials in optoelectronic devices.
Conclusion
The doping of anthracene and tetracene in biphenyl system shifts the fluorescence emission from blue region to green region considerably. The efficient two step excitation energy transfer is proposed from biphenyl host to AN and then to TN. XRD analysis confirms the homogeneous solid solution formation of host and guest material. SEM estimation reveals the average crystallite size of 140 nm. TGA-DSC analysis confirmed thermal stability of compounds. CV analysis showed that, tricomponent luminophors are promising candidates as n-type materials for Organic electronics. So, overall study confirms their use in Optoelectronic devices.
References
Posch P, Thelakkat M, Schmidt HW (1999) Perylenediimides with electron transport moieties for electroluminescent devices. Synth Met 102:1110–1112
Uekawa M, Miyamoto Y, Ikeda H, Kaifu K, Ichi T, Nakaya T (1999) A novel diamine compound containing carbazole groups for organic electroluminescent devices. Thin Solid Films 352:185–188
Pope M, Kallmann HP, Mangnante P (1963) Electroluminescence in organic crystals. J Chem Phys 38:2042.s
Nikl M (2000) Wide band gap scintillation materials: Progress in the technology and material understanding. Phys Status Solidi A 178:595–620
Patil SR, Patwari SB (1999) Red shift in fluorescence of naphthalene doped anthracene and perylene. J Lumin 82(2):115–119
Jadhav S, Pujari S, Patil S (2000) Photophysics of exciplex emission of crystalline biphenyl doped pyrene. Ind J Pure and Appl Phys 43
Pujari SR, Bhosale PN, Rao PMR, Patil SR (2002) Sensitized monomer fluorescence and excitation energy transfer in perylene-doped phenanthrene in crystalline and in polymer matrix. Mater Res Bull 37:439–448
Lincker F, Heinrich B, De Bettignies R, Rannou P, Pecaut J, Grevin B, Pron A, Donnio B, Demadrille R (2011) Fluorenone core donor–acceptor–donor π-conjugated molecules end-capped with dendritic oligo(thiophene)s: synthesis, liquid crystalline behaviour, and photovoltaic applications. J Mater Chem 21:5238
Friend RH, Gymer RW, Holmes, AB, Burroughes JH , Marks RN, Taliani C , Bradley DDC, Santos DAD, Bredas JL, Logdlund M, Salaneck WR, Study on the optoelectronic properties of UV luminescent polymer: ZnO Nanoparticles Dispersed PANI, Nature 397 (1999), 121, 128
Schab-Balcerzak E, Siwy M, Filapek M, Kula S, Malecki G, Laba K, Lapkowski M, Janeczek H, Domanski M (2015) New core-substituted with electron-donating group 1,8-naphthalimides towards optoelectronic applications. J Lumin 166:22–39
Balamurugan N, Arulchkkaravarthi A, Ramasamy P (2006) Scintillation characteristics on anthracene-doped naphthalene crystal for 137Cs-γ ray source. Nuclear Instruments and Methods in Physics Research Section A: Accelerators , Detectors and Associated Eqipment 568:767–771
Jadhav SA, Absorption and fluorescence studied in multicomponent organic systems, dissertation, (Dec.2000) Shivaji University, Kolhapur, Maharashtra-India
Pujari SR Preparation and characterization of green light emitting naphthalene luminophors. DAV Int J Sci 1(1):17
Wang H, Yang Z, Xie Z, Wang H, Wang B, Ma Y (2014) The thermodynamic characteristics of organic crystal growth by physical vapor transport: towards high-quality and color-tunable crystal preparation. Cryst Eng Comm 16:4539–4545
Mane KG, Nagore PB, Pujari SR (Sept.2018) Synthesis, photophysical, electrochemical and thermal investigation of anthracene doped 2-Naphthol luminophors and their thin films for optoelectronic devices. J Fluoresc 28(5):1023–1028
Gharge MN, Bhattar SL, Kolekar GB, Patil SR (2008) Structural and photophysical aspects of perylene- doped anthracene crystalline powders prepared by microwave heating. Ind J Chem A 47:1642–1648
Kalita PK, Sarma BK, Das HL (2000) Structural characterization of vacuum evaporated ZnSe thin films. Bull Mater Sci 23(4):313–317
Shaikh AM, Sharma BK, Kamble RM (2015) Synthesis, photophysical, electrochemical and thermal studies of triarylamines based on benzo[g]quinoxalines. J Chem Sci 127(9):1571–1579
Acknowledgements
The authors express sincere thanks to Prin. Dr. V. P. Ubale, Dr. D.S. Sutrave, Mr. Sangam Gaikwad of Electronics Dept. of D.B.F. Dayanand College of Arts and Science, Solapur, IIT Madras, SAIF and Instrumentation Center, Solapur University, Solapur, Maharashtra-India.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mane, K.G., Nagore, P.B. & Pujari, S.R. Synthesis, Photophysical, Electrochemical and Thermal Study of Biphenyl Luminophors: Green Light Emitting Materials. J Fluoresc 29, 177–183 (2019). https://doi.org/10.1007/s10895-018-2325-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2325-1