Abstract
Large-scale implementation of transgenic crop varieties raises concerns about possible nontarget effects on other organisms. This study examines the effects of genetic modification on plant volatile production and its potential impact on arthropod population dynamics. We compared herbivore-induced volatile emissions from Bacillus thuringiensis Berliner (Bt) maize plants to those from a nontransformed isoline following exposure to various types of leaf damage. When equal numbers of Helicoverpa zea Boddie (Lepidoptera: Noctuidae) larvae fed on Bt and non-Bt maize, volatile emissions were significantly lower in the transgenic plants, which also exhibited less leaf damage. When damage levels were controlled by adding more larvae to Bt plants, the plants' volatile emissions increased but displayed significant differences from those of nontransgenic plants. Significantly higher amounts of linalool, β-myrcene, and geranyl acetate were released from transgenic maize than from non-Bt plants. Manipulating the duration of feeding by individual larvae to produce similar damage patterns resulted in similar volatile profiles for Bt and non-Bt plants. Controlling damage levels more precisely by mechanically wounding leaves and applying larval regurgitant likewise resulted in similar emission patterns for Bt and non-Bt maize. Overall, changes in the herbivore-induced volatile profiles of Bt maize appeared to be a consequence of altered larval feeding behavior rather than of changes in biochemical plant defense pathways. The implications of these findings for understanding the impacts of plant-mediated cues on pest and natural enemy behavior in transgenic crop systems are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetically modified (GM) crops designed to resist insect herbivores are rapidly being adopted in many parts of the world. Transgenic maize (Zea mays L.) engineered to express delta-endotoxins from the soil bacterium Bacillus thuringiensis Berliner (Bt) has been commercially available since 1996 and now accounts for 35% of all maize grown in the US (National Agricultural Statistics Service, 2005). Large-scale implementation of transgenic crop technology has sparked substantial debate over the social, economic, and ecological implications of GM agriculture. Concerns about the ecology of insect-resistant transgenic crops have often focused on resistance development by pests and potential negative effects on natural enemies (Gould, 1998; Shelton et al., 2002). These issues are intricately linked to one another because natural enemies can influence the rate that pest populations adapt to resistant plants (Gould et al., 1991). The mechanisms underlying possible unintended ecological effects of transgenic crops warrant increased research attention.
One way in which GM crops might affect pest and natural enemy population dynamics is by altering plant defense mechanisms, including herbivore-induced plant volatiles. Volatile compounds released by plants can influence the behavior of herbivores searching for oviposition sites (Anderson and Alborn, 1999; De Moraes et al., 2001) and foraging natural enemies (Dicke et al., 1990; Dicke and van Loon, 2000; Turlings et al., 1990; De Moraes and Mescher, 2004). The ecological interactions mediated by these induced plant volatiles are often complex and can be quite sophisticated; for example, volatile blends can be keyed to particular herbivore species and attract species-specific parasitoids (De Moraes et al., 1998). Thus, these interactions may be sensitive to any changes in plant biochemistry that alter plant responses.
Volatile responses might be altered in GM crops through unanticipated phenotypic changes in plant defense systems because of pleiotrophic effects or insertional mutations caused by incorporating a foreign gene (Schuler et al., 1999a). Significant phenotypic changes induced by genetic modification are not without precedent: the production of lignin, a major structural component of plants, increased in vascular tissues by 33–97% in Bt maize over non-Bt isolines (Saxena and Stotzky, 2001). Similar changes to complex biochemical pathways involved in plant defense might disrupt or alter the ability of plants to recognize and respond to herbivores, leading to significant differences between crop lines.
Induced volatile responses in transgenic crops might also be influenced by changes in interactions between herbivores and plants. The presence of Bt toxin changes the behavior of some insects. Lepidopteran larvae, for example, can detect and avoid plant parts or artificial diet containing varying forms of the toxin (Ashfaq et al., 2001; Gore et al., 2002, 2005). Such behavioral changes may alter herbivore feeding patterns, changing patterns and intensity of plant tissue damage and the exposure of the plant to oral secretions of herbivores. Plant volatile emissions are likely to be affected by such changes (Schmelz et al., 2003; Pare et al., 2005).
Whereas qualitative and quantitative changes in volatile emissions have been shown for undamaged Bt cotton and mechanically damaged GM tomatoes (Smith et al., 1996; Yan et al., 2004), the effects of genetic engineering on herbivore-induced plant volatiles remain largely unexplored. No change in parasitoid attraction was found between Bt and non-Bt oilseed rape plants equally damaged by Bt-resistant herbivores, suggesting a similarity in the volatiles produced by each plant, but no mechanism was elucidated (Schuler et al., 1999b). Further understanding of these effects will be accomplished most readily in plant species, such as maize, where the molecular and biochemical bases of induced volatile production are well understood. In response to herbivore feeding on maize, specific blends of compounds from the lipoxygenase, terpenoid biosynthesis, and shikimic acid pathways are released both locally and systemically (Turlings and Tumlinson, 1992). Whereas many of these compounds are not released in response to mechanical damage, the application of herbivore regurgitant induces a volatile response that closely resembles actual feeding (Turlings et al., 1990).
In this study, we investigated how induced volatiles varied between GM maize with the Bt cry1A(b) gene inserted and a nontransformed isoline. The endotoxin produced by the modified gene is targeted toward the European corn borer (Ostrinia nubilalis Hübner), but is also effective at increasing developmental times and mortality rates in other lepidopteran larvae, including the corn earworm (Helicoverpa zea Boddie) (Wiseman et al., 1999). H. zea is an economically important pest of maize kernels, but can also cause damage to whorl-stage maize plants by foliar feeding (Capinera, 2000). We first compared volatiles from undamaged plants to determine if Bt and non-Bt maize plants differed in their constitutive release of chemicals. Then, we performed several experiments comparing volatile induction by H. zea feeding on Bt and non-Bt maize plants, manipulating larvae numbers, damage amounts, and feeding patterns. To further control damage levels and patterns, we mimicked herbivore feeding by mechanically wounding leaves and applying regurgitant collected from larvae fed either with Bt or non-Bt maize.
Methods and Materials
Plants and Insects
Both Bt and non-Bt maize seeds were obtained from Dekalb (Monsanto Co., St. Louis, MO, USA). The Bt maize DKC61-25 contains the Monsanto Event 810, with the cry1A(b) gene. The nearest isoline of the Bt hybrid, DKC61-24, was used for the non-Bt plants. Plants were grown in a growth chamber (14-hr photophase; 25:22°C day/night, 65% RH) in pots (16 × 17-cm diam) filled with a peat-based general-purpose potting soil with micronutrients added.
H. zea larvae were reared from eggs (USDA/ARS, Tifton, GA, USA) on an artificial casein-based diet in plastic diet cups (30 ml, Solo Cup Co., Urbana, IL, USA) in incubators (14-hr photophase, 25°C, 60% RH). Larvae were starved for 24 hr before experiments. Third instar larvae were placed on plants for all experiments.
Volatile Collection and Analysis
We collected volatiles in a greenhouse from potted, intact 2- to 3-wk-old maize plants using a closed push/pull system (Analytical Research Systems, Gainesville, FL, USA). About 3 in. above the soil line, a Teflon® base consisting of two sliding plates (that when pushed together left a hole for the stalk to pass) was secured around the plant. A cotton ball was wrapped around the stalk to fill space between the base and plant and to allow air to exit. A cylindrical glass chamber (46 cm tall, 8-cm diam) was placed over the plant, resting on the Teflon® base. Air was pumped into the chamber (3.0 l/min) through Teflon® tubing and pulled out of the chamber (1.0 l/min) through side ports and across traps containing 40 mg SuperQ® (Alltech, Deerfield, IL, USA). Two separate volatile collections were made daily: one during the light phase for 12 hr, and the other during the dark phase for 8 hr.
Traps were eluted with 150 μl of dichloromethane, and n-octane (80 ng) and nonyl acetate (400 ng) were added to each sample as internal standards. Volatiles were analyzed by gas chromatography with a Hewlett-Packard model 5890 GC. Samples were injected in 1-μl aliquots with a splitless injector held at 240°C. The column (15 m × 0.25 mm i.d., HP-1) was maintained at 60°C for 2 min, and then increased 4°C/min to 180°C, where it was held for 10 min. Quantifications of compounds were made relative to the internal standard using Enhanced ChemStation software (Agilent Technologies, Palo Alto, CA, USA). Identifications were confirmed with mass spectrometry and by comparing retention times with those of known standards.
Undamaged Plants
Volatiles were collected from undamaged Bt and non-Bt maize to determine baseline emissions. Collections were made from four plants of each type. Means for Bt and non-Bt maize for this and all other experiments were compared with Student's t test using Minitab v. 14.1 (State College, PA, USA) (Sokal and Rohlf, 1995).
Equal Number of Larvae Per Plant
We tested the induced responses of maize when exposed to an equal number of larvae on each plant type to approximate likely field conditions. Two third instars were added to each chamber containing individual Bt or non-Bt plants and allowed to feed freely on plants during 4 d of volatile collecting. Each day, dead or molting larvae were replaced with ones that had been starved for 24 hr. These comparisons were made on six Bt and six non-Bt plants.
To avoid disturbing the plants while larvae were feeding, we assessed herbivore damage while plants remained inside volatile collection chambers. Each morning during the 4-d feeding experiment, we estimated the size of damage holes on unfurled leaf blades and through the enumerated layers of the whorled cylinder by comparison with a guide composed of five different-sized circles of known area. From the number and estimated size of holes, we calculated the total leaf area removed.
Equal Amount of Herbivore Damage
To determine if the amount of larval feeding affects induced responses of Bt and non-Bt maize, we added H. zea larvae to Bt plants daily to achieve equivalent damage on both plant types. On the first day of the experiment, 8–10 third instars were added to each Bt maize plant, and one or two larvae were added to each non-Bt plant. Each day, more third instars (∼5) were added to Bt maize through the tops of chambers to replace ones that had died. By the end of the 4-d sampling period, most larvae had reached their fourth instar. As a control, the tops of the non-Bt chambers were vented for an equal time. Each day, we assessed and estimated the amount of plant damage as above. Three plants of each type were used for comparisons.
Equal Pattern and Amount of Herbivore Damage
Individual larvae on non-Bt plants feed longer and cause greater localized damage. Because the feeding pattern (i.e., the number and size of damage holes) may influence induced responses, we manipulated the duration of larval feeding, resulting in the consumption of an equal amount of leaf tissue in a similar pattern on each plant type. Third instars placed on Bt and non-Bt maize plants were monitored continuously as they fed, and individual larvae were removed once they consumed an area of leaf tissue equivalent in size to the damage a Bt plant would typically incur from one larva (∼5 mm2). The number of larvae on each plant was manipulated to achieve similar amounts of damage across plants (∼60 mm2). After all larvae were removed, usually about 3 hr after the first larvae were placed on the plants, we collected volatiles in a growth chamber for the next 3 d by using a collection system similar to the one used in the other experiments (Analytical Research Systems). Air was pumped into the chambers at 2 l/min and pulled out through traps at 0.8 l/min. A total of five Bt plants and four non-Bt plants were used as replications in this experiment. When volatile collections were finished, we cut plants at the base and digitally scanned leaves to quantify leaf area removed (SigmaScan Pro v. 5.0, SPSS Inc., Chicago, IL, USA).
Mechanical Damage and Regurgitant Application
To control the pattern and amount of damage more precisely than was possible using natural herbivory, we artificially simulated herbivory by mechanically damaging leaves and applying larval regurgitant. We collected regurgitant from fourth instar H. zea by probing the preoral cavity with a pipette tip and drawing in the fluid released from the mouth. Larvae in diet cups had either been fed with foliage from non-Bt maize for 24 hr or foliage from Bt maize for 6 hr after having been starved for 18 hr prior to collecting. The shorter feeding time was necessary for those fed with Bt maize because the larvae would become inactive soon after eating the transgenic leaves, at which point regurgitant could not be obtained. Regurgitant was kept on ice while being collected and boiled for 3 min to stop enzyme activity (Mori et al., 2001). Samples were stored at −80°C until needed. Preliminary experiments showed no clear differences between the volatile profiles of Bt or non-Bt plants induced by regurgitant collected from larvae fed with either Bt leaves or non-Bt leaves (data not shown). Because of this similarity in responses and the difficulties involved in collecting regurgitant from larvae exposed to the Bt toxin, regurgitant collected from non-Bt fed larvae was used to compare volatiles from Bt and non-Bt plants with artificial herbivory.
Volatiles were collected from mechanically damaged plants with and without regurgitant. Plants were damaged by scraping leaf tissue from the upper side of leaves with a razor blade in a 1 × 2 cm rectangle. Each day, the glass volatile collection chambers were temporarily removed, and three leaves on each plant were damaged, with regurgitant (10 μl) applied to each damaged spot. The chambers were replaced, and volatiles were collected. Three Bt and three non-Bt plants were used for each experiment (with and without regurgitant).
Results
Undamaged Plants
We found no differences in the total amount of volatiles released or in quantities of individual compounds in the emissions from undamaged Bt and non-Bt maize (Table 1).
Equal Numbers of Larvae Per Plant
When equal numbers of H. zea larvae were fed on both maize isolines, non-Bt plants suffered 4 to 22 times more damage by the end of the experiment (Student's t test, t 6 = −3.53, P = 0.012). By the third day, the amount of damage as well as the amount of volatiles released was significantly greater on non-Bt plants (Table 1). Damage to Bt maize typically occurred only on the first day larvae that were placed on the plants, because after initial feeding, larvae often would be inactive but still alive for one or two more days. On non-Bt plants, larvae would continue to feed for the 4 d of the experiment, causing extensive damage.
Equal Amount of Herbivore Damage
When we equalized the amount of herbivory each day, there was a significant difference in the total amount of volatiles released by day 3 (Table 1). Four compounds constituted a major proportion of this difference: (Z)-3-hexenyl acetate, linalool, β-myrcene, and geranyl acetate (Table 2). The pattern of damage inflicted by each larvae differed between plant lines, however, with larvae on Bt plants feeding in short bouts and leaving many small, scattered holes, whereas larvae on non-Bt plants fed more or less continuously, creating large areas devoid of leaf tissue (Fig. 1).
Equal Pattern and Amount of Herbivore Damage
Manipulating the amount of time larvae were allowed to feed resulted in plants with similar numbers of damage holes, leaf area removed, and perimeters of damaged areas (Table 3). In this case, the two maize isolines emitted similar blends in terms of total volatiles released and proportions of individual compounds (Table 1).
Mechanical Damage and Regurgitant Application
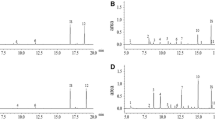
The volatiles released by Bt and non-Bt plants after applying larval regurgitant did not differ substantially from one another quantitatively or qualitatively (Table 1). The volatile profile of maize plants with artificial herbivory closely approximated that of plants exposed to actual larvae damage (Fig. 2). However, regurgitant-treated plants did not release some “green leafy volatiles” (Turlings et al., 1998) and had a weaker induction of β-farnescene and bergamotene (Fig. 2). Plants that were wounded with a razor blade appeared to release fewer volatile compounds and smaller quantities of the ones that were emitted relative to those damaged by herbivores.
Chromatograms of volatile compounds emitted on day 3 from non-Bt maize plants subjected to feeding by H. zea larvae, mechanical damage, mechanical damage with regurgitant applied, and no damage. Major compounds (with a threshold emission of 2 ng/hr) are labeled as follows: (1) unknown 1; (2) β-myrcene; (3) (Z)-3-hexenyl acetate; (4) linalool; (5) (E)-4-8-dimethyl-1,3,7-nonatriene; (6) unknown 2; (7) indole; (8) unknown 3; (9) unknown 4; (10) geranyl acetate; (11) bergamotene; (12) (E)-β-farnescene; (13) (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene; IS and IS2, internal standards (n-octane and nonyl acetate).
Discussion
In our experiments, genetic modification did not appear to alter the volatile profile of undamaged maize (Table 1). However, undamaged Bt cotton plants emitted unique compounds and different proportions of typical compounds when compared to non-Bt cotton (Yan et al., 2004), hinting at the diversity of effects genetic modification can have on different plant species.
Undamaged Bt maize plants do not appear to influence the ovipositional preferences of some Lepidopteran pests (Hellmich et al., 1999; Liu et al., 2002), suggesting that an equal number of larvae would initially be expected on each plant type. When we allowed equal numbers of larvae to feed on plants continuously for 4 d, non-Bt maize received up to 22 times more damage and emitted significantly more volatiles than Bt maize (Table 1). In the field, similar reductions in induced volatile emissions could cause areas planted with Bt maize to be less attractive to natural enemies that rely on herbivore-induced plant cues to find suitable hosts. Sked (2003) showed that fields sown with Bt maize had one third the numbers of Macrocentrus cingulum, a parasitoid specialist on the European corn borer, as non-Bt plots. Such reductions in population size could conceivably lead to local extirpation of natural enemies. At the same time, reduced volatile emissions could attract herbivores seeking a suitable oviposition site. For example, O. nubilalis oviposits in the field preferentially on undamaged maize plants compared to those infested with conspecific larvae (Harmon et al., 2003). More pest egg masses laid on Bt plants emitting fewer volatiles than severely damaged non-Bt maize could increase the possibility of resistance emerging within pest populations (Hellmich et al., 1999).
If an ovipositional preference for Bt plants did arise in the field, the greater numbers of larvae might produce an amount of damage closer to that incurred by non-Bt plants. Our results indicate that even with an equal amount of damage, volatile emissions still varied between Bt and non-Bt plants (Table 2). Linalool, β-myrcene, and geranyl acetate were released in significantly greater quantities from Bt maize. These compounds have been implicated in natural enemy attraction through behavioral studies and antennal electrophysiological responses (Turlings et al., 1991; Rose et al., 1998; Gouinguene et al., 2005).
Although damage amounts were equalized by increasing the number of larvae on Bt plants, these larvae exhibited a different feeding behavior, and thus created a different pattern of damage than larvae feeding on non-Bt plants. Damage holes were more numerous but smaller on Bt maize, increasing the perimeter of damaged areas and maximizing the contact zone of oral secretions and exposed plant tissue. When the feeding of individual larvae was manipulated to achieve similar damage patterns, differences in volatile emissions between Bt and non-Bt maize were no longer evident, suggesting that these differences resulted from the altered feeding behavior of larvae on Bt plants, rather than physiological changes in plant response resulting from genetic modification.
When the damage pattern and amount were standardized, either by manipulating the feeding duration of larvae or by applying larval regurgitant to mechanically damaged leaves, the induced volatile emissions of Bt and non-Bt maize were similar. These results suggest that Bt and non-Bt fields subjected to similar amounts and patterns of feeding damage, as might occur if pest populations developed resistance to the Bt maize, would be equally attractive to natural enemies. Similarly, the parasitic wasp Cotesia plutellae was found to be equally attracted to Bt oilseed rape plants fed upon by Bt-resistant hosts (Plutella xylostella) and wild-type plants equally damaged by the same herbivore, suggesting no change in the composition of the induced volatiles (Schuler et al., 1999b).
In summary, observed differences in induced volatile profiles between Bt and non-Bt maize appear to result from different amounts and patterns of feeding damage inflicted. Individual larvae on transgenic plants fed in short bouts, causing less damage and leaving distinctive damage patterns relative to conventional plants. When we controlled for these differences in the amount and pattern of feeding damage inflicted, differences in the volatile profiles of Bt and non-Bt plants disappeared, suggesting that these differences are a consequence of altered larvae feeding behavior on Bt plants rather than of changes in biochemical plant defense pathways. As reduced herbivory is the goal of insect-resistant transgenic crops, the consequent reductions in volatile emissions may have important implications for sustainable pest management.
References
Anderson, P. and Alborn, H. 1999. Effects on oviposition behaviour and larval development of Spodoptera littoralis by herbivore-induced changes in cotton plants. Entomol. Exp. Appl. 92:45–51.
Ashfaq, M., Young, S. Y., and McNew, R. W. 2001. Bollworm (Lepidoptera: Noctuidae) development and movement on Bacillus thuringiensis-treated cotton leaves. J. Entomol. Sci. 36:23–33.
Capinera, J. 2000. Featured Creatures: Corn earworm. University of Florida, Report no. EENY-145.
De Moraes, C. M., Lewis, W. J., Pare, P. W., Alborn, H. T., and Tumlinson, J. H. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573.
De Moraes, C. M., Mescher, M. C., and Tumlinson, J. H. 2001. Caterpillar-induced nocturnal plant volatiles repel nonspecific females. Nature 410:577–580.
De Moraes, C.M. and Mescher, M.C. 2004. Biochemical crypsis in the avoidance of natural enemies by an insect herbivores. Proc. Natl. Acad. Sci. USA 101:8993–8997.
Dicke, M., Vanbeek, T. A., Posthumus, M. A., Bendom, N., Vanbokhoven, H., and Degroot A. E. 1990. Isolation and identification of volatile kairomone that affects acarine predator–prey interactions—Involvement of host plant in its production. J. Chem. Ecol. 16:381–396.
Dicke, M. and Van Loon, J. J. A. 2000. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 97:237–249.
Gore, J., Leonard, B. R., Church, G. E., and Cook, D. R. 2002. Behavior of bollworm (Lepidoptera: Noctuidae) larvae on genetically engineered cotton. J. Econ. Entomol. 95:763–769.
Gore, J., Adamczyk, J. J., and Blanco, C. A. 2005. Selective feeding of tobacco budworm and bollworm (Lepidoptera: Noctuidae) on meridic diet with different concentrations of Bacillus thuringiensis proteins. J. Econ. Entomol. 98:88–94.
Gouinguene, S., Pickett, J. A., Wadhams, L. J., Birkett, M. A., and Turlings, T. C. J. 2005. Antennal electrophysiological responses of three parasitic wasps to caterpillar-induced volatiles from maize (Zea mays mays), cotton (Gossypium herbaceum), and cowpea (Vigna unguiculata). J. Chem. Ecol. 31:1023–1038.
Gould, F. 1998. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu. Rev. Entomol. 43:701–726.
Gould, F., Kennedy, G. G., and Johnson, M. T. 1991. Effects of natural enemies on the rate of herbivore adaptation to resistant host plants. Entomol. Exp. Appl. 58:1–14.
Harmon, J. P., White, J. A., and Andow, D. A. 2003. Oviposition behavior of Ostrinia nubilalis (Lepidoptera: Crambidae) in response to potential intra- and interspecific interactions. Environ. Entomol. 32:334–339.
Hellmich, R. L., Higgins, L. S., Witkowski, J. F., Campbell, J. E., and Lewis, L. C. 1999. Oviposition by European corn borer (Lepidoptera: Crambidae) in response to various transgenic corn events. J. Econ. Entomol. 92:1014–1020.
Liu, Y. B., Tabashnik, B. E., Dennehy, T. J., Carriere, Y., Sims, M. A., and Meyer, S. K. 2002. Oviposition on and mining in bolls of Bt and non-Bt cotton by resistant and susceptible pink bollworm (Lepidoptera: Gelechiidae). J. Econ. Entomol. 95:143–148.
Mori, N., Alborn, H. T., Teal, P. E. A., and Tumlinson, J. H. 2001. Enzymatic decomposition of elicitors of plant volatiles in Heliothis virescens and Helicoverpa zea. J. Insect Physiol. 47:749–757.
National Agricultural Statistics Service. 2005. Acreage. Agricultural Statistics Board, U.S. Department of Agriculture, Report no. Cr Pr 2–5 (6-05).
Pare, P. W., Farag, M. A., Krishnamachari, V., Zhang, H. M., Ryu, C. M., and Kloepper, J. W. 2005. Elicitors and priming agents initiate plant defense responses. Photosynth. Res. 85:149–159.
Rose, U. S. R., Lewis, W. J., and Tumlinson, J. H. 1998. Specificity of systemically released cotton volatiles as attractants for specialist and generalist parasitic wasps. J. Chem. Ecol. 24:303–319.
Saxena, D. and Stotzky, G. 2001. Bt corn has a higher lignin content than non-Bt corn. Am. J. Bot. 88:1704–1706.
Schmelz, E. A., Alborn, H. T., Banchio, E., and Tumlinson, J. H. 2003. Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216:665–673.
Schuler, T. H., Poppy, G. M., Kerry, B. R., and Denholm, I. 1999a. Potential side effects of insect-resistant transgenic plants on arthropod natural enemies. Trends Biotechnol. 17:210–216.
Schuler, T. H., Potting, R. P. J., Denholm, I., and Poppy, G. M. 1999b. Parasitoid behaviour and Bt plants. Nature 400:825–826.
Shelton, A. M., Zhao, J. Z., and Roush, R. T. 2002. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu. Rev. Entomol. 47:845–881.
Sked, S. 2003. Host–parasitoid interactions between Macrocentrus cingulum Brischke (Hymenoptera: Braconidae) and its host, Ostrinia nubialis Hubner (Lepidoptera: Crambidae): Implications for ECB management. Master's Thesis. The Pennsylvania State University, University Park.
Smith, R. M., Marshall, J. A., Davey, M. R., Lowe, K. C., and Power, J. B. 1996. Comparison of volatiles and waxes in leaves of genetically engineered tomatoes. Phytochemistry 43:753–758.
Sokal, R. R. and Rohlf, F. J. 1995. Biometry: The Principles and Practice of Statistics in Biological Research. W.F. Freeman and Co., New York.
Turlings, T. C. J. and Tumlinson, J. H. 1992. Systemic release of chemical signals by herbivore-injured corn. Proc. Natl. Acad. Sci. USA 89:8399–8402.
Turlings, T. C. J., Tumlinson, J. H., and Lewis, W. J. 1990. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253.
Turlings, T. C. J., Tumlinson, J. H., Heath, R. R., Proveaux, A. T., and Doolittle, R. E. 1991. Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J. Chem. Ecol. 17:2235–2251.
Turlings, T. C. J., Lengwiler, U. B., Bernasconi, M. L., and Wechsler, D. 1998. Timing of induced volatile emissions in maize seedlings. Planta 207:146–152.
Wiseman, B. R., Lynch, R. E., Plaisted, D., and Warnick, D. 1999. Evaluation of Bt transgenic sweet corn hybrids for resistance to corn earworm and fall armyworm (Lepidoptera: Noctuidae) using a meridic diet bioassay. J. Entomol. Sci. 34:415–425.
Yan, F., Bengtsson, M., Anderson, P., Ansebo, L., Xu, C., and Witzgall, P. 2004. Antennal response of cotton bollworm (Helicoverpa armigera) to volatiles in transgenic Bt cotton. J. Appl. Entomol. 128:354–357.
Acknowledgments
We thank J. Tooker, M. C. Mescher, J. Tumlinson, C. Frost, C. Delphia, and J. Runyon for helpful comments and J. Saunders, E. Bogus, A. Conrad, K. Cortellini, and M. Peiffer for technical support. This project was supported by the David and Lucile Packard Foundation and the Beckman Foundation. Jennifer Dean is supported under a National Science Foundation Graduate Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dean, J.M., De Moraes, C.M. Effects of Genetic Modification on Herbivore-Induced Volatiles from Maize. J Chem Ecol 32, 713–724 (2006). https://doi.org/10.1007/s10886-006-9040-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9040-0