Abstract
Transgenic soybean plants (RR) engineered to express resistance to glyphosate harbor a variant of the enzyme EPSPS (5-enolpyruvylshikimate-3-phosphate synthase) involved in the shikimic acid pathway, the biosynthetic route of three aromatic amino acids: phenylalanine, tyrosine, and tryptophan. The insertion of the variant enzyme CP4 EPSPS confers resistance to glyphosate. During the process of genetic engineering, unintended secondary effects are likely to occur. In the present study, we quantified volatile organic compounds (VOCs) emitted constitutively or induced in response to herbivory by the soybean looper Chrysodeixis includens in transgenic soybean and its isogenic (untransformed) line. Since herbivore-induced plant volatiles (HIPVs) are known to play a role in the recruitment of natural enemies, we assessed whether changes in VOC profiles alter the foraging behavior of the generalist endoparasitic larval parasitoid, Meteorus rubens in the transgenic line. Additionally, we assessed whether there was a difference in plant quality by measuring the weight gain of the soybean looper. In response to herbivory, several VOCs were induced in both the conventional and the transgenic line; however, larger quantities of a few compounds were emitted by transgenic plants. Meteorus rubens females were able to discriminate between the odors of undamaged and C. includens-damaged plants in both lines, but preferred the odors emitted by herbivore-damaged transgenic plants over those emitted by herbivore-damaged conventional soybean plants. No differences were observed in the weight gain of the soybean looper. Our results suggest that VOC-mediated tritrophic interactions in this model system are not negatively affected. However, as the preference of the wasps shifted towards damaged transgenic plants, the results also suggest that genetic modification affects that tritrophic interactions in multiple ways in this model system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Co-evolution of plants with herbivores has resulted in plant recognition of foreign molecules and cellular damage that activates a number of induced responses to herbivory in plants (Hare 2011). These induced responses include changes in specialized morphological structures such as trichomes, as well as in chemical compounds that directly influence the colonization and performance of herbivores (Karban and Baldwin 1997; Mithöfer and Boland 2012). In addition, plants can defend themselves indirectly by recruiting natural enemies of the attacking herbivore (Arimura et al. 2009), by offering an increase in resources such as extrafloral nectar (Turlings and Wäckers 2004), or through the emission of herbivore-induced plant volatiles (HIPVs) that attract predators and parasitoids of the herbivore. Herbivore-induced plant volatiles comprise an array of compounds from different biosynthetic pathways, some of which can be synthesized de novo in response to herbivory (Dudareva et al. 2006). During the last two decades, HIPVs have been studied extensively from molecular to community levels because manipulation of this ecological mechanism offers the possibility to improve biological control and develop novel, environmentally-friendly, pest management strategies (Kaplan 2012; Turlings and Ton 2006).
The development of soybeans resistant to the herbicide glyphosate through genetic modification has changed soybean production globally, including in Brazil, where the use of transgenic plants has been authorized since 2005. Roundup Ready® (RR) soybean plants express a variant of the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS). This enzyme participates in the shikimic acid pathway, the biosynthetic route of three aromatic amino acids: phenylalanine, tyrosine, and tryptophan (Maeda and Dudareva 2012). In addition to their role in the synthesis of proteins for plant development (Hermann and Weaver 1999), these aromatic amino acids are precursors of secondary metabolites involved in plant defense (Buchanan et al. 2000). Tryptophan is the precursor of indole, a volatile compound induced in response to herbivory in various plant species, including maize and soybean (Rostás and Eggert 2008; Turlings et al. 2005). In maize, this compound is essential in priming maize plants (Erb et al. 2015), and recently it has been demonstrated that indole acts as a direct defense against herbivores (Veyrat et al. 2016). L-Phenylalanine is the precursor of volatile phenylpropanoids/benzenoids, such as methyl salicylate (MeSA), which also is induced by soybean plants in response to herbivory (Michereff et al. 2011; Rostás and Eggert 2008). Methyl salicylate has important ecological functions in arthropod-plant interactions by acting as an infochemical for predators (De Boer and Dicke 2004) and by preventing plant colonization by aphids (Mallinger et al. 2011). Moreover, it is a mobile signal for the development of systemic acquired resistance against pathogens (Park et al. 2007). Salicylic acid (SA), its precursor, is involved in the activation of direct and indirect defenses against pathogens and phloem-feeding insects (Loake and Grant 2007; Zarate et al. 2007 and references therein). Recent studies have shown that the insertion of a gene that expresses an insensitive form of the EPSPS to confer resistance to glyphosate affects soybean metabolism. Altered expression of seed protein (Brandão et al. 2010; Garcia-Villalba et al. 2008), as well as altered accumulation of lignin in roots compared to conventional lines already has been reported (Zonetti et al. 2012). Cheng et al. (2008) did not find any significant differences in gene expression in soybean leaves between conventional and transgenic lines. However, the authors reported down-regulation of genes involved in cysteine protease inhibitor activity and dihydroflavonol-4-reductase activity in transgenic lines, possibly as an unintended consequence of genetic transformation (Cheng et al. 2008). According to Arruda et al. (2013), genetic modification itself contributes to alterations in a variety of soybean plant traits in a cascading manner. Based on this, not only primary but also secondary metabolism may be modified due to the insertion of the gene for CP4 EPSPS.
Because the behavior and performance of herbivores, and indirectly of natural enemies, is influenced by primary and secondary plant metabolism, any variation in the physical and chemical traits may affect plant-insect interactions. In transgenic maize plants engineered to expresss delta-endotoxins from Bacillus thuringiensis, emissions of HIPVs are altered, possibly as a result of a combination of altered insect feeding and changes in carbon allocation, but no negative effects on the recruitment of two parasitoids have been reported (Dean and De Moraes 2006; Moraes et al. 2011; Turlings et al. 2005). Recently, we have shown that herbivory by the velvet bean caterpillar, Anticarsia gemmatalis (Lepidoptera: Erebidae) induces much larger emissions of individual volatile compounds in transgenic plants expressing resistance to glyphosate compared with plants from the isogenic line (Strapasson et al. 2016). However, no negative effect on the foraging of the predatory bug, Podisus nigrispinus (Hemiptera: Pentatomidae) was observed.
In this study, we addressed whether transgenic soybean plants that harbor the gene for CP4 EPSPS resistance to glyphosate emit a VOC profile different from the isogenic line in response to feeding by the soybean looper, Chrysodeixis includens (Lepidoptera; Noctuidae). We also assessed whether any variation in HIPV emissions between lines affects the behavior of the parasitoid, Meteorus rubens (Hymenoptera: Braconidae). Finally, we tested the performance of the soybean looper on both transgenic and isogenic soybean lines. The soybean looper was regarded as a secondary pest of soybean crops in Brazil until 2003. Since then, this species has become a major soybean pest, likely due to elimination of natural enemies because of excessive pesticide sprayings, and due to the expansive planting of soybean in monoculture (Bueno et al. 2007). Meteorus rubens is a gregarious larval endoparasitoid of various species of noctuids. In Southern Brazil, it commonly is found in soybean fields parasitizing the soybean looper (Strapasson et al. unpublished data).
Methods and Materials
Living Material
Soybean seeds (Glycine max) from a transgenic cultivar (CD 202 RR) and its isogenic line (CD 202) were provided by COODETEC (Cascavel, Paraná, Brazil). Germinating seeds were placed in the dark for 4–5 d at 25 °C. After this period, seeds were planted in plastic pots (top 6 × 6 cm; bottom 4.5 × 4.5 cm; height 5.5 cm) filled with commercial substrate (Vida Verde, São Paulo, Brazil). Plants were kept under artificial lighting (14:10 h L:D regime), and watered as needed. They were used in the experiments when they reached the V3 phenological stage as described by Fehr et al. (1971), approximately 5 wk. after planting. A colony of C. includens was started in the laboratory from eggs purchased commercially (BUG, Piracicaba, São Paulo, Brazil), and kept in plastic pots containing a bean-based artificial diet (Parra 2001). Larvae were kept under controlled conditions of 25 ± 1 °C and a photoperiod of 14:10 L:D until pupation. Pupae were kept in vermiculite and, just prior to emergence, were transferred to PVC tubes (15 cm) lined with white paper as an oviposition substrate. As adults emerged, they were fed on a honey-based solution [honey, sorbic acid, methyl paraben, sugar, and distilled water, then mixed with beer (3:1 v/v)] according to Hoffman Campo et al. (1985). Every 2–3 d, eggs were collected to start a new cycle. Meteorus rubens used in the bioassays were recovered from parasitized larvae of the soybean looper collected in the experimental station “Gralha Azul” from the Pontificia Universidade Católica do Paraná - PUCPR (Fazenda Rio Grande, Paraná, Brazil). Larvae were kept as previously described until the emergence of the parasitoids. Wasps were identified by Dr. Eduardo Shimbori (Universidade Federal de São Carlos, São Carlos, Brazil) and Dr. Helmuth Aguirre (University of Wyoming, Laramie, USA). Adults were maintained on a 10 % honey solution until they were used in the bioassays.
VOC Collection and Analyses
Volatiles were collected from undamaged (control) and C. includens-damaged plants (treatment) from both, CD 202 and CD 202 RR. Herbivore induction was accomplished by infesting each plant with 10 3rd-instar larvae for a 24-h period. Prior to sampling, larvae were removed, and the unpotted plants were wrapped in aluminum foil in order to collect VOCs from the above ground parts only. Four plants (one per treatment) were sampled simultaneously. For this, each plant was placed in a sealed 1-L chamber. Humidified, charcoal-filtered air was pumped into each chamber at a rate of 1 L/min through Teflon tubes. VOCs were trapped onto ca. 20 mg HayeSep® Q 80–100 mesh in a glass tube for 24 h. Trapped VOCs were eluted with 300 μl of twice-distilled HPLC-grade hexane, and 10 μl of tetradecane (50-ppm solution) were added as an internal standard (IS). Samples were concentrated to 100 μl, and 1 μl of the extract was injected automatically in splitless mode (injector temperature 250 °C), and analyzed by GC-MS (Shimadzu QP 2010 Plus) with an RTX-5 column (30 m × 0.25 mm i.d., 0.25 mm film thickness; Restek, Bellefonte, PA, USA). The column oven temperature was held at 40 °C for 1 min, increased to 250 °C at 7 °C min−1, and held for 10 min. Helium was the carrier gas at a column head pressure of 170 kPa. The quantification of individual compounds was carried out based on the peak area of the IS. Emissions were expressed as ng/g dry weight/24 h. Quantitation of dry weight (DW) was obtained by cutting and drying the above ground plant parts for 48 h at 60 °C. Tentative identification of individual compounds was carried out by comparing the mass spectrum to those in reference libraries (NIST 27 and NIST 147), and by comparing their calculated Kovats Indexes (KI) with those reported in the literature. GC co-injections with synthetic compounds also were conducted for (Z)-3-hexen-1-ol, (Z)-3-hexenyl acetate, octanal, 1-octen-3-ol, (Z)-β-ocimene, β-linalool, decanal, methyl salicylate, indole, (Z)-jasmone, β-caryophyllene, and α-farnesene. A total of 40 plants, 10 from each treatment (N = 10) were sampled.
Behavioral Response of M. rubens
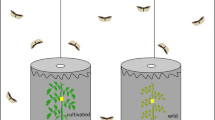
The responses of M. rubens were tested in dual-choice bioassays in a Y-tube olfactometer (Ø = 2 cm; main arm =18 cm; smaller arms =13 cm). A humidified, charcoal-filtered airflow of 1 L/min was equally split in two and channeled to each small arm. At the distal ends of the small arms, odor sources consisting of 20 μl of the extract (trapped soybean plant VOCs eluted in hexane and concentrated to 100 μl as previously described) or hexane were placed on a piece of filter paper (1 × 1 cm). At the end of the main arm of the olfactometer, 3–4-d-old mated female parasitoids were introduced individually and observed for a period of 10 min. We considered that an insect had responded when it passed the halfway point of one of the small arms, and remained there for at least 5 s; otherwise, the insect was considered as non-responsive, and excluded from the data analysis. The odor source was replaced, and the olfactometer was rotated 180°, after each observation. Every 5 observations, the position of the odor sources was switched. The following tests were conducted: a) undamaged vs. herbivore-damaged plants (isogenic line), b) undamaged vs. herbivore-damaged plants (transgenic line), 3) herbivore-damaged plants from the isogenic line vs. the herbivore-damaged plants from the transgenic line. The bioassays were conducted between 0900 and 1700 h at 25 °C over a period of 6 d.
Feeding Performance of C. includens
One-d-old larvae were weighed and kept singly in plastic pots under controlled conditions of 25 ± 1 °C and a photoperiod of 14:10 L:D. For 7 d, they were fed on leaves excised from either CD 202 (isogenic line) or CD 202 RR (transgenic line) plants (at the V3 stage). After this period, they were weighed again. The initial and final weights were recorded (N = 19 and N = 22 for CD 202 and CD 202 RR, respectively). Larvae that died before completing the bioassay were not included in the data analysis.
Statistical Analyses
Individual VOCs were log transformed (X + 1) and subjected to a Principal Component Analysis (PCA). Analysis of Variance (ANOVA) followed by Contrast Analysis was performed for those PCs that explained the main percentage variance to visualize differences between treatments. For a more detailed analysis, individual compounds and total VOC emissions were analyzed using one-way ANOVA followed by a Tukey HSD post hoc test. The preference of M. rubens was analyzed by performing a Chi-squared test. The performance of the soybean looper was checked for normality and analyzed by performing a t-test to compare the means between weight gain (final – initial weight) by the larvae fed on leaves of the transgenic or the isogenic line. Data were analyzed in the “R” Environment (R Development Core Team, 2006), except for the analysis of feeding preference that was performed on SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
VOCs Emitted by Transgenic and Isogenic Soybean Plants
Volatiles detected in the headspace of soybean plants from the various treatments and comparisons between treatments are presented in Table 1. Herbivory induced the emission of (Z)-3-hexenyl-2-methylbutyrate and (Z)-jasmone, plus two unidentified compounds (1 and 2) in the isogenic line; and (Z)-3-hexen-1-ol, 3-octanone, β-linalool, (Z)-3-hexenyl-2-methylbutyrate, (Z)-jasmone, β-caryophyllene, α-caryophyllene, and TMTT, plus three unidentified compounds (1–3) in the transgenic line. The PC1 explained 85.42 % of the variance. There were significant differences between treatments (ANOVA F = 4.76; P = 0.007), and the VOC emissions of transgenic C. includens-damaged plants differed from the rest of the treatments (P < 0.05), mainly because of the variation of α-farnesene, indole, (Z)-β-ocimene, and (Z)-3-hexen-1-ol (Figs. 1, 2). There were differences in the total amount of VOCs between treatments (ANOVA F = 18.357; P ≤ 0.001). However, the total induced VOC emissions in transgenic plants did not differ from those in conventional plants (Tukey HSD post hoc P = 0.138). From those compounds that were significantly increased in response to herbivory (Z)-β-ocimene (Tukey HSD post hoc P = 0.027), β-linalool (Tukey HSD post hoc P = 0.007), and indole (Tukey HSD post hoc P = 0.026) were emitted in larger quantities by transgenic than by conventional plants.
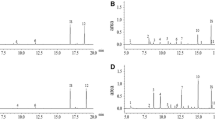
Typical total ion chromatograms (TIC) of volatile organic compounds (VOCs) emitted by an undamaged soybean plant of the isogenic a and transgenic line b, and a Chrysodeixis includens-damaged soybean plant from the isogenic c and transgenic line d. Major VOCs detected are: 1) (Z) 3-hexen-1-ol; 2) (Z)-3-hexenyl acetate; 3) (Z)-β-ocimene; 4) unidentified compound 2; 5) indole; and 6) α-farnesene. The X denotes contaminants in the sample. IS denotes the internal standard. Intensities are not normalized for dry weight of the plants
Scores of individual plants from undamaged (●) and herbivore-damaged soybean plants (x) from the isogenic line, undamaged plants (◊) and herbivore-damaged plants (+) from the transgenic line, and volatile organic compounds (VOCs) projected on the first (PC1) and second principal components (PC2). Vectors show the loading of each (numbered) compound and the length represents the relative magnitude of its contribution to the different treatments. Analyzed VOCs: 1) (Z)-3-hexen-1-ol; 2) 1-octen-3-ol; 3) 3-octanone; 4) octanal; 5) (Z)-3-hexenyl acetate; 6) (Z)-β-ocimene; 7) β–linalool; 8) nonanal; 9) unidentified compound 1; 10) unidentified compound 2; 11) methyl salicylate; 12) decanal; 13) (Z)-3-hexenyl 2-methylbutyrate; 14) indole; 15) unidentified compound 3; 16) (Z)-jasmone; 17) β-caryophyllene; 18) α-caryophyllene; 19) α-farnesene; and 20) TMTT
Behavioral Preference of M. rubens
Meteorus rubens discriminated between the odors from undamaged and C. includens-damaged plants from the isogenic line (X 2 = 9.00; P = 0.003) and the transgenic line (X 2 = 12.10; P < 0.001). However, when we compared the odors from herbivore-induced plants of both lines, M. rubens preferred the odors induced in transgenic plants (X 2 = 10.53; P = 0.001) (Fig. 3).
Choice of mated Meteorus rubens females toward the odors from undamaged (U) and Chrysodeixis includens-damaged plants (H) in the transgenic (T) and isogenic line (I), and between the odors of herbivore-damaged plants emitted by both lines. The asterisks denote de level of significance (P < 0.01 *, P < 0.001 **). The numbers on the left side of the graph show the total of number of insects that responded to any of the odors (N); the number of insects that did not respond is shown in brackets
Feeding Performance of the Soybean Looper
There was no difference between the weight increase of larvae fed on leaves from transgenic (Mean ± SEM, 11.36 ± 1.80) and leaves from conventional (Mean ± SEM, 8.59 ± 1.20) plants over one week (T = 1.135; P > 0.05).
Discussion
There is evidence that genetic transformation of plants to express the delta endotoxins of Bacillus thuringiensis (Bt) or resist the pathogen Venturia inaequalis can result in variation of HIPVs compared to conventional plants (Dean and De Moraes 2006; Turlings et al. 2005; Vogler et al. 2010). Studies using Bt-plants suggest that changes in VOC profiles may be the result of carbon allocation due to genetic transformation (Turlings et al. 2005), in addition to expected altered feeding patterns as a result of the Bt-toxin (Dean and De Moraes 2006; Turlings et al. 2005). Here, we demonstrated that, under controlled conditions of light and temperature, the emissions of VOCs induced in response to herbivory by the soybean looper are larger in transgenic plants expressing resistance to glyphosate than in non-transgenic plants. These results are in agreement with a previous study that showed that the emissions of VOCs in response to herbivory by the velvet bean moth are larger in transgenic RR soybean plants than in the isogenic line (Strapasson et al. 2016). Since the same soybean lines were used in both experiments, variation in the magnitude of individual compounds induced by the two species may be due to distinctive feeding behaviors and/or different oral elicitors present in the velvet bean moth and the soybean looper (Erb et al. 2012 and references therein). However, the question of why transgenic plants that express resistance to glyphosate emit greater amounts of VOCs in response to herbivory by two lepidopteran larvae remains unanswered. Considering the fact that transgenic RR plants express a variant of the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), an enzyme that participates in the biosynthesis of tryptophan, tyrosine, and L-phenylalanine via the shikimic acid pathway, a possible explanation would be that the insensitive EPSPS altered the synthesis of VOC precursors. This may be the case of indole, a tryptophan-derived volatile, whose emission is directly related to the activity of EPSPS (D’Alessandro et al. 2006). However, this may not explain why transgenic plants emitted larger amounts of terpenoids, which are synthesized from isopentenyl diphosphate and its allylic isomer via the mevalonic acid (MVA) pathway and the methyl erythritol phosphate (MEP) pathway (Dudareva et al. 2006).
A central event in the activation of plant responses to abiotic and biotic stress is the accumulation of phytohormones, mainly jasmonic acid (JA), salicylic acid (SA), and ethylene (ET) (Bari and Jones 2009). From these, JA is the main signaling molecule responsible for triggering induced responses (including HIPV emissions) in response to attack by mites and chewing insects such as lepidopteran caterpillars (Arimura et al. 2009; Menzel et al. 2014 for a review). Expression of JA-dependent genes relies, however, on a positive or negative interaction (cross-talk) between signaling pathways involved in plant defense (Pieterse et al. 2012). Cross-talk between SA and JA has been widely studied. Usually, accumulation of SA reduces the accumulation of JA and vice-versa, even though positive and neutral cross-talk between these two phytohormones also has been reported (Agrawal et al. 2014; Pieterse et al. 2012 and references therein). Considering the fact that the aromatic amino acid L-phenylalanine is precursor of SA (Dudareva et al. 2006), a second explanation might be that genetic transformation somehow affected the cross-talk between signaling pathways and, in turn, JA-dependent induced responses. This effect on cross-talk may have directly affected induction of volatiles or another trait that directly affected herbivore feeding behavior, which in turn altered the emissions of volatile compounds.
Herbivore-induced plant volatiles are reliable cues for parasitoids (see Clavijo McCormick et al. 2012 for a review), which can cope with natural genetic variation in plant VOC bouquets (Kappers et al. 2011). Our results from the bioassays showed that M. rubens is able to discriminate between herbivore-induced and constitutively-emitted volatiles in both transgenic and conventional lines. This is not surprising, as herbivory in both lines resulted in clear qualitative and quantitative changes compared with the constitutive VOC blend. However, when M. rubens was challenged to discriminate between the HIPVs emitted by the conventional line and those emitted by the transgenic line, there was a clear preference of the wasps for the odors from the transgenic line. These results suggest a shift in the preference of the wasps, and that tritrophic interactions are affected in multiple ways, in this model system at least. VOC-mediated plant-insect interactions are complex, and the preference of natural enemies for particular genotypes within one plant species may be due to the abundance of individual compounds or changes in VOC ratios (Bruce et al. 2005). That is, natural enemies can respond differently to HIPVs depending on the amount of individual or total VOCs emitted by the plant (Girling et al. 2011; Horiuchi et al. 2003; Shiojiri et al. 2012). For example, the predatory mite, Phytoseiulus persimilis is more attracted to the odors emitted by cucumber plants infested with its prey, Tetranychus urticae, in varieties that emit larger quantities of (E)-β-ocimene, TMTT, and two other unknown compounds (Kappers et al. 2011). In our study, when we compared different groups of plants, the volatile emissions in response to herbivory from transgenic plants differed from the other three groups. Clearly, herbivore-damaged transgenic plants emitted larger quantities of α-farnesene, indole, (Z)-β-ocimene, and (Z)-3-hexen-1-ol. Therefore, our results suggest that changes in the concentrations of a few compounds may have modified the preference of the parasitic wasps. To our knowledge, this is the first study assessing the behavioral response of this Neotropical parasitoid to plant volatiles. Nothing is known about the innate response of M. rubens or its ability for associative learning. Associative learning is an important process in the foraging behavior of parasitoids, and their ability to learn allows them to distinguish between specific volatiles (De Jong and Kaiser 1991). Specialist parasitoids depend strongly on host-derived stimuli and particular plant VOC compounds that evoke invariable responses, but are likely influenced by associative learning (Clavijo McCormick et al. 2012). In contrast, generalist parasitoids such as M. rubens that must cope with variable VOC bouquets, benefit from associative learning (Ngumbi et al. 2012). Nevertheless, responses of parasitoids to volatiles may depend not only on the degree of specialization of the parasitoid, but also on the herbivore. Since we used naïve individuals in our experiments, further studies addressing associative learning would be interesting to understand better the behavior of M. rubens. Host traits can affect parasitoid performance. For example, host weight can influence parasitoid development (Hardy et al. 1992). However, we did not find any evidence that feeding on leaves of the transgenic line can affect weight gain of the soybean looper. These results suggest that the performance of wasps that develop in larvae fed on RR plants may not be negatively affected. However, as we did not follow the development of the parasitoid, these results are not conclusive and deserve further investigation.
Our results suggest that, in the studied model system, VOC-mediated tritrophic interactions are not negatively affected in plants engineered to express resistance to glyphosate. However, the shift in the preference of the wasps towards herbivore-induced odors from RR transgenic plants suggests that tritrophic interactions are altered. In addition to tritrophic interactions, altered volatile emissions may affect other ecological interactions mediated by airborne signal molecules. Indole, for instance, which was emitted in much larger quantities by damaged transgenic plants, is a signal molecule for priming maize plants (Erb et al. 2015) and can directly affect herbivore performance and behavior (Veyrat et al. 2016). Since genetic expression within and between conventional and transgenic lines are likely to exist (Cheng et al. 2008) further studies need to be conducted with other lines.
References
Agrawal AA, Hastings AP, Patrick ET, Knight AC (2014) Specificity of herbivore-induced hormonal signaling and defensive traits in five closely related milkweeds (Asclepias spp. J Chem Ecol 40:717–729
Arimura G, Matsui K, Takabayashi J (2009) Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 50:911–923
Arruda MAZ, Azevedo RA, Barbosa HS, Mataveli LRV, Oliveira SR, Arruda SCC, Gratão PL (2013) Comparative studies involving transgenic and non-transgenic soybean: What is going on? In: Board J (ed) A comprehensive survey of international soybean research - Genetics, physiology, agronomyand nitrogen relationships. Intech press., Croácia, pp. 583–613
Bari R, Jones JD (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488
Brandão AR, Barbosa HS, Arruda MAZ (2010) Image analysis of two-dimensional gel electrophoresis for comparative proteomics of transgenic and non-transgenic soybean seeds. J Proteome 73:1433–1440
Bruce TJ, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274
Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and molecular biology of plants. American Society of Plant Physiologists. Rockville, Maryland
Bueno RCOF, Parra JRP, Bueno AF, Moscardi F, Oliveira JRG, Camillo MF (2007) Sem barreira. Rev Cultivar 93:12–15
Cheng KC, Beaulieu J, Iquira E, Belzile FJ, Fortin MG, Strömvik MV (2008) Effect of transgenes on global gene expression in soybean is within the natural range of variation of conventional cultivars. J Agric Food Chem 56:3057–3067
Clavijo McCormick AL, Unsicker S, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310
D’Alessandro M, Held M, Triponez Y, Turlings TC (2006) The role of indole and other shikimic acid derived maize volatiles in the attraction of two parasitic wasps. J Chem Ecol 32:2733–2748
De Boer JG, Dicke M (2004) The role of methyl salicylate in prey searching behavior of the predatory mite Phytoseiulus persimilis. J Chem Ecol 30:255–271
De Jong R, Kaiser L (1991) Odor learning by leptopilina boulardi a specialist parasitoid hymenoptera eucoilidae. J Insect Behav 4:743–750
Dean JM, De Moraes CM (2006) Effects of genetic modification on herbivore-induced volatiles from maize. J Chem Ecol 32:713–724
Dudareva N, Negre F, Nagegowda DA, Orlova I (2006) Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci 25:417–440
Erb M, Meldau S, Howe GA (2012) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17:250–259
Erb M, Veyrat N, Robert CA, Xu H, Frey M, Ton J, Turlings TC (2015) Indole is an essential herbivore-induced volatile priming signal in maize. Nat Commun 6:6273
Fehr W, Caviness C, Burmood DT, Pennington JS (1971) Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci 11:929–931
Garcia-Villalba R, Leon C, Dinelli G, Segura-Carretero A, Fernandez-Gutierrez A, Garcia-Canas V (2008) Comparative metabolomic study of transgenic versus conventional soybean using capillary electrophoresis-time-of-flight mass spectrometry. J Chromatogr A 1195:164–173
Girling RD, Stewart-Jones A, Dherbecourt J, Staley JT, Wright DJ, Poppy GM (2011) Parasitoids select plants more heavily infested with their caterpillar hosts: a new approach to aid interpretation of plant headspace volatiles. Proc R Soc B 278:2646–2653
Hardy ICW, Griffiths NT, Godfray HC (1992) Clutch size in a parasitoid wasp: a manipulation experiment. J Anim Ecol 61:121–129
Hare JD (2011) Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu Rev Entomol 56:161–180
Hermann KM, Weaver LM (1999) The shikimate pathway. Annu Rev Plant Physiol Plant Mol Biol 50:473–503
Hoffman Campo CB, Oliveira EB, Moscardi F (1985) Criação massal de lagarta da soja (Anticarsia gemmatalis). Embrapa CNPSo 23p
Horiuchi JI, Arimura GI, Ozawa R (2003) A comparison of the responses of Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae) to volatiles emitted from lima bean leaves with different levels of damage made by T. urticae or Spodoptera exigua (Lepidoptera. Appl Entomol Zool 38:109–116
Kaplan I (2012) Trophic complexity and the adaptive value of damage-induced plant volatiles. PLoS Biol 10:e1001437
Kappers IF, Hoogerbrugge H, Bouwmeester HJ, Dicke M (2011) Variation in herbivory-induced volatiles among cucumber (Cucumis sativus L.) varieties has consequences for the attraction of carnivorous natural enemies. J Chem Ecol 37:150–160
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Loake G, Grant M (2007) Salicylic acid in plant defence—the players and protagonists. Curr op. Plant Biol 10:466–472
Maeda H, Dudareva N (2012) The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu Rev Plant Biol 63:73–105
Mallinger RE, Hogg DB, Gratton C (2011) Methyl salicylate attracts natural enemies and reduces populations of soybean aphids (Hemiptera: Aphididae) in soybean agroecosystems. J Econ Entomol 104:115–124
Menzel TR, Weldegergis BT, David A, Boland W, Gols R, van Loon JJ, Dicke M (2014) Synergism in the effect of prior jasmonic acid application on herbivore-induced volatile emission by lima bean plants: transcription of a monoterpene synthase gene and volatile emission. J Exp Bot 65:4821–4831
Michereff MFF, Laumann RA, Borges M, Michereff-Filho M, Diniz IR, Neto ALF, Moraes MCB (2011) Volatiles mediating a plant-herbivore-natural enemy interaction in resistant and susceptible soybean cultivars. J Chem Ecol 37:273–285
Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63:431–450
Moraes MCB, Laumann RA, Aquino MFS, Paula DP, Borges M (2011) Effect of Bt genetic engineering on indirect defense in cotton via a tritrophic interaction. Transgenic Res 20:99–107
Ngumbi E, Jordan M, Fadamiro H (2012) Comparison of associative learning of host-related plant volatiles in two parasitoids with different degrees of host specificity, Cotesia marginiventris and Microplitis croceipes. Chemoecology 22:207–215
Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318:113–116
Parra JRP (2001) Técnicas de Criação de Insetos para Programas de Controle Biológico. FEALQ, Piracicaba
Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:489–521
Rostás M, Eggert K (2008) Ontogenetic and spatio-temporal patterns of induced volatiles in Glycine max in the light of the optimal defence hypothesis. Chemoecology 18:29–38
Shiojiri K, Ozawa R, Matsui K, Sabelis MW, Takabayashi J (2012) Intermittent exposure to traces of green leaf volatiles triggers a plant response. Sci Rep 2:378
Strapasson P, Pinto-Zevallos DM, Zarbin PHG (2016) Soybean (Glycine max) plants genetically modified to express resistance to glyphosate: can they modify airborne signals in tritrophic interactions? Chemoecology 26:7–14
Turlings TCJ, Ton J (2006) Exploiting scents of distress: the prospect of manipulating herbivore-induced plant odors to enhance the control of agricultural pests. Curr Opin Plant Biol 9:421–427
Turlings TCJ, Wäckers FL (2004) Recruitment of predators and parasitoids by herbivore-damaged plants. In: Cardé RT, Millar J (eds) advances in insect chemical ecology. University Press, Cambridge, pp. 21–75
Turlings TC, Jeanbourquin PM, Held M, Degen T (2005) Evaluating the induced-odour emission of a Bt maize and its attractiveness to parasitic wasps. Transgenic Res 14:807–816
Veyrat N, Robert CAM, Xu H, Frey M, Ton J, Turlings TCJ, Erb M (2016) Herbivore intoxication as a potential primary function of an inducible volatile plant signal. J Ecol 104:591–600
Vogler U, Rott AS, Gessler C, Dorn S (2010) Comparison between volatile emissions from transgenic apples and from two representative classically bred apple cultivars. Transgenic Res 19:77–89
Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143:866–875
Zonetti PC, Suzuki LS, Bonini EA, Ferrarese MLC, Ferrarese-Filho O (2012) High temperatures on root growth and lignifications of transgenic-resistant soybean. Agrociencia 46:557–565
Acknowledgments
The authors thank Dr. Marco Antonio Root de Oliveira and Mrs. Franciele Bebber from COODETEC-Cascavel who provided us with the seed material; Dr. Eduardo Shimbori (Universidade Federal de São Carlos) and Dr. Helmuth Aguirre (University of Wyoming, Laramie) for identification of M. rubens; Prof. Leandro S. Souto for support in the statistical analyses, and Profs. Jeffrey Aldrich and Martin Pareja for improving the draft version of the manuscript. Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq Processo N° 401928/2012-8, Coordenacão de Pessoal de Aperfeiçoamento de Nível Superior (CAPES), and Instituto Nacional de Ciências e Tecnologia de Semioquímicos na Agricultura (INCT) are acknowledged for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strapasson, P., Pinto-Zevallos, D.M., Da Silva Gomes, S.M. et al. Volatile Organic Compounds Induced by Herbivory of the Soybean Looper Chrysodeixis includens in Transgenic Glyphosate-Resistant Soybean and the Behavioral Effect on the Parasitoid, Meteorus rubens . J Chem Ecol 42, 806–813 (2016). https://doi.org/10.1007/s10886-016-0740-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0740-9