Abstract
After herbivore attack, plants release a plethora of different volatile organic compounds (VOCs), which results in odor blends that are attractive to predators and parasitoids of these herbivores. VOCs in the odor blends emitted by maize plants (Zea mays) infested by lepidopteran larvae are well characterized. They are derived from at least three different biochemical pathways, but the relative importance of each pathway for the production of VOCs that attract parasitic wasps is unknown. Here, we studied the importance of shikimic acid derived VOCs for the attraction of females of the parasitoids Cotesia marginiventris and Microplitis rufiventris. By incubating caterpillar-infested maize plants in glyphosate, an inhibitor of the 5-enolpyruvylshikimate-3-phospate (EPSP) synthase, we obtained induced odor blends with only minute amounts of shikimic acid derived VOCs. In olfactometer bioassays, the inhibited plants were as attractive to naive C. marginiventris females as control plants that released normal amounts of shikimic acid derived VOCs, whereas naive M. rufiventris females preferred inhibited plants to control plants. By adding back synthetic indole, the quantitatively most important shikimic acid derived VOC in induced maize odors, to inhibited plants, we showed that indole had no effect on the attraction of C. marginiventris and that M. rufiventris preferred blends without synthetic indole. Exposing C. marginiventris females either to odor blends of inhibited or control plants during oviposition experiences shifted their preference in subsequent olfactometer tests in favor of the experienced odor. Further learning experiments with synthetic indole showed that C. marginiventris can learn to respond to this compound, but that this does not affect its choices between natural induced blends with or without indole. We hypothesize that for naïve wasps the attractiveness of an herbivore-induced odor blend is reduced due to masking by nonattractive compounds, and that during oviposition experiences in the presence of complex odor blends, parasitoids strongly associate some compounds, whereas others are largely ignored.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants that are attacked by herbivorous arthropods are known to release a complex blend of volatile organic compounds (VOCs). These herbivore-induced plant volatiles (HIPVs) are exploited by predators and parasitoids as foraging signals that help them locate their herbivorous prey or hosts (Turlings and Wäckers, 2004; Arimura et al., 2005). At present, more than 1000 low-molecular-weight organic compounds have been reported to be emitted from plants, including alkanes, alkenes, alcohols, ketones, aldehydes, ethers, esters, and carboxylic acids (Dudareva et al., 2004; Niinemets et al., 2004). Although some of these compounds are constitutively emitted by undamaged, healthy plants, considerably higher amounts are emitted after herbivore damage and various HIPVs may even be synthesized de novo in response to damage (Turlings et al., 1990, 1998; Paré and Tumlinson, 1997). Some HIPVs are specific to certain plant taxa, for example, sulfur containing compounds in Allium plants (Dugravot et al., 2004) or glucosinolate breakdown products in Brassicaceae species (Scascighini et al., 2005), but others are common to many species (Fritzsche Hoballah et al., 2002; van den Boom et al., 2004). Common compounds include “green-leaf volatiles” (C6 aldehydes, alcohols, and derivatives), cyclic and acyclic terpenoids, phenolic compounds, and nitrogenous compounds (Dicke, 1999; Paré and Tumlinson, 1997). These derive from at least three pathways. Green leaf volatiles are products of the enzymatic activity of hydroperoxide lyase (HPL), a component of the lipoxygenase (LOX) pathway, which results in multiple rearrangement of (Z)-3-hexenal (Bate and Rothstein, 1998; Blee, 1998). Terpenoids are synthesized via the isopentenyl pyrophosphate (IPP) intermediate following the classical mevalonate pathway or via an alternative IPP pathway with glyceraldehyde-3-phosopate and pyruvate identified as the direct precursors of IPP (Lichtenthaler et al., 1997). Finally, aromatic compounds, such as methyl salicylate (MeSA) and indole, are formed via the shikimic acid pathway (Bennett and Wallsgrove, 1994; Paré and Tumlinson, 1997).
In maize plants, the mechanisms of biosynthesis, induction, and release of HIPVs are well characterized (Turlings et al., 1998; Frey et al., 2000; Shen et al., 2000; Schnee et al., 2002; Gouinguené et al., 2003; Schmelz et al., 2003a,b,c; Köllner et al., 2004; Lawrence and Novak, 2004; Ruther and Kleier, 2005), and the ecological significance of these compounds in tritrophic signaling has been demonstrated in laboratory and field experiments (Turlings et al., 1990; Bernasconi et al., 1998; Hoballah and Turlings, 2001; Fritzsche Hoballah et al., 2002; Rasmann et al., 2005). In particular, the role of green leaf volatiles and terpenoids in attracting natural enemies of the herbivores has been investigated in various experiments (D’Alessandro and Turlings, 2005; Hoballah and Turlings, 2005). Yet, it remains unclear which compounds are essential for attraction (D’Alessandro and Turlings, 2006). One group of compounds that has hardly been studied in the context of parasitoid attraction are the shikimic acid derived VOCs.
The main shikimic acid derived VOC released by maize seedlings after infestation with larvae of Spodoptera moths is indole (Turlings et al., 1998; D’Alessandro and Turlings, 2005). This compound is induced after treatment of maize seedlings with volicitin [N-(17- hydroxylinolenoyl)-l-glutamine], a fatty acid-amino acid conjugate found in the regurgitate of Spodoptera larvae (Alborn et al., 1997; Turlings et al., 2000). Indeed, jasmonic acid, which is involved in the induction of HIPVs in maize (Schmelz et al., 2003a), also appears to be an integral part of volicitin-mediated induction of indole (Frey et al., 2000). Frey et al. (2004) identified an enzyme in maize, indole-3-glycerol phosphate lyase (Igl), which converts indole-3-glycerol phosphate to free indole. This differs from the enzyme BX1, which catalyzes the conversion of indole-3-glycerol phosphate to indole to form the direct defense compounds 2,4-dihydroxy-2H-1,4-benzoxazin-3(4H)-one (DIBOA) and 2,4-dihydroxy-7-methoxy-2H-,1,4-benzoxazin-3(4H)-one (DIMBOA), or tryptophane synthase, which produces the amino acid tryptophane (Frey et al., 1997). The selective activation of the evolutionarily similar genes igl and bx1 suggests that the plants are capable of selecting direct or indirect defense mechanisms depending on the type of stress they are exposed to. Therefore, volatile indole was expected to be a key compound in the attraction of natural enemies of the herbivores.
Here we studied the importance of shikimic acid derived HIPVs, in particular indole, in attracting females of two parasitoid species, Cotesia marginiventris (Cresson) (Hymenoptera: Braconidae) and Microplitis rufiventris (Kokujev) (Hymenoptera: Braconidae). Both attack early instars of numerous lepidopteran moths, including many pests, and are known to use plant-provided VOCs in host location (Gouinguené et al., 2003; Hoballah and Turlings, 2005). We manipulated the volatile blend emitted by maize seedlings (Zea mays var. Delprim) that had been fed upon by larvae of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) by incubating the plants in glyphosate [N-(phosphonomethyl)-glycine]. This compound inhibits the enzyme 5-enolpyruvylshikimate-3-phospate (EPSP) synthase (Haslam, 1993; Schönbrunn et al., 2001) and strongly reduces the amounts of shikimic acid derived VOCs. Attraction of the odor from these inhibited plants was compared to that of the natural blends emitted by control plants. Subsequently, we tested an inhibited blend against an inhibited blend to which we added back a natural amount of synthetic indole, the major shikimic acid derived HIPVs.
C. marginiventris females, like many other female parasitoids, are able to learn and associate plant VOCs with the presence of suitable hosts during oviposition experiences (Turlings et al., 1993; D’Alessandro and Turlings, 2005). We therefore compared the responses of naïve and experienced female parasitoids, and in a series of learning experiments with C. marginiventris females, we estimated how well the wasps can learn to associate indole with host presence.
Methods and Materials
Insects and Insect Treatments
The caterpillar S. littoralis (Boisduval) (Lepidoptera: Noctuidae) and the solitary endoparasitoids, C. marginiventris (Cresson) (Hymenoptera: Braconidae) and M. rufiventris (Kokujev) (Hymenoptera: Braconidae) were reared as previously described (Turlings et al., 2004). Adult parasitoids were kept in plastic cages at a male/female ratio of approximately 1:2 and were provided with moist cotton wool and honey as food source. Cages were kept in incubators (C. marginiventris, 25 ± 1°C; M. rufiventris, 23 ± 1°C; 16:8 hr L/D) and transferred to the laboratory 30 min before the experiments. We tested mated 2- to 4-d-old naive and experienced females. The latter were given experiences by allowing them to oviposit 3–5 times into second instar S. littoralis. A metal screen was attached to the top opening (2 cm diam) of an odor source vessel of the same type as used in the olfactometer (see below). Approximately 20 larvae were placed on the screen, and individual wasps were allowed to oviposit in one or two larvae, while they were exposed to the odor of the source that was placed inside the vessel. This source was either an infested control plant, an infested inhibited plant, or synthetic indole only. Airflow and concentrations of volatiles were the same as during the olfactometer bioassays (see below). Naïve females were neither exposed to the volatiles nor given any oviposition experience before testing. The different groups of wasps were kept separately in small plastic boxes with moist cotton wool and honey and released into the olfactometer 1–3 hr after the oviposition experiences.
Plants and Odor Sources
Maize (Z. mays, var. Delprim) was sown in plastic pots (10 cm high, 4 cm diam) with commercial potting soil (Ricoter Aussaaterde, Aarberg, Switzerland) and placed in a climate chamber (23 ± 2°C, 60% r.h., 16:8 hr L/D, and 50,000 lm/m2). Plants used for the experiments were 10–12 d old and had 3 fully developed leaves. The evening before the experiments, plants were cut with a razor blade at soil level, while the stem was held under water to prevent air entering the vascular system. Subsequently, they were placed in a vial (8 ml) filled with either deionized water (control plant) or in a 1 mM glyphosate [N-(phosphonomethyl)-glycine; Fluka, Buchs, Switzerland] solution (inhibited plant). Vials were wrapped in aluminum foil, and one vial containing a single plant was placed in an open odor source vessel of the olfactometer (described by Turlings et al., 2004). Two hr after incubation, plants were infested with 20 second instar Spodoptera, which were released in the whorl of the youngest leaf. After infestation, plants were kept under laboratory conditions (25 ± 2°C, 40 ± 10% r.h., 16:8 hr L/D, and 8000 lm/m2) and were used for the experiments the following day, between 10 a.m. and 4 p.m.
To check whether the treatment of the plants with the inhibitor affected the larval feeding behavior, plants were collected after the bioassays, and the leaves were scanned into Adobe Photoshop 6.0. The total leaf area removed during the 24-hr feeding period was compared for the different treatments based on differences in pixels that indicated tissue removal.
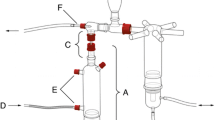
Indole (purity ≥ 99%; Fluka) was released from a device consisting of a 2-ml glass vial that contained 500 mg synthetic indole (Fig. 1), which was connected, via a glass capillary, to a Teflon tube placed between two glass tubes connecting the top of an odor source vessel to an olfactometer arm. Preliminary experiments with various synthetic volatile compounds (e.g., MeSA, linalool, indole) showed that at room temperature this device allowed the constant release of pure compounds, and that their release rates could be controlled by adjusting the length and the diameter of the capillary tube (Duran, Hirschmann EM). The release rate was calibrated to the lower range of amounts of indole that was found for infested maize plants. Vials were prepared freshly the evening before the experiments and connected the following morning to the odor source vessels used for training or testing.
Olfactometer Bioassays
All odor sources were tested for attractiveness to parasitoids in a four-arm olfactometer (described by D’Alessandro and Turlings, 2005) as indicated in Table 1. Cleaned and humidified air entered the odor source vessel at 1.2 l/min (adjusted by a manifold with four flowmeters; Analytical Research System, Gainesville, FL, USA) via Teflon tubing and carried the VOCs through to the olfactometer compartment. Half of the air (0.6 l/min/olfactometer arm) was pulled out via a volatile collection trap that was attached to the system above the odor source vessels (see “Collection and analyses of VOCs”). Incoming and outgoing air were balanced by a Tygon tube connected to a vacuum pump via another flow meter and a pressure gauge. Empty arms were connected to empty vessels and carried clean, humidified air only.
Wasps were released in groups of six into the central part of the olfactometer, and after 30 min the wasps that had entered an arm of the olfactometer were counted and removed. Wasps that did not enter an arm after this time were removed from the central part of the olfactometer and considered as “no choice.” Experiments were replicated on eight different days, and for each replicate a total of six groups of six wasps were tested during a 3-hr sampling period, alternating between groups of naive and experienced wasps in experiments where three different groups were tested (total of 96 wasps/group), or only naïve wasps in the other experiments (total 288 wasps). Ten neon tubes attached to a metal frame above the olfactometer provided approximately 7000 lm/m2 at the height of the odor source vessels. All bioassays were carried out between 10 a.m. and 4 p.m.
Collection and Analyses of VOCs
VOCs of each odor source were collected during the olfacotemeter bioassay on a Super-Q trap (25 mg, 80–100 mesh; Alltech Associates, Deerfield, IL, USA, described by Heath and Manukian, 1992). Each trap was attached horizontally to the elbow of the olfactometer and connected via Tygon tubing to a flowmeter (Analytical Research System) and a vacuum pump. Air carrying the volatiles was pulled through each trap for 3 hr at a rate of 0.6 l/min during each behavioral bioassay. Afterwards, the traps were extracted with 150 μl dichloromethane (Suprasolv; Merck, Dietikon, Switzerland), and 200 ng of n-octane and n-nonyl acetate (Sigma, Buchs, Switzerland) in 10 μl dichloromethane were added to the samples as internal standards. All extracts were stored at −76°C until analyses. Traps were washed with 3 ml dichloromethane before they were reused for a next collection.
VOCs of the experiments with control and inhibited plants were analyzed with a gas chromatograph (Agilent 6890 Series GC system G1530A) coupled to a mass spectrometer that operated in electron impact mode (Agilent 5973 Network Mass Selective Detector; transfer line 230°C, source 230°C, ionization potential 70 eV, scan range 33–280 amu). A 2-μl aliquot of each sample was injected in the pulsed splitless mode onto an apolar capillary column (HP-1, 30 m, 0.25 mm ID, 0.25 μm film thickness; Alltech Associates). Helium at constant flow (0.9 ml/min) was used as carrier gas. After injection, the column temperature was maintained at 40°C for 3 min and then increased to 100°C at 8°C/min and subsequently to 200°C at 5°C/min followed by a postrun of 5 min at 250°C. The detected volatiles were identified by comparison of their mass spectra with those of the NIST 02 library, by comparison of their spectra and retention times with those of authentic standards, and by comparison of retention times with those in previous analyses (D’Alessandro and Turlings, 2005). Compounds that were not identified by comparing retention times and spectra with those of pure standards are indicated in Fig. 2 with superscript N, and their identity should be considered tentative. Twelve samples per treatment were analyzed in the SIM mode (ion 117, qualifier 95), and indole was quantified based on a calibration curve with known amounts of synthetic indole. All other compounds were only quantified in the full scan range based on comparison of their peak area with those of the internal standards (n-octane for compounds 1–14, n-nonyl acetate for compounds 14–27). A total of 18 samples were injected per treatment.
Mean amount (±SE) (ng) of major VOCs recollected from cut Spodoptera-induced maize seedlings during 3 hr. Control plants were incubated in water, inhibited plants were incubated in a 1 mM glyphosate solution. Asterisks above bars indicate significant differences (t-test on log(x + 1) transformed data, P < 0.05) in the amount of a specific compound. N = 18 per treatment. The compounds are as follows: 1, (Z)-3-hexenal; 2, (E)-2-hexenal; 3, (Z)-3-hexen-1-ol; 4, (Z)-2-penten-1-ol acetate; 5, β-myrcene; 6, (Z)-3-hexenyl acetate; 7, (E)-2-hexenyl acetate; 8, (Z)-β-ocimeneN; 9, linalool; 10, (3E)-4,8-dimethyl-1,3,7-nonatriene (DMNT); 11, benzyl acetate; 12, phenethyl acetate; 13, indole; 14, methyl anthranilate; 15, geranyl acetate; 16, unknown sesquiterpenoid; 17, (E)-β-caryophyllene; 18, (E)-α-bergamotene; 19, unknown sesquiterpenoid; 20, (E)-β-farnesene; 21, unknown sesquiterpenoid; 22, unknown sesquiterpenoid; 23, β-sesquiphellandreneN; 24, (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene (TMTT). The compounds are ordered in accordance with their retention on a nonpolar capillary column
Statistical Analyses
The functional relationship between parasitoids’ behavioral responses and the different odor sources offered in the four-arm olfactometer was examined with a log-linear model (a generalized linear model, GLM). As the data did not conform to simple variance assumptions implied in using the multinomial distribution, we used quasi-likelihood functions to compensate for the overdispersion of wasps within the olfactometer (Turlings et al., 2004). The model was fitted by maximum quasi-likelihood estimation in the software package R (R: A language and Environment for Statistical Computing, Version 1.9.1, Vienna, Austria, 2006, ISBN 3-900051-07-0 http://www.R-project.org), and its adequacy was assessed through likelihood ratio statistics and examination of residuals. We tested “treatment” effects (=odor sources) for naive and experienced wasps separately, and we included “release” as an explanatory variable to avoid “pseudo-replications.” In addition, we tested if there was a significant effect of “experience” and an interaction between “treatment × experience.”
The amounts of VOCs were analyzed by using t-tests. Amounts of VOCs that were not normally distributed were log(x + 1) transformed prior to analysis. The amounts of indole quantified in the single ion mode were analyzed with a Kruskal–Wallis test. Differences between the treatments were analyzed with the Tukey’s test. Differences between the removed leaf areas after caterpillar feeding were analyzed with a t-test. All analyses were run on SigmaStat (Version 2.03).
Results
VOCs of Control and Inhibited Plants
Herbivore-infested maize seedlings that were incubated with their cut stem in water (control plants) released a volatile blend consisting of 24 detectable VOCs, including green leaf volatiles, terpenoids, and shikimic acid derivatives (Fig. 2). Seedlings that were incubated in a 1 mM glyphosate solution released only trace amounts of indole and methyl anthranilate and had strongly reduced amounts of other shikimic acid derived VOCs (t-test: benzyl acetate, t 34 = 1.741, P = 0.092; phenethyl acetate, t 34 = 11.218, P < 0.001). The amounts of VOCs derived from other biochemical pathways were similar to those of the control plants, except for the somewhat reduced amounts of (Z)-3-hexen-1-ol (t 34 = 2.306, P = 0.027) and 3-Z-hexen-1-ol acetate (t 34 = 2.577, P = 0.014). In addition to the compounds quantified in Fig. 2, we also detected trace amounts of (E)-nerolidol, (Z)-jasmone, and some minor, unidentified compounds. These VOCs were not included in quantification analyses.
The single ion mode analyses of indole revealed that during the 3-hr bioassay periods we collected 835.44 ± 135.91 ng from control plants and only 0.55 ± 0.17 ng from inhibited plants. In bioassays where synthetic indole was added to the air stream with the odor of an inhibited plant, we detected 192.77 ± 11.46 ng. There was a significant difference between the amounts released by these three treatments (Kruskal–Wallis followed by Tukey test, H 2 = 30.294, P < 0.001).
Inhibitor treatment did not affect the larvae’s feeding rate; the leaf areas removed by the larvae during a 24-hr feeding period were similar for control plants (4.73 ± 0.40 cm2) and for inhibited plants (4.81 ± 0.38 cm2) (t-test: t 20 = −0.15, P = 0.882).
Attractiveness of Inhibited vs. Control Plants
Neither naïve nor experienced C. marginiventris females significantly distinguished between VOC blends emitted by Spodoptera-induced maize seedlings with strongly reduced amounts of shikimic acid derived VOCs (see above) and VOCs emitted by control plants (Fig. 3a, GLM, naive: F 1,15 = 0.78, P = 0.39; experienced on inhibited blend: F 1,15 = 3.91, P = 0.067; experienced on control blend: F 1,15 = 0.43, P = 0.52). Yet, the type of experience had a significant effect on the choice of the wasps (F 1,60 = 4.566, P = 0.037), implying that C. marginiventris females were able to detect the difference between the two odor sources. The responsiveness (the proportion of wasps entering an arm with one of the two treatments) was high and similar for all treatments, and only few wasps entered an empty arm (Fig. 3a).
Importance of shikimic acid derived VOC for the attraction of two parasitoid species. (a) Choice of C. marginiventris females and (b) M. rufiventris females between arms carrying VOCs of Spodoptera-induced maize seedlings that were either incubated in water (control plants) or in a 1 mM glyphosate solution (inhibited plants). Pretreatment of the wasps (=type of experience) is indicated on the left. Pie charts indicate overall responsiveness (=number of wasps entering the different types of arms). GLMs were performed in order to test for differences between the two odor arms within one group of wasps as well as to compare the types of experiences. ***P < 0.001, **P < 0.01, *P < 0.05, n.s. = no significant difference, P > 0.05
Naive and experienced females of M. rufiventris significantly preferred the inhibited blend (Fig. 3b, GLM, naive: F 1,15 = 11.04, P = 0.005; experienced on inhibited blend: F 1,15 = 26.19, P < 0.001; experienced on control blend: F 1,15 = 6.19, P = 0.025 ). As with C. marginiventris, the type of experience had an effect on the choice of the wasps (F 1,60 = 7.073, P = 0.010). The responsiveness was high, and none of the M. rufiventris wasps entered an empty arm.
Role of Indole
Indole was the major shikimic acid derived VOC released by Spodoptera-induced maize seedlings (Fig. 2). We tested its role in the attraction of the two parasitoid species by comparing the attractiveness of HIPV blends released by two inhibited plants, whereby we added synthetic indole back to one of the blends. The amounts of indole added (192.77 ± 11.46 ng/3 hr) fell within the lower ranges of indole detected in a natural induced maize blend (see above). C. marginiventris did not distinguish between the two blends (Fig. 4a, GLM: F 1,47 = 0.87, P = 0.36). In contrast, M. rufiventris significantly preferred the inhibited blend without synthetic indole (Fig. 4b, F 1,47 = 43.16, P < 0.001). Results for both wasps are consistent with those from the previous experiment, showing an insignificant role of shikimate-derived compounds for C. marginiventris attraction, whereas they have a repellent effect on M. rufiventris.
Importance of indole for the attraction of two parasitoid species. (a) Choice of naïve C. marginiventris females and (b) naïve M. rufiventris females between arms carrying Spodoptera-induced maize VOCs of inhibited plants and of inhibited plants to which synthetic indole was added. See Fig. 3 for further explanations
Learning of Indole
When C. marginiventris females were given a choice between one arm with synthetic indole (192.77 ± 11.46 ng/3 hr) and three arms with clean air only, they neither showed an innate (naive wasps) attraction towards indole (Fig. 5a, GLM: F 1,47 = 0.60, P = 0.44 ), nor were they attracted to indole after having experienced a natural blend that contained similar amounts of indole (F 1,47 = 0.29, P = 0.60). However, if they were exposed to pure indole during oviposition experiences they preferred an arm carrying indole over arms with clean air (F 1,47 = 11.62, P = 0.001), although the type of experience had only a marginal effect on their choices (F 1,124 = 2.919, P = 0.090) and the overall responsiveness was rather low (Fig. 5a).
Importance of indole in learning experiments by C. margininventris. (a) Choice of females with different learning experiences between arms carrying synthetic indole and empty arms with clean air only. (b) Choice of females with different learning experiences between arms carrying Spodoptera-induced maize VOCs of control and inhibited plants. See Fig. 3 for further explanations
In an additional experiment, we tested whether exposing the wasps to indole during ovipositions increases the attraction towards an induced maize blend containing indole (control plant) compared to a blend with only trace amounts of indole (inhibited plant). As in the experiments above, naive wasps and wasps that had experienced the control blend did not distinguish between the two blends (Fig. 5b, GLM: F 1,15 = 0.062, P = 0.81; F 1,15 = 1.92, P = 0.19, respectively). Interestingly, even an experience with pure synthetic indole did not result in a change in preference (F 1,15 = 3.70, P = 0.074), and the type of experience did not have a significant effect on the wasps’ choice (F 1,60 = 0.214, P = 0.646).
Discussion
One way of studying the importance of individual VOCs for the attraction of natural enemies is to compare the attractiveness of an incomplete with a normal blend of HIPVs (D’Alessandro and Turlings, 2005). By restoring the incomplete blend with synthetic compounds that are missing, the importance of the added compounds can be confirmed (de Boer and Dicke, 2004). Here, we used glyphosate, an inhibitor of the enzyme EPSP synthase (Haslam, 1993; Schönbrunn et al., 2001), to inhibit the production of shikimic acid derived HIPVs. As expected, this inhibitor treatment resulted in a strong reduction of the emission of indole, methyl anthranilate, phenethyl acetate, and benzyl acetate, whereas the amounts of most other compounds, except two green-leaf volatiles, were not affected by the inhibitor treatment (Fig. 2). Treating plants with glyphosate normally results in lower amounts of chorismate, a precursor for the production of salicylic acid (SA) (Shah, 2003). SA has been shown to synergistically or antagonistically interact with jasmonic acid (Thaler et al., 2002; Bostock, 2005), an important hormone involved in the induction of maize VOCs (Schmelz et al., 2003c). We speculate that SA does not play a major role in induced volatile emissions in this maize variety. Indeed, MeSA, the volatile form of SA, was only occasionally detected in trace amounts in our control plants.
Glyphosate-treated plants are also expected to contain lower levels of phenolic compounds, which should have a positive effect on herbivores. However, we did not observe any difference in the amount of leaf damage inflicted by Spodoptera larvae between glyphosate-treated and glyphosate-untreated plants. A possible negative effect of glyphosate through direct toxicity has been ruled out in ecotoxicological risk assessment studies with arthropods (Giesy et al., 2000). Furthermore, glyphosate is not readily volatilized and is primarily degraded by microbial metabolism in the soil (Tu et al., 2001). Indeed, we did not detect additional VOCs from glyphosate-treated seedlings, making glyphosate a suitable inhibitor to study the attractiveness of shikimic acid derived VOCs.
Attractiveness of Shikimic Acid Derived VOCs
It is generally assumed that HIPVs are exploited by natural enemies of the herbivores in order to locate their host or prey (Dicke, 1999; Turlings and Wäckers, 2004), but which compounds of a volatile blend are actually important in the foraging behavior of natural enemies is not yet known for most tritrophic systems (Dicke and van Loon, 2000). Previous studies on caterpillar-induced maize volatiles showed that qualitative differences in the odor blend may be more important for the attraction of parasitic wasps than quantitative differences (Fritzsche Hoballah et al., 2002). Indeed, a blend of induced maize volatiles with reduced amounts of the three major sesquiterpenoids, (E)-β-caryophyllene, (E)-α-bergamotene, and (E)-β-farnesene, was equally attractive to naive C. marginiventris as control blends with high amounts of these compounds, whereas removing some minor, polar compounds from the blend rendered it completely unattractive to C. marginiventris (D’Alessandro and Turlings, 2005). Here, we provide another example showing that some common HIPVs, i.e., shikimic acid derived volatiles, are not involved in the initial attraction of two parasitoids to host infested plants (Figs. 3 and 4). Indole, which is one of the dominating compounds in Spodoptera-induced maize volatiles (Turlings et al., 1998; D’Alessandro and Turlings, 2005), might even be repellent or might mask the attractiveness of compounds used for host location. Interestingly, several studies on maize volatiles indicate specific induction of volatile indole by herbivore-derived elicitors and not by excision stress or mechanical damage (Frey et al., 2000; Schmelz et al., 2003b). However, in other studies, many of the common herbivore-induced VOCs, including indole and several terpenoids, have been detected in analyses of VOCs released by plants exposed to other forms of stresses, as for example mechanical wounding (van den Boom et al., 2004), exposure to other VOCs (Ruther and Fürstenau, 2005), or infection by microorganism (Huang et al., 2003). Hence, the emission of various volatiles can be induced by a number of enemies and stresses, yet natural enemies are able to discriminate between different forms of stresses (Takabayashi et al., 1995; De Moraes et al., 1998; de Boer et al., 2004; Vuorinen et al., 2004). Selection must have favored parasitoids with an ability to distinguish between host- and nonhost-related compounds and an innate response to compounds that are specifically correlated with host presence is the most likely mechanism that allows them to make such distinctions (Vet and Dicke, 1992). We hypothesize that naïve females of generalist parasitic wasps are attracted only to a few key compounds within a complex blend of volatiles, and that most other compounds within such a blend contribute little to its initial attractiveness. They may mask the attractive compounds, or may even be repellent. Still, these compounds may become attractants when the wasps have associated them with host presence.
Importance of Learning
One way for parasitoids to deal with highly complex and variable blends is their ability to learn by association (Turlings et al., 1993; Vet et al., 1995). It is assumed that such learning processes are specifically important for generalist wasps such as C. marginiventris and M. rufiventris, parasitizing various host, feeding on different plant species (Vet and Dicke, 1992; Steidle and van Loon, 2003). Indeed, C. marginiventris shows a keen ability for associative learning, whereas this form of learning is less clear for M. rufiventris (D’Alessandro and Turlings, 2005; Hoballah and Turlings, 2005; Tamò et al., 2006). Here again, C. marginiventris showed a significant shift in its preference in favor of the blend that it had experienced during multiple ovipositions (Fig. 3a). The response of M. rufiventris also changed significantly after experience, but it maintained a significant preference for the odor of inhibited plants even after having experienced the odor of control plants (Fig. 3b).
The predatory mites (Phytoseiulus persimilis) have been found to strongly associate MeSA with the presence of their prey if they are reared in the presence of a complex blend of herbivore-induced VOCs that includes MeSA (de Boer et al., 2004). Similarly, Vet et al. (1998) found that the Drosophila parasitoid Leptopilina heterotoma learned to discriminate between odors from substrates that were qualitatively different, but failed to discriminate when differences were small, unless unrewarding experiences provided evidence of the absence of hosts in one of the substrates. That some compounds are more important than others for associative learning was found for the parasitoid M. croceipes. After conditioning to a complex mixture, females of this species established a hierarchy among various components, with some of them accounting for a major part of the behavioral activity evoked by the mixture (Meiners et al., 2003). In our study, C. marginiventris was able to learn and subsequently respond to pure synthetic indole; however, this learning of indole had no effect on the females’ responses to natural, complex blends with or without indole (Fig. 5). Apparently, indole is not a compound that is strongly associated during learning processes, especially if it is not offered in a complex volatile environment. Although indole is strongly induced after Spodoptera infestation on maize plants, it is also found in a variety of other stress-induced plant volatile blends (see above), and may not provide foraging parasitic wasps with specific information on the presence or absence of hosts.
In summary, we studied the role of shikimic acid derived VOCs, in particular indole, in the host searching behavior of two parasitoid species, C. marginiventris and M. rufiventris. This group of VOCs forms a substantial part of the volatile blend released by maize seedlings in response to feeding by lepidopteran larvae, but the results show that they are not important for the attraction of the two wasp species tested here. Attraction of C. marginiventris was not affected by the presence or absence of indole, the major shikimic acid derived VOC, whereas this compound was repellent rather than attractive to M. rufiventris. Learning of indole during oviposition experiences did not greatly alter these responses. Hence, this study suggests that parasitoids do not use all herbivore-induced VOCs for habitat and host location to a similar degree, but rather pay selective attention to a few compounds. Identifying these key compounds seems crucial for a good understanding of the host searching process in parasitoids and for the development of strategies to increase the efficiency of natural enemies for the control of pest insects (Turlings and Ton, 2006).
References
Alborn, T., Turlings, T. C. J., Jones, T. H., Stenhagen, G., Loughrin, J. H., and Tumlinson, J. H. 1997. An elicitor of plant volatiles from beet armyworm oral secretion. Science 276:945–949.
Arimura, G., Kost, C., and Boland, W. 2005. Herbivore-induced, indirect plant defences. BBA-Mol. Cell Biol. L. 1734:91–111.
Bate, N. J. and Rothstein, S. J. 1998. C-6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 16:561–569.
Bennett, R. N. and Wallsgrove, R. M. 1994. Secondary metabolites in plant defense-mechanisms. New Phytol. 127:617–633.
Bernasconi M. L., Turlings T. C. J., Ambrosetti L., Bassetti P., and Dorn S. 1998. Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis. Entomol. Exp. Appl. 87:133–142.
Blee, E. 1998. Phytooxylipins and plant defense reactions. Prog. Lipid Res. 37:33–72.
Bostock, R. M. 2005. Signal crosstalk and induced resistance: Straddling the line between cost and benefit. Annu. Rev. Phytopathol. 43:545–580.
D’Alessandro, M. and Turlings, T. C. J. 2005. In situ modification of herbivore-induced plant odors: A novel approach to study the attractiveness of volatile organic compounds to parasitic wasps. Chem. Senses 30:739–753.
D’Alessandro, M. and Turlings, T. C. J. 2006. Advances and challenges in the identification of volatiles that mediate interactions among plants and arthropods. Analyst 131:24–32.
De Boer, J. G. and Dicke, M. 2004. The role of methyl salicylate in prey searching behavior of the predatory mite Phytoseiulus persimilis. J. Chem. Ecol. 30:255–271.
De Boer, J. G., Posthumus, M. A., and Dicke, M. 2004. Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J. Chem. Ecol. 30:2215–2230.
De Moraes C. M., Lewis W. J., Paré P. W., Alborn H. T., and Tumlinson J. H. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573.
Dicke, M. 1999. Evolution of induced indirect defense of plants, pp. 62–88 in R. Tollrian and C. D. Harvell (eds.). The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton, NJ.
Dicke, M. and van Loon, J. J. A. 2000. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 97:237–249.
Dudareva, N., Pichersky, E., and Gershenzon, J. 2004. Biochemistry of plant volatiles. Plant Physiol. 135:1893–1902.
Dugravot, S., Thibout, E., Abo-Ghalia, A., and Huignard, J. 2004. How a specialist and a non-specialist insect cope with dimethyl disulfide produced by Allium porrum. Entomol. Exp. Appl. 113:173–179.
Frey, M., Chomet, P., Glawischnig, E., Stettner, C., Grun, S., Winklmair, A., Eisenreich, W., Bacher, A., Meeley, R. B., Briggs, S. P., Simcox, K., and Gierl, A. 1997. Analysis of a chemical plant defense mechanism in grasses. Science 277:696–699.
Frey, M., Stettner, C., Paré, P. W., Schmelz, E. A., Tumlinson, J. H., and Gierl, A. 2000. An herbivore elicitor activates the gene for indole emission in maize. Proc. Natl. Acad. Sci. USA 97:14801–14806.
Frey, M., Spiteller, D., Boland, W., and Gierl, A. 2004. Transcriptional activation of igl, the gene for indole formation in Zea mays: A structure–activity study with elicitor-active n-acyl glutamines from insects. Phytochemistry 65:1047–1055.
Fritzsche Hoballah, M. E., Tamó, C., and Turlings, T. C. J. 2002. Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: Is quality or quantity important? J. Chem. Ecol. 28:951–968.
Giesy, J. P., Dobson, S., and Solomon, K. R. 2000. Ecotoxicological risk assessment for roundup (R) herbicide. Rev. Environ. Contam. T. 167:35–120.
Gouinguené, S., Alborn, H., and Turlings, T. C. J. 2003. Induction of volatile emissions in maize by different larval instars of Spodoptera littoralis. J. Chem. Ecol. 29:145–162.
Haslam, E. 1993. Shicimic Acid: Metabolism and Metabolites. Wiley, Chichester, UK.
Heath, R. R. and Manukian, A. 1992. Development and evaluation of systems to collect volatile semiochemicals from insects and plants using a charcoal-infused medium for air purification. J. Chem. Ecol. 18:1209–1226.
Hoballah M. E. F. and Turlings, T. C. J. 2001. Experimental evidence that plants under caterpillar attack may benefit from attracting parasitoids. Evol. Ecol. Res. 3:553–565.
Hoballah, M. E. and Turlings, T. C. J. 2005. The role of fresh versus old leaf damage in the attraction of parasitic wasps to herbivore-induced maize volatiles. J. Chem. Ecol. 31:2003–2018.
Huang J., Cardoza Y. J., Schmelz E. A., Raina R., Engelberth J., and Tumlinson, J. H. 2003. Differential volatile emissions and salicylic acid levels from tobacco plants in response to different strains of Pseudomonas syringae. Planta 217:767–775.
Köllner, T. G., Schnee, C., Gershenzon, J., and Degenhardt, J. 2004. The sesquiterpene hydrocarbons of maize (Zea mays) form five groups with distinct developmental and organ-specific distribution. Phytochemistry 65:1895–1902.
Lawrence, S. D. and Novak, N. G. 2004. Maize genes induced by herbivory and volicitin. J. Chem. Ecol. 30:2543–2557.
Lichtenthaler, H. K., Rohmer, M., and Schwender, J. 1997. Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Physiol. Plant. 101:643–652.
Meiners, T., Wäckers, F., and Lewis, W. J. 2003. Associative learning of complex odors in parasitoid host location. Chem. Senses 28:231–236.
Niinemets, U., Loreto, F., and Reichstein, M. 2004. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 9:180–186.
Paré, P. W. and Tumlinson, J. H. 1997. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 114:1161–1167.
Rasmann, S., Köllner, T. G., Degenhardt, J., Hiltpold, I., Toepfer, S., Kuhlmann, U., Gershenzon, J., and Turlings, T. C. J. 2005. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434:732–737.
Ruther, J. and Fürstenau B. 2005. Emission of herbivore-induced volatiles in absence of a herbivore—Response of Zea mays to green leaf volatiles and terpenoids. Z. Naturforsch. C 60:743–756.
Ruther, J. and Kleier, S. 2005. Plant–plant signaling: Ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-hexen-1-ol. J. Chem. Ecol. 31:2217–2222.
Scascighini, N., Mattiacci, L., D’Alessandro, M., Hern, A., Rott, A. S., and Dorn, S. 2005. New insights in analysing parasitoid attracting synomones: Early volatile emission and use of stir bar sorptive extraction. Chemoecology 15:97–104.
Schnee, C., Köllner, T. G., Gershenzon, J., and Degenhardt, J. 2002. The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-beta-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiol. 130:2049–2060.
Schmelz, E. A., Alborn, H. T., Banchio, E., and Tumlinson, J. H. 2003a. Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216:665–673.
Schmelz, E. A., Alborn, H. T., Engelberth, J., and Tumlinson, J. H. 2003b. Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize. Plant Physiol. 133:295–306.
Schmelz, E. A., Alborn, H. T., and Tumlinson, J. H. 2003c. Synergistic interactions between volicitin, jasmonic acid and ethylene mediate insect-induced volatile emission in Zea mays. Physiol. Plant. 117:403–412.
Schönbrunn, E., Eschenburg, S., Shuttleworth, W. A., Schloss, J. V., Amrhein, N., Evans, J. N. S., and Kabsch, W. 2001. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. Proc. Natl. Acad. Sci. USA 98:1376–1380.
Shah, J. 2003. The salicylic acid loop in plant defense. Curr. Opin. Plant Biol. 6:365–371.
Shen, B. Z., Zheng, Z. W., and Dooner, H. K. 2000. A maize sesquiterpene cyclase gene induced by insect herbivory and volicitin: Characterization of wild-type and mutant alleles. Proc. Natl. Acad. Sci. U. S. A. 97:14807–14812.
Steidle, J. L. M. and van Loon, J. J. A. 2003. Dietary specialization and infochemical use in carnivorous arthropods: Testing a concept. Entomol. Exp. Appl. 108:133–148.
Takabayashi, J., Takahashi, S., Dicke, M., and Posthumus, M. A. 1995. Developmental stage of herbivore Pseudaletia separata affects production of herbivore-induced synomone by corn plants. J. Chem. Ecol. 21:273–287.
Tamò, C., Ricard, I., Held, M., Davison, A. C., and Turlings, T. C. J. 2006. A comparison of naive and conditioned responses of three generalist endoparasitoids of lepidopteran larvae to host-induced plant odors. Anim. Biol. 56:205–220.
Thaler J. S., Karban R., Ullman D. E., Boege K., and Bostock R. M. 2002. Cross-talk between jasmonate and salicylate plant defense pathways: Effects on several plant parasites. Oecologia 131:227–235.
Tu, M., Hurd, C., and Randall, J. M. 2001. Weed control methods handbook: Tools and techniques for use in natural areas. Chapter 7e, ver. April, 2001. The Nature Conservancy, Davis, CA. http://tncweeds.ucdavis.edu/handbook.html.
Turlings, T. C. J. and Wäckers, F. 2004. Recruitment of predators and parasitoids by herbivore-injured plants, pp. 21–75, in R. T. Cardé and J. G. Millar (eds.). Ad Insect Chem Ecol. Cambridge University Press, Cambridge. Curr. Opin. Plant Biol.
Turlings, T. C. J. and Ton, J. 2006. Exploiting scents of distress: The prospect of manipulating herbivore-induced plant odors to enhance the control of agricultural pests. Curr. Opin. Plant Biol. 9:421–427.
Turlings, T. C. J., Tumlinson, J. H., and Lewis, W. J. 1990. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253.
Turlings, T. C. J., Wäckers, F. L., Vet, L. E. M., Lewis, W. J., and Tumlinson, J. H. 1993. Learning of host-finding cues by hymenopterous parasitoids, pp. 51–78, in D. R. Papaj and A. C. Lewis (eds.). Insect Learning. Ecological and Evolutionary Perspectives. Chapman & Hall, New York.
Turlings, T. C. J., Lengwiler, U. B., Bernasconi, M. L., and Wechsler, D. 1998. Timing of induced volatile emissions in maize seedlings. Planta 207:146–152.
Turlings, T. C. J., Alborn, H. T., Loughrin, J. H., and Tumlinson, J. H. 2000. Volicitin, an elicitor of maize volatiles in oral secretion of Spodoptera exigua: Isolation and bioactivity. J. Chem. Ecol. 26:189–202.
Turlings, T. C. J., Davison, A. C., and Tamó, C. 2004. A six-arm olfactometer permitting simultaneous observation of insect attraction and odor trapping. Physiol. Entomol. 29:45–55.
Van den Boom, C. E. M., Van Beek, T. A., Posthumus, M. A., De, Groot A., and Dicke, M. 2004. Qualitative and quantitative variation among volatile profiles induced by Tetranychus urticae feeding on plants from various families. J. Chem. Ecol. 30:69–89.
Vet, L. E. M. and Dicke, M. 1992. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 37:141–172.
Vet, L. E. M., Lewis, W. J., and Cardé, R. T. 1995. Parasitoid foraging and learning, pp. 65–101 in R. T. Cardé and W. J. Bell (eds.). Chemical Ecology of Insects. Chapman & Hall, New York.
Vet, L. E. M ., De Jong A. G., Franchi E., and Papaj, D. R. 1998. The effect of complete versus incomplete information on odor discrimination in a parasitic wasp. Anim. Behav. 55:1271–1279.
Vuorinen, T., Nerg, A. M., and Holopainen, J. K. 2004. Ozone exposure triggers the emission of herbivore-induced plant volatiles, but does not disturb tritrophic signalling. Environ. Pollut. 131:305–311.
Acknowledgment
We thank the members of the Evolutionary Entomology laboratory at the University of Neuchâtel for their continuous support and stimulating discussions on behavioral and chemical aspects of this study. We also thank Yves Borcard for parasitoid rearing and Syngenta (Stein, Switzerland) for the weekly shipments of S. littoralis eggs and artificial diet. We are grateful to Ingrid Ricard and Anthony Davison for statistical advice. This project was funded by the Swiss National Science Foundation (grant 31-058865.99) and the Swiss National Centre of Competence in Research “Plant Survival.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Alessandro, M., Held, M., Triponez, Y. et al. The Role of Indole and Other Shikimic Acid Derived Maize Volatiles in the Attraction of Two Parasitic Wasps. J Chem Ecol 32, 2733–2748 (2006). https://doi.org/10.1007/s10886-006-9196-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9196-7