Abstract
Purpose
To compare clinical outcomes following transfer of euploid blastocysts of varying quality biopsied on day 5 versus day 6.
Methods
Retrospective cohort study to evaluate embryo transfer outcomes for women undergoing autologous cryopreserved next generation sequencing euploid single embryo transfer from 10/2015 to 2/2022 at an academic IVF program. The primary outcome was live birth rate (LBR). Secondary outcomes included ongoing pregnancy rate (OPR), implantation rate (IR), and miscarriage rate (SAB rate).
Results
Five hundred and fifty-five transfers from 418 patients were analyzed. Euploid embryos biopsied on day 5 resulted in higher LBR compared to those biopsied on day 6 (62.3% vs. 49.6%; aRR 0.81 95% CI 0.65–0.996). When stratified by biopsy day and blastocyst quality, there was no difference in IR, OPR, and SAB rate for good, fair, and poor quality blastocysts biopsied on day 5 versus day 6. However, day 5 good quality embryos were associated with a higher LBR compared to day 6 good quality embryos (74.3% vs. 51.3%; aRR 0.69; 95% CI 0.48–0.999). There were no significant differences in LBR for fair and poor quality embryos biopsied on day 5 versus day 6.
Conclusion
Overall LBR are higher for euploid embryos biopsied on day 5 versus day 6. When stratified by embryo quality and day of biopsy, LBR are significantly higher for good quality day 5 versus day 6 embryos. When choosing between multiple euploid embryos, day 5 biopsied good quality embryos should be preferentially selected for transfer over day 6 embryos of the same quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the rapid advancements in technology for assisted reproduction, preimplantation genetic testing for aneuploidy (PGT-A) has become a frequently utilized ART procedure to genetically screen embryos prior to transfer [1]. While much is known regarding outcomes following the transfer of PGT-A embryos, there are limited data to suggest how to choose which euploid embryo to transfer when multiple euploid embryos are available. Capalbo et al. demonstrated no difference in implantation rate for euploid embryos based on embryo morphology, with equivalent implantation for excellent, good, average, and poor euploid embryos [2]. Gonzalez et al. also noted no difference in implantation, pregnancy, and live birth rates by embryo quality [3]. In contrast, Irani et al. and Zhao et al. both demonstrated increased pregnancy rates for euploid embryos with higher morphologic scores [4, 5]. Regarding day of embryo biopsy, Capalbo et al. showed that day of biopsy did not affect implantation rate; embryos biopsied and frozen on day 5 had an implantation rate of 48.8% compared to 51.2% for day 6 embryos [2]. Gonzalez et al. also noted no significant difference in implantation rates (OR 0.6 95%CI 0.4–1.2) and live birth rates (OR 0.3 95%CI 0.1–1.3) when comparing euploid embryos biopsied on day 5 versus day 6 [3]. Alternatively, Irani et al. demonstrated lower implantation and live birth rates for euploid blastocysts biopsied and frozen on day 6 as compared to those on day 5 (44.6% vs. 66.7% and 44.8% vs. 60.4%, respectively) [6].
In all prior studies, except for the Gonzalez et al. study, PGT-A was performed using array comparative genome hybridization (aCGH). With advancements in karyotype testing technology, most preimplantation genetic testing is currently performed using next generation sequencing (NGS), which offers improved accuracy in assessing incidence of ploidy compared to aCGH [7].
In this study, we aim to investigate how day of trophectoderm biopsy and freeze affects clinical outcomes, specifically implantation, ongoing pregnancy, and live birth rates for embryos tested with NGS. We hypothesize that NGS-tested euploid embryos that were biopsied and frozen on day 5 have improved clinical outcomes compared to those of equivalent morphologic quality biopsied and frozen on day 6. Additionally, we hypothesize that embryos biopsied on day 5 with lower morphologic scores are associated with at least equivalent clinical outcomes compared to those biopsied on day 6 with higher morphologic scores.
Materials and methods
This study was approved by the Partners HealthCare Institutional Review Board (Protocol Number 2020P000630).
Cycle selection
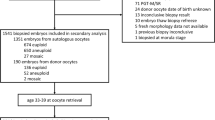
This was a retrospective cohort study of cryopreserved NGS euploid single embryo transfers performed from 10/2015 to 2/2022 at the Center for Infertility and Reproductive Surgery at Brigham and Women’s Hospital, Boston, Massachusetts. Embryos from autologous IVF or ICSI cycles were included. Cycles were excluded if (i) egg donation or in vitro maturation was used, (ii) embryos were imported from another institution, (iii) embryos were frozen at the two pronuclear (2pn) or cleavage stage then thawed and cultured to blastocyst stage for biopsy, and (iv) embryos were thawed then biopsied and refrozen.
Clinical protocols
Standard controlled ovarian hyperstimulation and monitoring protocols were used. Gonadotropin doses were determined based on age, serum antimullerian hormone levels, antral follicular count, body mass index (BMI), and previous response to stimulation. Ovarian stimulation was performed with the use of exogenous gonadotropins (Gonal-F, EMD-Serono; Menopur, Ferring Pharmaceuticals). Pituitary suppression was attained with the use of GnRH antagonist (Cetrotide, EMD-Serono) or GnRH agonist (leuprolide acetate, Abbott Laboratories). Gonadotropin dosage was adjusted according to each patient’s response to stimulation, which was monitored with the use of transvaginal ultrasound and serial E2 levels. When at least two follicles reached a mean diameter of 18 mm, final oocyte maturation was triggered with the use of hCG (Pregnyl, Arganon Pharmaceuticals or Novarel, Ferring Pharmaceuticals) and/or GnRH agonist (leuprolide acetate, Abbott Laboratories). The dose of hCG was tailored based on serum E2 levels on the day of trigger and number of follicles. Patients considered to be at risk for ovarian hyperstimulation syndrome (OHSS) were given 5000 units of hCG or a combination trigger (leuprolide acetate 40 units with 1500 units hCG). Ultrasound-guided oocyte retrieval was performed under intravenous general anesthesia 36–37 h after trigger.
Patients with regular menstrual cycles were offered ‘‘natural’’ cryopreserved embryo transfer (CET) cycles. Luteinizing hormone (LH) levels were monitored during the follicular phase to identify the LH surge with urinary testing at home and serum LH confirmation, or with daily serum LH measurements starting on cycle day 10. Blastocyst transfer was performed 6 days after the LH surge. Most patients received vaginal progesterone supplementation (Crinone; Allergan Pharmaceuticals), which was initiated 3 days after LH surge.
Anovulatory patients, and those desiring hormonal-regulated cycles, underwent ‘‘programmed’’ cycles. Estradiol (E2) was supplemented with tablets (Estrace, Allergan Pharmaceuticals) given orally or vaginally, or by using estrogen patches (Climara, Sandoz vs. Bayer Pharmaceuticals). Following at least 14 days of E2 administration, and once adequate endometrial thickness was achieved (at least 7 mm), daily intramuscular progesterone (50 mg, AuroMedics Pharma LLC) was initiated. Embryos were transferred after 6 days of exposure to exogenous progesterone. Patients with a serum progesterone level of < 20 ng/mL on the day of transfer increased their daily progesterone dose by 50–100%.
Laboratory protocols
All laboratory procedures were conducted in a well-established high-complexity embryology lab with highly trained embryologists with a minimum of 5-year experience and bi-annual quality assurance training. There were no changes in laboratory staff during the study period. All gametes and embryos were cultured at 37 °C in a dry benchtop incubator under an atmosphere of CO2 (5–6%), O2 (5%), and N2 (89–90%) at a pH of 7.3. IVF or ICSI was performed 4–6 or 3–5 h after oocyte retrieval, respectively, followed by a fertilization check 16–18 h after fertilization. A single step medium (25 μL microdrops, Global Total, IVFOnLine, Guelph, Ontario, Canada under mineral oil) was used to culture 2pn zygotes (one zygote/drop).
Embryos were evaluated on day 3 between 66 and 69 h post-insemination, underwent assisted hatching using laser pulses (ZILOS-tk laser; Hamilton Thorne) and then moved to individual fresh 25-μL microdrops of equilibrated Global Total medium for culture to day 5/6. Embryo morphology was evaluated on day 5 between 112 and 115 h and scored according to the stage of development and, if at the blastocyst stage, by quality of the inner cell mass (ICM) and trophectoderm (TE) [8]. The stages and grades of those blastocysts eligible for biopsy in our program are shown in Supplemental Table 1. Expanding blastocysts and any blastocyst with a “C” grade for both the ICM and TE, or a “D” for either the ICM or TE were considered ineligible. Embryos that were ineligible for biopsy or freeze on day 5 were left in culture and re-evaluated on day 6.
The embryos were biopsied on day 5 or day 6 once biopsy criteria were met. Biopsies were performed using standard techniques by embryologists certified to perform the procedure. Briefly, the embryo was immobilized with the use of a holding pipette and three to five cells were aspirated by means of a biopsy pipette with an internal diameter of 20–30 µm. The biopsied specimens were exposed to wash buffer, and the cells were placed in 0.2-mL polymerase chain reaction tubes with a 2–3 µL lysis buffer. The specimens were stored at either − 20 °C or − 80 °C (depending on the predetermined genetic testing lab) prior to being sent for analysis. Specimens were sent to well-established genetic testing labs with internal quality control (Cooper Genomics, Reprogenetics or RGI). The biopsied blastocysts were frozen by standard closed-system vitrification technique (Cryolock straws and vitrification media, Irvine Scientific, Santa Ana, CA). Embryos that were determined by PGT-A to be euploid were eligible for transfer.
Outcome measures
The primary outcome was live birth rate defined as the birth of at least one viable live born infant per embryo transfer. Secondary outcomes included ongoing pregnancy rate per embryo transferred defined as evidence of an intrauterine pregnancy with fetal cardiac activity at discharge of care to the patient’s primary obstetrician (7–8 weeks gestational age), implantation rate, and miscarriage rate. Implantation rate was defined as the number of gestational sacs divided by the number of embryos transferred. Miscarriage rate was defined as the loss of pregnancy after confirmation of a gestational sac.
Statistical analysis
Means and standard deviations were generated for continuous variables and frequencies and proportions for categorical variables. Statistical significance was assessed using Wilcoxon rank sum tests for continuous variables and chi square tests for categorical variables. Relative risks (RR) and 95% confidence intervals (CI) were generated using log-binomial regression and were adjusted for age and body mass index (BMI). When the algorithm did not converge, relative risks were adjusted for age alone. Generalized estimating equations were used to account for patients contributing more than one cycle. An alpha of 0.05 was considered statistically significant. All statistical analyses were performed with SAS® version 9.4 (Cary, NC, USA).
Results
A total of 551 embryo transfers from 418 patients were included. Demographic characteristics of the cycles involving transfers following day 5 biopsy (n = 353) and day 6 biopsy (n = 198) are shown in Table 1. Aside from a higher AMH for the day 5 biopsy group compared to the day 6 group (p = 0.02), the two groups had similar population and cycle characteristics. Aneuploidy rates by day of biopsy were similar for the cycles contributing euploid embryos to the study population (day 5: 27.1% SD = 31.2, day 6: 30.2% SD = 31.2).
Analysis by day of biopsy
Live birth rates were higher for embryos biopsied on day 5 compared to those biopsied on day 6 when combining all transfers irrespective of blastocyst quality (62.3% vs. 49.6%; aRR 0.81 95% CI 0.65–0.996). There was no significant difference in implantation, ongoing pregnancy, and miscarriage rates when comparing day 5 to day 6 euploid embryos (implantation rate: 70.0% vs. 63.1%, RR 0.90, 95%CI 0.80–1.02; ongoing pregnancy rate: 65.4% vs. 56.6%, aRR 0.87, 95%CI 0.75–1.003; miscarriage rate: 7.4% vs. 7.1%, aRR 0.98, 95%CI 0.52–183; day 5 versus day 6, respectively) (Table 2).
Analysis by blastocyst quality
When cycles were stratified by embryo quality without regard to day of biopsy, poor quality embryos resulted in statistically significantly lower implantation (RR 0.70, 95%CI 0.53–0.90), ongoing pregnancy (aRR 0.67, 95%CI 0.51–0.88) and live birth (aRR 0.70, 95%CI 0.50–0.97) rates compared with good quality embryos (Table 3). Both fair and poor quality embryos had significantly higher miscarriage rates compared to good quality embryos (fair: 9.5% vs. 1.9%, aRR 4.94, 95%CI 1.53–16.0); poor: 8.6% vs. 1.9%, aRR 4.40. 95% CI 1.13–17.1).
Analysis by blastocyst quality and day of biopsy
Stratification by blastocyst quality and then comparison of outcomes by day of embryo biopsy, revealed a similar trend of improved live birth rate for day 5 embryos compared with day 6 embryos for good quality embryos (Table 4); day 5 good quality embryos had a higher live birth rate compared to day 6 good quality embryos (74.3% vs. 51.3%, aRR 0.69, 95%CI 0.48–0.999). There was no significant difference in implantation, ongoing pregnancy, and miscarriage rates for good quality embryos (IR 75.0% vs. 65.5%, RR 0.87, 95%CI 0.71–1.07; OPR 75.0% vs. 61.9%, aRR 0.83, 95%CI 0.67–1.02; SAB rate: 1.4% vs. 2.4%, aRR 1.95, 95%CI 0.18–21.5, respectively). Live birth rates were also higher for fair and poor quality embryos biopsied on day 5 compared to those biopsied on day 6, but the differences were not significant (fair: 61.4% vs. 54.6%, aRR 0.90, 95%CI 0.70–1.17; poor: 51.9% vs. 37.9%, aRR 0.68, 95%CI 0.39–1.20). There were no significant differences in implantation, ongoing pregnancy, and miscarriage rates for both fair and poor quality embryos biopsied on day 5 compared to those biopsied on day 6.
Analysis of day 5 fair and poor quality blastocysts compared with day 6 good quality blastocysts
In addition to the above analyses, it was of interest to investigate outcomes of fair and poor quality day 5 embryos compared with those associated with good quality day 6 embryos (Supplemental Table 2). Fair quality day 5 embryos and good quality day 6 embryos had equivalent rates of implantation, (71.1% vs. 65.5%, RR 1.09, 95%CI 0.91–1.29) ongoing pregnancy (64.7% vs. 61.9%, aRR 1.04, 95%CI 0.86–1.25), and live birth (61.4% vs. 51.3%, RR 1.16, 95%CI 0.85–1.60). Similarly, poor quality day 5 embryos and good quality day 6 embryos had equivalent rates of implantation, (50.0% vs. 65.5%, RR 0.76, 95%CI 0.52–1.12) ongoing pregnancy (50.0% vs. 61.9%, aRR 0.82, 95%CI 0.56–1.18), and live birth (51.9% vs. 51.3%, RR 0.96, 95%CI 0.60–1.55). However, the miscarriage rate for poor quality day 5 embryos was higher than that of good quality day 6 embryos (12.5% vs. 2.4%; RR 5.25, 95%CI 1.02–26.9).
Discussion
In this study, we investigated the associations between day of trophectoderm biopsy, morphological quality, and clinical outcomes for NGS-tested euploid single embryo transfer cycles. We observed higher live birth rates for embryos biopsied on day 5 compared to those biopsied on day 6. When stratified by embryo quality, we found a 23% higher live birth rate for good quality day 5 embryos compared to good quality day 6 embryos. There was no statistical difference in implantation, ongoing pregnancy, or miscarriage rates between embryos biopsied on day 5 versus day 6, even when stratified by embryo quality.
Taken together, these results indicate that both day of embryo biopsy and morphologic score are important considerations when selecting which high quality euploid embryo to transfer. These results support our hypothesis that good quality embryos biopsied and frozen on day 5 have improved live birth rates compared to embryos that are biopsied and frozen on day 6. When considering morphologic grade, the fact that fair quality day 5 embryos perform similarly to good quality day 6 embryos supports our hypothesis that lower quality day 5 embryos are associated with at least equivalent outcomes to good quality day 6 embryos. Poor quality day 5 embryos had similar ongoing pregnancy and live birth rates as day 6 good quality embryos, but a significantly higher miscarriage rate (12.5% vs. 2.4%, RR 5.25, 95%CI 1.02–26.9). However, this difference should be interpreted with caution given the small sample sizes and wide confidence interval.
PGT-A has become an increasingly common approach to select the “best” embryo for transfer. Pregnancy rates are higher in good prognosis patients undergoing PGT-A with the transfer of one euploid embryo compared to embryo selection based on morphology alone [9, 10]. However, the transfer of a euploid embryo does not ensure a pregnancy. In addition, there may be a difference in pregnancy rates between euploid embryos dependent on blastocyst development rate and morphologic scores. While some studies demonstrate equivalent implantation and ongoing pregnancy rates when comparing euploid embryos with differential development rates and morphology [2, 11], other studies demonstrate poorer outcomes for day 6 embryos and those with lower morphologic scores [5, 6]. These previous studies assessed embryo ploidy status with aCGH. NGS detects ploidy status more accurately than aCGH [7]; therefore, most embryo chromosomal analysis is currently performed on NGS platforms. The study by Gonzalez et al. analyzed embryo transfer outcomes for euploid embryos tested on NGS platforms, but the authors report outcomes by day of biopsy and embryo quality separately, not together [3]. Additionally, the study is relatively small (179 blastocysts compared to our 551) and the authors did not control for BMI in their analysis. Elevated BMI has been correlated with lower live birth and higher miscarriage rates with euploid embryo transfer [12]. Given discordant outcomes reported in the literature and the increased use of NGS, we compared clinical outcomes for NGS euploid embryos by both day of biopsy and blastocyst morphology.
Our findings demonstrate statistically similar implantation and ongoing pregnancy rates, but significantly higher live birth rates for euploid embryos biopsied on day 5 compared to those biopsied on day 6 (implantation rate 70% vs. 63%, ongoing pregnancy rate 65% vs. 57%, live birth rate 62% vs. 50%). When untested embryos are transferred in fresh autologous cycles, day 5 embryos have higher implantation and pregnancy rates than day 6 embryos [13, 14]. The proposed physiologic explanation for this difference is the presence of embryo-endometrial asynchrony with day 6 embryo transfers. However, a similar trend has been noted in CET cycles, with increased pregnancy and live birth rates noted for more rapidly advancing embryos [14,15,16,17]. Some studies have demonstrated higher aneuploidy rates in day 6 embryos compared to day 5 embryos, which may account for these proposed differences [2, 18]. However, when evaluating only euploid embryo transfers, data on clinical outcomes are inconsistent. Some studies report equivalent implantation and pregnancy rates for euploid embryos biopsied and frozen on day 5 versus day 6 [2, 3, 18], while other studies demonstrate improved outcomes with transfer of day 5 embryos [6]. While we observed no statistically significant difference in implantation and pregnancy rates between day 5 and day 6 embryos in our study, there was a significant improvement in live birth with transfer of a euploid embryo biopsied on day 5 over an embryo biopsied on day 6.
Many studies have demonstrated the importance of blastocyst morphologic scoring in embryo selection for transfer. The three features correlated with pregnancy and live birth are stage (i.e., degree of blastocyst expansion and hatching status), grade of the inner cell mass, and grade of the trophectoderm [19, 20]. The rationale for using morphologic scoring in the absence of PGT-A to select an embryo for transfer is to increase the likelihood of selecting a euploid embryo [2]. While high-quality embryos are more likely to be euploid, aneuploid embryos can also be high quality and euploid embryos can be of low quality [2, 21]. PGT-A increases the likelihood of selecting a euploid embryo for transfer and although it is not a perfect test due to false positives and false negatives, based on the results of our study and others, we should not disregard morphologic grading when selecting between multiple euploid embryos. Blastocyst morphology impacts clinical outcomes even when transferring a euploid embryo [4, 5]. In our study, good quality euploid embryos had significantly higher live birth rates compared to poor quality euploid embryos. This contradicts the findings by Gonzalez et al. who demonstrated equivalent ongoing live birth rates for euploid embryos regardless of morphologic score [3].
When stratified by both embryo quality and day of biopsy, day 5 good quality embryos had a significantly higher live birth rate compared to good quality day 6 embryos (74.3% vs. 51.3%). There were no significant differences in clinical outcomes for fair or poor quality embryos when comparing those biopsied on day 5 versus day 6. Fair and poor quality day 5 embryos had equivalent implantation, ongoing pregnancy, and live birth rates as compared with good quality day 6 embryos, but poor quality day 5 embryos had higher miscarriage rates. Our findings for good quality embryos are consistent with the results from Irani et al. using aCGH for embryo ploidy analysis [6], whereas our findings for lower quality embryos are more consistent with the results from Capalbo et al. and Gonzalez et al. [2, 3]. Both developmental rates and morphologic scores should be considered when choosing between multiple good quality euploid embryos for transfer and can be considered when choosing among multiple lower quality euploid embryos.
When designing a protocol for selecting which euploid embryo should be transferred, one option would be to prioritize transferring all day 5 embryos first over day 6 embryos. However, given the higher miscarriage rate for poor quality day 5 embryos as compared to good quality day 6 embryos, we argue for a more conservative approach. Based on the findings we present, we propose choosing to transfer good quality day 5 embryos as first priority, followed by good quality day 6 embryos or fair quality day 5 or 6 embryos as second priority, day 5 poor quality embryos selected as third priority, and day 6 poor quality embryos as fourth priority (Supplemental Table 3). In addition to considering clinical outcomes, the higher risk of degeneration post-warming [22] is a further argument for reserving poor quality embryos for transfer only after higher quality euploid embryos have been transferred.
Our study is limited in its retrospective design and limited sample size for stratified outcomes. While the study was powered to detect a 22% difference in implantation rate between day 5 and day 6 embryos, the comparison groups for poor quality day 5 versus day 6 embryos were small following stratification. A larger retrospective study or prospective cohort study would offer additional insight into factors associated with improved clinical outcomes. Long-term studies evaluating the association between day of embryo biopsy, morphology, and perinatal/childhood outcomes are also indicated. With the ever-advancing field of reproductive medicine, trophectoderm biopsy may become obsolete as it is replaced by non-invasive genetic testing platforms [23]. However, in the setting of current aneuploidy testing and embryo selection, our findings argue for meticulous assessment of morphologic characteristics when selecting between euploid embryos for transfer.
Conclusion
Previous studies on untested embryos demonstrate that higher morphology scores and earlier blastulation are associated with improved clinical outcomes. However, data are mixed regarding the impact of morphology and developmental rate for euploid embryo transfer. The results of our study support the use of morphological grading when choosing between multiple euploid embryos for transfer and that when day 5 and day 6 biopsied euploid embryos of the same quality are available, that day 5 embryos are preferentially selected for transfer.
Data availability
All data and materials support the published claims and comply with field standards.
Code availability
All statistical analyses were performed with SAS® software version 9.4.
References
Maxwell SM, Grifo JA. Should every embryo undergo preimplantation genetic testing for aneuploidy? A review of the modern approach to in vitro fertilization. Best Pract Res Clin Obstet Gynaecol. 2018;53:38–47.
Capalbo A, Rienzi L, Cimadomo D, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–81.
Viñals Gonzalez X, Odia R, Naja R, et al. Euploid blastocysts implant irrespective of their morphology after NGS-(PGT-A) testing in advanced maternal age patients. J Assist Reprod Genet. 2019;36(8):1623–9.
Irani M, Reichman D, Robles A, et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril. 2017;107(3):664–70.
Zhao Y-Y, Yu Y, Zhang X-W. Overall Blastocyst quality, trophectoderm grade, and inner cell mass grade predict pregnancy outcome in euploid blastocyst transfer cycles. Chin Med J (Engl). 2018;131(11):1261–7.
Irani M, Palermo GD, Xu K, et al. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil Steril. 2018;110(1):95-102.e1.
Maxwell SM, Colls P, Hodes-Wertz B, et al. Why do euploid embryos miscarry? A case-control study comparing the rate of aneuploidy within presumed euploid embryos that resulted in miscarriage or live birth using next-generation sequencing. Fertil Steril. 2016;106(6):1414-1419.e5.
Bakkensen JB, Brady P, Carusi D, et al. Association between blastocyst morphology and pregnancy and perinatal outcomes following fresh and cryopreserved embryo transfer. J Assist Reprod Genet. 2019;36(11):2315–24.
Yang Z, Liu J, Collins GS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5(1):24.
Scott RT, Upham KM, Forman EJ, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100(3):697–703.
Piccolomini MM, Nicolielo M, Bonetti TCS, et al. Does slow embryo development predict a high aneuploidy rate on trophectoderm biopsy? Reprod Biomed Online. 2016;33(3):398–403.
Cozzolino M, García-Velasco JA, Meseguer M, et al. Female obesity increases the risk of miscarriage of euploid embryos. Fertil Steril. 2021;115(6):1495–502.
Shapiro BS, Daneshmand ST, Garner FC, et al. Contrasting patterns in in vitro fertilization pregnancy rates among fresh autologous, fresh oocyte donor, and cryopreserved cycles with the use of day 5 or day 6 blastocysts may reflect differences in embryo-endometrium synchrony. Fertil Steril. 2008;89(1):20–6.
Barrenetxea G, De Larruzea AL, Ganzabal T, et al. Blastocyst culture after repeated failure of cleavage-stage embryo transfers: a comparison of day 5 and day 6 transfers. Fertil Steril. 2005;83(1):49–53.
Ferreux L, Bourdon M, Sallem A, et al. Live birth rate following frozen-thawed blastocyst transfer is higher with blastocysts expanded on Day 5 than on Day 6. Hum Reprod. 2018;33(3):390–8.
Haas J, Meriano J, Laskin C, et al. Clinical pregnancy rate following frozen embryo transfer is higher with blastocysts vitrified on day 5 than on day 6. J Assist Reprod Genet. 2016;33(12):1553–7.
Tubbing A, Shaw-Jackson C, Ameye L, et al. Increased live births after day 5 versus day 6 transfers of vitrified-warmed blastocysts. J Assist Reprod Genet. 2018;35(3):417–24.
Taylor TH, Patrick JL, Gitlin SA, et al. Comparison of aneuploidy, pregnancy and live birth rates between day 5 and day 6 blastocysts. Reprod Biomed Online. 2014;29(3):305–10.
Dokras A, Sargent IL, Gardner RL, et al. Human trophectoderm biopsy and secretion of chorionic gonadotrophin. Hum Reprod. 1991;6(10):1453–9.
Gardner DK, Lane M, Stevens J, et al. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8.
Minasi MG, Colasante A, Riccio T, et al. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31(10):2245–54.
Cimadomo D, Capalbo A, Levi-Setti PE, et al. Associations of blastocyst features, trophectoderm biopsy and other laboratory practice with post-warming behavior and implantation. Hum Reprod. 2018;33(11):1992–2001.
Huang L, Bogale B, Tang Y, et al. Noninvasive preimplantation genetic testing for aneuploidy in spent medium may be more reliable than trophectoderm biopsy. Proc Natl Acad Sci U S A. 2019;116(28):14105–12.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Catherine Gordon, Andrea Lanes, and Ann Thomas. The first draft of the manuscript was written by Catherine Gordon and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gordon, C.E., Lanes, A., Thomas, A. et al. Day of trophectoderm biopsy and embryo quality are associated with outcomes following euploid embryo transfer. J Assist Reprod Genet 39, 2539–2546 (2022). https://doi.org/10.1007/s10815-022-02613-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02613-x