Abstract
Asperococcus J.V. Lamouroux is a genus of brown algae in the Chordariaceae family (Order Ectocarpales). This concise review compiles the published literature on Asperococcus taxonomy, morphology, geographical distribution, ecology, life cycles, cultivation, biochemical composition and bioactive properties, emphasizing its commercial potential. This genus, which is mainly distributed in temperate-cold latitudes, is represented by six species of which A. fistulosus, A. ensiformis and A. bullosus have been the most studied. Species of this genus have membranous saccate or filiform thalli with prominent sori protruding from the surface. The life cycle is heteromorphic, alternating between a macroscopic sporophyte and a microscopic branched gametophyte. They inhabit tidal pools or different depths in the subtidal zone. Some species have been reported as epiphytic and epizoic organisms. Although there is no record of the commercialization of Asperococcus species, the cultivation of A. ensiformis from the Argentine Patagonian coasts was recently assessed and the biochemical composition of some species was studied, revealing a commercial potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genus Asperococcus includes a group of brown algae with a heteromorphic life cycle, belonging to the family Chordariaceae, Order Ectocarpales (Peters and Ramírez 2001). Six species of Asperococcus are flagged as taxonomically accepted in Algaebase (Guiry and Guiry 2023), however, the genus has a complex nomenclatural history that has led to uncertainty about the identity of the species that it encompasses. Asperococcus species are widespread around the world, however they have received less attention than other species of Chordariaceae and the literature on the genus is somewhat fragmented and scarce. The only available information for some species is the original description which is several decades old.
In recent years Asperococcus has been valued as a source of biologically active compounds (Zubia et al. 2009; Poza et al. 2022) which are of great interest to the pharmaceutical industry. Considering the global trend towards the production of macroalgae through aquaculture, the culture of some species of Asperococcus was studied, allowing the development of techniques that guarantee sustainable management of the natural populations (FAO 2020; Poza et al. 2022).
Historical background of the genus
The name Asperococcus means “rough seed” (asper = rough; kokkoç = seed or grain) and was established by J.V. F. Lamouroux to distinguish a seaweed that resembled the thallus of Ulva, but with prominent reproductive structures. Lamouroux (1813) described the fronds of Asperococcus as fistulous, generally cylindrical and rarely compressed, with a less bright color than Ulva. He clearly pointed out that Asperococcus could not be confused with Ulva because in the former, isolated and scattered “seeds” protruded from the surface of the frond when mature, giving the thallus a rough texture. Hence, he transferred the species Ulva rugosa of Linnaeus to the genus Asperococcus (as Asperococcus rugosus). In the same publication of 1813, Lamouroux designated the species Asperococcus bullosus from the Mediterranean, without including a formal description.
Botanists and phycologists through time have adopted different classifications for Asperococcus, placing the genus in different orders and families. Agardh (1820) was the first to classify this genus among the brown algae, in the order Fucoideae, however, he established the genus Encoelium to include the plants studied by Lamouroux with three species, Encoelium echinatus (= A. rugosus), Encoelium bullosum (= A. bullosus) and Encoelium sinuosum. In a later publication, Greville (1830) rescued the name Asperococcus indicating that the generic name given by Lamouroux should be preferred over Encoelium because it describes the most striking characteristic of these algae, the surface roughness given by the prominent sori. Other authors placed the genus in the Dictyoteae (Greville 1830; Hooker 1833; Harvey 1857), Punctariaceae (Kylin 1907), Encoeliaceae (MacCaughey 1918), Ectocarpales (Fritsch 1945) and Dictyosiphonales (Wynne 1982). The most recent classification adopted for this genus is based on the multilocus phylogeny of the Phaeophyceae proposed by Silberfeld et al. (2010), in which Asperococcus is classified in the subclass Fucophycidae, order Ectocarpales, and family Chordariaceae (Guiry and Guiry 2023).
More than 40 species names of Asperococcus appear in the phycological literature, but the majority are considered synonyms of the three most common species A. fistulosus, A. ensiformis and A. bullosus. Many different generic or specific names have been given to the same species by different authors, resulting in a plethora of synonyms for some species. An example is that of A. bullosus, which was given that name in continental Europe (Cabioc´h et al. 2006), but the same alga was named Asperococcus turneri in Britain (Fletcher 1987; Morton 1994). Currently, only six species of Asperococcus are taxonomically accepted in Algaebase (Guiry and Guiry 2023): Asperococcus bullosus J.V. Lamouroux, Asperococcus ensiformis (Delle Chiaje) M.J. Wynne, Asperococcus fistulosus (Hudson) W.J. Hooker, Asperococcus scaber Kuckuck, Asperococcus norvegicus Kylin and Asperococcus tortilis Suhr (Table 1), and many still have uncertain taxonomic status. According to Greville (1830), A. fistulosus (= A. rugosus, in Lamouroux 1813) was once considered the type of the genus; however, it has never been formally designated, and currently A. bullosus is regarded as the type non designatus (Guiry and Guiry 2023).
Morphology
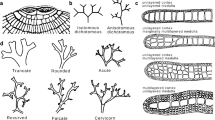
The macroscopic thallus of Asperococcus is the sporophyte, which has a simple morphology. The sporophytes may arise solitarily, but more frequently they appear in clumps or groups attached to the substratum by a small disc (Fig. 1a). The fronds may be tubular, filiform or elongate-saccate, solid, completely hollow or highly compressed in some parts with a ribbon-like shape. In some species, the hollow part of the fronds is irregularly inflated. The fronds are characterized by a slightly tapering or rounded apex, and a basal part that tapers gradually to the attachment disc, forming a sort of short stipe (Fig. 1b,c). The most remarkable feature of this genus is the prominent sori, scattered on the surface of the fertile thalli (Fig. 1b,c). Although the size of the thallus is variable, some species reach large sizes up to 1 m long and 5 cm wide.
Morphological characteristics of Asperococcus (on the example of A. ensiformis). a) Macroscopic sporophytes in nature. Scale bar: 1 cm (Photo by Gonzalo Bravo www.proyectosub.org.ar). b) Pressed specimen of A. ensiformis from the BBB Herbarium of the Universidad Nacional del Sur. Scale bar: 2 cm. c) Fresh specimen showing sori as dark dots. Scale bar: 1 cm. d) Surface view of the cortical cells. Scale bar: 25 µm. e) Cross-section of the thallus. Scale bar: 50 µm. f) Surface view of a frond showing scattered sori. Scale bar: 100 µm. 5) Detail of a sorus protruding from the surface, with unilocular sporangia (arrow) and paraphyses (arrowhead)
The structure of the thallus is parenchymatous, polystichous, with diffuse growth. It is formed internally of a cortex of assimilatory pigmented cells with numerous chloroplasts, each with one or two pyrenoids, and a medulla of larger colorless cells (Fig. 1d,e). Phaeophycean hairs are scattered on the surface of the thallus, solitary or in groups, usually related to the sorus.
The macroscopic sporophytes have unilocular sporangia grouped in sori that are prominent and may be visible to the naked eye (Fig. 1c,f). Both types of sporangia (unilocular and plurilocular) can be found in the same sorus on one plant or on different plants. The paraphyses are multicellular. They are straight, club-shaped or pyriform, and are mixed with the sporangia (Fig. 1g). The microscopic thallus (gametophyte) is filamentous and forms a disc from which erect filaments arise, bearing plurilocular gametangia.
Several authors have provided descriptions of A. fistulosus, A. ensiformis, A. bullosus and A. scaber (Greville 1830; Sauvageau 1895; Fritsch 1945; Asensi 1972; Womersley 1987; Schneider and Searles 1991; Nelson and Knight 1995; D'Archino and Nelson 2006; Cormaci et al. 2012; Bárbara et al. 2015), whereas little is known about A. tortilis and A. norvegicus, of which only the original description is available.
The following descriptions summarize the main morphological characters found on the macroscopic thallus of the six species taxonomically accepted in Algaebase.
Asperococcus bullosus J.V. Lamouroux
(Greville 1830; Fritsch 1945; Womersley 1987; Nelson and Knight 1995; D'Archino and Nelson 2006; Cormaci et al. 2012).
Thalli are medium to dark brown, flabby, gelatinous, irregularly inflated to bullose, up to 100 cm long and 12 cm wide. The base of the thallus tapers abruptly to a short stipe 2 mm to 10 mm long. The holdfast is 0.5–2 mm wide. The membrane is formed of 3 to 5 layers of cells. The cortex is formed of one layer of angular and isodiametric cortical cells, 20–35 µm in diameter in surface view, with numerous chloroplasts, each with 1 or 2 pyrenoids. The medulla is formed of 1 to 3 layers of large colorless cells. In the center of the membrane there is a large hollow cavity. Phaeophycean hairs are 16–20 µm in diameter, they grow singly or in groups from a basal meristem and each has a basal sheath. Unilocular sporangia are sessile, pyriform or clavate in transversal section, 30–40 µm long and 20–30 µm in diameter, and are located in rounded or elongated sori that measure 100 to 350 µm across. Plurilocular sporangia are lanceolate, ovoid or conical, 40–60 µm long and 30–40 µm in diameter, sessile or pedunculated. They are usually in the same sorus as the unilocular sporangia. Paraphyses are clavate, 2–4 cells long, with the apical cell rich in physodes.
Asperococcus ensiformis (Delle Chiaje) M.J. Wynne
(Womersley 1987; Sauvageau 1895; Asensi 1972; D'Archino and Nelson 2006; Cormaci et al. 2012; pers. obs.)
The thalli are yellowish, medium brown or olive-greenish, membranous, elongate-clavate or attenuated at the apex and flattened like a ribbon. Several fronds arise from a small discoid holdfast, 1–3 mm in diameter. They are up to 80 cm long and 4 cm broad, attenuating at the base to a short stipe 3–10 mm long. The fronds have the same width throughout the thallus. The membrane is formed of 1 or 2 layers of subquare cortical cells, or rectangular in surface view, measuring 13–20 µm × 8–12 µm, with numerous chloroplasts, each with one pyrenoid, and a medulla of 1 to 3 large rectangular colorless cells, measuring 60–90 µm × 25–45 µm. In cross-section, the thallus is strongly compressed in the center, forming a narrow cavity because the medullary cells are close together and are linked by irregular strands of long, cylindrical, colorless cells; while the margins are hollow, giving the thallus a flattened appearance. Plurilocular sporangia are rare, cylindrical or conical, 9–15 µm × 35 µm, sessile or pedunculated, and grouped in sori. Unilocular sporangia are rounded in surface view, 26–43 µm × 40–60 µm, globose or pyriform in cross-section, sessile or occasionally pedunculate, located in circular or irregularly shaped sori. Paraphyses are 2–4 cells long, cylindrical or clavate, 13–23 µm × 58–87 µm. Phaeophycean hairs in groups, 8–12 µm in diameter with a basal meristem and a basal sheath.
Asperococcus fistulosus (Hudson) W.J. Hooker
(Greville 1830; Fritsch 1945; Womersley 1987; Schneider and Searles 1991; Cormaci et al. 2012).
Thalli are light to middle brown, flabby, cylindrical or with sparse bulges, up to 60 cm long and 30 mm broad, attenuating at the base. The attachment disc is 100–300 µm across. The membrane is formed of 2–4 layers of cells, with 1 layer of angular cortical cells, isodiametric or rectangular, 1 to 3 layers of large medullary cells and a large cavity in the center. Cortical cells are 15–40 µm across and 10–44 µm long in surface view, with numerous chloroplasts, each with 1 or 2 pyrenoids. Phaeophycean hairs are solitary or in groups, 14–20 µm in diameter, with a basal sheath. Unilocular sporangia are located in rounded to elongated sori and they are pyriform to globular, sessile, rounded in surface view and pyriform in cross-section, 45–100 µm long and 40–70 µm in diameter. Paraphyses are relatively straight or cylindrical, 3–8 cells long, 18–36 µm × 18–47 µm and rarely branched.
Asperococcus scaber Kuckuck
(Kuckuck 1899; Fritsch 1945; Cormaci et al. 2012; Bárbara et al. 2015).
The thallus is minute, cylindrical, and slightly attenuated at both ends, or elongate and clavate, up to 1 cm long and 0.5 mm broad, with a pseudo discoid basal portion. Cortical cells are angular in surface view and measure 10–21 µm × 10–27 µm, with numerous chloroplasts, each with 1 or 2 pyrenoids. In cross-section the thallus is compact, without a central hollow cavity; instead, the axes are occupied by 4 to 6 large colorless cells, surrounded by 1–2 layers of small pigmented cortical cells. Phaeophycean hairs are frequent, with a basal sheath. Plurilocular sporangia are frequent, measuring 24–36 µm × 12–20 µm and are grouped in irregular sori. They are cylindrical or conical, and rarely associated with multicellular paraphyses of 2–3 cells long. Unilocular sporangia are rare, spheric or pyriform, pedunculated, measuring 45–45 µm × 30–35 µm.
Asperococcus norvegicus Kylin
(Kylin 1910)
Thalli are simple, solid, cylindrical, filiform, thin, up to 3 cm long and 0.75 mm. In cross-section, the fronds are composed of 4 long and large central cells surrounded by an external layer composed of 1–2 small isodiametric cells. The basal portion has a Myrionema-like growth. The plurilocular sporangia, which are linear or linear-lanceolate, 30–55 µm long and 11–15 µm wide, are arranged in minute, scattered sori along with hairs and some 2–5 articulated paraphyses.
Asperococcus tortilis Suhr
(Suhr 1836).
The thalli are bright orange-yellow, coriaceous, cylindrical, tubular, and spirally twisted, simple, up to 10 cm long. The thalli emerge from a very small disc and are filiform at the base, spreading toward the apex. The apices are closed in young specimens and open and slashed in older ones. Sporangia are located in rounded sori.
Table 2 summarizes the main morphological differences between the six recognized species of Asperococcus. The species are mainly distinguished according to the shape of the thallus and the degree of compression of the membrane (e.g., flattened, compressed, terete, bullose, cylindrical, etc.). Asperococcus ensiformis and A. bullosus are the largest representatives within the genus. Asperococcus ensiformis is morphologically distinguished from A. bullosus by the ribbon-like shape of the thallus that is highly compressed in the center, and hollow at the margins. In addition, it usually lacks plurilocular sporangia. On the contrary, A. bullosus is saccate, bullose, with an intestine-like shape. Asperococcus scaber and A. norvegicus are the smallest representatives. Both species can be differentiated from the others because they have a compact cylindrical thallus, without a hollow central cavity; however, they share several morphological aspects. In both species, the substrate attachment structure consists of a disc with marginal growth similar to Myrionema, they present a solid thallus formed by 4 large and elongated inner cells and an outer layer of isodiametric cells. Asperococcus scaber sometimes has a third middle layer of isodiametric cells, and A. norvegicus is usually larger than A. scaber. A. norvegicus has not been described by any other author than Kylin (1910) and to date there is no molecular evidence to determine whether A. norvegicus could be conspecific with A. scaber. Asperococcus fistulosus shares some characteristics with A. tortilis, for example, the cylindrical thallus, attenuated at the base with a large cavity in the center, however A. tortilis is distinguished by the thallus twisted into a spiral.
Molecular phylogeny
The family Chordariaceae is the largest and most diverse lineage within the order Ectocarpales (van den Hoek et al. 1995). However, to date only a few genera included in this family have been studied with molecular tools (Silberfeld et al. 2014; Kawai and Hanyuda 2021). The characteristics that allow the inclusion of Asperococcus species within the family Chordariaceae are the presence of discoid plastids and life cycles with heteromorphic alternation between haploid microscopic filamentous gametophytes and diploid macroscopic sporophytes, or direct asexual cycles, which regenerate diploid macrothalli (e.g., van den Hoek et al. 1995; Peters and Ramírez 2001). However, according to the current classification, the family Chordariaceae includes a large number of genera with a great variety of morphologies of which many are suspected to be polyphyletic (Silberfeld et al. 2014; Kawai and Hanyuda 2021). The phylogeny of the family should be revised with multimarker approaches.
Regarding the genus Asperococcus, there are 35 DNA sequences with the names A. bullosus and A. fistulosus available in Genbank (Table 3), however no species delimitations or phylogenetic studies based on DNA markers have been published specifically for this genus. Most of the public sequences are partial sequences of mitochondrial cox1 and chloroplast rbcL markers obtained from specimens collected in Norway, Britain, France, Malta, Australia, Chile and Japan, or from cultured specimens. Many of these sequences appear in phylogenetic studies of Phaeophyceae taxa, which have shown that Asperococcus is close to Chordariaceae genera, such as Punctaria, Leathesia, Striaria, Delamarea and Hecatonema (Siemer et al. 1998; Cho et al. 2003; Silberfeld et al. 2010; Kawai and Hanyuda 2021).
In Fig. 2 we present a Maximum Likelihood tree constructed with 11 rbcL sequences with the name Asperococcus and 95 rbcL sequences of species representative of families of Ectocarpales, all of which were retrieved from GenBank. The tree shows high or full support (100% or 98% bootstrap percentage, BP) for five of the six families of Ectocarpales delimited by Peters and Ramírez (2001) and Racault et al. (2009), Adenocysaceae, Acinetosporaceae, Ectocarpaceae, Petrospongiaceae and Scytosiphonaceae, but the family Chordariaceae was not well resolved in this phylogeny.
Maximum likelihood tree phylogenetic based on 106 rbcL sequences of Ectocarpales obtained from Genbank. Four sequences of species representative of Fucales were used as outgroups. Values above branches are Bootstrap Percentages (> 70%). Analysis was conducted with RAxML-NG with the CIPRES web service (Miller et al. 2010)
Asperococcus sequences formed a monophyletic group with two sequences of Striaria attenuata, that were highly supported (93% BP). This close relationship between Asperococcus and Striaria has been demonstrated by several authors (Cho et al. 2003; Kawai et al. 2019; Kawai and Hanyuda 2021). It is worth noting that the sequences representing Asperococcus are separated from genera of the Scytosiphonaceae, which sometimes used to be confused with Asperococcus due to some morphological similarities.
Within the Asperococcus group, two clades were highly supported with 97 and 100% BP, which may represent two of the known species of Asperococcus. Clade 1 included sequences from Norway and Britain named A. fistulosus and clade 2 included sequences from Norway, Japan, France, and Malta named A. bullosus. A sequence from Australia named A. bullosus (MN510448) segregated from both clades. Intraclade distances were between 0 and 0.09% in clade 1 and between 0.08% and 0.84% in clade 2. These values show that the genetic variability between specimens from clade 1 is lower than the variability within clade 2, which is expected given that the sequences from A. bullosus represent a wider distribution range, whereas those of A. fistulosus are from Norway and Britain. Distances between members of clades 1 and 2 were between 1.8% and 2.1%, whereas distances between the sequence MN510448 and clades 1 and 2 were between 2.1 and 2.5%.
The Australian sequence MN510448, named A. bullosus, clearly represents a third species. Considering the geographical distribution of the genus and the morphology of the six species of Asperococcus, this third species that appears in the ML tree could correspond to a specimen of A. ensiformis, a species that has been recorded in at least two coasts of Australia (Womersley 1987).
The Norwegian Asperococcus specimens, previously analyzed by Bringloe et al. (2019), segregate into two groups, which correspond to A. fistulosus and A. bullosus. Surprisingly, no molecular evidence of the species A. norvegicus can be derived from the tree, since all the sequences from Norwegian specimens, including the one from Bergen (MN184557) that is the type locality of A. norvegicus, are segregated in either the A. bullosus or A. fistulosus clades. The only morphological characterization available of A. norvegicus is the original description of Kylin (1910), which describes it as short and narrow (up to 3 cm long and up to 0.75 mm wide), solid and filiform, with plurilocular sporangia, with no mention of the presence of unilocular sporangia. It is unlikely that authors of the published sequences may have confused the morphology of A. norvegicus with that of A. fistulosus or A. bullosus, given the evident differences with the other two species that are larger, hollow, and somewhat inflated. An alternative explanation would be that the specimen studied by Kylin in 1910 was a young immature individual of A. fistulosus, given that A. fistulosus is a common seaweed in the northeast Atlantic and Baltic Sea (references in the section Geographic distribution). However, we cannot question the taxonomic validity of A. norvegicus unless a more comprehensive study of this species is conducted. Bringloe et al. (2019) already denoted that Asperococus species need further scrutiny because there are cryptic groups around the world. In order to elucidate the identity of the Asperococcus species, the genus needs an urgent revision based on molecular markers and morphology of the representative specimens of the distribution range reported for the species.
Life cycle
Asperococcus is characterized by a heteromorphic life cycle, alternating between a macroscopic sporophytic thallus (diploid phase) and a microscopic branched gametophytic thallus (haploid phase) (Asensi 1972; D'Archino and Nelson 2006; Poza et al. 2022); and depending on the species, plurilocular sporangia may or may not be present on the sporophytic thallus.
To date, a few studies have described the life cycle of some species of the genus, e.g., A. bullosus was studied by Clayton (1982) as A. turneri; A. ensiformis was studied by Asensi (1972) as Haloglossum compressum and recently by Poza et al. (2022); and A. fistulosus was studied by Knight et al. (1935) and Pedersen (1984).
In the life cycle of A. bullosus, both a direct and an indirect (or sexual) cycle can be distinguished. In the direct cycle, diploid spores from the plurilocular sporangia germinate, first forming a prostrate microscopic phase, known as plethysmothalli and constituting the basal part of the sporophyte (Fig. 3). Studies of A. bullosus populations from the Australian coasts revealed that plethysmothalli have the ability to reproduce and regenerate by means of plurilocular sporangia during the cold season. Then, during the warm season these thalli develop the young Asperococcus sporophyte (Chapman 1962; Clayton 1982). In the indirect cycle, unilocular sporangia release haploid spores (generated by meiosis) that give rise to the microscopic gametophytes, which produce plurilocular gametangia when mature. If the gametes produced by these gametangia fuse, the zygote develops into a plantule from which a new macroscopic plant arises, but if there is no fusion then they merely develop parthenogenetically into a new gametophytic generation (Chapman 1962; Pedersen 1984).
In A. ensiformis the haploid spores from the unilocular sporangia exhibit unipolar germination and develop a germ tube with transverse divisions, forming uniseriate branched filaments that develop into a prostrate microscopic gametophyte with occasional hyaline hairs (Fig. 3). Gametophytes give rise to plurilocular, terminal or intercalary gametangia. The female gametes (3.7–4.5 μm in diameter) settle first, becoming spherical, and are subsequently fertilized by male gametes (1–2.5 μm in diameter). The zygotes exhibit bipolar germination, forming two germ tubes. Some germlings form a little branched protonema with globose cells, from which, one or two erect laminae emerge, corresponding to the erect part of young sporophyte. The erect laminae can also emerge from a single basal cell, instead of a protonema. The erect lamina forms a uniseriate filament of cylindrical cells by transverse divisions and, subsequently, longitudinal divisions occur, forming a pseudoparenchymatous tissue with intercalary growth. On the other hand, although Sauvageau (1895) mentioned that the sporophyte of A. ensiformis rarely presents plurilocular sporangia, these structures have not been reported since by any other authors. In populations of A. ensiformis on the Patagonian coast, the presence of plurilocular sporangia has never been reported.
Knight et al. (1935) and Pedersen (1984) found that the life cycle of A. fistulosus depends on the behavior of the haploid spores from the unilocular sporangia. On one hand, once the haploid spores settle, they may form a prostrate thallus from which a macroscopic sporophyte emerges. This phenomenon, known as heteroblasty, is common in some Ectocarpus species where meiospores develop directly into haploid partheno-sporophytes (Coelho et al. 2020). On the other hand, the haploid spores may give rise to a filamentous microscopic phase (possibly gametophytic) that forms plurilocular reproductive structures and regenerates parthenogenetically (Knight et al. 1935; Pedersen 1984). However, these authors did not record the syngamy and assumed that the sexual cycle could be repressed.
Geographic distribution
Asperococcus is a genus mostly distributed in temperate-cold latitudes, with higher occurrences in the northeast Atlantic and Mediterranean zone. The species A. bullosus, A. ensiformis, and A. fistulosus have a wider world distribution compared to the other Asperococcus species. Asperococcus norvegicus was reported by Athanasiadis (1996) at Bergen on the Norwegian coast and A. tortilis is restricted to Indian Ocean Island (Silva et al. 1996) (Fig. 4).
Asperococcus scaber has a wider distribution, but is restricted to the northeast Atlantic marine zone (Newton 1931; Fletcher 1987; Hardy and Guiry 2003; Guiry 2012; Brodie et al. 2015; Anon 2017; Burel et al. 2019), the Mediterranean area (Giaccone 1978; Ribera et al. 1992), and the West African marine zone (Gallardo et al. 2016) (Fig. 4).
According to the phycological literature the most common species, A. bullosus, A. ensiformis and A. fistulosus, have a much wider distribution range than the other three species. Asperococcus bullosus has been reported in the Arctic zone (Nielsen and Gunnarsson 2001; Bringloe et al. 2019), in the northeast Atlantic (Wollny 1881; Hauck 1889; Adams 1908; Cotton 1912; Kuckuck 1912; Lyle 1920; Newton 1931; Rees 1931; Miranda 1934; Feldmann 1954; De Mesquita Rodrigues 1963; Ardré 1970; Flores-Moya et al. 1995; Bartsch and Kuhlenkamp 2000; Báez et al. 2001; Hardy and Guiry 2003; Bárbara et al. 2005; Valenzuela Miranda 2005; Peña and Bárbara 2008, 2010; Araújo et al. 2009; Bunker et al. 2010, 2017; Cires Rodriguez and Cuesta Moliner 2010; Silberfeld et al. 2010; Guiry 2012; Brodie et al. 2015; Anon 2017), in the Mediterranean (Feldmann 1937; Diannelidis 1953; Giaccone 1969, 1978; Cinelli 1971; Güven and Öztig 1971; Coppejans 1972; Feoli and Bressan 1972; Cinelli et al. 1976; Boudouresque and Perret 1977; Ercegovic 1980; Güner et al. 1985; Rodríguez Prieto et al. 1993; Borg et al. 1998; Furnari et al. 1999; Aysel et al. 2005; Taşkin et al. 2008; Antolic et al. 2010; Bunker et al. 2010; Joher et al. 2012; Ould-Ahmed et al. 2013; Tsiamis et al. 2013; Burel et al. 2019; Taşkın et al. 2019; Bartolo et al. 2021; Boudouresque et al. 2022), in the Baltic Sea (Nielsen et al. 1995; Kontula and Fürhapter 2012; Nielsen and Lundsteen 2019), in West Africa (Kylin 1907, 1947; Price et al. 1978; Seagrief 1984; Silva et al. 1996; Gil-Rodríguez et al. 2003; John et al. 2004; Moro et al. 2011; Afonso-Carrillo 2014; Gallardo et al. 2016; Sabri et al. 2021), in the northeast Pacific (Pedroche et al. 2008; Pedroche and Sentíes 2020), in the northwest Pacific (Holmes 1896; Okamura 1936; Segawa 1981; Yoshida et al. 1990, 2015), in the South Pacific (MacCaughey 1918) and in the Australia and New Zealand area (Womersley 1987; Huisman and Walker 1990; Adams 1994; Nelson and Knight 1995; Huisman 1997, 2000; 2019; Nelson 1999; D'Archino and Nelson 2006; Harper et al. 2012; Bringloe et al. 2019; Nelson et al. 2021). Asperococcus bullosus was also reported as A. turneri and as A. turneri var. profundus in the Arctic (Irvine 1982), in the northeast Atlantic (Fletcher 1987) in the Mediterranean (Rodríguez and Femenías 1889; Gerloff and Geissler 1974; Munda 1979; Ribera Siguán 1983; Verlaque 1984; Ribera Siguán and Gómez Garreta 1985; Athanasiadis 1987; Ballesteros 1989), and West Africa (Price et al. 1978; Haroun et al. 2002; Gil-Rodríguez et al. 2003; John et al. 2004; Moro et al. 2011).
Asperococcus ensiformis shares a distribution range with A. bullosus in the northeast Atlantic (Bárbara et al. 2005; Peña and Bárbara 2008; Araújo et al. 2009; Cires Rodriguez and Cuesta Moliner 2010; Bermejo et al. 2012; Guiry 2012; Brodie et al. 2015; Burel et al. 2019), the Mediterranean (Taşkin et al. 2008; Temniskova et al. 2008; Boudouresque et al. 2022), Baltic Sea (Kontula and Fürhapter 2012; Nielsen and Lundsteen 2019), West Africa (Afonso-Carrillo 2014; Gallardo et al. 2016) and Australia and New Zealand (Harper et al. 2012; Nelson et al. 2021). Asperococcus ensiformis is the only Asperococcus species reported in the South Atlantic on the Patagonian coast by Asensi (1972, as Haloglossum compressum) and Boraso de Zaixso (2013).
Nevertheless, A. ensiformis was cited as A. compressus in the Arctic (Rueness 1997; Brattegard and Holthe 1997; Fredriksen et al. 2019), in the northeast Atlantic (Heape 1888; Adams 1908; Newton 1931; Blackler 1951; Cullinane 1971; Guiry 1978; De Valéra et al 1979; Whelan and Cullinane 1985; Fletcher 1987; Morton 1994; Coppejans and Kling 1995; Bárbara et al. 2005; Brodie et al. 2015), in the Mediterranean (Feldmann 1937; Giaccone 1969; Edwards et al. 1975; Ercegovic 1980; Ribera et al. 1992; Flores-Moya et al. 1995; Furnari et al. 1999; Rindi et al. 2002), in the Baltic Sea (Nielsen et al. 1995) in the West Africa area (Price et al. 1978; Stegenga et al. 1997; Gil-Rodríguez et al. 2003; John et al. 2004; Moro et al. 2011; Gallardo et al. 2016), in the South Atlantic zone (Asensi and Küpper 2012) and Australia and New Zealand (Womersley 1987).
Asperococcus fistulosus is widely distributed. This species was reported in the Arctic (Caram and Jónsson 1972), northeast Atlantic (Adams 1907; Cotton 1912, 1913; Newton 1931; De Valéra et al. 1979; Maggs 1983; Whelan and Cullinane 1985; Fletcher 1987; Morton 1994; Bartsch and Kuhlenkamp 2000; Hardy and Guiry 2003; Brodie et al. 2015; Bunker et al. 2017), West Atlantic (Neto et al. 2001; Haroun et al. 2002; Gil-Rodríguez et al. 2003; John et al. 2004; Moro et al. 2011; Afonso-Carrillo 2014; Ferreira et al. 2018), Mediterranean (Gerloff and Geissler 1974; Ribera et al. 1992; Furnari et al. 1999; Rindi et al. 2002; Taşkin et al. 2008), Baltic Sea (Nielsen et al. 1995; Kontula and Fürhapter 2012; Nielsen and Lundsteen 2019), in the wider Caribbean and North Carolina (USA) (Schneider and Searles 1991), in the northwest Atlantic from Virginia to New Hampshire (Humm 1979; Schneider et al. 1979), in the northeast Pacific (Pedroche et al. 2008), and on the Australia and New Zealand coasts (Womersley 1987).
Asperococcus bullosus and A. ensiformis, were also reported by D'Archino and Nelson (2006) as introduced species in New Zealand, and A. ensiformis (cited as A. compressus) was reported in Australia by Womersley (1987). Asperococcus bullosus, a native species from Europe, was first recorded in New Zealand waters at Stewart Island by Lindauer (1957) and later, in the late 1980s and early 1990s, it was recorded in South Island (D'Archino and Nelson 2006). In Australia, A. bullosus was found from Rottnest Island (West Australia) to Pittwater, Sydney (NSW) and also on the north and east coasts of Tasmania. Asperococcus ensiformis (cited as A. compressus) was observed solely at Flinders and Port Phillip (Victoria), and A. fistulosus was found from Kangaroo Island (South Australia) to Apollo Bay (Victoria), and in southeastern Tasmania (Womersley 1987).
Given that Asperococcus species are predominantly distributed in the northern hemisphere, and its presence in the southern hemisphere (Australia and New Zealand) is related to possible introduction, the records of A. ensiformis in the Atlantic Patagonian coast could represent another potential introduction.
Ecological aspects
Asperococcus species have been found in the intertidal zone, in intertidal pools (Fritsch 1945; Blackler 1951) and at different depths in the subtidal zone, reaching depths of up to 46 m (Cinelli 1971; Borg et al. 1998; Bárbara et al. 2005; D'Archino and Nelson 2006; Taşkin et al. 2008; Bartolo et al. 2021).
Several checklists reported the presence of other genera of Chordariaceae coexisting with Asperococcus, of which the most represented are Acrospongium, Acrothrix, Adriogloia, Botrytella, Chilionema, Chordaria, Cladosiphon, Climacosorus, Corynophlaea, Cylindrocarpus, Delamarea, Dermatocelis, Dictyosiphon, Elachista, Endodictyon, Eudesme, Giraudia, Halothrix, Hecatonema, Isthmoplea, Kuetzingiella, Laminariocolax, Leathesia, Leblondiella, Leptonematella, Litosiphon, Mesogloia, Microcoryne, Microspongium, Mikrosyphar, Myriactula, Myriocladia, Myrionema, Myriotrichia, Nemacystus, Phaeostroma, Protectocarpus, Punctaria, Saundersella, Sauvageaugloia, Spermatochnus, Sphaerotrichia, Spongonema, Stictyosiphon, Stilophora, Streblonema, Striaria and Ulonema (Haroun et al. 2002; Bárbara et al. 2005; Antolic et al. 2010; Kontula and Fürhapter 2012; Sabri et al. 2021).
Some species of Asperococcus have been reported as epiphytic and epizoic organisms. Asperococcus ensiformis (cited as A. compressus) was found growing on shells together with Chorda filum (Blackler 1951) and on fronds of Cystoseira barbata (Taşkin et al. 2008). Also, the basal holdfast of A. ensiformis was associated with Urospora, Enteromorpha (Ulva), Bangia and Ectocarpus spp. (Rees 1931). This author even highlighted the association with Bangia-Urospora, outside of the usual tidal level where Asperococcus occurs. On the Atlantic Patagonian coasts A. ensiformis (cited as Haloglossum compressum, Asensi 1972) was observed in lower intertidal pools, associated with calcareous algae such as Jania.
Asperococcus fistulosus was reported as an epiphyte on Fucus, Himanthalia lorea, Laminaria saccharina, Punctaria latifolia and Cystoseira and Cladophora thalli. Furthermore, it was observed bearing other seaweeds, such as Myriotrichia filiformis, Myriotrichia clavaeformis, Sphacelaria cirrosa, Ceramium rubrum, Ceramium strictum, filamentous diatoms and Ectocarpus sp. (Rees 1931). This species was also cited as an epiphyte on seagrasses. In South Australia it was collected from tidal flats on Heterozostera and on the brown seaweed Scaberia (Womersley 1987).
Asperococcus bullosus was also reported by Taşkin et al. (2008), growing as an epiphyte on the seagrass Posidonia oceanica on Turkish coasts. Furthermore, it was observed growing on Posidonia sinuosa, Posidonia australis and Amphibolis antarctica on the Australian coast, and as an epiphyte on Heterozostera in low intertidal pools in Tasmanian coastal areas (Womersley 1987). In New Zealand, many of the A. bullosus thalli found at Rangaunu harbor were attached to fragments of Zostera (Nelson and Knight 1995).
Seaweed phenology, like in terrestrial plants, is susceptible to temperature mediated changes, such as shifts in the timing of their reproduction. Also, temperature can affect individual performance regarding photosynthesis, growth, and reproduction rates, and it determines the tolerance or survival limits (Breeman 1990; Eggert 2012). Possible northern boundaries of seaweeds have been determined, whose ranges are established by lethally low winter temperatures, or by summer temperatures too low for growth and/or reproduction, while southern boundaries are established by lethally high summer temperatures, or by winter temperatures too high for induction of the crucial step in the life cycle (Breeman 1990). These thermal thresholds are commonly related to the tolerance of the hardiest life history stage, the microthallus stage, and temperature requirements for reproduction and growth. In particular, seaweeds are extremely sensitive to the effect of temperature on their distributional range. However, changes in temperature, such as warming, may favor opportunistic and tolerant seaweeds by increasing their competitive ability (Dukes 2007).
Some studies indicated the occurrence of A. bullosus, A. ensiformis and A. fistulosus macrothalli during summer and autumn in the northeast Atlantic zone, bearing mature unilocular sporangia (Harvey 1857; Heape 1888; Blackler 1951; Peña and Bárbara 2008). In southern latitudes, such as the Patagonian coast in the southwest Atlantic, A. ensiformis macrothalli were reported in summer, mostly in February and March (Asensi 1972).
Cultivation
Current state and perspectives
Traditionally seaweed production came mainly from the harvest of wild populations (FAO 2018, 2020). However, in recent decades, the worldwide demand of seaweed has increased notably due to its use in the food, cosmetic, nutraceutical and pharmaceutical industries, as well as for the production of organic fertilizers and biofuel (Cai et al. 2021; Grand View Research 2021). This has generated the need to develop algae cultures on a large scale to ensure sustainable management of the natural populations.
This situation encouraged the study of a large number of species with industrial potential or nutraceutical benefits, such as bioactive foods or superfoods (Chopin and Tacon 2021).
To date, there are no published records of commercialization of Asperococcus, but A. fistulosus was included in a list of seaweed species that could be used for human consumption (Drennan 2016; Mendes et al. 2022) and recently, a study evaluated the optimal conditions for the cultivation of A. ensiformis from the Patagonian coasts of Argentina (Poza et al. 2022).
Spore-based culture
Seeding spores or zygotes on collectors has been a traditional method for the production of seaweed seedlings for mass cultivation (Critchley and Ohno 1998). Due to the heteromorphic life cycle of Asperococcus, the cultures can be started from spores released from pluri or unilocular sporangia, depending on the species. The ones that present both types of spores (haploid and diploid), such as A. bullosus, present two ways to generate sporophytes; asexually through a direct cycle or sexually following an indirect cycle (Chapman 1962; Pedersen 1984). Other species, such as A. ensiformis, follow an indirect cycle, interspersed with a sexual phase (gametophytes), that ensures genetic recombination and a greater variability in the population (Asensi 1972; Poza et al. 2022). The culture from spores is advantageous because it can be started from a small amount of fertile biomass, it provides wide genetic variability and could be highly productive due to the vigor of the germlings produced from spores (Hales and Fletcher 1990; Peteiro et al. 2016; Gupta et al. 2018; Hu et al. 2021). Culture studies of A. ensiformis were carried out under different temperatures and nutrient conditions where the viability of spores, zygotes and germlings was evaluated (Poza et al. 2022). It was observed that the number of settled spores was higher than the number of settled zygotes, generating a high number of gametophytic microscopic stages, and also that the subsequent survival of sporophytes and gametophytes germlings was around 40%.
However, when the cultivation starts from fertile fronds (spores), it depends on the seasonality and the availability of fertile specimens in the natural beds (Macchiavello et al. 2010; Camus and Buschmann 2017; Gupta et al. 2018; Hu et al. 2021). Therefore, the management and conservation of banks of microscopic forms (gametophyte germlings in Asperococcus) could represent an alternative for aquaculture development of rare species, since it allows the long-term preservation of more productive or diverse strains, which will then be used to generate new macroscopic sporophytes, avoiding the dependence on a continuous supply of fertile fronds from natural beds (Wade et al. 2020; Schoenrock et al. 2021).
Culture methods
Asperococcus ensiformis has been successfully cultivated on seeding ropes under controlled environmental conditions in the laboratory (Poza et al. 2022). Cultivation on ropes is a traditional method of cultivation that consists in producing macroalgae on ropes. This can be started from spores released from mature sporophytes extracted from natural populations or from gametes obtained from banks of microscopic forms (gametophytes in Asperococcus), which can then be transferred to the sea once they reach a certain size (Peteiro 2018). This technique is very effective and low cost, because it only requires a reduced biomass of fertile fronds to start the cultivation, and also because the thalli grown on ropes have lower production costs, due to the shorter time required in the hatchery and smaller hatcheries (Macchiavello et al. 2010; Camus and Buschmann 2017). Another hatchery technique is the free-floating system (Westermeier et al. 2006), which allows larger individuals to be stocked. However, it has higher production costs, due to a greater need for water and nutrients.
The culture procedure used for A. ensiformis in Poza et al. (2022) was an adaptation from the methodologies established for other species of brown algae with heteromorphic life cycles, where the production of seedlings on rope under laboratory conditions is divided into two steps: a first step where a culture of microscopic gametophytes is settled, and another stage where macroscopic thalli are produced under controlled environmental conditions.
Briefly the method used involves (i) collection of fertile thalli from nature, which are easily identified by the prominent sori visible as dark dots; (ii) thallus conditioning, to remove epiphytes and possible pathogens, and also to stimulate the maturation of the sporangia; (iii) laboratory incubation, which consists of fixing the fertile fragments to a mesh with an interlaced polypropylene rope, allowing the release of the spores and their settlement on the rope. Then the meshes are placed in transparent containers that allow the passage of light and they are kept in the laboratory under controlled abiotic conditions for 5 months, until the seedlings can be moved to the sea. This technique makes it possible to inoculate several meters of rope in a small area.
The collections, transportation and conditioning of fertile thalli used for cultivation are critical stages since the subsequent health of the culture will largely depend on them.
Some authors recommend transporting the fertile fronds at low temperatures, on moist paper towels and in black bags to avoid light. They also recommend never submerging the thalli in seawater since it could cause premature sporulation (Oliveira et al. 1995; Redmond et al. 2014; Forbord et al. 2018).
Regarding thallus conditioning to remove epiphytes and pathogens, different methodologies are mentioned in the literature; in Poza et al. (2022) the fertile sporophytes of A. ensiformis were first brushed and then immersed in freshwater with 0.5% chlorine for 10 s and rinsed with freshwater. Treatment with surface disinfectants allows the removal of epiphytes, animals, and microorganisms. Other surface disinfectants normally used are copper sulfate, which can remove epiphytic algae including phytoplankton and even fungi, and Betadine (an iodine-based antiseptic) which can also eliminate a wide range of microorganisms (Sulu et al. 2004; Redmond et al. 2014; Forbord et al. 2018).
To stimulate the maturation of the sporangia and the subsequent release of spores, the cleaned thalli have to be subjected to stress conditions generated by desiccation and darkness (Forbord et al. 2018; Suebsanguan et al. 2021). In Poza et al. (2022) the disinfected thalli of A. ensifomis were dried with paper towels and preserved in the dark at 8 °C for 12 h, without leaving the surface withered.
The early post-settlement phases of spores and zygotes of macroalgae is critical for the successful establishment of populations, so several studies have focused on evaluating the optimum temperature and radiation conditions, and nutrient concentration that affect the settlement and recruitment in macroalgae cultures (Vadas et al. 1992; Bogaert et al. 2016; Camus and Buschmann 2017; Lind and Konar 2017; Poza et al. 2018, 2022; Suebsanguan et al. 2021).
Temperature conditions
In the cultivation of A. ensifomis, the effect of temperature on the early stages of gametophytes and sporophytes was evaluated by Poza et al. (2022). A temperature range of 8–12 °C was favorable for the growth of A. ensiformis gametophytes on seed-rope with densities that reached 74 ± 2 individuals/2 mm. On the other hand, they observed that the sporophytes were able to develop in a wider temperature range of 8–16 °C, with densities that reached 13 ± 1 individuals/2 mm of rope. Seaweeds with a wide geographical range, such as A. ensiformis, exhibit a wide tolerance range to different temperatures, as they experience great seasonal temperature changes under natural conditions, which represents an advantage when it comes to cultivation (Eggert 2012).
Nutrient enrichment
Another determining factor is the flow of nutrients received at the hatchery stage, since proper handling reduces production costs and minimizes the amount of time and energy used for the production of the young sporophytes (Camus and Buschmann 2017; Charrier et al. 2017; Boderskov et al. 2022). There is evidence that certain nutrients, such as nitrate and phosphate, not only affect the growth in the brown algae, but also gametogenesis (Harries 1932; Amsler and Neushul 1990; Liu et al. 2016; Boderskov et al. 2022). Also, nutrient variations can affect the chemotaxis of spores and zygotes, which actively detect nutritionally favorable microhabitats for settlement and subsequent algal growth and survival (Amsler and Neushul 1990). Therefore, selection of the seawater to be used at the hatchery stage is essential because some nutrients may be limited under natural conditions (Peteiro et al. 2016) and external nutrient sources are needed to complete a successful hatchery operation in those situations.
Provasoli culture medium (PES) is an artificial medium that has traditionally been used for growing macroalgae, containing nitrogen and phosphorus in relatively high concentrations, macro and micronutrients, vitamins, and a chelating solution for poorly soluble ions. The use of artificial media continues to play an important role in overcoming nutrient supply problems because it allows the amounts of macro and micronutrients or other compounds to be manipulated according to the need (Berges et al. 2001; Camus and Buschmann 2017). Seaweed extracts are currently being tested as biostimulants for the hatchery stage, as an alternative to reduce the quantities of Provasoli used or, in some cases, to replace it (Robertson-Andersson et al. 2006; Hurtado et al. 2012; Vatsos and Rebours 2015; Umanzor et al. 2019, 2020).
The effect of biostimulant was tested in the Asperococcus culture combined with the traditional PES culture medium (Poza et al. 2022). These authors cultivated gametophytes and sporophytes of A. ensiformis by the seeding rope method using the traditional PES medium and also a biostimulant based on seaweed extracts called Kelpak® (Kelp Products Pty Ltd., Simon's Town, South Africa). They observed that the growth of A. ensiformis gametophytes was favored by the Povasoli culture medium at concentrations of 20 mL PES L−1 of seawater with densities that reached 104 ± 5 individuals/2 mm. However, the highest sporophyte density and greater growth of the thallus was achieved with the combination of 5 mL PES L−1 plus 1 mL L−1 of Kelpak, with 21 ± 1 individuals/2 mm and 6 ± 2 mm in length after 45 days of incubation. Kelpak is a liquid concentrate derived from the stipes and laminae of the subtidal kelp Ecklonia maxima, that contains phytohormones (auxins and cytokinins) (Stirk and van Staden 1997), alginate, amino acids, and small amounts of macro and microelements (Lötze and Hoffman 2016).
Initially the seaweed extracts gained popularity as agricultural supplies because of the presence of fucoidans and other sulfate esters or alginates, which provide a plant response similar to that produced by the application of composted seaweed (Milton 1964). Currently there are few studies that evaluate the effect of biostimulants on the growth of brown algae (Umanzor et al. 2019). The promotion of the use of algae-based biostimulants is not only focused on reducing the production costs, but also because previous studies highlighted their function as growth enhancers and improvers of the health status of the cultures (Robertson-Andersson et al. 2006; Hurtado et al. 2012; Vatsos and Rebours 2015; Umanzor et al. 2019).
Although the commercial cultivation of macroalgae does not use seaweed extracts as biostimulants nowadays, more and more studies are focusing on analyzing their effects on macroalgae cultivation with very promising results. For example, studies carried out on commercial red seaweeds, such as Kappaphycus alvarezii, revealed that the use of commercial biostimulants derived from brown seaweeds (Acadian®) increased the growth rate, shoot formation and yield of carrageenans (Hurtado et al. 2012; Loureiro et al. 2014a, b; Hurtado and Critchley 2020). Similarly, in Eucheumatopsis isiformis cultures supplemented with Kelpak®, Acadian®, and Ocean Organics biostimulant products, a larger number of shoots per propagule was observed (Umanzor et al. 2020). Also, brown algae cultures, such as the sporophytes of Saccharina spp. supplemented with biostimulants (Acadian), showed blades more than three times thicker than those without any supplementation, and their growth capacity was enhanced (Umanzor et al. 2019).
Chemical composition and bioactive properties
Seaweeds have been used for food since ancient times, especially in the Asia–Pacific region (Wells et al. 2017). Human consumption of algae varies from country to country, for example in Japan it ranges from 9.6 to 11 g of macroalga per day (MHLW 2014). The use of algae for human consumption and food additives is increasing due to the growing awareness about its health benefits (Wells et al. 2017). Seaweeds contain 10 to 20 times more minerals than terrestrial plants and they are a good source of proteins, minerals, vitamins, amino acids, lipids, fatty acids, polysaccharides, and carotenoids (Arasaki and Arasaki 1983). In addition, a large number of compounds isolated from different algae species have biological activity, as antiviral, antioxidant, antifungal, antidiabetic, neuroprotector, among others (Kumar et al. 2008).
Regarding the direct consumption of Asperococcus species as human food, there is only one record that mentions A. fistulosus, called thin sausage weed, as a possible edible seaweed, but this species has not been traditionally consumed (Drennan 2016).
Information about the chemical composition of Asperococcus species is really scarce and data on proximal composition, pigments and polysaccharides have only been recorded for A. ensiformis from the Atlantic Patagonian coasts in Argentina (Poza et al. 2022).
The protein content determined in samples from that population of A. ensiformis was around 2.8% dry weight, a value higher than the protein content reported for other brown algae from the same coastal area, such as Macrocystis pyrifera, Undaria pinnatifida and Leathesia marina (Viera et al. 2013; Poza et al. 2018). Although it is a low value, it is important to note that brown algae usually have a low protein content (3–15%) when they are compared with green and red seaweeds (Fleurence 1999).
Asperococcus ensiformis from the Patagonian coasts also has lower values of total carbohydrate content (6.45 ± 0.50 mg (100 mg)−1 organic matter) in comparison to other brown algae from the same area, e.g., M. pyrifera, U. pinnatifida (Viera et al. 2013) and L. marina (Poza et al. 2018), and it is the only record of total carbohydrate content for species of Asperococcus.
The ash content refers to the inorganic residue remaining after ignition in biological material and represents the amount of total minerals and trace elements (Liu 2019). It is an important parameter of seaweed nutritional quality since high ash content in most algae prevents their use as human food and hampers the inclusion of intact seaweed in animal diets (Austic et al. 2013; Bikker et al. 2020). However, since edible brown seaweeds usually contain high amounts of K, Na, Ca, Mg, Fe, Zn, Mn, P, a controlled intake of small portions (between 8 and 10 g dw) can supply most of the recommended daily intake of some essential minerals (Rupérez 2002; Mišurcová et al. 2011). The ash content recorded for A. ensiformis from the Atlantic Patagonian coast was higher than 40% dry weight (Poza et al. 2022), a high value compared to most land plants (Rupérez 2002; Liu 2019) and also to other seaweeds, which present values ranging from 20 to 35% (Verma et al. 2017). Moreover, brown seaweeds usually show values of ash content between 20 and 40% (Pereira 2011; Kumar et al. 2015).
Another compound studied in a species of Asperococcus was the iodine content. Iodine content is an important quality parameter to evaluate in edible seaweeds, since a regular consumption of iodine-rich seaweeds, such as kelps, could severally affect thyroid function; however, the occasional intake (once or twice a week) or their use as a dietary supplement is unlikely to be harmful (Smyth 2021). Iodine was measured in A. echinatus from Woods Hole (USA), showing very low values of I2 (0.056 mg g−1 alga) (Grimm 1952). The iodine content of seaweed and its chemical form varies with different species and also varies depending on factors, such as season, geographical location, parts of the alga and growth stages, but the iodine content is generally greater in kelps than in other algae groups (Teas et al. 2005; Mussig 2009; Roleda et al. 2018).
The cell walls of brown algae are mainly composed of alginic acid, cellulose, and sulfated polysaccharides in an average weight ratio 3:1:1 (Kloareg and Mabeau 1987). These polysaccharides are arranged in two layers, the innermost is a skeleton of cellulose microfibrils that provides rigidity to the cell, and the outermost is mainly made of alginic acid and fucoidans conforming an amorphous matrix in which the set of microfibrils is embedded (Davis et al. 2003; Ponce and Stortz 2020).
Alginic acid (or alginate) constitutes between 18.9 to 66.7% of the dry weight in brown algae (Li et al. 2021). This polysaccharide is responsible for the springiness and flexibility required by the algae to withstand the forces of the ocean and waves. It also helps to prevent desiccation and facilitates the bonding of salts present in the seawater, particularly Ca2+, Na+, Mg2+, Sr2+, and Ba2+ ions (Haug 1964).
Alginate is a linear polymer of residues of mannuronic (M) and glucuronic acid (G) connected through 1,4-glycosidic linkages (Brownlee et al. 2005). The blocks are composed of consecutive G residues, consecutive M residues, and alternating M and G residues (Lee and Mooney 2012). The M/G ratio varies among different algal species, as well as with season, age, tissue type, and geographic location (Kraemer and Chapman 1991; Donati et al. 2003). In general, alginates that are rich in G residues exhibit stronger stiffness and gelling properties, so they are widely used in the food and cosmetic industry (Kim et al. 2018).
Alginate yield of A. ensiformis from the Atlantic Patagonian coast is around 30% (Poza et al. 2022), a yield similar to kelp species, such as Macrocystis pyrifera, Laminaria hyperborea, Laminaria digitata and Saccharina japonica, which are industrially important species for alginate production (Peteiro 2018).
Fucoidans are a group of fucose-rich sulfated polysaccharides, usually built of a backbone of α-linked L-fucose residues accompanied by variable amounts of other monosaccharides, like galactose, xylose, mannose, rhamnose, and glucuronic acid (Ale and Meyer 2013; Ponce and Stortz 2020). Fucoidans are believed to play a key role in cell wall architecture, by cross-linking alginates and cellulose microfibrils (Kloareg et al. 1986). They would also help to retain water in the fronds by preventing desiccation during the emersion period, since when the fronds are in contact with seawater, the ester sulfate groups are associated with magnesium ions that are highly hydrated (Percival 1979). Proof of this is that brown seaweeds growing in the upper parts of the shores have a high fucoidan content, whereas subtidal species contain very little or no fucoidans (Carlberg et al. 1978).
A yield of 18.5% of fucoidans was found in A. ensiformis from the Atlantic Patagonian coast, being the first record of fucoidans yield in Asperococcus species (Poza et al. 2022). This yield of fucoidans is high compared to other brown algae inhabiting the same shores, such as Leathesia marina and Scytosiphon lomentaria (Feldman et al. 1999; Ponce et al. 2019), but similar to those yields found in Undaria pinnatifida from the same coastal area (Viera et al. 2013).
Fucoidans have demonstrated a number of promising biological activities, including immunomodulatory, antitumor (Ale et al. 2011), anti-inflammatory (Cumashi et al. 2007), antithrombotic, and anticoagulant (Wang et al. 2010) activity. Such bioactivities have been related to their chemical structure, i.e., monosaccharide composition, sulfate content, position of sulfate ester groups, and molecular weight (Li et al. 2008).
The pigment profile of brown algae is mainly composed of chlorophylls a and c; carotenes, principally α and β carotene; and xanthophylls, mainly neoxanthins A and B, fucoxanthin and violaxanthin (Garcia-Perez et al. 2022). Fucoxanthin is the pigment responsible for the characteristic golden-brown color of this group of algae (Kumar et al. 2013) and its biological function is to enhance light harvesting due to its ability to absorb blue-green light (450–570 nm) which is poorly absorbed by the chlorophylls (Kim et al. 2011). It also may function as a photoprotective pigment under overexposure to solar radiation (Frank et al. 1997; Griffiths et al. 2016).
Pigment composition varies significantly between populations and also between individuals as a result of the influence of local environmental conditions. Therefore, adjustments in the chlorophyll a + c concentration reflect the acclimatation to the in-situ radiation and are applied in order to balance light harvesting and photoprotection (Marambio et al. 2017). Concentrations of photosynthetic pigments in Asperococcus species have only been recorded for A. ensiformis from Atlantic Patagonian coasts, being chlorophyll a the most abundant pigment (Poza et al. 2022). Asperococcus ensiformis also shows high levels of fucoxanthin (Poza et al. 2022), similar to those found in other phaeophycae, such as Sargassum linearifolium and Spatoglossum asperum (Verma et al. 2017).
Pigments from brown seaweed have been used as coloring agents in food products and in animal feed, and also to improve the appearance of pet foods and dairy products (Aryee et al. 2018). In addition, numerous studies show the beneficial impact of pigments on human health, for example fucoxanthin has been demonstrated to have antidiabetic and anti-obesity properties, blood-pressure lowering properties and anticancer, antioxidant, immune modulatory, antiangiogenic, antimalarial and anti-inflammatory activities (Brown et al. 2014; Namvar et al. 2014; Déléris et al. 2016; Pangestuti and Siahaan 2018).
Regarding biological activity, data on the antioxidant activity have only been obtained for A. ensiformis from the Atlantic Patagonian coast and for A. bullosus from the Britany coasts. A. ensiformis shows high radical scavenging activity, with an IC50 of 9.54 mg mL−1 and vitamin C equivalent of 41.84 mg VCE per 100 g of dry algae, whereas A. bullosus presented lower IC50 values for radical scavenging activity and low antioxidant activity evaluated using another methodology (β-carotene–linoleic acid system and reducing activity) (Zubia et al. 2009; Poza et al. 2022).
Numerous studies indicated a direct relationship between the antioxidant activity and the total content of phenolic compounds (Velioglu et al. 1998; Kim et al. 2005; Connan et al. 2006; Zhang et al. 2007; Le Lann et al. 2008). Phenolic compounds are secondary metabolites made up of an aromatic benzene ring with one or more hydroxyl substituents, they have a protective role against abiotic stressors such as UV radiation (de la Coba et al. 2009). The postulated protective mechanism involves the ability to donate a phenolic hydrogen as well as the stabilization of the resulting antioxidant radical through electron delocalization and/or intramolecular hydrogen bonding or further oxidation (Frankel and Meyer 2000). In this regard, total phenolic content data of A. ensiformis and A bullosus has been recorded in the literature (Zubia et al. 2009; Poza et al. 2022). However, no profile of the main phenolic compounds has yet been published for the genus.
Regarding antitumoral activity, there is only one paper evaluating the cytotoxic activity of A. bullosus from the Britany coasts against different tumoral cells lines, Daudi (human Burkitt’s lymphoma), Jurkat (human leukaemic T cell lymphoblast) and K562 (human chronic myelogenous leukaemia); however, this study did not detect any antitumoral activity (Zubia et al. 2009).
Conclusions
Asperococcus is a genus of Chordariaceae little studied in terms of its biology, ecological dynamics, and phenology, despite its wide distribution in most oceans around the world. Species of this genus have simple thalli and can be easily distinguished in the field from other brown intertidal seaweeds by the notorious sori. Although a plethora of species names and synonyms for Asperococcus can be found in the literature, only a few species have been thoroughly described and the phylogenetic relationships between them are still uncertain. Given the simple morphology of the different species, the taxonomy of this group needs a critical revision on the basis of molecular data. The life cycle of Asperococcus has only been studied in A. ensiformis, A. bullosus and A. fistulosus, however there are some aspects of the cycle that need further study, for example the coexistence of unilocular and plurilocular sporangia in the macroscopic sporophyte.
Although there is no tradition of cultivating species of this genus, the results of cultivation trials carried out with A. ensiformis under laboratory conditions have shown that it is feasible to cultivate this seaweed by the rope seeding method, which is traditionally used for large brown algae with heteromorphic life cycles. To determine the actual potential of the species for mariculture, the performance of the sporophytes transplanted in the sea should be evaluated.
A few studies have evaluated the chemical compositions and bioactive properties of Asperococcus species, which have shown similar performance and characteristics to other commercially important brown algae; however, the scarce literature on this topic shows that further studies are needed on the uses and implementation of the species. Given that the species of Asperococcus are widely distributed and present promising features for the successful development of nursery stages, these seaweeds should be regarded as a promising alternative source of seaweed bioproducts, such as alginates and fucoidans.
Data Availability
All data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, for any investigator who requires it.
References
Adams J (1907) The seaweeds of the Antrim coast. Scient Pap Ulster Fish Biol Ass 1:29–37
Adams J (1908) Algae. In: Cole GAJ, Praeger RL (eds) Handbook to the city of Dublin and the surrounding district. Ponsonbody and Gibbs, Dublin, pp 102–108
Adams NM (1994) Seaweeds of New Zealand. An Illustrated Guide. Canterbury University Press, Christchurch
Afonso-Carrillo J (2014) Lista actualizada de las algas marinas de las islas Canarias. Soc Esp Ficol, Las Palmas
Agardh CA (1820) Species algarum rite cognitae, cum synonymis, differentiis specificis et descriptionibus succinctis. Volumen primum. Pars prima. ex officina Berlingiana, Lundae [Lund], pp 1–168
Ale MT, Maruyama H, Tamauchi H, Mikkelsen JD, Meyer AS (2011) Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int J Biol Macromol 49:331–336
Ale MT, Meyer AS (2013) Fucoidans from brown seaweeds: an update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv 3:8131–8141
Amsler CD, Neushul M (1990) Chemotactic effects of nutrients on spores of the kelps Macrocystis pyrifera and Pterygophora californica. Mar Biol 102:557–564
Anon (2017) Inventaire national du Patrimoine naturel. Paris Muséum National d’Histoire Naturelle. http://wwwinpn.mnhn.fr/espece/cd_nom/72505; accessed August 2022
Antolic B, Špan A, Žuljevic A, Nikolic V, Grubelic I, Despalatovic M, Cvitkovica I (2010) Checklist of the benthic marine macroalgae from the eastern Adriatic coast: II. Heterokontophyta: Phaeophyceae. Acta Adriat 51:9–33
Arasaki S, Arasaki T (1983) Low calorie, high nutrition vegetables from the sea to help you look and feel better. Japan Publications, Tokyo
Araújo R, Bárbara I, Tibaldo M, Berecibar E, Díaz-Tapia P, Pereira R, Santos R, Sousa-Pinto I (2009) Checklist of benthic marine algae and cyanobacteria of northern Portugal. Bot Mar 52:24–46
Ardré F (1970) Contribution à l’étude des algues marines du Portugal. I flore. Port Acta Biol Sér B Sist 10:137–555
Aryee AN, Agyei D, Akanbi TO (2018) Recovery and utilization of seaweed pigments in food processing. Curr Opin Food Sci 19:113–119
Asensi AO (1972) Dos géneros de algas pardas nuevos para Patagonia y el resultado de su cultivo “in vitro”: (Feldmannia y Haloglossum. Phaeophyta). Darwiniana 17:358–377
Asensi AO, Küpper FC (2012) Seasonal periodicity and reproduction of brown algae (Phaeophyceae) at Puerto Deseado (Patagonia). Bot Mar 55:217–228
Athanasiadis A (1987) A survey of the seaweeds of the Aegean Sea with taxonomic studies on species of the tribe Antithamnieae (Rhodophyta). Ph.D. Thesis, University of Gothenburg, 174 p
Athanasiadis A (1996) Taxonomisk litteratur och biogeografi av Skandinaviska rödalger och brunalger. Algologia, Göteborg
Austic RE, Mustafa A, Jung B, Gatrell S, Lei XG (2013) Potential and limitation of a new defatted diatom microalgal biomass in replacing soybean meal and corn in diets for broiler chickens. J Agric Food Chem 61:7341–7348
Aysel V, Erduğan H, Dural-Tarakçı B, Okudan EŞ (2005) Marine algae and seagrasses of Giresun Shores (Black Sea, Turkey). J Black Sea/Medit Environ 11:271–285, 2:183–192
Báez JC, Conde F, Flores-Moya A (2001) Notas corológicas del macrofitobentos de Andalucía (España). V. Acta Bot Malacit 26:193–196
Ballesteros E (1989) Contribució al coneixement algològic de la Mediterrània Espanyola; VIII. Addicions a la flora balear. Fol Bot Misc 6:65–70
Bárbara I, Cremades J, Calvo S, López-Rodríguez MC, Dosil J (2005) Checklist of the benthic marine and brackish Galician algae (NW Spain). Anal Jardin Bot Mad 62:69–100
Bárbara I, De Clerck O, García-Redondo V, Peña V, García-Fernández A, Peteiro C, Sánchez N (2015) Fragmentos taxonómicos, corológicos, nomenclaturales y fitocenológicos (253–263). Acta Bot Malacit 39:207–237
Bartolo AG, Zammit G, Russell H, Peters AF, Küpper FC (2021) DNA barcoding of marine algae from Malta: new records from the Central Mediterranean. Acta Bot Croat 80:176–183
Bartsch I, Kuhlenkamp R (2000) The marine macroalgae of Helgoland (North Sea): an annotated list of records between 1845 and 1999. Helgol Mar Res 54:160–189
Berges JA, Fraklin MJ, Harrson PJ (2001) Evolution of an artificial seawater medium: improvements in enriched seawater, artificial water over the last two decades. J Phycol 37:1138–1145
Bermejo R, Pérez-Lloréns JJ, Vergara JJ, Hernández I (2012) Notas corológicas del macrofitobentos marino de Andalucía (España). X. Acta Bot Malacit 37:163–165
Bikker P, Stokvis L, van Krimpen MM, van Wikselaar PG, Cone JW (2020) Evaluation of seaweeds from marine waters in Northwestern Europe for application in animal nutrition. Anim Feed Sci Tech 263:114460
Blackler H (1951) An algal survey of Lough Foyle, north Ireland. Proc R Ir Acad 54B:97–139
Boderskov T, Rasmussen MB, Cassard CH, Sevnsgaard J, Enevoldsen LN, Bruhn A (2022) Comparing effects of nutrient sources approved for organic seaweed production on hatchery stage development of sugar kelp, Saccharina latissima. Algal Res 61:102602
Bogaert K, Beeckman T, De Clerck O (2016) Abiotic regulation of growth and fertility in the sporophyte of Dictyota dichotoma (Hudson) J.V. Lamouroux (Dictyotales, Phaeophyceae). J Appl Phycol 28:2915–2924
Boraso de Zaixso AL (2013) Elementos para el estudio de las macroalgas de Argentina. Con colaboración de J.M. Zaixso. Editorial Universitaria de la Patagonia (EDUPA), Chubut, Argentina 204 p
Borg JA, Howege HM, Lanfranco E, Micallef S, Mifsud C, Schembri PJ (1998) The macrobenthic species of the infralittoral to circalittoral transition zone off the northeastern coast of Malta (Central Mediterranean). Xjenza 3:16–24
Boudouresque CF, Perret M (1977) Inventaire de la flore marine de Corse (Méditerranée): Rhodophyceae, Phaeophyceae, Chlorophyceae et Bryopsidophyceae. Bibl Phycol 25:1–171
Boudouresque CF, Perret-Boudouresque M, Blanfuné A (2022) Diversity of marine and brackish macrophytes in the Port-Cros National Park (Provence, France, Mediterranean Sea): Taxa and research effort over space and time. Diversity 14:329
Brattegard T, Holthe T (1997) Distribution of marine, benthic macroorganisms in Norway: A tabulated catalogue. Director Nat Manag, Trondheim, Norway, p 409
Breeman AM (1990) Expected effects of changing seawater temperatures on the geographic distribution of seaweed species. In: Beukema JJ, Wolff WJ, Brouns JJWM (eds) Expected effects of climatic change on marine coastal ecosystems. Kluwer, Dordrecht, pp 69–76
Bringloe TT, Sjøtun K, Saunders GW (2019) A DNA barcode survey of marine macroalgae from Bergen (Norway). Mar Biol Res 15:580–589
Brodie J, Wilbraham J, Pottas J, Guiry MD (2015) A revised check-list of the seaweeds of Britain. J Mar Biol Assoc UK 96:1005–1029
Brown ES, Allsopp PJ, Magee PJ, Gill CI, Nitecki S, Strain CR, McSorley EM (2014) Seaweed and human health. Nutr Rev 72:205–216
Brownlee IA, Allen A, Pearson JP, Dettmar PW, Havler ME, Atherton MR, Onsøyen E (2005) Alginate as a source of dietary fiber. Crit Rev Food Sci Nutr 45:497–510
Bunker FSD, Brodie JA, Maggs CA, Bunker AR (2010) Seasearch guide to seaweeds of Britain and Ireland. Marine Conservation Society, UK
Bunker FSD, Brodie JA, Maggs CA, Bunker AR (2017) Seaweeds of Britain and Ireland. Wild Nature Press, Plymouth
Burel T, Le Duff M, Gall EA (2019) Updated check-list of the seaweeds of the French coasts, Channel and Atlantic Ocean. An aod-les cahiers naturalistes de l’Observatoire marin 7:1–38
Cabioc'h J, Floc'h JY, Le Toquin A, Boudouresque CF, Meinesz A, Verlaque M (2006) Guide des Algues des Mers D'Europe. Delachaux et Niestlé, Paris
Cai J, Lovatelli A, Aguilar-Manjarrez J, Cornish L, Dabbadie L, Desrochers A, Diffey S, Garrido GE, Geehan J, Hurtado A, Lucente D, Mair G, Miao W, Potin P, Przybyla C. Reantaso Melba, Roubach R, Tauati M, Yuan X (2021) Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. In: FAO Fisheries and Aquaculture Circular, no 1229. FAO, Rome
Camus C, Buschmann AH (2017) Macrocystis pyrifera aquafarming: Production optimization of rope-seeded juvenile sporophytes. Aquaculture 468:107–114
Caram B, Jónsson S (1972) Nouvelle inventaire des algues marines de l’Islande. Acta Bot Isl 1:5–31
Carlberg GE, Percival E, Rahman MA (1978) Carbohydrates of the seaweeds, Desmarestia ligulata and D. firma. Phytochemistry 17:1289–1292
Chapman VJ (1962) The Algae. Palgrave Macmillan, London
Charrier B, Abreu MH, Araújo R, Coates JC, De Clerck O, Katsaros C, Robaina RR, Wichard T (2017) Furthering knowledge of seaweed growth and development to facilitate sustainable aquaculture. New Phytol 216:967–975
Cho TO, Cho GY, Yoon HS, Boo SM (2003) New records of Myelophycus cavus (Scytosiphonaceae, Phaeophyceae) in Korea and the taxonomic position of the genus on the basis of a plastid DNA phylogeny. Nova Hedwigia 76:3–4
Chopin T, Tacon AGJ (2021) Importance of seaweeds and extractive species in global aquaculture production. Rev Fish Sci Aquac 29:139–148
Cinelli F (1971) Alghe bentoniche di profundità raccolte alla punta S. Pancrazio nell’Isola di Ischia (Golfo di Napoli). Gior Bot Ital 105:207–236
Cinelli F, Drago D, Furnari G, Giaccone G, Scammacca B, Solazzi A, Sortino M, Tolomio C (1976) Flora marina dell’Isola di Linosa (Arcipelago delle Pelagie). Mem Biol Mar Oceanogr 6:141–172
Cires Rodriguez E, Cuesta Moliner C (2010) Checklist of benthic algae from the Asturias coast (North of Spain). Bol Cien Nat RIDEA 51:135–212
Clayton MN (1982) Life history studies in the Ectocarpales (Phaeophyta): contributions toward the understanding of evolutionary processes. Bot Mar 25:111–116
Coelho SM, Peters AF, Müller D, Cock JM (2020) Ectocarpus: an evo-devo model for the brown algae. EvoDevo 11:19
Connan S, Delisle F, Deslandes E, Ar Gall E (2006) Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot Mar 49:34–46
Coppejans E (1972) Resultats d’une étude systématique et écologique de la population algale des côtes rocheuses du Dramont, Saint Raphaël (Var, France). Biol Jaarb Dodonaea 40:153–180
Coppejans E, Kling R (1995) Flora algologique des côtes du Nord de la France et de la Belgique. Jardin Botanique National de la Belgique, Meise
Cormaci M, Furnari G, Catra M, Alongi G, ad Giaccone G (2012) Flora marina bentónica del Mediterraneo: Phaeophyceae. Boll Accad Gioenia Sci Nat 45:1-508
Cotton AD (1912) Clare island Survey: Marine algae. Proc R Ir Acad 31B:1–178
Cotton AD (1913) Marine algae. Ir Nat 22:195–198
Critchley AT, Ohno M (1998) Seaweed resources of the world. Japan International Cooperation Agency, Yokosuka
Cullinane JP (1971) Frequency and distribution of seaweeds in Cork harbour, 1966–7. Ir Nat’ J 17:6–8
Cumashi A, Ushakova NA, Preobrazhenskaya ME, D'Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI, Usov AI, Ustyuzhanina NE, Grachev AA, Sanderson CJ, Kelly M, Rabinovich GA, Iacobelli S, Nifantiev NE; Consorzio Interuniversitario Nazionale per la Bio-Oncologia, Italy (2007) A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 17:541-552
D’Archino R, Nelson WA (2006) Marine brown algae introduced to New Zealand waters: First record of Asperococcus ensiformis (Phaeophyta, Ectocarpales, Chordariaceae). N Z J Mar Freshw Res 40:599–604
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
de la Coba F, Aguilera J, Figueroa FL, de Galvez MV, Herrera E (2009) Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J Appl Phycol 21:161–169
Déléris P, Nazih H, Bard JM (2016) Seaweeds in human health. In: Fleurence J, Levine I (eds) Seaweed in health and disease prevention. Academic Press, London, pp 319–367
De Mesquita Rodrigues JE (1963) Contribuição para o conhecimento das Phaeophyceae da Costa portuguesa. Mem Soc Brot 16:5–124
De Valéra M, Pybus C, Casley B, Webster A (1979) Littoral and benthic investigations on the west coast of Ireland X. Marine algae of the northern shores of the Burren, Co. Clare. Proc R Ir Acad 79B:259–269
Diannelidis T (1953) Contribution à la connaissance des algues marines des Sporades du Nord (Cyanophyceae, Chlorophyceae, Phaeophyceae, Rhodophyceae). Prak Hellenic Hydrobiol Inst 6:41–84
Donati I, Vetere A, Gamini A, Skajak-Bræk G, Coslovi A, Campa C, Paoletti S (2003) Galactose-substituted alginate: preliminary characterization and study of gelling properties. Biomacromol 4:624–631
Drennan F (2016) Seaweed bushcraft guide: seaweed in season. Bushcraft J 9:60–82
Dukes JS (2007) Tomorrow's plant communities: different but how? New Phytol 176:235–237
Edwards PE, Bird E, Cotgreave G, Cossind A, Crompton K, Fowler K, Herdson D, Hudson J (1975) Marine phytobenthos of the Castellabate (Cilento) Natural Park, Salerno, Italy. Phytocoenologia 1:403–426
Eggert A (2012) Seaweed responses to temperature. In: Wiencke C, Bischof K (eds) Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization. Springer, Heidelberg, pp 135–156
Ercegovic A (1980) Étude comparative de la végétation des basses eaux et de celle des eaux profondes de l’Adriatique centrale. Acta Adriat 21:11–40
FAO (2020) El estado mundial de la pesca y la acuicultura (SOFIA). In: La sostenibilidad en acción. FAO, Rome, p 223
FAO (2018) The global status of seaweed production, trade and utilization, vol 124. Globefish Research Programme, Rome, p 120
Feldmann J (1937) Les algues marines de la côte des Albères I-III Cyanophycées, Chlorophycées, Phéophycées. Rev Algol 9:141–335
Feldmann J (1954) Inventaire de la flore marine de Roscoff. Algues, champignons, lichens et spermatophytes. Trav Stat Biol Roscoff, Suppl 6:1–152
Feldman SC, Reynaldi S, Stortz CA, Cerezo AS, Damonte EB (1999) Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine 6:335–340
Feoli E, Bressan G (1972) Affinità floristica dei tipi di vegetazione bentonica della Cala di Mitigliano (Massa Lubrense, Napoli). Gior Bot Ital 106:245–256
Ferreira SJ, Gonçalves Silva JJ, Araújo R (2018) Marine algae collection in the Herbarium of the Funchal Natural History Museum (MADM) with new records from the archipelago of Madeira. Bol Mus Munic Funchal 68:31–52
Fletcher RL (1987) Seaweeds of the British Isles. 3. Fucophyceae (Phaeophyceae) 1. British Museum (Natural History), London
Fleurence J (1999) Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol 10:25–28
Flores-Moya A, Soto J, Sánchez A, Altamirano M, Reyes G, Conde F (1995) Check-list of Andalusia (S. Spain) seaweeds. I. Phaeophyceae. Acta Bot Malac 20:5–18
Forbord S, Steinhovden KB, Rød KK, Handå A, Skjermo J (2018) Cultivation protocol for Saccharina latissima. In: Charrier B, Wichard T, Reddy CRK (eds) Protocols for macroalgae research. CRC Press, Boca Raton, pp 38–50
Frank H, Chynwat V, Desamero R, Farhoosh R, Erickson J, Bautista J (1997) On the photophysics and photochemical properties of carotenoids and their role as light–harvesting pigments in photosynthesis. Pure Appl Chem 69:2117–2124
Frankel EN, Meyer AS (2000) The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J Sci Food Agric 80:1925–1941
Fredriksen S, Karsten U, Bartsch I, Woelfel J, Koblowsky M, Schumann R, Moy SR, Steneck RS, Wiktor JM, Hop H, Wiencke C (2019) Biodiversity of benthic macro- and microalgae from Svalbard with special focus on Kongsfjorden. In: Hop H, Wiencke C (eds) The Ecosystem of Kongsfjorden, Svalbard. Springer, Cham pp 331–371
Fritsch FE (1945) The Structure and Reproduction of the Algae. Volume 2. Foreword, Phaeophyceae, Rhodophyceae, Myxophyceae. Cambridge University Press, Cambridge
Furnari G, Cormaci M, Serio D (1999) Catalogue of the benthic marine macroalgae of the Italian coast of the Adriatic Sea. Bocconea 12:1–214
Gallardo T, Bárbara I, Afonso-Carrillo J, Bermejo R, Altamirano M, Gómez A, Barceló MC, Rull J, Ballesteros E, De la Rosa J (2016) Nueva lista crítica de las algas bentónicas marinas de España. Algas 51:7–52
Garcia-Perez P, Lourenço-Lopes C, Silva A, Pereira AG, Fraga-Corral M, Zhao C, Xiao J, Simal-Gandara J, Prieto MA (2022) Pigment composition of nine brown algae from the Iberian Northwestern coastline: influence of the extraction solvent. Mar Drugs 20:113
Gerloff J, Geissler U (1974) Eine revidierte Liste der Meeresalgen Griechenlands. Nova Hedwigia 22:721–793
Giaccone G (1969) Raccolte di fitobenthos sulla banchina continentale Italiana. Gior Bot Ital 103:485–514
Giaccone G (1978) Revisione della flora marina del Mare Adriatico. World Wildlife Fund Italia, Trieste, p 118
Gil-Rodríguez MC, Haroun R, Ojeda Rodríguez A, Berecibar Zugasti E, Domínguez Santana P, Herrera Morán B (2003) Proctoctista. In: Moro L, Martín JL, Garrido MJ, Izquierdo I (eds) Lista de especies marinas de Canarias (algas, hongos, plantas y animales). Consejería de Política Territorial y Medio Ambiente del Gobierno de Canarias, Las Palmas, pp 5–30
Grand View Research (2021) Market Research Report. Commercial Seaweed Market Size, Share & Trends Analysis Report By Product (Brown, Red, Green), By Application (Human Consumption, Animal Feed, Agriculture), By Form (Leaf, Powdered, Flakes), By Region, And Segment Forecasts, 2022 - 2030. https://www.grandviewresearch.com/industry-analysis/commercial-seaweed-market; accessed on September 2022
Greville RK (1830) Algae britannicae, or, descriptions of the marine and other inarticulated plants of the British Islands, belonging to the order Algae. Maclachlan & Stewart, Edinburgh
Griffiths M, Harrison S, Smit M, Maharajh D (2016) Major commercial products from micro and macroalgae. In: Bux F, Chisti Y (eds) Algae biotechnology: Products and processes. Springer, New York, pp 269–300
Grimm M (1952) Iodine content of some marine algae. Pac Sci 6:318–323
Guiry MD (1978) A consensus and bibliography of Irish seaweeds. J. Cramer, Vaduz
Guiry MD (2012) A catalogue of Irish seaweeds. Gantner Verlag, Liechtenstein
Guiry MD, Guiry GM (2023) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. https://www.algaebase.org; accessed 10 May 2023
Güner H, Aysel V, Sukatar A, Öztürk M (1985) Turkyie Ege denizi florasi. I. Mavi-yesil, yesil, esmer alger kapali tohumlular. Doga Bilim Dergisi, Ser A 9:272–282
Gupta V, Trivedi N, Simoni S, Reddy CRK (2018) Marine macroalgal nursery: A model for sustainable production of seedlings for large scale farming. Algal Res 31:463–468
Güven KC, Öztig F (1971) Über die marinen Algen an den Küsten der Türkei. Bot Mar 14:121–128
Hales JM, Fletcher RL (1990) Studies on the recently introduced brown alga Sargassum muticum (Yendo) Fensholt. V. Receptacle initiation and growth, and gamete release in laboratory culture. Bot Mar 33:241–249
Hardy FG, Guiry MD (2003) A check-list of the seaweeds of Britain and Ireland. British Phycological Society, London
Haroun RJ, Gil-Rodríguez MC, Díaz de Castro J, Prud'homme van Reine WF (2002) A checklist of the marine plants from the Canary Islands (Central Eastern Atlantic Ocean). Bot Mar 45:139–169
Harper MA, Cassie Cooper V, Chang FH, Nelson WA, Broady PA (2012) Phylum Ochrophyta: brown and golden-brown algae, diatoms, silicioflagellates, and kin. In: Gordon DP (ed) New Zealand inventory of biodiversity, Volume Three. Kingdoms Bacteria, Protozoa, Chromista, Plantae, Fungi. Canterbury University Press, Christchurch, pp 114-163
Harries R (1932) An investigation by cultural methods of some of the factors influencing the development of the gametophytes and the early stages of the sporophytes of Laminaria digitata, L. saccharina, and L. cloustoni. Ann Bot 46:893–928
Harvey, (1857) Synopsis of the British Seaweeds, compiled from Professor Harvey’s Phycologia Britannica. Lovell Reeve, London
Hauck F (1889) Algas do norte de Portugal. Algues marines. Bol Soc Brot 7:136–158
Haug A (1964) Composition and properties of alginates. Rep Norw Inst Seaweed Res 30:10–25
Heape W (1888) Preliminary report upon the fauna and flora of Plymouth Sound. J Mar Biol Assoc UK 1:153–193
Holmes EM (1896) New marine algae from Japan. J Linn Soc Bot 31:248–260
Hooker WJ (1833) Div. I. Inarticulatae. In: Hooker WJ (ed) The English Flora of Sir James Edward Smith. Class XXIV. Cryptogamia. Vol. V. (or Vol. II of Dr. Hooker's British flora). Part I. Comprising the Mosses, Hepaticae, Lichens, Characeae and Algae. Longman, Brown, Green & Longmans Paternoster-Row, London
Hu ZM, Shan TF, Zhang J, Critchley AT, Choi HG, Yotsukura N, Liu FL, Duan DL (2021) Kelp aquaculture in China: a retrospective and future prospects. Rev Aquac 13:1324–1351
Huisman JM (1997) Marine benthic algae of the Houtman Abrolhos Islands, Western Australia. In: Wells FE (ed) The Marine Flora and Fauna of the Houtman Abrolhos Islands, Western Australia. Western Australian Museum, Perth, pp 177–237
Huisman JM (2019) Marine plants of Australia, Revised. UWA Publishing, Crawley WA
Huisman JM (2000) Marine plants of Australia. University of Western Australia Press, Nedlands
Huisman JM, Walker DI (1990) A catalogue of the marine plants of Rottnest Island, Western Australia, with notes on their distribution and biogeography. Kingia 1:349–459
Humm HJ (1979) The marine algae of Virginia. University Press of Virginia, Charlottesville, Virginia, p 267
Hurtado AQ, Critchley AT (2020) Time for applications of biostimulants in phyconomy. In: Torres DM, Kraan S, Dominguez H (eds) Seaweed extracts for enhanced cultivation of seaweeds (SEECS). Sustainable Seaweed Technologies. Elsevier, London pp 103–127
Hurtado AQ, Joe M, Sanares RC, Fan D, Prithiviraj B, Critchley AT (2012) Investigation of the application of Acadian Marine Plant Extract Powder (AMPEP) to enhance the growth, phenolic content, free radical scavenging, and iron chelating activities of Kappaphycus Doty (Solieriaceae, Gigartinales, Rhodophyta). J Appl Phycol 24:601–611
Irvine DEG (1982) Seaweeds of the Faroes 1: The flora. Bull Br Mus Bot 10:109–131
Joher S, Ballesteros E, Cebrian E, Sánchez N, Rodriguez-Prieto C (2012) Deep-water macroalgal-dominated coastal detritic assemblages on the continental shelf off Mallorca and Menorca (Balearic Islands, Western Mediterranean). Bot Mar 55:485–497
John DM, Prud’homme van Reine WF, Lawson GW, Kostermans TB, Price JH (2004) A taxonomic and geographical catalogue of the seaweeds of the western coast of Africa and adjacent islands. Beih Nova Hedwigia 127:1–339
Kawai H, Hanyuda T (2021) Discovery of a novel brown algal genus and species Setoutiphycus delamareoides (Phaeophyceae, Ectocarpales) from the Seto Inland Sea, Japan. Sci Rep 11:13901
Kawai H, Hanyuda T, Kim SH, Ichikawa Y, Uwai S, Peters AF (2016) Cladosiphon takenoensis sp. nov. (Ectocarpales s.l. Phaeophyceae) from Japan. Phycol Res 64:212–218
Kawai H, Hanyuda T, Ahibata K, Kamiya M, Peters AF (2019) Proposal of a new brown algal species, Mesogloia japonica sp. nov. (Chordariaceae, Phaeophyceae), and transfer of Sauvageaugloia ikomae to Mesogloia. Phycologia 58:63–69
Kim S, Baek SK, Song KB (2018) Physical and antioxidant properties of alginate films prepared from Sargassum fulvellum with black chokeberry extract. Food Packag Shelf Life 18:157–163
Kim SM, Shang YF, Um BH (2011) A preparative method for isolation of fucoxanthin from Eisenia bicyclis by centrifugal partition chromatography. Phytochem Anal 22:322–329
Kim SJ, Woo S, Yun H, Yum S, Choi E, Do JR, Jo JH, Kim DG, Lee SC, Lee TK (2005) Total phenolic contents and biological activities of Korean seaweed extracts. Food Sci Biotechnol 14:798–802
Kloareg B, Demarty M, Mabeau S (1986) Polyanionic characteristics of purified sulphated homofucans from brown algae. Int J Biol Macromol 8:380–386
Kloareg B, Mabeau S (1987) Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcata and Laminaria digitata. J Exp Bot 38:1573–1580
Knight M, Blackler MCH, Parke MW (1935) Notes on the life-cycle of species of Asperococcus. Proc Trans Liverpool Biol Soc 48:79–97
Kontula T, Fürhapter K (2012) Checklist of Baltic Sea macrophyte species. In: Kontula T, Haldin J (eds) Checklist of Baltic Sea macro-species. Baltic Sea Environment Proceedings No. 130. Helsinki Commission, Helsinki, pp 9–12
Kraemer GP, Chapman DJ (1991) Biomechanics and alginic acid composition during hydrodynamic adaptation by Egregia menziesii (Phaeophyta) juveniles. J Phycol 27:47–53
Kuckuck P (1912) Beiträge zur Kenntnis der Meeresalgen. Helgol Wiss Meeresunters 5:116–227
Kuckuck P (1899) Beiträge zur Kenntnis der Meeresalgen. 5–9. Helgol Wiss Meeresunters 3:11–81
Kumar S, Sahoo D, Levine I (2015) Assessment of nutritional value in a brown seaweed Sargassum wightii and their seasonal variations. Algal Res 9:117–125
Kumar CS, Ganesan P, Suresh PV, Bhaskar N (2008) Seaweeds as a source of nutritionally beneficial compounds - A review. J Food Sci Technol 45:1–13
Kumar SR, Hosokawa M, Miyashita K (2013) Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar Drugs 11:5130
Kylin H (1907) Studien über die Algenflora der schwedischen Westküste. Akademische Abhandlung. K.W. Appelbergs Buchdruckeri, Upsala
Kylin (1910) Zur Kenntnis der Algenflora der norwegischen Westküste. Arkiv för Botanik 10:1–37
Kylin H (1947) Die Phaeophyceen der schwedischen Westküste. Acta Lund 43:1–99
Lamouroux JVF (1813) Essai sur les genres de la famille des Thalassiophytes non articulées. Annales du Muséum d’Histoire Naturelle, Paris
Le Lann K, Jegou C, Stiger-Pouvreau V (2008) Impact of different conditioning treatments on total phenolic content and antioxidant activities in two Sargassacean species: comparison of the frondose Sargassum muticum (Yendo) Fensholt and the cylindrical Bifurcaria bifurcata R. Ross. Phycol Res 56:238–245
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37:106–126
Li Y, Zheng Y, Zhang Y, Yang Y, Wang P, Imre B, Wong ACY, Hsieh YSY, Wang D (2021) Brown algae carbohydrates: structures, pharmaceutical properties, and research challenges. Mar Drugs 19:620
Li B, Lu F, Wei X, Zhao R (2008) Fucoidan: structure and bioactivity. Molecules 13:1671–1695
Lind AC, Konar B (2017) Effects of abiotic stressors on kelp early life-history stages. Algae 32:223–233
Lindauer VW (1957) A descriptive review of the Phaeophyceae of New Zealand. Trans Roy Soc N Z 85:61–74
Liu K (2019) Effects of sample size, dry ashing temperature and duration on determination of ash content in algae and other biomass. Algal Res 40:101486
Liu F, Pang SJ, Gao SQ (2016) Growth performance of unialgal gametophytes of the brown alga Saccharina japonica in mass culture conditions. J Appl Phycol 28:1145–1152
Lötze E, Hoffman EW (2016) Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J Appl Phycol 28:1379–1386
Loureiro RR, Reis RP, Berrogain FD, Critchley AT (2014a) Effects of a commercial extract of the brown alga Ascophyllum nodosum on the biomass production of Kappaphycus alvarezii (Doty) Doty ex P. C Silva and its carrageenan yield and gel quality cultivated in Brazil. J Appl Phycol 26:763–768
Loureiro RR, Reis RP, Marroig RG (2014b) Effect of the commercial extract of the brown alga Ascophyllum nodosum Mont. on Kappaphycus alvarezii (Doty) Doty ex P.C. Silva in situ submitted to lethal temperatures. J Appl Phycol 26:629–634
Lyle L (1920) The marine algae of Guernsey. J Bot 58:1–53
MacCaughey (1918) Algae of the Hawaiian Archipelago. II. Bot Gaz 65:121–149
Macchiavello J, Araya E, Bulboa C (2010) Production of Macrocystis pyrifera (Laminariales: Phaeophyceae) in northern Chile on spore-based culture. J Appl Phycol 22:691–697
Maggs CA (1983) A seasonal study of seaweed communities on subtidal maërl (unattached coralline algae). Progr Underwater Sci 9:27–40
Marambio J, Rodriguez JP, Mendez F, Ocaranza P, Rosenfeld S, Ojeda J, Rautenberger R, Bischof K, Terrados J, Mansilla A (2017) Photosynthetic performance and pigment composition of Macrocystis pyrifera (Laminariales, Phaeophyceae) along a gradient of depth and seasonality in the ecoregion of Magellan, Chile. J Appl Phycol 29:2575–2585
Mendes MC, Navalho S, Ferreira A, Paulino C, Figueiredo D, l Silva D, Gao F, Gama F , Bombo G, Jacinto R , Aveiro SS , Schulze PSC, Gonçalves AT, Pereira H, Gouveia L, Patarra RF , Abreu MH, Silva JL, Navalho J, Varela JCS, Speranza GL (2022) Algae as food in Europe: An overview of species diversity and their application. Foods 11:1-36
MHLW (2014) The National Health and Nutrition Survey in Japan, 2004–2014. The Ministry of Health, Labour and Welfare. http://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html; accessed September 2022
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE). GCE, New Orleans, LA, pp 1–8
Milton RF (1964) Liquid seaweed as a fertilizer. Proc Int Seaweed Symp 4:428–431
Mišurcová L, Machů L, Orsavová J (2011) Seaweed minerals as nutraceuticals. Adv Food Nutr Res 64:371–390
Miranda F (1934) Materiales para una flora marina de las Rías Bajas Gallegas. Bol Soc Esp Hist Nat 34:165–180
Moro L, Martín JL, Garrido MJ, Izquierdo I (2011) Listado preliminar de especies marinas de Canarias. Observatorio Ambiental Granadilla, Tenerife
Morton O (1994) Marine Algae of Northern Ireland. Ulster Museum, Belfast
Munda IM (1979) Some Fucacean associations from the vicinity of Rovinj, Istrian Coast, Northern Adriatic. Nova Hedwigia 31:607–666
Mussig K (2009) Iodine-induced toxic effects due to seaweed consumption. In: Preedy V, Burrow G, Watson R (eds) Comprehensive handbook of iodine. Academic Press, London, pp 897–908
Namvar F, Baharara J, Mahdi AA (2014) Antioxidant and anticancer activities of selected Persian Gulf algae. Ind J Clin Biochem 29:13–20
Nelson WA (1999) A revised checklist of marine algae naturalised in New Zealand. N Z J Bot 37:355–359
Nelson WA, D'Archino R, Neill KF, Robinson NM (2021) Introduced marine macroalgae: new perspectives on species recognition and distribution in New Zealand. Bot Mar 64:379–393
Nelson WA, Knight GA (1995) Asperococcus bullosus - A new record for Northern New Zealand of an adventive marine brown alga. Tane 35:121–125
Neto AI, Cravo DC, Haroun RT (2001) Checklist of the benthic marine plants of the Madeira Archipelago. Bot Mar 44:391–414
Newton L (1931) A Handbook of the British Seaweeds. The Trustees of the British Museum of, Natural History, London
Nielsen R, Gunnarsson K (2001) Seaweeds of the Faroe islands. An annotated checklist. Fródskarrit 49:45–108
Nielsen R, Kristiansen A, Mathiesen L, Mathiesen H (1995) Distributional index of the benthic marine macroalgae of the Baltic Sea area. Acta Bot Fenn 155:1–70
Nielsen R, Lundsteen S (2019) Danmarks havalger Bind 2 Brunalger (Phaeophyceae) og Grønalger (Chlorophyta). Sci Danica B 8:1–476
Okamura K (1936) Nippon kaisô shi. Uchida Rokakuho, Tokyo
Oliveira EC, Paula EJ, Plastino EM, Petti R (1995) Metodologías para cultivo no axénico de marcroalgas marinas in vitro. In: Alveal K, Ferrario ME, Oliveira EC, Sar E (eds) Manual de Métodos Ficológicos. Universidad de Concepción, Concepción, pp 430–447
Ould-Ahmed N, Gómez Garreta A, Ribera Siguan MA, Bouguedoura N (2013) Checklist of the benthic marine macroalgae from Algeria. I. Phaeophyceae. Anal Jardin Bot Mad 70:136–143
Pangestuti R, Siahaan EA (2018) Seaweed-derived carotenoids. In: Qin Y (ed) Bioactive seaweeds for food applications. Academic Press, London, pp 95–107
Pedersen PM (1984) Studies on primitive brown algae (Fucophyceae). Opera Bot 74:1–76
Pedroche FF, Sentíes A (2020) Diversidad de macroalgas marinas en México. Una actualización florística y nomenclatural. Cymbella 6:4–55
Pedroche PF, Silva PC, Aguilar Rosas LE, Dreckmann KM, Aguilar Rosas R (2008) Catálogo de las algas bentónicas del Pacífico de México II. Phaeophycota. Universidad Autónoma Metropolitana; Universidad Autónoma de Baja California; University of California, Mexico DF
Peña V, Bárbara I (2008) Biological importance of an Atlantic European maerl bed off Benencia Island (Northwest Iberian Peninsula). Bot Mar 51:493–505
Peña V, Bárbara I (2010) Seasonal patterns in the maërl community of shallow European Atlantic beds and their use as a baseline for monitoring studies. Eur J Phycol 45:327–343
Percival E (1979) The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Brit Phycol J 14:103–117
Pereira L (2011) A review of the nutrient composition of selected edible seaweeds. In: Pomin VH (ed) Seaweed: ecology, nutrient composition and medicinal uses. Nova Science, London, pp 15–47
Peteiro C (2018) Alginate production from marine macroalgae, with emphasis on kelp farming. In: Rehm BHA, Moradali MF (eds) Alginates and their biomedical applications. Springer, Singapore pp 27–66
Peteiro C, Sánchez N, Martínez B (2016) Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlantic coast of Southern Europe: An overview. Algal Res 15:9–23
Peters AF, Ramírez ME (2001) Molecular phylogeny of small brown algae, with special reference to the systematic position of Caepidium antarcticum (Adenocystaceae, Ectocarpales). Cryptogam Algol 22:187–200
Peters AF, Couceiro L, Tsiamis K, Küpper FC, Valero M (2015) Barcoding of cryptic stages of marine brown algae isolated from incubated Ssubstratum reveals high diversity in Acinetosporaceae (Ectocarpales Phaeophyceae). Cryptogam Algol 36:3–29
Ponce NMA, Flores ML, Pujol CA, Becerra MB, Navarro DA, Córdoba O, Damonte EB, Stortz CA (2019) Fucoidans from the Phaeophyta Scytosiphon lomentaria: chemical analysis and antiviral activity of the galactofucan component. Carbohydr Res 478:18–24
Ponce NMA, Stortz CA (2020) A comprehensive and comparative analysis of the fucoidan compositional data across the Phaeophyceae. Front Plant Sci 11:556312
Poza AM, Fernández C, Gauna MC, Parodi ER (2018) Biochemical properties and culture optimization of Leathesia marina (Phaeophyceae). Algal Res 33:379–388
Poza AM, Fernández C, Latour EA, Raffo MP, Dellatorre FG, Parodi ER, Gauna MC (2022) Optimization of the rope seeding method and biochemical characterization of the brown seaweed Asperococcus ensiformis. Algal Res 64:102668
Price JH, John DM, Lawson GW (1978) Seaweeds of the western coast of tropical Africa and adjacent islands: a critical assessment. II. Phaeophyta. Bull Br Mus Nat Hist Bot 6:87–182
Racault MFL, Fletcher RL, De Reviers B, Cho GY, Boo SM, Parente MI, Rousseau F (2009) Molecular phylogeny of the brown algal genus Petrospongium Nägeli ex Kütz (Phaeophyceae) with evidence for Petrospongiaceae fam. nov. Cryptogam Algol 30:111–123
Redmond S, Green L, Yarish C, Kim J, Neefus C (2014) New England Seaweed Culture Handbook-Nursery Systems. Connect Sea Grant CTSG-14–01 http://seagrant.uconn.edu/publications/aquaculture/handbook.pdf
Rees TK (1931) Preliminary observations on the Phaeophyceae of Lough Hyne (Ine). J Ecol 14:439–448
Ribera MA, Gómez Garreta A, Gallardo T, Cormaci M, Furnari G, Giaccone G (1992) Check-list of Mediterranean seaweeds. I. Fucophyceae (Warming 1884). Bot Mar 35:109–130
Ribera Siguán MA (1983) Estudio de la flora bentónica marina de las islas Baleares. PhD Thesis, Universidad de Barcelona, Spain, p 636
Ribera Siguán MA, Gómez Garreta A (1985) Catálogo de la flora bentónica marina de las Islas Baleares: II (Phaeophyceae, Chlorophyceae). Collect Bot 16:25–41
Rindi F, Sartoni G, Cinelli F (2002) A floristic account of the benthic marine algae of Tuscany (Western Mediterranean Sea). Nova Hedwigia 74:201–250
Robertson-Andersson DV, Leitao D, Bolton JJ, Anderson RJ, Njobeni A, Ruck K (2006) Can kelp extract (KELPAK®) be useful in seaweed mariculture? J Appl Phycol 18:315–321
Rodríguez Y, Femenías JJ (1889) Algas de las Baleares. Anales Soc Esp Hist Nat 18:199–274
Rodríguez Prieto C, Boudouresque ChF, Marcot-Coqueugniot J (1993) Nouvelles observations sur les algues marines du Parc Naturel Regional de Corse. Trav Sci Parc Nat Rég Rés Corse Fr 41:53–61
Roleda MY, Skjermo J, Marfaing H, Jónsdóttir R, Rebours C, Gietl A, Stengel DB, Nitschke U (2018) Iodine content in bulk biomass of wild-harvested and cultivated edible seaweeds: inherent variations determine species-specific daily allowable consumption. Food Chem 254:333–339
Rueness J (1997) Algae. In: Brattegard T, Holthe T (eds) Distribution of marine, benthic macro-organisms in Norway: a tabulated catalogue. Preliminary edition. Directorate for Nature Management Research report, 1997(1). Direktoratet for Naturforvaltninhg, Trondheim, pp 1–321
Rupérez P (2002) Mineral content of edible marine seaweeds. Food Chem 79:23–26
Sabri H, Cherifi O, Maarouf A, Bahammou N, Boundir Y (2021) The checklist and the ecological index of the brown seaweeds from Essaouira coastline (Morocco). JASES 4:406–417
Sauvageau C (1895) Sur les sporanges pluriloculaires de l’Asperococcus compressus Griff. J Bot 9:1–3
Schneider CW, Searles RB (1991) Seaweeds of the Southeastern United States: Cape Hatteras to Cape Canaveral. Duke University Press, Durham
Schneider CW, Suyemoto MM, Yarish C (1979) An annotated checklist of Connecticut seaweeds. Bull Connecticut State Geol Nat Hist Surv 108:1–20
Schoenrock KM, McHugh TA, Krueger-Hadfield SA (2021) Revisiting the ‘bank of microscopic forms in macroalgal-dominated ecosystems. J Phycol 57:14–29
Seagrief SC (1984) A catalogue of South African green, brown and red marine algae. Mem Bot Surv S Afr 47:1–72
Segawa S (1981) Genshoku Nihon kaiso zukan. Hoikusha Publishing Co., Ltd, Osaka
Siemer BL, Stam WT, Olsen JL, Pedersen P (1998) Phylogenetic relationships of the brown algal orders Ectocarpales, Chordariales, Dictyosiphonales, and Tilopteridales (Phaeophyceae) based on large subunit and spacer sequences. J Phycol 34:1038–1048
Silberfeld T, Leigh JW, Verbruggen H, Cruaud C, de Reviers B, Rousseau F (2010) A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): Investigating the evolutionary nature of the ‘‘brown algal crown radiation”. Mol Phylogenet Evol 56:659–674
Silberfeld T, Rousseau F, de Reviers B (2014) An updated classification of brown algae (Ochrophyta, Phaeophyceae). Cryptogam Algol 35:117–156
Silva PC, Basson PW, Moe RL (1996) Catalogue of the benthic marine algae of the Indian ocean. University California Publications in Botany, Berkeley
Smyth PPA (2021) Iodine, seaweed, and the thyroid. Eur Thyroid J 10:101–108
Stegenga H, Mol I, Prud’homme van Reine WF, Lokhorst GM (1997) Checklist of the marine algae of the Netherlands. Gorteria Suppl 4:3–57
Stirk W, van Staden J (1997) Comparison of cytokinin- and auxin-like activity in some commercially used seaweed extracts. J Appl Phycol 8:503–508
Suebsanguan S, Strain EMA, Morris RL, Swearer SE (2021) Optimizing the initial cultivation stages of kelp Ecklonia radiata for restoration. Restor Ecol 29:1–9
von Suhr JN (1836) Beiträge zur Algenkunde. Nr. 2. Flora 19:337–350
Sulu R, Kumar L, Hay C, Pickering T (2004) Kappaphycus seaweed in the Pacific: review of introductions and field testing proposed quarantine protocols. Secretariat of the Pacific Community, Noumea, p 85
Tan IH, Druehl LD (1993) Phylogeny of the Northeast Pacific brown algal (Phaeophycean) orders as inferred from 18S rRNA gene sequences. Hydrobiologia 260:699–704
Taşkın E, Akbulut A, Yıldız A, Şahin B, Şen B, Uzunöz C, Solak C, Başdemir D, Sevik F, Sönmez F, Açkgöz I, Pabuccu K, Öztürk M, Alp MT, Albay M, Çakır M, Özbay Ö, Can Ö, Akçaalan R, Atıcı T, Koray T, Özer T, Karan T, Aktan Y, Zengin ZT (2019) Türkiye suyosunları listesi (Turkey algae list). Ali Nihat Gökyiğit Vakfı Yayını, İstanbul
Taşkin E, Öztürk M, Kurt O, Öztürk M (2008) The check-list of the marine algae of Turkey. Ecem Kirtasiye, Manisa
Teas J, Pino S, Critchley A, Braverman LE (2005) Variability of iodine content in common commercially available edible seaweeds. Thyroid 14:836–841
Temniskova D, Stoyneva MP, Kirjakov IK (2008) Red list of the Bulgarian algae. I. Macroalgae. Phytol Balc 14:193–206
Tsiamis K, Panayotidis P, Economou-Amilli A, Katsaros C (2013) Seaweeds of the Greek coasts. I. Phaeophyceae. Mediterr Mar Sci 14:141–157
Umanzor S, Shin S, Marty-Rivera M, Augyte S, Yarish C, Kim JK (2019) Preliminary assessment on the effects of the commercial seaweed extract, AMPEP, on growth and thermal tolerance of the kelp Saccharina spp. from the Northwest Atlantic. J Appl Phycol 31:3823–3829
Umanzor S, Jang S, Antosca R, Critchley AT, Yarish C, Kim JK (2020) Optimizing the application of selected biostimulants to enhance the growth of Eucheumatopsis isiformis, a carrageenophyte with commercial value, as grown in land-based nursery systems. J Appl Phycol 32:1917–1922
Vadas RL, Johnson S, Norton TA (1992) Recruitment and mortality of early post-settlement stages of benthic algae. Br Phycol J 27:331–351
Valenzuela Miranda S (2005) Catálogo de las algas recogidas por Faustino Miranda en las costas atlánticas de Francia. NACC 14:5–12
van den Hoek C, Mann DG, Jahns HM (1995) algae. An Intoduction to Phycology. Cambridge University Press, Cambridge
Vatsos IN, Rebours C (2015) Seaweed extracts as antimicrobial agents in aquaculture. J Appl Phycol 27:2017–2035
Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem 46:4113–4117
Verlaque M (1984) Biologie des juvéniles de l'oursin herbivore Paracentrotus lividus (Lamarck): séléctivité du broutage et impact de l'espèce sur les communautés algales de substrat rocheux en Corse (Méditerranée, France). Bot Mar 27:401–424
Verma P, Kumar M, Mishra G, Sahoo D (2017) Multivariate analysis of fatty acid and biochemical constituents of seaweeds to characterize their potential as bioresource for biofuel and fine chemicals. Bioresour Technol 226:132–144
Viera M, Donati E, Plaza-Cazón J (2013) Dynamic Cr (III) uptake by Macrocystis pyrifera and Undaria pinnatifida biomasses. Electron J Biotechnol 16:8
Wade R, Augyte S, Harden M, Nuzhdin S, Yarish C, Alberto F (2020) Macroalgal germplasm banking for conservation, food security, and industry. PLoS Biol 18:e3000641
Wang J, Zhang Q, Zhang Z, Song H, Li P (2010) Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol 46:6–12
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol 29:949–982
Westermeier R, Patiño D, Piel MI, Maier I, Mueller DG (2006) A new approach to kelp mariculture in Chile: production of free-floating sporophyte seedling from gametophyte culture of Lessonia trabeculata and Macrocystis pyrifera. Aquac Res 37:164–171
Whelan PM, Cullinane JP (1985) The algal flora of a subtidal Zostera bed in Ventry Bay, Southwest Ireland. Aquat Bot 23:41–51
Wollny R (1881) Die Meeresalgen von Helgoland. Hedwigia 20:1–8
Womersley HBS (1987) The marine benthic flora of Southern Australia Part II. South Australian Government Printing Division, Adelaide
Wynne MJ (1982) Phaeophyceae. In: Parker SP (ed) Synopsis and classification of living organisms. McGraw-Hill, New York, pp 115–125
Yoshida T, Nakajima Y, Nakata Y (1990) Check-list of marine algae of Japan (revised in 1990). Jpn J Phycol 38:269–320
Yoshida T, Suzuki M, Yoshinaga K (2015) Checklist of marine algae of Japan (Revised in 2015). Jpn J Phycol 63:129–189
Zhang WW, Duan XJ, Huang HL, Zhang Y, Wang BG (2007) Evaluation of 28 marine algae from the Qingdao coast for antioxidative capacity and determination of antioxidant efficiency and total phenolic content of fractions and subfractions derived from Symphyocladia latiuscula (Rhodomelaceae). J Appl Phycol 19:97–108
Zubia M, Fabre MS, Kerjean V, Le Lann K, Stiger-Pouvreau V, Fauchon M, Deslandes E (2009) Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem 116:693–701
Acknowledgements
The authors wish to thank Dr. Michael Guiry for his guidance on taxonomic and nomenclatural aspects of this review, to Gonzalo Bravo for providing the photographs of A. ensiformis in nature, and to Rosemary Scoffield MSc for checking the grammar of the manuscript.
Funding
This work had the support of the Secretaría General de Ciencia y Tecnología, Universidad Nacional del Sur under grant number PGI 24/ZB88.
Author information
Authors and Affiliations
Contributions
Poza, Ailen M.: Writing—Original Draft, Review and Editing; performed the literature search. Croce, M. Emilia: Writing—Original Draft, Review and Editing; performed the literature search; data analysis. Fernández, Carolina: Writing—Original Draft, Review and Editing; performed the literature search. Parodi, Elisa R: Review. Gauna, M. Cecilia: Writing—Original Draft, Review and Editing; Project administration search; performed the literature search.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poza, A.M., Croce, M.E., Fernández, C. et al. A concise review of the genus Asperococcus (Phaeophyceae: Chordariaceae). J Appl Phycol 35, 2069–2093 (2023). https://doi.org/10.1007/s10811-023-02983-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-02983-4