Abstract
Upon herbivory, plants activate complex biochemical pathways that result in an array of defense responses including the emission of a novel blend of volatile organic compounds (VOCs). These compounds mediate the recruitment of predators and parasitoids that exert biological control of the attacking herbivore. Genetic manipulation of a particular trait to improve agricultural plant varieties may affect other traits as a result of possible pleiotropy or insertional mutations, which in turn can affect the interaction of the plant with other organisms. Changes in herbivore-induced VOC emissions are known to occur in transgenic plants engineered to express resistance to insects (mainly Bt-plants), not only as a result of modified insect behavior but also as a result of altered resource allocation. Transgenic glyphosate-resistant plants express a variant of the enzyme EPSPS (5-enolpyruvylshikimate-3-phosphate synthase) that is insensitive to the herbicide glyphosate. This enzyme is essential in metabolic routes that result in the synthesis of amino acids and secondary metabolites. We addressed whether the constitutive and Anticarsia gemmatalis-induced emissions of VOCs from a transgenic soybean line differ from those of the isoline, and whether changes may interfere in the foraging behavior of the predatory bug Podisus nigrispinus. Analyses showed that both herbivory and genotype influenced VOC emissions. In addition, the genotype affected the herbivore-induced VOC emission. Larger emissions were measured in the transgenic line than the non-transgenic line upon herbivory. The bioassays showed that P. nigrispinus significantly discriminated only between the odors of undamaged and damaged plants of the non-transgenic line. No preference was observed for herbivore-damaged plants of any of the two lines over the other. The results from this study suggest that despite a greater emission of volatiles the predators are less able to discriminate between herbivore-damaged and undamaged transgenic plants. This condition does not necessarily increase the preference of the predator for damaged non-transgenic plants over transgenic plants. This study opens possibilities for new studies of chemical ecology in tritrophic systems to assess the effect of transgenic glyphosate-resistant plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant volatile organic compounds (VOCs) drive a number of ecological interactions between plants and the surrounding community. In plant–insect interactions, they mediate the attraction and orientation of foraging females to a suitable host for oviposition (Gouinguené and Städler 2006; Proffit et al. 2011) and pollinators to odorous flowers (Raguso 2008). In addition, the induced emission of novel compounds upon oviposition or feeding (Pinto-Zevallos et al. 2013) results in the attraction of natural enemies (e.g., predators and parasitoids) (McCormick et al. 2012), the attraction or repellence of other herbivores (Xiao et al. 2012) and the induction of defenses and allelopathy in neighboring plants (Arimura et al. 2010). Thus, plant VOCs affect population dynamics in complex food webs.
Since the introduction of transgenic crops in the early 1990s, the adoption of engineered lines has steadily increased in several countries (FAO 2011). Nevertheless, concerns about the possible adverse impact on human health and on the environment are still highly topical. Non-target effects include genetic drift, development of “super weeds” or insect resistance (Ferry and Gatehouse 2009). Genetic manipulation of a particular trait may affect others as a result of possible pleiotropy or insertional mutations (Schuler et al. 1999). During the last few years several studies have focused on the impact of the Bt gene on plant VOC emissions, and the possible effects on herbivores and carnivores (Yan et al. 2004; Turlings et al. 2005; Dean and De Moraes 2006; Himanen et al. 2009). More recently, a few studies have also assessed the effects of plants engineered to express resistance to Venturia inaequalis, a causal agent of apple scab disease, on volatile induction and the response of a parasitoid to herbivore-induced VOCs (Vogler et al. 2009, 2010). To our knowledge, however, no study has assessed the ecology of VOC-mediated interactions between plants genetically modified to express resistance to glyphosate [(N-(phosphonomethyl) gly] and higher trophic levels.
Glyphosate-resistant plants express a bacterial variant of the enzyme EPSPS (5-enolpyruvylshikimate-3-phosphate synthase) that is insensitive to glyphosate. EPSPS catalyzes the formation of 5-enolpyruvylshikimate 3-phosphate from shikimate 3-phosphate and phosphoenolpyruvate, in the shikimate pathway. Products of this metabolic route are the aromatic amino acids l-phenylalanine, tyrosine, and tryptophan which are precursors for the synthesis of compounds involved in plant growth, reproduction and plant responses to stress (Maeda and Dudareva 2012 for a review on biochemical pathways). Glyphosate targets this enzyme and inhibits the synthesis of these compounds that leads the plant to death. Therefore, the expression of a glyphosate-insensitive EPSPS in transgenic plants confers resistance to the broad spectrum post-emergence herbicide. Tryptophan is the precursor of indole which is known to be induced upon herbivory in some plant species including maize and soybean (Turlings et al. 2005; Rostás and Eggert 2008). l-Phenylalanine is the precursor of phenylpropanoid volatiles such as methyl salicylate (MeSA), which is also induced in soybean plants (Rostás and Eggert 2008). Genetically modified soybean plants expressing resistance to glyphosate can obviously develop normally. However, possible changes in the plant metabolism are likely to occur. For example, a comparative metabolomic study has shown that slight qualitative variations in a few metabolites in seeds between a transgenic and a non-transgenic line do occur (García-Villalba et al. 2008). In another study, differences in the expression of 10 proteins between transgenic and non-transgenic soybean seeds were also identified (Brandão et al. 2010). More recently, it was shown that the accumulation of lignin in roots is higher in transgenic glyphosate-resistant soybean plants (Zonetti et al. 2012). According to Arruda et al. (2013) genetic modification itself contributes for changes in a variety of traits of soybean plants, producing alterations, in a cascade manner, to the metabolism. Therefore, it may be possible that such changes may have implications in plant–insect interactions. In this study, we addressed whether genetically modified soybean plants that express a variant of the EPSPS emit similar constitutive and herbivore-induced VOC profiles as its isoline, and whether any alteration of the herbivore-induced VOC emissions can modify the behavior of a carnivore. As model system we used soybean plants CD202 and CD202RR that express CP4- EPSPS, Anticarsia gemmatalis (Lepidoptera: Erebidae) an oligophagous species and has great economical interest as it affects the soybean crop worldwide and Podisus nigrispinus (Hemiptera: Pentatomidae) one of the major predators of A. gemmatalis found in soybean crops in Brazil.
Materials and methods
Living material
Soybean (Glycine max) seeds (CD202 and CD202RR) were provided by COODETEC (Cascavel, Paraná, Brazil). Seeds were germinated at 25 °C in complete darkness. Three to 4 days after germination, seeds were individually sown in plastic pots (top 6 × 6 cm; bottom: 4.5 × 4.5 cm; height: 5.5 cm) on a commercial substrate (Vida Verde, São Paulo, Brazil). Plants were grown under artificial lighting with a light:dark regime of 14:10 h until they reach the V3 stage (Fehr et al. 1971) (approximately 5 weeks after sowing) when they were used for VOC analyses or behavioral tests. Plants were watered as required. Anticarsia gemmatalis was reared in controlled conditions of temperature (25 ± 1 °C) and photoperiod (14:10 L:D). Adults were maintained in PVC tubes lined with paper to support oviposition, and were fed on a honey-based solution (honey, sorbic acid, methyl paraben, sugar and distilled water) mixed with beer (3:1 v/v), which is used in mass rearing of A. gemmatalis (Campo et al. 1985). Eggs were collected every 2 or 3 days and placed in plastic boxes. Larvae were fed on a bean-based artificial diet (Parra 2001), and transferred to a plastic box filled with vermiculite to support pupation. Eggs of Podisus nigrispinus were obtained from a stock at the Federal University of Sergipe (Sergipe, Brazil). As the nymphs emerged, they were fed on third- or fourth-instar Spodoptera frugiperda larvae until they were used in the experiments. P. nigrispinus were also kept in controlled conditions of temperature (25 ± 1 °C) and photoperiod (14:10 L:D).
Volatile collection and analysis
VOCs were collected from undamaged (control) and herbivore-damaged plants of CD202 and CD202RR (four treatments). To damage the plant and induce the emission of VOCs each plant was infested when they reached the V3 stage with 10 third-instar larvae for 24 h. Even though A. gemmatalis oviposits isolatedly, 10 larvae per plant were used to ensure the induction of measurable VOCs. Larvae and frass were removed before sampling. Prior to sampling plants were removed from the pots and the roots and substrate were carefully wrapped in aluminum foil to reduce the emission of volatiles from below ground parts or the substrate. Then, each plant was individually enclosed in an airtight 1-L glass chamber. A humidified, charcoal-filtered airflow of 4 L/min was channeled through a glass tube and split four ways, resulting in an airflow of 1 L/min coming into four chamber. Therefore, this system allowed us to sample four plants (one replicate of each treatment) simultaneously. VOCs were trapped onto ca. 20 mg HayeSep® Q 80–100 mesh in a glass tube for a period of 24 h. After sampling, the upper part of the plants were cut and dried for 48 h at 60 °C to quantify the dry weight (DW). Trapped VOCs were eluted with 300 µL of double-distilled HPLC-grade hexane, and 10 µL of tetradecane (50 ppm) was added as an internal standard (IS). Samples were concentrated to 100 μL, and 1 µL of the extract was injected automatically in splitless mode (injector temperature 250 °C) and analyzed by GC–MS instrument (Shimadzu QP 2010 Plus) with an RTX-5 column (30 m × 0.25 mm i.d., 0.25 mm film thickness; Restek, Bellefonte, PA, USA). Helium was the carrier gas at a column head pressure of 170 kPa. The quantification of individual compounds was achieved on the basis of the peak area of the IS. Emissions were expressed as ng g DW−1 24 h−1. Compounds were identified by comparing their mass spectra with those from NIST mass spectrum libraries (NIST 27 and NIST 147) and by comparing the calculated Kovats Indices (KI) with those reported in the literature. In addition the mass spectra and retention times of (Z)-3-hexen-1-ol, (Z)-3-hexenyl acetate, octanal, 1-octen-3-ol, (Z)-β-ocimene, β-linalool, decanal, methyl salicylate, indole, (Z)-jasmone, β-caryophyllene and α-farnesene were compared with the mass spectra of synthetic compounds. A total of eight replicates (N = 8) for the various treatments were collected and analyzed, except for undamaged transgenic plants for which only six of the collected samples were analyzed (N = 6). All replicates were taken over a period of 8 days.
Odor preference of Podisus nigrispinus
The responses of P. nigrispinus were tested in dual-choice bioassays in a Y-tube olfactometer (Ø = 2 cm; main arm = 18 cm; smaller arms = 13 cm). A humidified charcoal-filtered airflow of 1.2 L/min was equally split into two parts, and pushed into 1-L chambers containing the odor sources. Thus, airflow of 0.6 L/min carried the odor sources through each small arm of the olfactometer towards the main arm. Each odor source consisted of a V3 stage soybean plant of either the transgenic or the non-transgenic line, uninfested or induced by A. gemmatalis as described above. Second- to third-instar nymphs, starved for 24 h were individually placed downwind at the end of the main arm and allowed to choose between the odor sources. Podisus nigrispinus nymphs were chosen for the experiments as they start to predate from the second instar (Vivan et al. 2002), and because the related species P. maculiventris actively respond in Y-tube olfactometer tests at this age (Bryant et al. 2014). We considered that an insect had responded when it passed the halfway point of one of the arms and remained there for at least 5 s. When a nymph did not make a decision during a 10-min period, it was considered as a non-responsive individual, and excluded from the data analysis. The olfactometer was rotated 180° after each observation. After every five observations position of the odor sources was switched and after every ten observations the plants were replaced for new ones, and the olfactometer were flushed with clean air for 2 min. The bioassays were conducted between 9 and 17 h at 25 °C, as they are more active during daytime (Torres et al. 2002). The following tests were conducted: (a) undamaged vs. herbivore-damaged plants (non-transgenic line); (b) undamaged vs. herbivore-damaged plants (transgenic line); (3) herbivore-damaged plants from the non-transgenic line vs. the herbivore-damaged plants from the transgenic line. The three different tests were conducted in the morning and in the afternoon on the same day to avoid any effect of the time and day over a period of 2 days.
Statistical analysis
Data analyses were processed with SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA). Data of VOC emissions were log transformed and analyzed with a MANOVA with genotype and herbivory and their interaction as main factors. The preference of the predators in the olfactometer was analyzed by applying the non-parametric Chi-square Test.
Results
Volatile emissions from soybean plants
Emissions of undamaged and herbivore-damaged soybean plants from CD202RR and CD202 are presented in Table 1. Genotype (MANOVA, Pillai’s Trace, P = 0.003), herbivory (MANOVA, Pillai’s Trace, P = 0.001) and the combination of both (MANOVA, Pillai’s Trace, P = 0.002) affected the VOC emission of soybean plants. Regardless of the genotype, herbivory by A. gemmatalis significantly increased or induced the emission of several aliphatic compounds, all terpenoids, all aromatic compounds, and two out of three unidentified compounds detected. Only the emissions of nonanal and decanal were not affected by the herbivore treatment (Table 2 for P values). Genotype had a significant effect on the emissions of (Z)-3-Hexen-1-ol (P = 0.001); 1-octen-3-ol (P = 0.022); (Z)-3-hexenyl acetate (P = 0.006), (Z)-β-ocimene (P < 0.001), β-linalool (P = 0.002); nonanal (P = 0.009); unidentified compound 1 (P < 0.001); unidentified compound 2 (P = 0.008); MeSA (P = 0.005); (Z)-hexenyl-2-methylbutyrate (P = 0.003); indole (P = 0.015) (Z)-jasmone (P = 0.001); β-caryophyllene (P = 0.004), α-caryophyllene (P = 0.004), α-farnesene (P = 0.017) and TMTT (P < 0.001). These effects are owed to variation in the induced emissions of transgenic plants compared to those from the non-transgenic line as the genotype had an effect on the herbivore-induced emission of soybean plants. With the exception of 3-octanone, 3-octanol and the unidentified compound 3, there was a significant interaction between the genotype and the herbivory treatment for all compounds. This suggests that the herbivore-induced emissions vary in the transgenic and the non-transgenic lines. Upon herbivory a greater emission of all the individual VOCs reported was found in the headspace of transgenic plants than non-transgenic plants (Fig. 1; Table 2 for P values).
Typical chromatograms from non-transgenic control (a), plants subjected to feeding by Anticarsia gemmatalis (b), transgenic soybean plants resistant to glyphosate control (c) and plants subjected to feeding by Anticarsia gemmatalis (d). Major compounds induced upon herbivory are: 1 (Z) 3-hexen-1-ol, 2 1-octen-3-ol, 3 4-hexen-1-ol, 4 (Z)-β-ocimene, 5 β-linalool, 6 unidentified compound 1, 7 unidentified compound 2, 8 MeSA, 9 (Z)-3-hexenyl-2-methylbutyrate, 10 indole, 11 (Z)-jasmone, 12 α-farnesene
Odor preference by Podisus nigrispinus
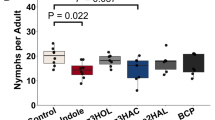
81.4 and 75 % of the nymphs observed in the olfactometer for comparing the odors of undamaged and herbivore-damaged plants from the isogenic line and the transgenic line, respectively, made a choice. Nymphs of P. nigrispinus were able to discriminate between undamaged and damaged plants of the non-transgenic line (P = 0.028) and they showed a trend to orientate towards damaged plants over undamaged plants of the transgenic line, even though the result was not statistically significant (P = 0.083). However, only 65.5 % of the nymphs observed made a choice when damaged plants of both of the lines were offered. Moreover, nymphs did not discriminate between herbivore-damaged plants of the non-transgenic line and from the transgenic line (P = 0.194) (Fig. 2).
Orientation of nymphs of Podisus nigrispinus towards the odors of undamaged- (U) or Anticarsia gemmatalis-damaged (H) plants in transgenic (T) plants and its isoline (I), and to the odors of herbivore-damaged plants from both of the lines. Asterisk shows the level of significance P < 0.05*, n.s. means “not significant”. Numbers on the left show the total of insects that responded in the test (N). The number of non-responsive insects is shown in brackets
Discussion
Our major finding is that herbivory by A. gemmatalis substantially increased the VOC emission of transgenic glyphosate-resistant plants compared to its isogenic line. Constitutive emissions of VOCs were not dramatically affected by the genetic modification. There are two possible explanations for our findings. The first explanation is that the induction and emission of VOC may have been altered in transgenic plants. According to Arruda et al. (2013) genetic modifications in glyphosate-resistant plants alter the metabolism of the whole plant. In fact there is evidence that the metabolism of the lignin content in roots of transgenic soybean plants is different compared with the isogenic line (Zonetti et al. 2012). In maize, Bt lines emit lower quantities of VOCs than non-transgenic lines as a result of not only reduced insect feeding but also altered carbon allocation in the plant (Dean and De Moraes 2006; Turlings et al. 2005). A second speculative explanation is that Anticarsia gemmatalis may have modified its feeding behavior, for example by increasing the duration of feeding on the transgenic plants what may have led to larger emissions of VOCs. This may be possible considering that metabolic changes in primary or secondary metabolism may have occurred (García-Villalba et al. 2008; Brandão et al. 2010; Zonetti et al. 2012; Arruda et al. 2013). Potential changes in primary and secondary metabolites in transgenic glyphosate-resistant plants that mediate plant–insect interactions deserve further investigation, and this is currently under study in our laboratory.
The results of the bioassays showed that nymphs of P. nigrispinus prefer the odors of herbivore-damaged plants to the odors from undamaged plants of the non-transgenic line. However, they are less able to discriminate between herbivore-damaged and undamaged transgenic plants. From the measurements of herbivore-induced VOCs, it is evident that the emissions of induced compounds (particularly terpenoids, (Z)-3-hexenyl acetate, (Z)-3 hexenyl-2-methyl butyrate and (Z)-jasmone) are several times larger in the transgenic plants than in the non-transgenic plants. This increment in VOC emissions was not a stimulus for P. nigrispinus to orientate towards the odors of damaged transgenic plants. Ratios between a few active compounds are crucial for insects to orientate towards suitable hosts (Bruce et al. 2005, 2010) and probably new ratios in the blend emitted by transgenic plants may have altered the perception of P. nigrispinus. P. nigrispinus is known to detect at least a few plant volatiles, but the response to specific blends and herbivore-induced VOCs remains unknown (Sant’ana and Dickens 1998). Moreover, compounds emitted in very low quantities may be important cues for discriminating odors by natural enemies (D’Alessandro et al. 2009) and they do not necessarily orientate towards plants emitting larger quantities of volatiles (Fritzsche-Hoballah et al. 2002; Bruce et al. 2010). In Bt maize, for instance, decreased emission of VOCs by transgenic plants does not affect the foraging behavior of parasitic wasps (Turlings et al. 2005). Based on the bioassays, we expected that nymphs would have preferred the odor of damaged plants emitted by the isogenic line over those emitted by the transgenic line. Instead, they did not discriminate between the odor sources. It may be possible that larger emissions of particular compounds may have led to volatile interference that affected the response of the insects (Soler et al. 2007). On the other hand, it has to be taken into account that P. nigrispinus, is a generalist insect that feeds on a wide range of preys within a wide range of crops (Torres et al. 2006). Generalist predators are known to respond differently to volatile compounds depending on previous experiences with the prey (Drukker et al. 2000) and to modulate their responses to different plant genotypes after associative learning (Glinwood et al. 2011). Previous behavioral studies conducted in a Y-tube olfactometer set-up assessing the responses of non-experienced P. maculiventris, a related species from P. nigrispinus have failed to establish a VOC-mediated interaction between undamaged plants and P. maculiventris-damaged plants (Vuorinen et al. 2004; Bryant et al. 2014). Likewise, Hippodamia convergens and three species of predatory mites failed to discriminate the odors of damaged plants and undamaged plants in behavioral studies conducted in Y-tube olfactometers (Himanen et al. 2005; Bryant et al. 2014). Podisus species may be able to discriminate the odors induced by their prey in particular cultivars (Vuorinen et al. 2004). Nevertheless, as generalist insects, their searching time and olfactory responses may benefit from associative learning. Thus, our results may reflect the lack of experience of the insects used in the bioassays.
The results of this study suggest that there is an altered interaction between the transgenic line and the herbivory of A. gemmatalis that leads to increased emissions of herbivore-induced VOCs. However, based on the results we are not able to conclude how such increases may affect the behavior of higher trophic levels. Further studies using more specialized carnivores or other herbivores and their parasitoids as model insects may be helpful to better understand whether our findings on herbivore-induced VOCs can affect the foraging of natural enemies or can support our results.
References
Arimura GI, Shiojiri K, Karban R (2010) Acquired immunity to herbivory and allelopathy caused by airborne plant emissions. Phytochemistry 71:1642–1649
Arruda MAZ, de Sousa Barbosa H, Mataveli LRV, Gratão PL, Azevedo RA, Arruda SCC, Oliveira SR (2013) Comparative studies involving transgenic and non-transgenic soybean: what is going on?. INTECH Open Access Publisher. http://cdn.intechopen.com/pdfs-wm/40087.pdf
Brandão AR, Barbosa HS, Arruda MAZ (2010) Image analysis of two-dimensional gel electrophoresis for comparative proteomics of transgenic and non-transgenic soybean seeds. J. Proteomics 73:1433–1440
Bruce TJA, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274
Bruce TJA, Midega CAO, Birkett MA, Pickett JA, Khan ZR (2010) Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol Lett 6:314–317
Bryant A, Coudron T, Brainard D, Szendrei Z (2014) Cover crop mulches influence biological control of the imported cabbageworm (Pieris rapae L., Lepidoptera: Pieridae) in cabbage. Biol Control 73:75–83
Campo CBH, Oliveira EB, Moscardi F (1985) Criação massal de lagarta da soja (Anticarsia gemmatalis). Embrapa CNPSo. 23p
D’Alessandro M, Brunner V, von Mérey G, Turlings TC (2009) Strong attraction of the parasitoid Cotesia marginiventris towards minor volatile compounds of maize. J Chem Ecol 35:999–1008
Dean JM, De Moraes CM (2006) Effects of genetic modification on herbivore-induced volatiles from maize. J Chem Ecol 32:713–724
Drukker B, Bruin J, Sabelis MW (2000) Anthocorid predators learn to associate herbivore-induced plant volatiles with presence or absence of prey. Physiol Entomol 25:260–265
Fehr W, Caviness C, Burmood DT, Pennington JS (1971) Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci 11:929–931
Ferry N, Gatehouse AMR (2009) Environmental impact of genetically modified crops. CABI International, Cambridge
Food and Agriculture Organization (2011) Genetically modified crops. http://www.fao.org/docrep/015/i2490e/i2490e04d.pdf. Accessed 4 Sept 2014
Fritzsche-Hoballah ME, Tamò C, Turlings TC (2002) Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid cotesia marginiventris: is quality or quantity important? J Chem Ecol 28:951–968
García-Villalba R, León C, Dinelli G, Segura-Carretero A, Fernández-Gutiérrez A, Garcia-Cañas V, Cifuentes A (2008) Comparative metabolomic study of transgenic versus conventional soybean using capillary electrophoresis–time-of-flight mass spectrometry. J Chromatogr A 1195:164–173
Glinwood R, Ahmed E, Qvarfordt E, Ninkovic V (2011) Olfactory learning of plant genotypes by a polyphagous insect predator. Oecologia 166:637–647
Gouinguené SP, Städler E (2006) Oviposition in Delia platura (Diptera, Anthomyiidae): the role of volatile and contact cues of bean. J Chem Ecol 32:1399–1413
Himanen S, Vuorinen T, Tuovinen T, Holopainen JK (2005) Effects of cyclamen mite (Phytonemus pallidus) and leaf beetle (Galerucella tenella) damage on volatile emission from strawberry (Fragaria × ananassa Duch.) plants and orientation of predatory mites (Neoseiulus cucumeris, N. californicus, and Euseius finlandicus). J Agric Food Chem 53(22):8624–8630
Himanen SJ, Nerg AM, Nissinen A, Pinto DM, Stewart CN Jr, Poppy GM, Holopainen JK (2009) Effects of elevated carbon dioxide and ozone on volatile terpenoid emissions and multitrophic communication of transgenic insecticidal oilseed rape (Brassica napus). New Phytol 181:174–186
Maeda H, Dudareva N (2012) The shikimate pathway and aromatic amino acid biosynthesis in plants. Ann Rev Plant Biol 63:73–105
McCormick AC, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310
Parra JRP (2001) Técnicas de Criação de Insetos para Programas de Controle Biológico. FEALQ, Piracicaba
Pinto-Zevallos DM, Hellén H, Hakola H, van Nouhuys S, Holopainen JK (2013) Induced defenses of Veronica spicata: variability in herbivore-induced volatile organic compounds. Phytochem Lett 6:653–656
Proffit M, Birgersson G, Bengtsson M, Reis R Jr, Witzgall P, Lima E (2011) Attraction and oviposition of Tuta absoluta females in response to tomato leaf volatiles. J Chem Ecol 37:565–574
Raguso RA (2008) Wake up and smell the roses: the ecology and evolution of floral scent. Annu Ver Ecol Evol Syst 39:549–569
Rostás M, Eggert K (2008) Ontogenetic and spatio-temporal patterns of induced volatiles in Glycine max in the light of the optimal defence hypothesis. Chemoecology 18:29–38
Sant’ana J, Dickens JC (1998) Comparative electrophysiological studies of olfaction in predaceous bugs, Podisus maculiventris and P. nigrispinus. J Chem Ecol 24:965–984
Schuler TH, Potting RP, Denholm I, Poppy GM (1999) Parasitoid behaviour and Bt plants. Nature 400:825–829
Soler R, Harvey JA, Kamp AF, Vet LE, Van der Putten WH, Van Dam NM, Stuefer JF, Gols R, Hordijk CA, Martijn Bezemer T (2007) Root herbivores influence the behaviour of an aboveground parasitoid through changes in plant‐volatile signals. Oikos 116(3):367–376
Torres JB, Evangelista WS, Barras R, Guedes RNC (2002) Dispersal of Podisus nigrispinus (Het., Pentatomidae) nymphs preying on tomato leafminer: effect of predator release time, density and satiation level. J Appl Entomol 126:326–332
Torres JB, Zanuncio JC, Moura MA (2006) The predatory stinkbug Podisus nigrispinus: biology, ecology and augmentative releases for lepidopteran larval control in Eucalyptus forests in Brazil. CAB Rev: Perspect Agric Vet Sci Nutr Nat 15:1–17
Turlings TC, Jeanbourquin PM, Held M, Degen T (2005) Evaluating the induced-odour emission of a Bt maize and its attractiveness to parasitic wasps. Transgenic Res 14:807–816
Vivan LM, Torres JB, Veiga AF, de Souza L, Zanuncio JC (2002) Comportamento de predação e conversão alimentar de Podisus nigrispinus sobre a traça-do-tomateiro. Pesquisa Agropecuária Brasileira 37:581–587
Vogler U, Rott AS, Gessler C, Dorn S (2009) Terpene-mediated parasitoid host location behavior on transgenic and classically bred apple genotypes. J Agric Food Chem 57:6630–6635
Vogler U, Rott AS, Gessler C, Dorn S (2010) Comparison between volatile emissions from transgenic apples and from two representative classically bred apple cultivars. Transgenic Res 19:77–89
Vuorinen T, Nerg AM, Ibrahim MA, Reddy GVP, Holopainen JK (2004) Emission of Plutella xylostella-induced compounds from cabbages grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiol 135(4):1984–1992
Xiao Y, Wang Q, Erb M, Turlings TCJ, Ge L, Hu L, Li J, Han X, Zhang T, Lu J, Zhang G, Lou Y (2012) Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol Lett 15:1130–1139
Yan F, Bengtsson M, Anderson P, Ansebo L, Xu C, Witzgall P (2004) Antennal response of cotton bollworm (Heliocoverpa armigera) to volatiles in transgenic Bt cotton. J Appl Entomol 128:354–357
Zonetti PC, Suzuki LS, Bonini EA, Ferrarese MLC, Ferrarese-Filho O (2012) High temperatures on root growth and lignifications of transgenic-resistant soybean. Agrociencia 46:557–565
Acknowledgments
The authors would like to thank Dr. James D. Blande for comments and improvement of the language, Dr. Marco Antonio Root de Oliveira and Mrs. Franciele Bebber from COODETEC-Cascavel who kindly provided us with the seed material; Prof. Adeney F. Bueno from EMBRAPA-Londrina and Prof. Braulio Santos for the eggs to start the rearing of A. gemmatalis, and Prof. Genésio Tâmara Ribeiro for the P. nigrispinus used in the bioassays. Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq Processo No. 401928/2012-8, Coordenacão de Pessoal de Aperfeiçoamento de Nível Superior (CAPES) and Instituto Nacional de Ciências e Tecnologia de Semioquímicos na Agricultura (INCT) are acknowledged for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Michael Heethoff.
Rights and permissions
About this article
Cite this article
Strapasson, P., Pinto-Zevallos, D.M. & Zarbin, P.H.G. Soybean (Glycine max) plants genetically modified to express resistance to glyphosate: can they modify airborne signals in tritrophic interactions?. Chemoecology 26, 7–14 (2016). https://doi.org/10.1007/s00049-015-0202-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-015-0202-9