Abstract
Purpose
In vitro fertilization with trophectoderm embryo biopsy and pre-implantation genetic screening with comprehensive chromosomal screening (PGS-CCS) for aneuploidy is becoming increasingly more popular. Embryos are cryopreserved and implanted in a subsequent frozen thawed embryo transfer cycle (FET). No studies have investigated differences in pregnancy outcomes by timing of trophectoderm biopsy relative to stages of blastocyst development.
Methods
Retrospective study of all patients (n = 363) at a single IVF center between January 1, 2013 and December 31, 2016 undergoing single embryo transfer with PGS-CCS where embryos were cryopreserved with subsequent FET. Embryo expansion and grading was assessed both at the time of biopsy and transfer. Pregnancy rates were analyzed by embryo expansion and embryo grading.
Results
Implantation, clinical pregnancy, and live birth rates improved significantly with increased embryo expansion at the time of embryo biopsy (P < 0.001). Pregnancy loss decreased with increases in embryo expansion prior to biopsy (P < 0.001). Superior live birth rates with PGS-CCS were seen when embryos were hatching at the time of biopsy (p < 0.001). For fresh and frozen embryo transfers without PGS-CCS, embryo expansion did not affect pregnancy outcomes.
Conclusions
PGS-CCS significantly increases implantation and live birth rates only if embryos are hatching at the time of biopsy. The embryo biopsy itself on a non-hatching embryo significantly damages the embryo in ways which are not reflected in future embryo expansion. IVF labs should wait until embryos hatch before performing trophectoderm biopsy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Pre-implantation genetic screening is a common technique used in assisted reproductive technology where embryo DNA undergoes comprehensive chromosomal screening (PGS-CCS) for aneuploidy prior to implantation. Currently, most IVF labs perform PGS-CCS on trophectoderm cells from blastocyst embryos [1]. Trophectoderm biopsy can provide multiple cells for chromosome analysis thereby reducing the possibility of diagnostic errors [2,3,4]. Studies reviewing trophectoderm biopsies and PGD-CCS correlate IVF aneuploidy with increasing maternal age [5] but also demonstrate high aneuploidy rates even in younger women [6, 7].
After optimal selection of an embryo based on morphology and chromosomal status, fresh embryo transfer can be performed during the same cycle or frozen embryo transfer (FET) can be performed in a subsequent thawed cycle. Most IVF centers have high survival and pregnancy rates from thawed embryos [8,9,10,11,12]. Cryopreservation of embryos allows sufficient time for PGS-CCS to improve embryo selection [13]. FET nearly eliminates the risk of ovarian hyperstimulation syndrome [14,15,16,17,18,19]. Additionally, FET demonstrates equivalent or higher implantation and ongoing pregnancy rates compared to fresh embryo transfer, hypothesized to result from improved embryo-endometrium synchrony and more physiological estradiol levels [13, 20,21,22,23,24,25,26]. Most importantly, perinatal outcomes including low birth weight, preterm delivery, and pre-eclampsia favor FET over fresh embryo transfer [26,27,28,29,30,31,32,33,34].
IVF with PGS-CCS and subsequent FET with elective single embryo transfer (eSET) has been advocated by many physicians and IVF centers as the optimal mode of treatment. A recent meta-analysis of three randomized controlled trials found that PGS-CCS improved implantation rates (RR of 1.29, 95% CI 1.15–1.45) and sustained implantation rates beyond 20 weeks (RR of 1.39, 95% CI 1.21–1.60) [35]. Another meta-analysis of PGS-CCS demonstrated higher implantation rates, higher live birth rates, and decreased multiple pregnancy rates in four randomized controlled trials as well as higher ongoing pregnancy rates and decreased miscarriage and multiple pregnancy rates in seven cohort studies [36]. FET and eSET after PGS-CCS can achieve high implantation rates (75%), pregnancy rates (80%), and live birth rates (77%) [13]. eSET after PGS-CCS showed comparable pregnancy rates relative to untested two-blastocyst transfer (60.7% vs 65.1%, CI 0.7–1.2) [37]. Similar results were seen by Scott et al., with the PGS-CCS eSET having 66.4% sustained implantation and 84.7% delivery rates [38]. Comparable results are seen with women of advanced maternal age [39] and across different methods used for DNA analysis [40].
Studies clearly favor blastocyst biopsy of trophectoderm cells over cleavage stage embryo biopsy [1, 41]. Cleavage stage biopsies were associated with higher rates of mosaicism [42] and embryo damage, [43] which likely contributed to the failure of early PGS studies to find clinical benefit [44]. Determining when to biopsy cleavage stage embryos was easy since almost all embryos reach the 8-cell stage as a cohort. However, blastocysts develop at varied rates of time where the embryo cavity expands and later trophectoderm cells herniate out as the embryo hatches. Most labs assess embryos on the morning of day 5. Many labs biopsy all embryos at the same time on the morning of day 5 even though some embryos may not have expanded yet and others may already be hatching. Embryologists are not always available for a biopsy (they may be in an egg retrieval, performing ICSI, or maybe the left for the day). And embryologists may not be in the office as late on weekends or holidays as they are during a normal work week. Some labs may biopsy day 5 embryos of a single patient at different times on day 5, and other labs may biopsy embryos only in the morning of day 5 and day 6 but not at other times of the day. Protocols may vary depending on whether days 5 and 6 are weekdays or weekends or based on the time of the egg retrieval and how that impacts the time of day that embryos are assessed on day 5.
There are no studies to date which have assessed the optimal time to perform trophectoderm biopsy with respect to the expansion and growth of an embryo once it has already reached the blastocyst stage. The primary aim of our study is to evaluate whether the stage of development of trophectoderm biopsy for PGS-CCS affects pregnancy outcomes.
Methods
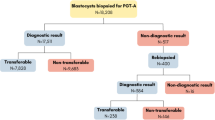
All cycles between January 1, 2013 and December 312,016 at Island Reproductive Services were examined. The practice consisted of two physicians who used standardized transfer techniques and one embryologist who performed all embryo grading and all embryo biopsies. This study included only IVF cycles in which all blastocyst embryos underwent trophectoderm biopsy for PGS-CCS with immediate cryopreservation. PGS-CCS was recommended to all patients regardless of prognosis, age, or diagnosis. Every embryo that reached the blastocyst stage was biopsied either on day 5 or day 6 based on embryologist availability. FET followed in a subsequent cycle. Analysis was restricted to cycles in which eSET was performed (n = 362). All pregnancy data resulted from day 5 embryos. Although some embryos were extended in culture to day 6, none of those are represented here since day 5 euploid embryos were always prioritized over day 6 embryos for embryo transfer. All FET were performed after oral 17B estradiol preparation to thicken the endometrium to at least 8 mm. A total of 429 cycles were initiated—32 cycles had no euploid embryos to transfer and 67 cycles had double embryo transfer and were excluded from analysis. No monozygotic twins occurred.
Oocyte retrieval was performed by transvaginal ultrasound-guided aspiration 35 h after hCG administration. All egg retrievals were performed between 6:30 and 9:30 AM. ICSI was performed for all oocytes 4–6 h after oocyte retrieval with fertilization check 16–18 h later. Embryos were cultured in an open culture system in group culture in 25-μl micro drops Global® Total® media (Life Global®, USA) with a change of media on day 3. Temperature was maintained at 37 °C with 40% humidity. Triple mix gas lines were used with 5% O2, 6% CO2, and 89% N2 concentrations. Every single embryo (regardless of PGC-CCS) underwent assisted laser hatching on day 3 (ZILOS-tk® laser, Hamilton Thorne).
Embryos were assessed by a single embryologist first at the time of trophectoderm biopsy (prior to cryopreservation) as well as at the time of FET [45]. Embryo expansion was characterized as non-expanded (blastocoel cavity filling 50–100% of the volume of the embryo), expanded (blastocoel cavity larger than the original embryo), hatching (trophectoderm cells extruding outside the zona pellucida), and hatched (complete extrusion of trophectoderm cells and inner cell mass from the original embryo). Inner cell mass was grades A (many cells, tightly packed), B (fewer cells, loosely packed), and C (few cells). Trophectoderm was grades A (many cells forming a tightly packed layer), B (fewer cell, more loosely packed), and C (few cells with gaps between them).
PGS was performed at the blastocyst stage by trophectoderm biopsy of 7–10 cells by a single embryologist with a noncontact 1.46 μ laser (ZYLOS-tk ®, Hamilton Thorne, MA, USA). Trophectoderm cells were aspirated by a 20 μ pipet (30° angled flat Humagen pipet, ORIGIO, Cooper Surgical, USA) and loaded into a PCR tube with 3.0 μl PBS. No day 3 biopsies were taken during the study period. Whole genome amplification was performed for all embryos by a single commercial laboratory using an Illumina CGH microarray. Only euploid embryos were transferred.
All embryos were cryopreserved by vitrification (Life Global®, USA) at the blastocyst stage to negative 196 °C. After thaw of frozen embryos for FET, embryos were held and warmed for approximately 4 h prior to FET. Embryo transfer was standardized for both physicians using Wallace ® transfer catheter under abdominal ultrasound guidance with the embryo loaded in 10 μl of media. The embryo was placed 1.5 cm from the fundus. Luteal support consisted of Crinone® 8% vaginally twice per day and Prometrium® 200 mg orally twice per day. No hCG was used for luteal support.
Cycle parameters and patient characteristics were tabulated for all patients. Clinical pregnancy was defined by fetal cardiac activity and implantation rates were defined as the number of fetal heartbeats per embryo transferred. Pregnancy loss included both biochemical pregnancies and miscarriages. Associations between embryo expansion and trophectoderm and inner cell mass scoring at the time of embryo biopsy and again at the time of embryo transfer were assessed by two-way ANOVA. Pregnancy outcomes (live birth rate, clinical pregnancy, pregnancy loss, and implantation) were assessed by ANOVA with Bonferroni correction, Kruskal-Wallis with Dunn’s test where appropriate, and Fisher’s exact test. Chi-squared and logistic regression were used to analyze the associations between trophectoderm grading, embryo expansion, and pregnancy outcomes. Stata v10 was used for statistical analysis and p value < 0.05 was considered statistically significant. Figures were created in GraphPad Prism. This study was approved by an institutional review board at Staten Island University Hospital and Northwell Health.
Results
Overall, patients undergoing FET after PGS-CCS with eSET had excellent pregnancy outcomes—clinical pregnancy rate was 46% and live birth rate was 42%. Data combined patients of all ages since only euploid embryos were transferred. Patients overall had appropriate ovarian reserve and normal endometrial thickness (Table 1). Patients in the PGS-CCS group were slightly younger; however, this did not impact any pregnancy outcomes (implantation, clinical pregnancy, live birth rate). Embryo grading and expansion were examined by two-way ANOVA. Increasing embryo expansion at the time of biopsy led to higher rates of implantation (SS 1.56, p = 0.02), clinical pregnancy (SS 1.58, p = 0.02), and live birth rates (SS 1.85, p = 0.007). Inner cell mass and trophectoderm grading at the time of biopsy did not affect any of these pregnancy outcomes (implantation SS 0.97 p = 0.2, clinical pregnancy SS 0.00 p = 0.17, live birth rate SS 0.5, p = 0.41). There was no statistical relationship between trophectoderm and inner cell mass grading and embryo expansion. All of these embryo parameters were now analyzed at the time of embryo transfer. Again, only embryo expansion affected implantation (SS 1.49, p = 0.04), clinical pregnancy (SS 1.51, p = 0.04), and live birth (SS 1.87, p = 0.01).
Pregnancy outcomes in PGS-CCS cycles with eSET were now stratified by embryo expansion at the time of embryo biopsy. Embryo expansion at biopsy was characterized as non-expanded (blastocoel cavity filling 50–100% of the volume of the embryo, n = 35), expanded (blastocoel cavity larger than the original embryo, n = 68), and hatching (trophectoderm cells extruding outside the zona pellucida, n = 259). Implantation, clinical pregnancy, and live birth rates drastically improved, and pregnancy loss decreased with increasing embryo expansion at the time of biopsy. Embryos that were hatching prior to biopsy had superior pregnancy outcomes (Fig. 1). Live birth rate was higher (OR 25.12, p < 0.001), clinical pregnancy was higher (OR 11.26, p < 0.001), and pregnancy loss was lower (OR 0.26, p < 0.001) (multivariable logistic regression) with more embryo expansion at biopsy. Dismal pregnancy outcomes resulted, despite PGS-CCS, when embryos were non-expanded at the time of biopsy. Specifically, no live birth resulted from a PGS-CCS euploid embryo that was non-expanded at biopsy.
Pregnancy outcomes with PGS-CCS by embryo expansion at time of embryo biopsy. *Statistical significance. Includes all age groups combined. Overall comparisons made by Kruskal-Wallis and post hoc Dunn test. All p values < 0.001 for comparisons between non-expanded and hatching as well as between expanded and hatching. No significant differences between non-expanded versus expanded embryos for any pregnancy outcomes
We wanted to determine whether these findings were attributable solely to embryo expansion at biopsy or whether further expansion (or lack thereof) by the time of FET-affected outcomes. Embryo expansion at the time of biopsy was compared to expansion later at FET. We examined whether pregnancy outcomes changed if embryos were non-expanded or expanded at the time of biopsy but subsequently expanded or were hatching by the time of FET. Thirty-five non-expanded embryos were biopsied. At the time of subsequent FET, 12 were still non-expanded, 4 had expanded, and 19 were hatching. Although some non-expanded embryos at biopsy later expanded and hatched prior to FET, no live births resulted from any of these embryos (Table 2). Of the 68 expanded embryos biopsied, 34 were expanded and 34 were hatching at the time of FET. Despite some live births from these embryos, pregnancy outcomes overall remained poor (Table 3).
In a final effort to distinguish embryo expansion effects from biopsy effects, pregnancy outcomes of PGS-CCS cycles were compared to fresh eSET without PGS-CCS during the same study period as well as to FET with eSET but without PGS-CCS. The purpose was not to make a comparison of pregnancy rates between the various groups, but only to see if non-biopsied non-expanded or expanded embryos led to pregnancies. There were 119 fresh eSET during the study period. All fresh transfers took place on day 5 and had similar luteal support to PGS-CCS cycles except that patients used Crinone® 8% vaginally once (not twice) daily for fresh cycles. For fresh eSET cycles, embryo expansion did not predict implantation (SS 0.80, p = 0.2), clinical pregnancy (SS 0.08, p = 0.2), or live birth rates (SS 0.73, p = 0.2) (two-way ANOVA). There was no interaction between embryo expansion and grade (p all > 0.5). Embryo expansion in these cycles did not affect pregnancy loss (SS 0.28, p = 0.33). There were 115 FET eSET during the study period without PGS-CCS. These were performed either to avoid OHSS, in patients with elevated progesterone levels prior to oocyte retrieval, or electively. All FET used day 5 embryos and had identical luteal support to PGS-CCS cycles. For FET eSET cycles without PGS-CCS, embryo expansion did not predict implantation (SS 0.49, p = 0.38), clinical pregnancy (SS 0.47, p = 0.40), or live birth rates (SS 0.95, p = 0.14) (two-way ANOVA). There was no interaction between embryo expansion and grade (p all > 0.5). Embryo expansion in these cycles did not affect pregnancy loss (SS 0.03, p = 0.90). No significant comparisons of pregnancy outcomes can be made between these non-matched groups. However, the fact that fresh and frozen non-biopsied non-hatching embryos led to many pregnancies—and virtually no euploid biopsied non-hatching embryos led to births—seems to implicate the biopsy as a source of embryo damage.
Finally, we recalculated the initial comparisons of implantation rates, live birth rates, and pregnancy loss for eSET between PGS-CCS and non-PGS-CCS cycles (fresh and frozen transfers). For this analysis, we only included PGS-CCS cycles where embryos were hatching prior to embryo biopsy. We see far superior pregnancy rates in all outcomes from PGS-CCS and eSET and no difference in pregnancy loss as a result of PGS-CCS (Fig. 2).
Pregnancy outcomes for PGS-CCS of hatching embryos only compared to fresh and frozen non-PGS-CCS cycles with elective single embryo transfer. *Statistical significance. Includes all age groups combined. PGS-CCS cycles only include outcomes for embryos which were hatching at the time of embryo biopsy. Non-expanded and expanded embryos were excluded from this analysis. Overall comparisons made by Kruskal-Wallis and post hoc Dunn test. All p values < 0.001 for comparisons between FET PGS-CCS and fresh transfer as well as FET PGS-CCS and FET without PGS-CCS. No differences in any pregnancy outcomes were seen between fresh transfer and FET without PGS-CCS
Discussion
The application of PGS to routine infertility care has evolved over the past 10 years. At first, PGS-CCS was reserved for women of advanced age, recurrent pregnancy loss, or recurrent IVF failures. Now, PGS-CCS is commonly employed for couples of all diagnoses and age. Multiple randomized control studies demonstrate routine benefit to PGS-CCS [35,36,37,38,39]. Day 3 biopsies were abandoned due to concerns over embryo damage from the biopsy itself and mosaicism [41,42,43,44]. However, from a logistic standpoint, day 3 biopsies were easy to perform since most embryos advanced to the 8-cell stage at similar predictable times. Embryologists could easily plan their day and biopsy most embryos at the same time (provided that egg retrievals took place at one time). Very little decision making took place in deciding when to perform the biopsy.
However, there is no consensus as to when to biopsy a blastocyst embryo. Although informally physicians may comment as to whether they biopsy non-expanded or non-hatching embryos or perform day 5 versus day 6 biopsies, that data is largely absent from the literature. From a research standpoint, most publications do not describe protocols for the timing of embryo biopsy and provide few details about embryo expansion and biopsy. Many centers probably do not have a formal policy guiding the timing of biopsy. From a practical standpoint, embryos continually expand throughout the day and night. Most embryologists are not in the lab 24 h a day to observe embryo expansion (closed culture system) or continually remove and replace embryos from incubators to follow changes (open culture system). Embryologists must work based on their schedule. Practices with multiple embryologists have more choices as to when to perform embryo biopsies, but most still choose certain times to assess embryos before they leave in the afternoon and when they arrive in the morning. Embryologists must choose whether to biopsy at different times on day 5 or whether to wait 12–20 more hours and assess again on day 6, risking increased fragility of an embryo that has fully hatched out.
We provide compelling evidence that biopsy should only be performed once an individual embryo is hatching. In our study, when PGS-CCS biopsy was performed prior to an embryo hatching, very poor pregnancy outcomes resulted. PGS-CCS conferred a large advantage in pregnancy rates over non PGS-CCS embryos, as long as PGS biopsy was not taken until hatching occurred. These outcomes appear to be dependent on the biopsy itself. Even if non-expanded or expanded embryos hatched after biopsy, they did not produce good outcomes. Our study was uniform in that one highly experienced embryologist graded all embryos and performed all interventions. A lab with a less experienced embryologist or with multiple embryologists performing biopsies would likely experience a wider array of outcomes even for hatching embryos. In fresh and frozen cycles without PGS-CCS, good outcomes were seen in non-expanded and non-hatching embryos. This suggests that the biopsy itself mechanically harmed non-hatching embryos, essentially negating any positive effect of PGS results.
We hypothesize multiple possibilities for embryo damage from the biopsy. Biopsy of a non-hatching embryo requires penetration of the zona of the embryo. After ICSI and assisted hatching, this is potentially a third time in 5 days that the zona is breached. It is possible that embryo damage can occur simply from repeated mechanical penetration of the zona which is avoided when embryos are hatching. Additionally, some blastocyst embryos will arrest in growth. Early biopsy may mask recognition of embryo arrest. This scenario is less plausible since we saw implantation and live births from non-expanded fresh and frozen embryos in our study that had not undergone PGS-CCS testing. Finally, as the embryo expands and hatches, visual differentiation between the inner cell mass and trophectoderm improves. Biopsy of non-hatching blastocysts likely damages the inner cell mass even in the hands of experienced embryologists.
The major limitation of our study is its retrospective nature. We did not anticipate such a discrepancy in pregnancy rates between embryos. We feel at this time the feasibility of a prospective study is limited until more retrospective data is collected. We encourage others to report similar data. We have since changed laboratory protocol to permit only embryo biopsy of hatching embryos. It would be unethical to knowingly harm embryos by randomizing patients to early biopsy without significantly more data collection. In addition, we combined data for patients of all ages. Declining pregnancy rates with advancing age are most attributable to increased rates of aneuploidy. The transfer of euploid embryos largely negates this problem which is why we have chosen not to group patients by age. Our smaller sample size precludes meaningful analysis by SART age groups. However, our data for the past 5 years (not shown) demonstrates similar implantation and live birth rates per embryo transfer in patients of all ages once a euploid embryo is found.
The decision of when to perform trophectoderm biopsy is commonly left to embryologist discretion. Many labs biopsy a patient’s embryos all at the same time for convenience of biopsy and shipping. As more patients and physicians commonly employ PGS in routine care, the sheer number of embryo biopsies will increase. Larger practices with multiple embryologists capable of performing biopsies at any time of day may already have protocols in place for biopsy timing. In fact, smaller practices with fewer resources may have difficulty performing biopsies on one patient’s embryos at different times (for instance morning of day 5, afternoon and/or evening of day 5, and morning of day 6). However, we suggest that all labs have a protocol for optimal results.
Conclusions
In a general population of infertility patients, PGS-CCS and eSET after FET can provide superior live birth rates. However, blastocyst embryos should not be biopsied until they are hatching as earlier biopsy seems to damage embryos and drastically lower pregnancy rates. If embryos are not hatching by the time of embryologist availability for trophectoderm biopsy on day 5, labs should strongly consider embryo culture to day 6 to allow extra time for hatching prior to biopsy.
Availability of data and material
All data is publicly available at https://osf.io/ under project “timing of PGD biopsy” under Michael Traub.
Abbreviations
- IVF:
-
In vitro fertilization
- PGS-CCS:
-
preimplantation genetic screening with complete chromosomal analysis
- FET:
-
frozen thawed embryo transfer
- eSET:
-
elective single embryo transfer
References
Kalma Y, Bar-El L, Asaf-Tisser S, Malcov M, Reches A, Hasson J, et al. Optimal timing for blastomere biopsy of 8-cell embryos for preimplantation genetic diagnosis. Hum Reprod. 2017;33:1–7. https://doi.org/10.1093/humrep/dex343.
Carson SA, Gentry WL, Smith AL, Buster JE. Trophectoderm microbiopsy in murine blastocysts: comparison of four methods. J Assist Reprod Genet. 1993;10(6):427–33.
Dokras A, Sargent IL, Gardner RL, Barlow DH. Human trophectoderm biopsy and secretion of chorionic gonadotrophin. Hum Reprod. 1991;6(10):1453–9.
Gianaroli L, Magli MC, Ferraretti AP. The in vivo and in vitro efficiency and efficacy of PGD for aneuploidy. Mol Cell Endocrinol. 2001;183(Suppl 1):S13–8.
Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656–63 e1. https://doi.org/10.1016/j.fertnstert.2013.11.004.
Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, et al. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod BioMed Online. 2012;24(6):614–20. https://doi.org/10.1016/j.rbmo.2012.02.009.
Harton GL, Munne S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100(6):1695–703. https://doi.org/10.1016/j.fertnstert.2013.07.2002.
Lopes AS, Frederickx V, Van Kerkhoven G, Campo R, Puttemans P, Gordts S. Survival, re-expansion and cell survival of human blastocysts following vitrification and warming using two vitrification systems. J Assist Reprod Genet. 2015;32(1):83–90. https://doi.org/10.1007/s10815-014-0373-2.
Li Z, Wang YA, Ledger W, Edgar DH, Sullivan EA. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: a population-based cohort study. Hum Reprod. 2014;29(12):2794–801. https://doi.org/10.1093/humrep/deu246.
Sifer C. Contribution of embryo vitrification procedure to ART efficiency. Gynecol Obstet Fertil. 2014;42(10):721–4. https://doi.org/10.1016/j.gyobfe.2014.07.031.
Van Landuyt L, Van de Velde H, De Vos A, Haentjens P, Blockeel C, Tournaye H, et al. Influence of cell loss after vitrification or slow-freezing on further in vitro development and implantation of human day 3 embryos. Hum Reprod. 2013;28(11):2943–9. https://doi.org/10.1093/humrep/det356.
Pavone ME, Innes J, Hirshfeld-Cytron J, Kazer R, Zhang J. Comparing thaw survival, implantation and live birth rates from cryopreserved zygotes, embryos and blastocysts. J Human Reprod Sci. 2011;4(1):23–8. https://doi.org/10.4103/0974-1208.82356.
Coates A, Kung A, Mounts E, Hesla J, Bankowski B, Barbieri E, et al. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randomized controlled trial. Fertil Steril. 2017;107(3):723–30 e3. https://doi.org/10.1016/j.fertnstert.2016.12.022.
Karacan M, Erdem E, Usta A, Arvas A, Cebi Z, Camlibel T. Gonadotropin-releasing hormone agonist triggering with concomitant administration of low doses of human chorionic gonadotropin or a freeze-all strategy in high responders. Saudi Med J. 2017;38(6):586–91. https://doi.org/10.15537/smj.2017.6.17717.
Zech J, Brandao A, Zech M, Lugger K, Neururer S, Ulmer H, et al. Elective frozen-thawed embryo transfer (FET) in women at risk for ovarian hyperstimulation syndrome. Reprod Biol. 2017;18:46–52. https://doi.org/10.1016/j.repbio.2017.12.004.
Delvigne A, Rozenberg S. Review of clinical course and treatment of ovarian hyperstimulation syndrome (OHSS). Hum Reprod Update. 2003;9(1):77–96.
Endo T, Honnma H, Hayashi T, Chida M, Yamazaki K, Kitajima Y, et al. Continuation of GnRH agonist administration for 1 week, after hCG injection, prevents ovarian hyperstimulation syndrome following elective cryopreservation of all pronucleate embryos. Hum Reprod. 2002;17(10):2548–51.
Martinez MC, Ruiz FJ, Garcia-Velasco JA. GnRH-agonist triggering to avoid ovarian hyperstimulation syndrome: a review of the evidence. Curr Drug Targets. 2013;14(8):843–9.
Prapas Y, Ravanos K, Petousis S, Panagiotidis Y, Papatheodorou A, Margioula-Siarkou C, et al. GnRH antagonist administered twice the day before hCG trigger combined with a step-down protocol may prevent OHSS in IVF/ICSI antagonist cycles at risk for OHSS without affecting the reproductive outcomes: a prospective randomized control trial. J Assist Reprod Genet. 2017;34(11):1537–45. https://doi.org/10.1007/s10815-017-1010-7.
Pontre JC, Ryan JP, Tan A, Hart RJ. The interval transfer of a frozen-thawed embryo is more successful than a fresh embryo transfer for women undergoing IVF with recurrent implantation failure after cleavage stage embryo biopsy. Aust N Z J Obstet Gynaecol. 2018. https://doi.org/10.1111/ajo.12798.
Roque M, Valle M, Guimaraes F, Sampaio M, Geber S. Freeze-all policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril. 2015;103(5):1190–3. https://doi.org/10.1016/j.fertnstert.2015.01.045.
Adeviye Ersahin A, Acet M, Ersahin SS, Dokuzeylul GN. Frozen embryo transfer prevents the detrimental effect of high estrogen on endometrium receptivity. J Turk German Gynecological Assoc. 2017;18(1):38–42. https://doi.org/10.4274/jtgga.2016.0186.
Wu K, Zhao H, Liu H, Li M, Ma S, Li C, et al. Day 3 ET, single blastocyst transfer (SBT) or frozen-thawed embryo transfer (FET): which is preferable for high responder patients in IVF/ICSI cycles? J Assist Reprod Genet. 2014;31(3):275–8. https://doi.org/10.1007/s10815-013-0156-1.
Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil Steril. 2013;99(2):389–92. https://doi.org/10.1016/j.fertnstert.2012.09.044.
Zhou F, Lin XN, Tong XM, Li C, Liu L, Jin XY, et al. A frozen-thawed embryo transfer program improves the embryo utilization rate. Chin Med J. 2009;122(17):1974–8.
Roy TK, Bradley CK, Bowman MC, McArthur SJ. Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers. Fertil Steril. 2014;101(5):1294–301. https://doi.org/10.1016/j.fertnstert.2014.01.046.
Maheshwari A, Raja EA, Bhattacharya S. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: an analysis of 112,432 singleton pregnancies recorded in the human fertilisation and embryology authority anonymized dataset. Fertil Steril. 2016;106(7):1703–8. https://doi.org/10.1016/j.fertnstert.2016.08.047.
Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375(6):523–33. https://doi.org/10.1056/NEJMoa1513873.
Ozgur K, Berkkanoglu M, Bulut H, Humaidan P, Coetzee K. Perinatal outcomes after fresh versus vitrified-warmed blastocyst transfer: retrospective analysis. Fertil Steril. 2015;104(4):899–907 e3. https://doi.org/10.1016/j.fertnstert.2015.06.031.
Sun L, Chen ZH, Yin MN, Deng Y. Pregnancy and obstetric outcomes of fresh embryo transfer versus frozen-thawed embryo transfer in women below 35 years of age. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2017;37(7):929–32.
Litzky JF, Boulet SL, Esfandiari N, Zhang Y, Kissin DM, Theiler RN, et al. Effect of frozen/thawed embryo transfer on birthweight, macrosomia, and low birthweight rates in US singleton infants. Am J Obstet Gynecol. 2018;218(4):433 e1–e10. https://doi.org/10.1016/j.ajog.2017.12.223.
Vidal M, Vellve K, Gonzalez-Comadran M, Robles A, Prat M, Torne M, et al. Perinatal outcomes in children born after fresh or frozen embryo transfer: a Catalan cohort study based on 14,262 newborns. Fertil Steril. 2017;107(4):940–7. https://doi.org/10.1016/j.fertnstert.2017.01.021.
Sha T, Yin X, Cheng W, Massey IY. Pregnancy-related complications and perinatal outcomes resulting from transfer of cryopreserved versus fresh embryos in vitro fertilization: a meta-analysis. Fertil Steril. 2018;109(2):330–42 e9. https://doi.org/10.1016/j.fertnstert.2017.10.019.
Van Heertum K, Weinerman R. Neonatal outcomes following fresh as compared to frozen/thawed embryo transfer in in vitro fertilization. Birth Defects Res. 2018;110(8):625–9. https://doi.org/10.1002/bdr2.1216.
Dahdouh EM, Balayla J, Garcia-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril. 2015;104(6):1503–12. https://doi.org/10.1016/j.fertnstert.2015.08.038.
Chen M, Wei S, Hu J, Quan S. Can comprehensive chromosome screening technology improve IVF/ICSI outcomes? A meta-analysis. PloS One. 2015;10(10):e0140779. https://doi.org/10.1371/journal.pone.0140779.
Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100(1):100–7 e1. https://doi.org/10.1016/j.fertnstert.2013.02.056.
Scott RT Jr, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100(3):697–703. https://doi.org/10.1016/j.fertnstert.2013.04.035.
Rubio C, Bellver J, Rodrigo L, Bosch E, Mercader A, Vidal C, et al. Preimplantation genetic screening using fluorescence in situ hybridization in patients with repetitive implantation failure and advanced maternal age: two randomized trials. Fertil Steril. 2013;99(5):1400–7. https://doi.org/10.1016/j.fertnstert.2012.11.041.
Keltz MD, Vega M, Sirota I, Lederman M, Moshier EL, Gonzales E, et al. Preimplantation genetic screening (PGS) with comparative genomic hybridization (CGH) following day 3 single cell blastomere biopsy markedly improves IVF outcomes while lowering multiple pregnancies and miscarriages. J Assist Reprod Genet. 2013;30(10):1333–9. https://doi.org/10.1007/s10815-013-0070-6.
Shinar S, Kornecki N, Schwartz T, Mey-Raz N, Amir H, Almog B, et al. Timing embryo biopsy for PGD - before or after cryopreservation? Gynecol Endocrinol. 2016;32(9):756–8. https://doi.org/10.1080/09513590.2016.1177010.
Linan A, Lawrenz B, El Khatib I, Bayram A, Arnanz A, Rubio C, et al. Clinical reassessment of human embryo ploidy status between cleavage and blastocyst stage by next generation sequencing. PLoS One. 2018;13(8):e0201652. https://doi.org/10.1371/journal.pone.0201652.
Cohen J, Wells D, Munne S. Removal of 2 cells from cleavage stage embryos is likely to reduce the efficacy of chromosomal tests that are used to enhance implantation rates. Fertil Steril. 2007;87(3):496–503. https://doi.org/10.1016/j.fertnstert.2006.07.1516.
Munne S, Cohen J, Simpson JL. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357(17):1769–70; author reply 70-1. https://doi.org/10.1056/NEJMc076314.
Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69(1):84–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study was approved by the Northwell Health IRB HS16-0380. The study did not require patient consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, S., Hobeika, E., Knochenhauer, E.S. et al. Pregnancy rates after pre-implantation genetic screening for aneuploidy are only superior when trophectoderm biopsy is performed on hatching embryos. J Assist Reprod Genet 36, 621–628 (2019). https://doi.org/10.1007/s10815-019-01400-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01400-5