Abstract
Objective
To study if the number of trophectoderm (TE) biopsied cells has an impact on implantation rates.
Design
A retrospective cohort study in a single-center study.
Setting
In vitro fertilization center.
Patients
Patients who underwent PGT-A from January 2013 to March 2016. In total, 482 vitrified/warmed single embryo transfers were included.
Interventions
None.
Main outcome measures
Clinical pregnancies rate, implantation rate.
Results
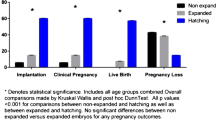
Overall, clinical pregnancies per embryo transfer were higher when a regular TE were biopsied compared to larger size biopsy cells (66% (175/267) vs 53% (115/215) (p < 0.005) respectively). Pregnancy rates were also analyzed according to embryo morphology at the moment of embryo biopsy, when a good-quality embryo was transferred the clinical outcome was 75% (81/108) in group 1 and 61% (60/99) in group 2 (p < 0.05). Data was also stratified by age in patients ≤ 35 years and > 35 years. The clinical pregnancy was 67% (51/76) in women ≤ 35 years and 65% (124/191) in women > 35 years when a regular size biopsy was performed. These results significantly reduced when a larger size biopsy was performed 54% (49/91) and 53% (66/124), respectively (p < 0.05). Further investigation indicated that miscarriage rate was similar between these groups (4% (7/182) in group 1 and 5% (6/121) in group 2).
Conclusions
These findings underscore that when a large amount of TE cells are biopsied, it may negatively affect implantation rates, but once implanted, the embryos have the same chance to miscarry or reach term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneuploidy in embryos increases with advancing maternal age [1, 2] resulting in lower implantation rates and higher pregnancy loss with age [3]. However, once a euploid embryo is replaced, they implant equally well at least up to age 42 [4].
Preimplantation genetic testing for aneuploidy (PGT-A) was proposed to select euploid embryos [5,6,7,8,9,10,11] with current comprehensive chromosome screening (CCS) techniques able to analyze all chromosomes consisting of either aCGH [12], SNPs array [13], qPCR [14], or NGS [15], which in combination with vitrification methods [16, 17], blastocyst biopsy (McArthur 2005), better blastocyst culture media, and deferred embryo transfer [18] have proved to improve clinical outcomes in randomized trials [3, 19,20,21].
Blastocyst biopsy, although an integral part of PGT-A, has not been standardized yet and there are few studies on the subject; Scott et al. showed that biopsy on embryos at cleavage stage has a negative effect resulting on reduction of the implantation rate while blastocyst stage biopsy resulted in the same implantation rate as non-biopsied blastocysts [20]. Another study confirmed that when there was no difference on embryologists performing TE biopsy at blastocysts stages in a standardized IVF setting, there was high consistency and reproducibility of CSS with optimal genetic and clinical outcomes [22]. However, a study has shown that TE quality may affect implantation potential [23] and more importantly that a large amount of TE cells biopsied may affect pregnancy rates [24].
In this study, we aimed to compare two sizes of blastocyst biopsy in patients treated in the same fertility group. The two sizes were approximately five cells biopsied (group 1) or about 10 cells biopsied (group 2), showing that smaller biopsies result in better implantation outcomes.
Material and methods
Patients
This retrospective single-center study included patients who underwent PGT-A from January 2013 to March 2016.
All patients received ovarian stimulation in a GnRH antagonist protocol, using a combination of either recombinant follicle-stimulating hormone plus recombinant luteinizing hormone (r-FSH and r-FSH/r-LH; Gonal® and Pergoveris®) or, highly purified follicle-stimulating hormone plus highly purified human menopausal gonadotropin (hp-FSH and hp-HMG; Bravelle® and Menopur®) starting from cycle day 2 or 3. Daily doses of 0.25 mg GnRH antagonist (Cetrotide®) were administered from day 6 of stimulation to the day of triggering. When two or more follicles reached 18 mm, either recombinant hCG 250 IU (r-hCG; Ovidrel®) or Triptorelin 0.2 mg (Gonapeptyl daily®) was used to trigger LH surge. The gonadotropin dose range used was 225–300 IU. The decision of the dose used was based on the antral follicle count and patient age. Transvaginal ultrasound and serum estradiol were used for monitoring ovarian stimulation. Follicular puncture was 36 h after triggering.
Embryo culture, embryo biopsy, and vitrification
Zygotes were cultured individually until day 5/6 in drops of 25 μL of Global Total® media (Lifeglobal, Canada). Cultures were under mineral oil at 37 °C. This embryo culture protocol was based on Sepulveda [25] with some modifications in the atmosphere culture conditions (7% CO2, 5% O2, and 88% N2).
The assisted hatching (AH) was performed with a laser on day 4; the multipulse function was used with the following laser software features (Lykos; Hamilton Thorne, Beverly, ME, USA): seven laser pulses per second, each pulse has a duration of 150 μs, AH was done from the internal edge of the zona pellucida to the outer edge. Embryo biopsy was done on hatching blastocyst on day 5 or 6, and was performed with the aid of a laser with a power setting of 100% and a potency ranging from 350 to 400 μs. Embryos were classified before biopsy according to the SART classification [26]. Biopsied cells were collected according to the genetic laboratory protocol.
Physicians in the center sent samples to two different genetic reference centers according to their personal preference. Cases sent to Reprogenetics (group 1) underwent aCGH and had regular size blastocyst biopsies (2–6 cells) while those sent to Natera (group 2), following specifications from Natera, had on average 10 cells biopsied, and the biopsies were analyzed by SNP-arrays. The same fully trained embryologists, with more than 2 years of embryo biopsy experience, performed embryo biopsy.
Biopsied Blastocysts were vitrified within 1 h after TE biopsy using a Cryotech vitrification kit (Cryotec, Japan) following the manufacturer’s protocols. Each blastocyst was vitrified individually in an open system using cryotec.
Regarding other confounding variables such as vitrification media and devices, embryo transfer technique, catheters, and syringes used for embryo transfer were the same during the period of the study.
Genetic analysis
For group 1, the biopsied trophectoderm cells were sent to Reprogenetics (Lima, Peru). Array CGH was used according to Gutierrez-Mateo [12] plus further updates on the chips and software from the manufacturer thereafter. Briefly, biopsies were collected in Eppendorf tubes containing 2 μl of non-sticking buffer and referred to the genetic laboratory. Samples were lysed and amplified using the SurePlex kit, according to the manufacturer’s instructions (BlueGnome, UK). Amplified samples were processed following the protocol of BlueGnome Cyto-Chip. Microarray chips were scanned, analyzed, and quantified as previously described by Gutierrez-Mateo [12]. Copy number ratios were analyzed using Cyto-Chip algorithm fixed setting in BlueFuse Software (BlueGnome).
For group 2, biopsied trophectoderm cells and parental blood samples were sent to Natera (USA) on dry ice. Briefly, DNA was isolated and amplified by using two protocols: Rubicon whole genome amplification with Sigma proteinase K buffer (PKB), and a modified multiple displacement amplification with PKB. For the second protocol, cells were placed in PKB (Arcturus PicoPure Lysis Buffer, 50 mM dithiothreitol), incubated at 56 °C for 1 h, and then heat inactivated at 95 °C for 10 min. Multiple displacement amplification reactions were incubated at 30 °C for 2.5 h and then 95 °C for 5 min. Genomic DNA from bulk tissue (Epicentre MasterAmp Buccal Swabs) was prepared using the DNeasy Blood and Tissue Kit (Qiagen). No-template controls (buffer blanks) were used for all amplification methods and all sample types and in all cases produced intensities commensurate to the noise floor of the data [27].
SNP genotyping and ploidy determination
Both, the amplified single cells and the parental tissue were genotyped using Illumina Infinium II genome-wide genotyping microarrays (HapMap CNV370Quad or CytoSNP-12 chips). For the tissue, the standard Infinium II protocol was used, whereas all single cells were genotyped using a modified Infinium II genotyping protocol, such that the entire protocol, from single cell lysis through array scanning, was completed in fewer than 24 h. Ploidy results are based on a novel statistical algorithm that makes use of high-throughput SNP measurements of parental and child samples to determine chromosome copy number of the child [27, 28].
Verification of biopsy size
Ten aneuploidy embryos were thawed with a warming kit (Cryotec, Japan) following the manufacturer’s protocol. After 2 hours, the re-expanded blastocysts were re-biopsied by an experience embryologist, classified by size, and a video was recorded. Other three blinded embryologists analyzed the video and performed counts of the number of cells biopsied.
Biopsied cells were exposed to a hypotonic solution (1% sodium citrate and 6 mg BSA/ml) for 2–5 min. TE cells were fixed to a glass slide into a previously marked position [5]. Slides were stained with DAPI (0.5 mg/ml; Calbiochem, USA) and nuclei were counted with a blinded operator under fluorescence microscopy (Leica). Finally, a correlation was calculated.
Endometrium preparation for embryo transfer
Vitrified-warmed embryos were transferred in an artificial endometrial priming cycle after priming of the endometrium with Oral Estradiol Valerate (2 mg administered three times a day) (Progynova; Bayer Schering Pharma). When an endometrial thickness of > 7 mm was reached, luteal phase support was started with the use of intravaginal micronized progesterone tablets (200 mg three times a day; Utrogestan; Biopas Laboratories). Single embryo transfer was scheduled 5 days later. ET was performed under ultrasound guidance with the use of a soft catheter.
Institutional review board approval
The nature of our study was retrospective because all biopsy were performed before the analysis of this data; the institutional review board approval was not requested. Nevertheless, all patients signed an informed consent for all procedures performed.
Statistical analysis
For categorical variables, Fisher exact test was used. Continuous variables did not show a normal distribution and therefore, Mann-Whitney U test was performed. Spearman rank correlation coefficients and corresponding p values were calculated. Subsequently, a step wise regression analysis was performed to identify which subset of variables correlated independently to clinical pregnancy. A p value < 0.05 was considered statistically significant.
Results
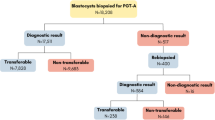
A total of 482 vitrified/warmed single embryo transfers were included in the analysis. In total, 2064 embryos were biopsied; 1071 embryos in group 1 (average of five cells) and 993 embryos in group 2 (average of 10 cells). The euploid blastocyst rate was comparable between groups (57% (608/1071) and 55% (554/993), p = 0.343; respectively). The average number of euploid blastocysts per stimulated cycle was also not significantly different between group 1 and group 2 (2.3 ± 1.5 vs 2.6 ± 1.6), as can be seen in Table 1.
In the single embryo transfer, the embryo quality might affect clinical outcomes; subsequently, we further compared the two groups according to embryo morphology. The biochemical pregnancy rate for good-quality embryos was 79% (85/108) for group 1 vs 64% (63/99) for group 2 (p < 0.05), and for fair-quality embryos was 61% (97/158) vs 50% (58/116) (p < 0.05). Clinical pregnancy rate for good- and fair-quality embryo again was higher for group 1 compared to Group 2 (75% (81/108) vs 61% (60/99) and 59% (94/159) vs 47% (55/116); respectively p < 0.05) (Table 2A).
Another confounder factor could be the age of the patient. Hence, data was classified according to the age groups, patients with ≤ 35 years and ≥ 35 years. The average age was 32.37 ± 2.3 vs 32.80 ± 2.3 (p = 0.233) and 40.6 ± 3.6 vs 39.1 ± 2.8 (p < 0.001) for groups 1 and 2 respectively.
Clinical outcomes are shown in Table 2B and showed to be significantly higher for group 1 for both age groups. However, miscarriage rate was similar between the groups, with 4% (7/182) for group 1 and 5% (6/121) for group 2.
To identify the variables that independently correlate with clinical pregnancy, which included the doctor effect, a univariate analysis was performed (Table 3). Finally, logistic regression analysis showed that total number of embryos biopsied per cycle, the biopsied protocols, and embryo quality were independent variables that predicted the clinical outcome.
Finally, to demonstrate the correlation between the biopsy size, the number of trophectoderm cells biopsied and the number of nuclei, aneuploid embryos were re-biopsied and their cells fixed for nuclei counting (Fig. 1) and confirmed a correlation among the biopsy sizes per groups.
Discussion
Usually, each IVF center chooses which PGT-A technique to use and follows a single biopsy protocol. However in our setting, SNPs array and aCGH were performed in parallel, and embryo manipulation was performed by the same embryologist applying two different biopsy techniques. This study compares the outcomes of two groups of patients who had undergone IVF treatments in the same center. Some underwent PGT-A with aCGH (group 1), on average, five blastocyst cells were biopsied, and some SNPs array, which required a larger amount of cells biopsied according to the reference PGD Lab (group 2). Physicians from the same center used similar stimulation protocols, with slight differences between them in a duration of stimulation which could have resulted in differences in aneuploidy rates [29]. However, this was not the case as shown in Table 1, and euploidy rates were similar in both groups. In addition, the multivariate statistical analysis showed that the factor “physician” was not a variable that independently correlated with the differences observed between PGT-A groups (Table 4).
There were significant differences in clinical outcomes between groups and there are two potential explanations for it, the biopsy size and the physician. Physicians still have a great deal of influence on the outcome of a cycle depending on their embryo transfer skills and thus the difference in pregnancy rates observed in this study could be related to differences between doctors and not differences between ours study groups (Table 4). Hormonal stimulation differences probably played a minor role, if any, since the transfers were on FET, thus all endometrium were in a natural state and because aneuploidy was similar. Furthermore, the multivariate statistical analysis showed that the physician was not a factor contributing to the difference in ART outcome.
Chromosome abnormality rates were similar between group 1 and group 2, despite maternal ages being higher in group 1 than in group 2, in the > 35 age group. This could be explained by differences in detecting abnormalities by one technique versus the other [12, 30, 31], but these differences are minor. More importantly, miscarriage rate was similar between groups, reassuring that both techniques had the same capability to detect aneuploidy compatible with implantation. In both groups, a single euploid embryo was replaced and therefore the most plausible reason for the decrease in implantation potential is the size of the embryo biopsy.
It is well known that biopsying two cells from day 3 embryos significantly reduces the implantation rates [32, 33]. Lately, other studies have shown that embryo biopsy on day 3 embryos impairs blastocyst formation and implantation potential, while this has not been reported for blastocyst biopsy [34]. Recently, it has been described that the number of biopsied blastocyst cells affects implantation when poor trophectoderm quality was biopsied with a larger amount of cells [24] than in our group 2. In our setting, an average of 10 cells biopsied clearly negatively affects embryo implantation compared to an average of five biopsied cells.
The TE is essential for implantation. Some studies have confirmed that blastocyst quality is directly related to successful implantation [23, 35,36,37,38,39] possibly explained by the TE function needed to invade the endometrium [40]. TE secreted hCG plays a role in the immunological tolerance facilitating the embryo implantation [41, 42] with embryos with good TE morphology secreting higher levels of hCG [23]. Regarding gene expression in TE biopsies, microarrays analysis showed a preponderance of gene expression of families involved in cell adhesion and cell communication [43, 44]. Overall, these studies demonstrate the importance of TE for a successful embryo implantation.
This study compared two PGT-A techniques that are being substituted by next-generation sequencing (NGS). It is important to notice that aCGH and NGS used the same whole genome amplification protocol, making our results extendible to other PGT-A platforms.
A major strength of the current study was the use of single blastocyst transfers, which eliminated the potential bias of multiple embryo transfer, as well as controlling for morphologic parameters and performing logistic regression to control for confounding variables. However, some limitations are present and need to be highlighted. Firstly, this study was not designed to provide evidence regarding the effect of biopsying a large amount of cells at blastocyst stage, although that has been covered by the Zhang et al. (2016) study. Secondly, patients were not randomized into each group.
In sum, our study suggests that there is a detrimental effect of the biopsy size on implantation produced by blastocyst biopsy. This raises a cautionary note to embryologists, and highlights that although one study showed no detrimental effect of blastocyst biopsy [34], the technique is not well standardized and it can still have a detrimental effect if performed incorrectly. Other aspects of blastocyst biopsy need to be standardized and more automation would be desirable.
References
Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64:382–91.
Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, et al. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod BioMed Online. 2012;24:614–20.
Dahdouh EM, Balayla J, Garcia-Velasco JA. Impact of blastocyst biopsy and comprehensive chromosome screening technology on preimplantation genetic screening: a systematic review of randomized controlled trials. Reprod BioMed Online. 2015;30:281–9.
Harper JC, Sengupta SB. Preimplantation genetic diagnosis: state of the art 2011. Hum Genet. 2012;131:175–86.
Munne S, Lee A, Rosenwaks Z, Grifo J, Cohen J. Diagnosis of major chromosome aneuploidies in human preimplantation embryos. Hum Reprod. 1993;8:2185–91.
Munne S, Magli C, Cohen J, Morton P, Sadowy S, Gianaroli L, et al. Positive outcome after preimplantation diagnosis of aneuploidy in human embryos. Hum Reprod. 1999;14:2191–9.
Munne S, Sandalinas M, Escudero T, Velilla E, Walmsley R, Sadowy S, et al. Improved implantation after preimplantation genetic diagnosis of aneuploidy. Reprod BioMed Online. 2003;7:91–7.
Colls P, Escudero T, Cekleniak N, Sadowy S, Cohen J, Munne S. Increased efficiency of preimplantation genetic diagnosis for infertility using “no result rescue”. Fertil Steril. 2007;88:53–61.
Verlinsky Y, Tur-Kaspa I, Cieslak J, Bernal A, Morris R, Taranissi M, et al. Preimplantation testing for chromosomal disorders improves reproductive outcome of poor-prognosis patients. Reprod BioMed Online. 2005;11:219–25.
Rubio C, Buendia P, Rodrigo L, Mercader A, Mateu E, Peinado V, et al. Prognostic factors for preimplantation genetic screening in repeated pregnancy loss. Reprod BioMed Online. 2009;18:687–93.
Hodes-Wertz B, Grifo J, Ghadir S, Kaplan B, Laskin CA, Glassner M, et al. Idiopathic recurrent miscarriage is caused mostly by aneuploid embryos. Fertil Steril. 2012;98:675–80.
Gutierrez-Mateo C, Colls P, Sanchez-Garcia J, Escudero T, Prates R, Ketterson K, et al. Validation of microarray comparative genomic hybridization for comprehensive chromosome analysis of embryos. Fertil Steril. 2011;95:953–8.
Schoolcraft WB, Treff NR, Stevens JM, Ferry K, Katz-Jaffe M, Scott RT Jr. Live birth outcome with trophectoderm biopsy, blastocyst vitrification, and single-nucleotide polymorphism microarray-based comprehensive chromosome screening in infertile patients. Fertil Steril. 2011;96:638–40.
Forman EJ, Tao X, Ferry KM, Taylor D, Treff NR, Scott RT Jr. Single embryo transfer with comprehensive chromosome screening results in improved ongoing pregnancy rates and decreased miscarriage rates. Hum Reprod. 2012;27:1217–22.
Wells D. Next-generation sequencing: the dawn of a new era for preimplantation genetic diagnostics. Fertil Steril. 2014;101:1250–1.
Cobo A, Vajta G, Remohi J. Vitrification of human mature oocytes in clinical practice. Reprod BioMed Online. 2009;19(Suppl 4):4385.
Van Landuyt L, Verpoest W, Verheyen G, De Vos A, Van de Velde H, Liebaers I, et al. Closed blastocyst vitrification of biopsied embryos: evaluation of 100 consecutive warming cycles. Hum Reprod. 2011;26:316–22.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. High ongoing pregnancy rates after deferred transfer through bipronuclear oocyte cryopreservation and post-thaw extended culture. Fertil Steril. 2009;92:1594–9.
Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5:24.
Scott RT Jr, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100:697–703.
Forman EJ, Upham KM, Cheng M, Zhao T, Hong KH, Treff NR, et al. Comprehensive chromosome screening alters traditional morphology-based embryo selection: a prospective study of 100 consecutive cycles of planned fresh euploid blastocyst transfer. Fertil Steril. 2013;100:718–24.
Capalbo A, Ubaldi FM, Cimadomo D, Maggiulli R, Patassini C, Dusi L, et al. Consistent and reproducible outcomes of blastocyst biopsy and aneuploidy screening across different biopsy practitioners: a multicentre study involving 2586 embryo biopsies. Hum Reprod. 2016;31:199–208.
Ahlstrom A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26:3289–96.
Zhang S, Luo K, Cheng D, Tan Y, Lu C, He H, et al. Number of biopsied trophectoderm cells is likely to affect the implantation potential of blastocysts with poor trophectoderm quality. Fertil Steril. 2016;105:1222–7 e4.
Sepulveda S, Garcia J, Arriaga E, Diaz J, Noriega-Portella L, Noriega-Hoces L. In vitro development and pregnancy outcomes for human embryos cultured in either a single medium or in a sequential media system. Fertil Steril. 2009;91:1765–70.
Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, Pomeroy KO, et al. Standardization of grading embryo morphology. Fertil Steril. 2010;94:1152–3.
Rabinowitz M, Ryan A, Gemelos G, Hill M, Baner J, Cinnioglu C, et al. Origins and rates of aneuploidy in human blastomeres. Fertil Steril. 2012;97:395–401.
Johnson DS, Gemelos G, Baner J, Ryan A, Cinnioglu C, Banjevic M, et al. Preclinical validation of a microarray method for full molecular karyotyping of blastomeres in a 24-h protocol. Hum Reprod. 2010;25:1066–75.
Munne S, Alikani M, Barritt J, Hesla J, Kaplan B, Alper M, et al. Egg donor aneuploidy rates significantly differ between fertility centers. Fertil Steril. 2014;102:0–354.
Treff NR, Su J, Kasabwala N, Tao X, Miller KA, Scott RT Jr. Robust embryo identification using first polar body single nucleotide polymorphism microarray-based DNA fingerprinting. Fertil Steril. 2010;93:2453–5.
Bisignano A, Wells D, Harton G, Munne S. PGD and aneuploidy screening for 24 chromosomes: advantages and disadvantages of competing platforms. Reprod BioMed Online. 2011;23:677–85.
Cohen J, Wells D, Munne S. Removal of 2 cells from cleavage stage embryos is likely to reduce the efficacy of chromosomal tests that are used to enhance implantation rates. Fertil Steril. 2007;87:496–503.
De Vos A, Staessen C, De Rycke M, Verpoest W, Haentjens P, Devroey P, et al. Impact of cleavage-stage embryo biopsy in view of PGD on human blastocyst implantation: a prospective cohort of single embryo transfers. Hum Reprod. 2009;24:2988–96.
Scott KL, Hong KH, Scott RT Jr. Selecting the optimal time to perform biopsy for preimplantation genetic testing. Fertil Steril. 2013;100:608–14.
Thompson SM, Onwubalili N, Brown K, Jindal SK, McGovern PG. Blastocyst expansion score and trophectoderm morphology strongly predict successful clinical pregnancy and live birth following elective single embryo blastocyst transfer (eSET): a national study. J Assist Reprod Genet. 2013;30:1577–81.
Van den Abbeel E, Balaban B, Ziebe S, Lundin K, Cuesta MJ, Klein BM, et al. Association between blastocyst morphology and outcome of single-blastocyst transfer. Reprod BioMed Online. 2013;27:353–61.
Ahlstrom A, Westin C, Wikland M, Hardarson T. Prediction of live birth in frozen-thawed single blastocyst transfer cycles by pre-freeze and post-thaw morphology. Hum Reprod. 2013;28:1199–209.
Hill MJ, Richter KS, Heitmann RJ, Graham JR, Tucker MJ, DeCherney AH, et al. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril. 2013;99:1283–9 e1.
Honnma H, Baba T, Sasaki M, Hashiba Y, Ohno H, Fukunaga T, et al. Trophectoderm morphology significantly affects the rates of ongoing pregnancy and miscarriage in frozen-thawed single-blastocyst transfer cycle in vitro fertilization. Fertil Steril. 2012;98:361–7.
Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–8.
Licht P, Russu V, Lehmeyer S, Wildt L. Molecular aspects of direct LH/hCG effects on human endometrium--lessons from intrauterine microdialysis in the human female in vivo. Reprod Biol. 2001;1:10–9.
Tsampalas M, Gridelet V, Berndt S, Foidart JM, Geenen V, Perrier d’Hauterive S. Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. J Reprod Immunol. 2010;85:93–8.
Parks JC, McCallie BR, Janesch AM, Schoolcraft WB, Katz-Jaffe MG. Blastocyst gene expression correlates with implantation potential. Fertil Steril. 2011;95:1367–72.
Jones GM, Cram DS, Song B, Kokkali G, Pantos K, Trounson AO. Novel strategy with potential to identify developmentally competent IVF blastocysts. Hum Reprod. 2008;23:1748–59.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guzman, L., Nuñez, D., López, R. et al. The number of biopsied trophectoderm cells may affect pregnancy outcomes. J Assist Reprod Genet 36, 145–151 (2019). https://doi.org/10.1007/s10815-018-1331-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1331-1