Abstract
We present a tritrophic analysis of the potential non-intended pleiotropic effects of cry1Ac gene derived from Bacillus thurigiensis (Bt) insertion in cotton (DeltaPine 404 Bt Bollgard® variety) on the emission of herbivore induced volatile compounds and on the attraction of the egg parasitoid Trichogramma pretisoum (Hymenoptera: Trichogrammatidae). Both the herbivore damaged Bt variety and its non-Bt isoline (DeltaPine DP4049 variety) produced volatiles in higher quantity when compared to undamaged plants and significantly attracted the egg parasitoids (T. pretiosum) when compared to undamaged plants. However, Trichogramma pretiosum did not differentiate between the transgenic and nontransgenic varieties, suggesting that the ratios between the compounds released by herbivory damaged -Bt cotton and herbivory damaged-nonBt cotton did not change significantly. Finally, no detrimental effect of the Bt genetic engineering was detected related to the volatile compounds released by Bollgard cotton on the behavior of the natural enemy studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The insertion of genes expressing insecticidal proteins (δ-endotoxin) from Bacillus thuringiensis (Bt) Berliner as Cry toxins in cultivated plants represents an innovation in pest control, which has been increasingly adopted on a large scale in world agriculture over the past decade (ISAAA 2006). This technology is considered an important tool in pest management by allowing the reduction in the number of applications of synthetic chemical pesticides and promoting more effective control against a variety of pest species throughout the whole crop cycle (Fitt and Wilson 2000; Sharma and Ortiz 2000; Wu 2001).
The Bt varieties available commercially in several countries provide protection against some pests in the orders Lepidoptera and Coleoptera (Estruch et al. 1996; Perlak et al. 1990, 2001). In March 2005 the Brazilian National Technical Commission on Biosafety (CTNBio, 2006) approved Bollgard® cotton expressing the Cry1Ac insecticidal protein (CTNBio 2006) for planting on a commercial scale, and since then several commercial varieties have been introduced. Cry1Ac expression in cotton was proposed to protect from attack by lepidopteran pests like budworm-bollworm complex (Heliothis virescens, Helicoverpa spp, Alabama argilacea, Pectinophora gossypella) and it was quickly incorporated in Brazilian agriculture, and in only 3 years the total cropped area reached 800 thousand hectares (http://www.conab.gov.br/conabweb/IA-nov09.pdf, MDM-Seeds 2008).
Despite the benefits of this technology, genetic engineering potentially can generate unpredictable unintended effects in non-target insects such as natural enemies (parasitoids or predators) of the pests, altering the natural biological control capacity existing in the agroecosystem. Parasitoids and predators are important natural enemies of many pest species and are used extensively in biological and integrated control programs. These insects use different cues to locate their hosts, and the volatile compounds released by the plants when damaged by herbivory are important cues used by these insects when foraging for their hosts (Turlings et al. 1990, 2005; Rose et al. 1996, 2004, 2005; Moraes et al. 2005, 2008, 2009).
Few studies have been conducted to evaluate the influence of the Bt genetic engineering on the secondary metabolism of the plants and its influence on parasitoids and predators. Yan et al. (2004) studied volatile composition of a transgenic Bt cotton variety (GK-97) and its parental variety (Simina No. 3) and showed that the profile of volatiles of both varieties was very similar. However, the transgenic variety produced higher quantities of two monoterpenes, α-pinene and β-pinene, and a minor compound that was not identified. In addition, the authors did not observe differences in the electrophysiological response of males and females of Helicoverpa armigera to the volatiles from Bt and non-Bt cotton. The authors did not conduct behavioral bioassays to evaluate if the blend of the compounds released by the Bt and non Bt cotton could affect the behavior of H. armigera. Therefore, they could not infer if the Bt genetic engineering caused some alteration of the chemical communication between the plant and its herbivore. Turlings et al. (2005) evaluated the volatile organic compounds released by a herbivore damaged Bt maize (Bt11, N4640Bt) and its parental variety (N4640). The chemical analysis comparing Bt maize and non-Bt maize showed that the same compounds were released, but that the non-Bt maize released higher quantities of the compounds when compared to Bt maize. In addition, the two species of parasitoids evaluated did not show preference in bioassays between Bt maize and non-Bt maize plants.
A better understanding of the interaction between transgenic plants, pests and parasitoids is necessary to limit disruption of biological control and to provide background knowledge essential for implementing measures for the conservation of natural enemy populations. The aim of this study was to understand whether Bt genetic engineering in cotton affects a volatile-mediated tritrophic interaction. Our system consisted of a transgenic (DeltaPine 404 Bt Bollgard®) and a non-transgenic (DeltaPine DP4049) cotton variety. First we compared the volatile chemical profiles of the two varieties in response to feeding behavior by the chewing larvae of Spodoptera frugiperda (Lepidoptera: Noctuidae). Second, we evaluated whether this modification affected the attraction of the egg parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) in laboratory bioassays.
Materials and methods
Plants and insects
Cotton varieties DP404 (Bt Bollgard®) and its non-Bt isoline DP4049 were planted in 300 mL plastic pots and grown in a controlled chamber (at 25°C with 16 h of photoperiod). Plants were used in experiments 30–35 days after germination.
Eggs of S. frugiperda were reared in the laboratory under 14 h photoperiod at 26.0 ± 0.5°C and 65 ± 10% relative humidity. Approximately 20 eggs glued on a strip of paper (3 × 6 cm), were placed in 250 mL plastic cups and two blocks (2 × 2 × 2 cm) of artificial diet were provided as food. When larvae reached the second instar they were separated and used in the bioassays (Schmidt et al. 2001).
Trichogramma pretiosum were reared in angled-neck 25 cm2 plastic tissue culture flasks (ICN Biomedicals, Irvine, CA, USA) on S. frugiperda or Anticarsia gemmatalis (Hübner) (Lepidoptera: Noctudiae) eggs under the same environmental conditions described for S. frugiperda. A droplet of pure honey was supplied in each flask as a food source. Adult parasitoids obtained with these procedures were kept for 24–48 h in the plastic cages described above for mating. Later, the females were separated individually into glass tubes (4 × 0.5 cm) for use in bioassays.
Volatile analysis
To evaluate the profile of defensive compounds released we compared four treatments: 1–Undamaged Bt cotton (UD-Bt); 2–herbivory damaged Bt cotton (by five S. frugiperda larvae) (HD-Bt), 3–undamaged nonBt cotton (UD-nonBt); 4–herbivory damaged nonBt cotton–(by five S. frugiperda larvae) (HD-nonBt). The damage lasted either 24, 48 or 72 h depending on the experiment (described below). A system with 12 independent glass chambers was run simultaneously for volatiles collection at 1,200 mL/min of air flux. The volatiles were captured in 8.0 cm long × 0.7 cm diameter glass tubes with 60 mg of Super Q adsorbent (Alltech 80/100 mesh–Alltech Associates, Inc.) during 24 h over 3 days in a total of 5 replicates per treatment. The volatile trap was extracted with 1 mL of hexane and the solvent evaporated under pure nitrogen to 200 μL. The compounds were quantified using 0.03 mg/mL of 16-hexadecanolide as an internal standard. The quantitative analysis was carried out in a gas chromatography flame ionization detector (Perkin Elmer, column DB-5, 30 m × 0.25 mm ID, 0.25 μm film) with the column maintained at 50°C for 2 min and then programmed at 5°C/min to 150°C and 10°C/min until 250°C (20 min) and helium was used as carrier gas. The qualitative composition of the chosen extracts was analysed using a QP-2010 Shimadzu quadruple mass spectrometer equipped with a DB-5 capillary column (30 m × 0.32 mm ID, 0.25 μm film) and splitless injector. The oven temperature was programmed at 50°C for 2 min and then on a ramp at 5°C/min to 150°C and 10°C/min until 250°C (held for 30 min). Ionization was by electron impact (70 eV, source temperature 250°C). Compound identification was carried out by comparison of spectra with library databases (NIST) and subsequent confirmation using authentic standards.
Bioassays
To compare the effect of the volatiles released by Bt and non-Bt varieties damaged and undamaged by herbivory of S. frugiperda larvae, bioassays were carried out using a dual-choice (Y-tube) olfactometer. The plants were placed in 1 L glass chambers and exposed to five S. frugiperda second instar larvae per plant. After the damage (24–72 h), the larvae were removed to avoid any interference of larvae volatiles with parasitoid behavior. The chambers were connected to the olfactometer by silicon tubes. An acrylic box with a Y-shaped cavity (27.5 × 21.0 cm) sandwiched between two glass plates was used as the bioassay olfactometer (Moraes et al. 2005). An air flow was generated by a push–pull system with aquarium air pumps, filtered using an activated charcoal filter (0.4 mL/h), passed through the glass chambers with the plants and then into the olfactometer. The olfactometer was illuminated from above by two fluorescent lamps (40 W) and from below by two infrared lamps (homogenous emission of wavelengths at 950 nm provided by 108 LEDs). For each bioassay, a single naïve (without plant and oviposition experience) T. pretiosum female (24–48 h in the adult stage) was introduced at the base of the Y-tube, and its behavior was monitored for 10 min. Insects that had not made a choice after 5 min were considered as non responders, and they were not included in the statistical analyses. To test for any bias between the two olfactometer arms, blank tests were carried out in each set of bioassays, presenting clean air through both arms. All bioassays were performed between 10:00 and 16:00 h in a room at 26.0 ± 1.0°C. During each bioassay, two behavioral parameters were recorded: first choice, measured as the arm of the olfactometer into which the insect entered first for at least 1 cm and remained for at least 20 s; and residence time, measured as the percentage of total bioassay time spent in each arm of the olfactometer. First, to test if the parasitoid recognizes and selectively responds to the volatiles of cotton varieties when compared clean air, the following combination of treatments were carried out: air versus air, UD-nonBt versus air, UD-Bt versus air, HD-nonBt versus air, HD-Bt versus air. Second, to test if the parasitoid selectively responds to one of the two varieties under study, the following combinations were tested: HD-nonBt versus UD-nonBt (N = 70), HD-Bt versus UD-Bt (N = 70), UD-Bt versus UD-nonBt (N = 70), HD-Bt versus HD-nonBt (N = 70), UD-Bt versus HD-nonBt (N = 70), HD-Bt versus UD-nonBt (N = 70).
Statistical analysis
The amount of the main compounds identified in each treatment (converted to natural logarithms) was compared by repeated measures split-plot ANOVA, fitting treatment as the explanatory variable and time as the within-subject (plant) factor.
The choices made by the parasitoids in the bioassays were analyzed by logistic regression and estimation of the probability of choosing the test odor. The model used a factor for the side (left or right) on which the test odor was presented to control for this variability. The hypothesis of no preference (50% first choice to each odor) was tested by means of a χ2 Wald test. The time spent in each odor field was analyzed by Wilcoxon’s matched-pairs test after arcsine transformation of the data. Previous results using HD vs. UD Bt and non Bt plants showed no differences in the response of the parasitoid over the time after herbivory damage (i.e. 24, 48 or 72 h) to cotton plants. The initial choice of the parasitoid was significantly biased to the HD plants in the three times evaluated (χ2 p < 0.05 for both varieties) and the time from the liberation of the insects in the olfactometer until the choice of one of the arms by the parasitoid was the same when the bioassays were conducted with plants in the three different periods of time considered (Kruskal–Wallis Anova H = 1.60 df = 2 p = 0.449 and H = 0.472 df = 2 p = 0.790 for Bt plants and NonBt plants respectively). Therefore, the statistical analyses of the plant-parasitoid bioassays were performed for the complete set of bioassays. The analyses were carried out using the R programming language (R Development Core Team 2008).
Chemicals
Super Q (80/100 mesh) was purchased from Alltech (PA, USA). The following chemicals were purchased from Sigma–Aldrich (Steinheim, Germany); α-Pinene (98%); camphene (75%); β-pinene (99%); myrcene (90%), (Z)-3-hexenyl acetate (98%), limonene (97%), (E)-β-ocimene, indole (98.5%), (E)-β-farnesene (90%). The compound β-caryophyllene (90%), was purchased from (TCI-America, Portland, US). (E,E)-α-farnesene was provide by Dr. J. Aldrich; (E)-4,8-Dimethylnona-1,3,7–triene and (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene were synthesized from geraniol and (E,E)-farnesol respectively, by oxidation to their corresponding aldehydes followed by Wittig methylenation (Leopold 1990) and were provide by Dr. M Birkett (from Rothamsted Research).
Results
Volatile analysis
Second instar larvae of Spodoptera frugiperda fed on Bt and non-Bt cotton in a similar way, that is, no differences were observed in the area damaged by larvae in each variety. In addition, as expected, all larvae survived after 3 days feeding on Bt cotton.
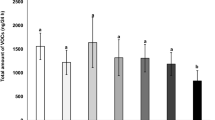
The total amount of volatiles released by cotton varieties was different among the treatments (F 3,52 = 4.876, p = 0.005). HD plants volatiles liberation was higher than UD plants. The effect of the time was not significant (F 1,52 = 0.0002, p = 0.988), however there was a significant interaction between treatment and time (F 3,52 = 3.119, p = 0.033) (Fig. 1). The graphical analyses showed that both varieties responded similarly to the herbivory attack with an increase of the total amount of volatiles released (Fig. 1).
The analysis of the extracts of each treatment did not show any qualitative difference in the chemical composition of the volatile blends released (Fig. 2). However, there was a significant difference in the quantities released when comparing damaged and undamaged plants, and this was observed for both the Bt and the non-Bt variety among the treatments and over the time (Fig. 2). In the Bt cotton variety, the following compounds were released in higher quantities elicited by herbivory along the 3 days: α-pinene (F 1,8 = 9.148, p = 0.016), β-pinene (F 1,8 = 13.730, p = 0.006), (E)-β-ocimene (F 1,8 = 14.122, p = 0.006) and (E)-β-caryophyllene (F 1,8 = 8.131, p = 0.021); while TMTT (F 1,8 = 5.42, p = 0.048) was released in significantly higher quantity only on the third day (Fig. 2a–c). Although the other compounds did not show statistical significance when compared to undamaged plants, an increase was apparent in all compounds analysed.
Qualitative and quantitative (mean and error standard) comparison of the volatile organic compounds released by cotton varieties in the different treatments over 3 days. a First day (24 h): Herbivory damage on Bt cotton (HD-Bt, n = 5), Undamaged Bt cotton (UD-Bt, n = 5), b Second day (48 h): HD-Bt (n = 5) and UD-Bt (n = 5), c Third day (72 h): HD-Bt (n = 5) and UD-Bt (n = 5), d First day: Herbivory damage (HD-non Bt, n = 5) and undamaged non-Bt cotton (UD-non Bt), e Second day: HD-non Bt (n = 5) UD-non Bt (n = 5), f Third day on HD-non Bt (n = 5) UD non-Bt (n = 5). Compound numbering is as follows: 1. α-pinene; 2. camphene; 3. β-pinene; 4. myrcene; 5. (Z)-3-hexenyl acetate; 6. limonene; 7. (E)-β-ocimene; 8. (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT); 9 Indole; 10. (E)-β-caryophyllene; 11. (E,E)-α-farnesene; 12. (E)-β-farnesene; 13. (E,E)-4,8,12-tetramethyl-1,3,7,11-tridecatetrene (TMTT). *0.05 > p>0.01, and **0.01 > p > 0.001
In the non-Bt variety only, (E)-β-ocimene was released in higher quantity throughout the 3 days following the onset of herbivore damage (F 1,8 = 20.733, p = 0.002) (Fig. 2d–f). The compounds TMTT (F 1,8 = 13.444, p = 0.005) and β-caryophyllene (F 1,8 = 8.733, p = 0.021) were released in higher quantities from the second day onwards (Fig. 2d–f). Several compounds showed an increase in their release when compared to undamaged nonBt plants, mainly on the third day (Fig. 2f), but statistical significance was marginal. This was the case for compounds such as α-pinene (F 1,8 = 3.647, p = 0.085), camphene (F 1,8 = 4.176, p = 0.071) and β-pinene (F 1,8 = 4.176, p = 0.071). When we compared the individual compounds released by the HD-Bt variety with those of the HD-nonBt variety and UD-Bt with UD-nonBt, we did not observe significant differences in the quality or quantity of the compounds released.
Bioassays
The parasitoid T. prestiosum showed a significant response (measured by both first choice and residence time) to cotton volatiles of undamaged plants from both Bt and nonBt varieties when compared against clean air (Fig. 3). The parasitoid also showed significant responses to herbivore-damaged plants from both varieties when tested against clean air (Fig. 3). When the nonBt was tested against the Bt variety the parasitoid showed no preference, whether both were herbivory damaged or not. In bioassays comparing undamaged vs. herbivore damaged plants T. pretiosum females showed a clear preference for the herbivore-damaged plants when measured by the first choice (Fig. 4a). This preference was evident for all the combinations tested. For residence time the cross-variety comparisons (UD-nonBt vs HD-Bt and UD-Bt vs HD-nonBt) residence time did not differ between the odour fields (Fig. 4b).
First choice (a) and residence time (b) of the parasitoid Trichogramma pretiosum in Y-tube olfactometer bioassays with two cotton varieties (DeltaPine 404 Bt Bollgard® and the parental variety N4640) undamaged (UD) or herbivore damaged by Spodoptera frugiperda larvae (HD) and clean air. * p < 0.05. Bars indicate the mean values of the parameters and lines are the 95% confidence intervals. The parameters were estimated from the behaviour of 50 females, numbers to the right of A indicate the number of insects tested that did not show response to any treatment
First choice (a) and residence time (b) of the parasitoid Trichogramma pretiosum in Y-tube olfactometer bioassays with two cotton varieties (DP404 Bt Bollgard and the isoline variety DP4049) undamaged (UD) or herbivore damaged by Spodoptera frugiperada larvae (HD). * p < 0.05. Bars indicate the mean values of the parameters and lines are the 95% confidence intervals. The parameters were estimated from the behaviour of 50 females, numbers to the right of figure A indicate the number of insects tested that did not show response to any treatment
Discussion
The total amount of volatiles released by both varieties evaluated in this study changed between the treatments and showed an interaction between time and treatment, which suggest an effect of herbivory caused by S. frugiperda, instead of an effect of the Bt gene. Both Herbivory damaged varieties showed an increase, and the undamaged varieties showed a decrease on the amount of total volatiles released. On the first day HD-Bt cotton released a larger quantity of α-pinene, β-pinene, (E)-β-ocimene and (E)-β-caryophyllene when compared to UD-Bt cotton, and this effect continued throughout the 3 days studied. On the first day the HD-nonBt cotton released only (E)-β-ocimene in larger quantity when compared to UD-nonBt cotton. However, along the following days it showed a similar profile of compounds compared to the Bt variety.
Yan et al. (2004) studied the volatile composition of the transgenic Bt cotton variety GK-97 and its parental non-Bt variety, Simina No. 3, and showed that the volatile composition of the Bt and the parental variety was very similar, with the transgenic variety producing higher quantities of two monoterpenes, α-pinene and β-pinene and a minor compound that was not identified. Turlings et al. (2005), working with two Bt cultivars of maize (Bt11, N4640Bt) and an isogenic line (4640), observed that the HD-nonBt cultivar released a higher quantity of volatile compounds when compared to HD-Bt maize. Vogler et al. (2009) also showed that the headspace volatiles of transgenic scab-resistant apple plants compared to two cultivars, an isogenic Gala and Florina, did not emit a qualitatively different profile of volatiles when submitted to herbivore-damage and/or fungal infection, but they observed differences in the amounts of terpenoid compounds released.
Previous studies with different cultivars of cotton have shown the importance of herbivore-induced volatiles in the attraction of natural enemies of herbivores (De Moraes et al. 1998; Paré and Tumlinson 1998; Rose and Tumlinson 2004, 2005; Bezemer et al. 2004; Olson et al. 2008). In a similar way the egg parasitoid T. pretiosum also responded to herbivore-damaged plants (24, 48 and 72 h after the feeding damage) when compared to undamaged plants. Herbivory damaged Bt plants had the same effect on the attraction of the parasitoid as did herbivore-damaged nonBt plants in the olfactometer bioassays, which corroborates results from previous studies (Schuler et al. 1999; Turlings et al. 2005). The egg parasitoid was used in this study because it is well known that parasitoids use different cues to find their hosts, including information from plants and insects that are not directly linked to their hosts, such as those from the plant-herbivore system (Vinson 1985; Laumann et al. 2009; Moraes et al. 2008, 2009). Using an egg parasitoid also allowed us to evaluate the influence of Bt plants on natural enemies that did not has direct contact Bt toxins.
The range of variability in odour emissions among different vegetal lines, considering the same crop, is a common standard considering the total amount produced (quantity), as well as the composition of the blend (quality) (Fritzsche-Hoballah et al. 2002). Therefore, this corroborates the idea that the parasitoid uses as information the ratio of the compounds more than the presence or absence of one or more compounds or the total amount released by the plants (Turlings et al. 2005; Moraes et al. 2008, 2009).
The data available to date relating Bt transgenes and induced volatile release by crop plants, including the data presented here, indicate that the quantities of the compounds changed, but the ratio between the compounds did not change enough to affect the behavior of herbivore natural enemies (Turlings et al. 2005). Parasitoids and predators are likely not to use one or two compounds from the blends emitted by the plants to find their hosts, but several compounds, and the ratio between the components is likely to be very important information (Rose and Tumlinson 2004; Pareja et al. 2009; Moraes et al. 2009; Turlings et al. 2005). We know that the main plant volatiles used by natural enemies are very similar and composed basically of green leafy volatiles (De Moraes et al. 1998; Turlings et al. 1998; Heil 2004; Kost and Heil 2006; Pareja et al. 2007; Williams et al. 2008; Ozawa et al. 2008), including monoterpenes, sesquiterpenes and homoterpenes (De Moraes et al. 1998; Turlings et al. 1998; Colazza et al. 2004; Zhu and Park 2005; Heil 2004; Rose and Tumlinson 2005; Kost and Heil 2006; Williams et al. 2008) and compounds derived from shikimic acid (Turlings et al. 1998; Zhu and Park 2005; D’Alessandro et al. 2006; Moraes et al. 2008, 2009). Further studies are necessary to identify which compounds or ratios between these compounds are essential in the induced blend for the attraction of the natural enemy, to understand how the natural enemies achieve this specific response to plant volatiles from different varieties in the context of a complex volatile blend, and how the Bt proteins affect the quantitative profile of volatile compounds (D’Alessandro et al. 2006; Maffei et al. 2007; Heil 2008; Heil and Ton 2008; van Dam and Poppy 2008).
Although the studies with Bt plants evaluating indirect defense suggest that there is no change in the abundance of natural enemies, this cannot be taken to mean there is no effect on these natural enemies as discussed above. For example, Schuler et al. (1999) reported that the larvae of Plutella xylostella susceptible to Bt oilseed rape died within 5 days after feeding on Bt plants, and the larvae of the parasitoid Cotesia plutellae need 7 days to finish their life cycle.
Most biosafety studies have compared transgenic crops to corresponding non-transgenic crops, taking into account the host/prey using as model predators and larval parasitoids (Romeis et al. 2006). The results of these previous studies showed a deleterious effect on predators and parasitoids; however, most of this effect was not provoked by Bt protein directly, but indirectly, because the Bt crops affected the quantity and quality of their food. Similar results were obtained in studies carried out in fields with Bt plants and nonBt plants (mainly maize and cotton) that also did not show differences in the abundance of parasitoids and predators, but reported a lower number of larvae and eggs parasitized, which was provoked due the lower abundance and quality of the host affected by the Bt protein (Romeis et al. 2006; Lundgren et al. 2009).
Egg parasitoids also need high quality hosts, although they do not ingest the Bt protein they can be affected by alterations in the environment, such as changes in the chemical profile of volatile organic compounds released by the plants, which are used as cues to locate their hosts (Romeis et al. 2006; Lundgren et al. 2009). To disturb the mechanisms involved in indirect defense, provoking changes in the ratios or quantities of the compounds produced by the plants, for example, the genetic engineering would have to alter primary carbon metabolism, such as photosynthesis and cellular respiration processes, and/or one of the biochemical routes of the shikimic, malonic or mevalonic acid pathways. These effects are not expected to occur due to the insertion of genes for Bt proteins in plants. Although, differences in volatile emission from plants submitted to different types of biotic stress have been reported when comparing transgenic plants to non transgenic plants (Turlings et al. 2005; Yan et al. 2004; Vogler et al. 2009). The differences in the chemical profile of volatiles when comparing Bt and nonBt plants could be an effect of energetic costs and not a direct effect of the Bt protein. The Bt plants use energy to produce the δ-protein, which may not affect primary carbon metabolism, but could affect the syntheses of secondary metabolites.
In this work, no pleiotropic effect of the Bt genetic engineering was observed on the indirect defense response in the cotton varieties studied. As expected, the volatiles were released in larger amount after herbivory elicitation and the transgeny significantly altered the chemical profile over time for some compounds during the indirect defense process. Despite this, the tritrophic communication as reflected in the foraging behavior by the egg parasitoid T. pretiosum was not affected. Further studies will be conducted to expand the analysis to field conditions, and with different cultivars opening the possibility to cover a broader spectrum of natural enemies.
References
Bezemer TM, Wagenaar R, van Dam MN, van Der Putten WH, Wackers FL (2004) Above- and below ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J Chem Ecol 30:53–67
Colazza S, McElfresh JS, Millar JG (2004) Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis. J Chem Ecol 30:945–964
CTNBio (2006) Parecer técnico conclusivo prévio No 513/2005. http://www.ctnbio.gov.br/index.php/content/view/3663.html (last entry in March 2007)
D’Alessandro M, Held M, Triponez Y, Turlings TCJ (2006) The Role of indole and other shikimic acid derived maize volatiles in the attraction of two parasitic wasps. J Chem Ecol 32:2733–2748
De Moraes CM, Lewid WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573
Estruch JJ, Gregory GW, Mullins MA, Nye GJ, Craig JA, Koziel MG (1996) Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc Nat Acad Sci USA 93:5389–5394
Fitt GP, Wilson LJ (2000) Genetic 0engineering in IPM: Bt cotton. In: Kennedy GG, Sutton TB (eds) Emerging technologies for integrated pest management, vol 1. APS Press, St. Paul, pp 108–125
Fritzsche-Hoballah ME, Tamo C, Turlings TCJ (2002) Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: is quality or quantity important? J Chem Ecol 28:951–968
Heil M (2004) Induction of two indirect defences benefits Lima bean (Phaseolus lunatus, Fabacea) in nature. J Ecol 92:527–536
Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178:41–61
Heil M, Ton J (2008) Long-distance signaling in plant defence. Trends in Plant Sci 13:264–272
ISAAA (2006) http://www.agenciabrasil.gov.br/noticias/2007/01/18/materia.2007-01-18.9381176603/view (last entry in August 2007)
Kost C, Heil M (2006) Herbivore-Induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol 94:619–628
Laumann RA, Aquino MF, Moraes MCB, Pareja M, Borges M (2009) Response of the Egg Parasitoids Trissolcus basalis and Telenomus podisi to Compounds from Defensive Secretions of Stink Bugs. J Chem Ecol 35(1):8–19
Leopold EJ (1990) Selective hydroboration of a 1, 3, 7-triene: homogeraniol. Org Synth 64:164–171
Lundgren JG, Gassmann AJ, Bernal J, Duan JS, Ruberson JR (2009) Ecological compatibility of GM crops and biological control. Crop Prot 28:1017–1030
Maffei ME, Mithofer A, Boland W (2007) Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release. Phytochemistry 64:2946–2959
MDM-Seeds (2008) (www.mdm-algodao.com.br; last entry in November 2008)
Moraes MCB, Laumann RA, Pires CSS, Sujii ER, Borges M (2005) Induced volatiles in soybean and pigeon pea plants artificially infested with the neotropical brown stink bug, Euschistus heros, and their effect on the egg parasitoid, Telenomus podisi. Entomol Exp Appl 115:227–237
Moraes MCB, Pareja M, Laumann RA, Hoffmann-Campo CB, Borges M (2008) Response of the parasitoid Telenomus podisi to induced volatiles from soybean damaged by stink bug herbivory and oviposition. J Plant Inter 3:111–118
Moraes MCB, Laumann RA, Pareja M, Sereno FTPS, Michereff MFF, Birket MA, Pickett JA, Borges M (2009) Attraction of the stink bug egg parasitoid Telenomus podisi to defence signals from soybean activated by treatment with cis-jasmone. Entomol Exp Appl 131:178–188
Olson DM, Davis RF, Wackers FL, Rains GC, Potter T (2008) Plant-herbivore-carnivore Interactions in cotton, Gossypium hirsutum:linking belowground and aboveground. J Chem Ecol 34:1341–1348
Ozawa R, Shiojiri K, Sabelis MW, Takabayashi J (2008) Maize plants sprayed with either jasmonic acid or its precursos, methyl linolenate, attract armyworm parasitoids, but the composition of attractants differs. Entomol Exp Appl 129:189–199
Paré PW, Tumlinson JH (1998) Cotton volatiles synthesized and released distal to the site of insect damage. Phytochemistry 47:521–526
Pareja M, Moraes MCB, Clark SJ, Birkett MA, Powell W (2007) Response of the aphid parasitoid Aphidius funebris to volatiles from undamaged and aphid-infested Centaurea nigra. J Chem Ecol 33:695–710
Pareja M, Mohib A, Birkett MA, Dufour S, Glinwood RT et al (2009) Multivariate statistics coupled to generalized linear models reveal complex use of chemicals cues by parasitoid. Anim Behav 77:901–909
Perlak FJ, Deaton RW, Armstrong TA et al (1990) Insect resistant cotton plants. Biotechnology 8:939–943
Perlak FJ, Oppenhuizen M, Gustafson K, Fuchs RL, Sims SR, Greenplate JT, Fischhoff DA (2001) Development and commercial use of Bollgard cotton in the USA–Nearly promises versus today′s reality. Plant J 27:489–501
R Development Core Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Romeis J, Meissle M, Bigler F (2006) Transgenic crops expressing Bacillus Thuringiensis toxins and biological control. Nat Biotechnol 24:63–71
Rose USR, Tumlinson JH (2004) Volatiles released from cotton plants in response to Helicoverpa zea feeding on cotton flower buds. Planta 218:824–832
Rose USR, Tumlinson JH (2005) Systemic induction of volatile release in cotton: How specific is the signal to herbivory? Planta 222:327–335
Rose USR, Manukian A, Heath RR, Tumlinson JH (1996) Volatile semiochemicals released from undamaged cotton leaves (a systemic response of living plants to caterpillar damage). Plant Physiol 111:487–495
Schmidt FGV, Monnerat RS, Borges M, Carvalho RS (2001) Metodologia de criação de insetos para avaliação de agentes entomapotogênicos. Embrapa-Circular-Técnica 11:1–20
Schuler TH, Roel PJ, Potting ID, Denholm I, Poppy GM (1999) Parasitoid behaviour and Bt plants. Nature 400:825–826
Sharma HC, Ortiz R (2000) Transgenics, pest management, and the environment. Current Science 79:421–437
Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic Wasps. Science 250:1252–1253
Turlings TCJ, Lengwiler UB, Bernasconi ML, Wechsler D (1998) Timing of induced volatile emissions in maize seedlings. Planta 207:146–152
Turlings TCJ, Jeanbourquin PM, Held M, Degen T (2005) Evaluating the induced-odour emission of a Bt maize and its attractiveness to parasitic wasps. Transgenic Res 14:807–816
van Dam NM, Poppy GM (2008) Why plant volatile analysis needs bioinformatics-detecting signal from noise in increasingly complex profiles. Plant Biol 10:29–37
Vinson SB (1985) The behaviour of parasitoids. In: Kertut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol 1. Pergamon Press, New York, pp 417–469
Vogler U, Rott AS, Gessler C, Dorn S (2009) Comparison between volatile emissions from transgenic apples and from two representative classically bred apple cultivars. Transgenic Res. doi: 10.1007/s11248-009-9294-8
Williams LIII, Rodriguez-Saona C, Castle SC, Zhu S (2008) EAG-Active herbivore–induced plant volatiles modify behavioral responses and host attack by an egg parasitoid. J Chem Ecol 34:1190–1201
Wu K (2001) IPM in cotton. In: Jia S (ed) Transgenic cotton, vol 1. Science Press, Beijing, China, pp 218–224
Yan F, Bengtsson M, Anderson P, Ansebo L, Xu C, Witzgall P (2004) Antennal response of cotton bollworm (Heliocoverpa armigera) to volatiles in transgenic Bt cotton. J Appl Entomol 128:354–357
Zhu J, Park KC (2005) MeSA, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempuctata. J Chem Ecol 31:1733–1746
Acknowledgments
We thank Isabela Girotti Crisi and Hélio Moreira for rearing the insects, and his work was funded by International Foundation for Science (IFS), National Council for Scientific and Technological Development (CNPq), Distrito Federal Research Foundation and Brazilian Agricultural Research Corporation (Embrapa). We are grateful to Dr. Mark Horn International Consultant at Embrapa genetic Resources and Biotechnology for comments that greatly improved the manuscript, Dr. Michael Birkett for providing synthetic standards (TMTT and DMNT) and Dr. Martin Pareja and the statistician joseane Padilha Silva for valuable comments and support on the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moraes, M.C.B., Laumann, R.A., Aquino, M.F.S. et al. Effect of Bt genetic engineering on indirect defense in cotton via a tritrophic interaction. Transgenic Res 20, 99–107 (2011). https://doi.org/10.1007/s11248-010-9399-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-010-9399-0