Abstract
An optimization study on concentration (viz. 0.01, 0.1, and 1.0 g L−1) and dipping time (i.e., 30 and 60 min) was conducted on three different color morphotypes (i.e., reddish brown, yellowish brown and purple) of the commercial carrageenophyte Kappaphycus alvarezii (Doty) Doty. The study tested the efficacy of Acadian Marine Plant Extract Powder (AMPEP) on the growth rate and occurrence of macro-epiphytes from August to November, representing the wet season of the Philippines. The optimum concentration and dipping time were obtained at 0.1 g L−1 and 30 min, respectively. These optimum parameters were then further verified in a commercial nursery using the yellowish brown morphotype. In another experiment, K. alvarezii (tambalang purple morphotype) and Kappaphycus striatum (Schmitz) Doty (sacol green morphotype) with, and without, AMPEP dippings were tested for their total phenolic content, free radical scavenging and iron chelating activities. Seaweed dipped in AMPEP demonstrated higher growth rates than the control. Lower concentrations (i.e., 0.01-0.1 g L−1) and shorter dipping time (e.g., 30 min) produced higher growth rates than the highest concentration (1.0 g L−1) and longer (60 min) dipping time. The presence of macro-epiphytes such as filamentous Ulva did not adversely affect the robust growth of the three color morphotypes of K. alvarezii. The lowest and highest growth rates obtained in a commercial seaweed nursery using the optimum concentration and dipping time of AMPEP were observed in July and January with 0.8% and 6.7% day−1, respectively. The antioxidant content of K. alvarezii (tambalang purple) and K. striatum (sacol green) responded differently to AMPEP dipping. The changes in total antioxidant activity followed almost the same trend as in phenolic content, in both K. alvarezii (tambalang purple) and K. striatum (sacol green), whereas, the iron chelating ability of both seaweeds with and without AMPEP dipping varied monthly. The results obtained for the use of AMPEP dips for commercial Kappaphycus cultivation demonstrated an effective management tool for improved farming protocols.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seaweeds in general are known to be good sources of nutrients, bioactive compounds and biostimulants. Several seaweeds have been used effectively as source of plant growth regulators in agricultural crops such as lettuce (Crouch et al. 1990), tomatoes (Crouch and Van Staden 1992), potatoes (Kowalski et al. 1999), barley (Rayorath et al. 2008a), canola (Ferreira 2002) bean, wheat, and maize (Blunden and Jenkins 1997), to name but a few. The extensive reviews of Khan et al. (2009) and more recently by Craigie (2011) clearly show the significant role of various seaweed extracts as biostimulants of plant growth and development. Among the brown seaweeds (Phaeophyta), Ascophyllum nodosum and its extracts are the most widely used in agriculture. This Pan-Atlantic alga grows abundantly along the coastlines of North America and Europe (Ugarte and Sharp 2001, Ugarte et al. 2006).
Seaweed extract from the kelp Ecklonia maxima: Kelpak has been used effectively for the tips of Gracilaria gracilis when grown under laboratory conditions. A single dose of 1:2,500 concentration, or in combination with aquaculture effluent water was used for the growth of the green, abalone feed species Ulva in a pilot-scale, commercial evaluation (Robertson-Anderson et al. 2006). In a previous study by the authors of this study, A. nodosum extract powder promoted early shoot formation in the tissue culture of three varieties of Kappaphycus (Hurtado et al. 2009, Yunque et al. 2011). The efficacy of Acadian Marine Plant Extract Powder (AMPEP) in limiting the growth of macro-epiphytes and Neosiphonia infestation was clearly demonstrated see Loureiro et al. 2009 and Borlongan et al. 2011, respectively. Recently, Loureiro et al. 2011 proposed a “vaccine-like” mode of action for the beneficial properties of AMPEP when used in Kappaphycus culture.
Since 2008, seaweed production in the Philippines (Neish 2008) has progressively declined, in part due to decreased productivity of the dominant cultivars in the farming areas, brought about by the inferior quality of cultivars, persistent occurrence of “ice-ice” and infestation by the red epiphyte Neosiphonia, poor agronomic practices, and insufficient capital to expand the farming areas. Three separate studies were conducted to test the efficacy of Ascophyllum seaweed extract (AMPEP) as a source of nutrients and growth regulators to boost biomass production, vigor, and health of Kappaphycus.
Materials and methods
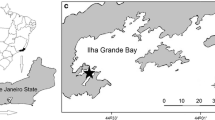
Three color morphotypes (i.e., reddish brown, yellowish brown and purple) of K. alvarezii (Doty) Doty were collected from a seaweed farm in Zamboanga City, Philippines (6°54′53.06″ N and 122°10′43.33″ E). Only young, robust and “ice-ice”—epi- and endophyte-free cultivars were selected. Five “seedlings” weighing 100 g each were individually tied with plastic straw and markers, dipped in AMPEP (a gift from Acadian Seaplants Limited, Nova Scotia, Canada; Hurtado et al. 2009) at three concentrations (i.e., 0.01, 0.1, and 1.0 g L−1 of seawater) for two exposure times (i.e., 30 and 60 min). While the “seedlings” were dipped in AMPEP at the desired concentration and time, manual stirring was done every 5–10 min to accelerate absorption. Immediately at the end of each dip time, the “seedlings” were tied at 20-cm intervals on a 20-m cultivation line (two ply, flat binder), provided with five floaters (20 cm diameter) tied 5 m apart to suspend the “seedlings” 0.75–1.0 m below the water surface. These were allowed to grow and sampled every 30 days for a period of 3 months from August to November 2010. Every month, the seaweeds were totally harvested, the epiphytes and surface water removed and weighed to measure their increments in biomass. As per normal vegetative propagation methods, new “seedlings” (i.e., robust and ‘ice-ice’—epiphyte–endophyte-free) were selected. The original biomass of 100 g of each color morphotype with five replicates was maintained for the subsequent growth period. The daily growth rate (DGR) was expressed as the percentage increase in fresh weight day−1 (Dawes et al. 1994) was calculated, as follows: daily growth rate (% day−1) = ln (W f/W i)/t × 100 where: W i—initial weight (in grams); W f—final weight at day t; t—number of culture days
Application of the optimized AMPEP concentration and dipping time of K. alvarezii (yellowish brown) in a commercial nursery
A polyethylene rope (PER #5) was cut into 45 cm pieces both ends were knotted and looped. One end of the loop was inserted into a 10 m cultivation line (PER #10). K. alvarezii (yellowish brown) “seedlings” weighing approximately 100-120 g each were attached individually into the cultivation line at 20 cm apart. The “seedlings” looped into the cultivation line were dipped in AMPEP at 0.1 g L−1 for 30 min using wide-opening plastic drums with constant manual stirring. The strings were taken to the cultivation site to be tied to a supporting frame. A modified “spider web” (100 m × 25 m) method (Hurtado et al. 2008) was employed for assessment of growth. The “seedlings” were left to for 21–25 days. Biomass increase (in kilograms) and daily growth rate of K. alvarezii was determined monthly for a period of 1 year. Likewise, the presence of macro-epiphytes was recorded.
Growth and phenolic content of K. alvarezii (tambalang purple) and K. striatum (sacol green) with AMPEP dipping versus control
Preparation of the “seedlings” and looping of K. alvarezii (tambalang purple) and K. striatum (sacol green) to a 3 m cultivation line followed the same procedure as described above. Three replicates of each strain were dipped in AMPEP (0.1 g L−1 at 30 min); another set of three replicates for each strain served as the control. A total of 12 lines were allowed to grow for a period of 45 days from September 2010 to February 2011. However, at the start of each month, a new set of cultivation lines containing the test plants was made to extend the 45 day growth cycle for a total 6 month period, representing the peak of southwest season and early northeast season production seasons for the Philippines. At the end of each 45-day cycle, all of the test plants were harvested, weighed, and the macro-epiphytes were identified and also weighed. Computation of average DGR was performed as above. Daily monitoring of seawater temperature and salinity were made at 08.00, 12.00, and 15.00 hours using a thermometer and an Atago refractometer.
Phenolic content of K. alvarezii (tambalang purple) and K. striatum (sacol green)
Total phenolic content was analyzed using the Folin–Ciocalteu method as described by Singh et al. (2002) with slight modifications. Kappaphycus extracts, or standard solutions, were pipetted into 2 mL tubes and to each 1.58 mL water and 100 μL 2 N Folin–Ciocalteu reagent was added. After 8 min incubation, 300 μL 20% (w/v) sodium carbonate solution was added to stop the reaction. The vortexed mixture was left at room temperature in the dark for 2 h and absorbance was read at 760 nm using a Hitachi U1100 spectrophotometer versus a blank (70% methanol). A standard calibration curve (20–500 mg L−1) was prepared using the same procedure as above. Total phenolics were expressed as gallic acid equivalents (in milligrams gallic acid per 100 g FW).
DPPH assay—free radical scavenging activity
Dried Kappaphycus (0.2 g) was homogenized in 4 mL MeOH using a mortar and pestle, centrifuged at 10,000×g for 10 min, and the pellet was re-extracted with 3 mL MeOH. Supernatants were combined and the total volume made up to 7 mL. The pellet was re-dissolved in 0.5 mL dichloromethane (DCM), re-homogenized and re-centrifuged at 10,000×g for 10 min. The pellet was re-extracted with 0.5 mL DCM, and the remaining supernatant was centrifuged, combined, and made up to 1 mL. Each extract was added to 2.85 mL of fresh DPPH solution (0.11 mM), and incubated for 6 h at 22°C. Absorbance was measured at 515 nm against either MeOH or DCM as a blank. The scavenging activity was calculated according to the equation:
Where A b is the absorption of blank sample, and A s is the absorption in the test sample. The results were expressed in micromolar Trolox equivalents per 100 g FW through a comparison against a standard curve (25–800 μM Trolox).
Iron chelating activity
The chelating activity of Kappaphycus samples with Fe2+ was determined according to Heimler et al. (2007). Each of the samples was diluted in 1.4 mL 250 mM acetate buffer (pH 4.75) and mixed with 25 μL 2 mM FeCl2 and 1 mL 70% MeOH. After a 20-min incubation at room temperature, 100 μL of 5 mM ferrozine was added to initiate the reaction. The mixture was shaken vigorously and the absorbance was read after 10 min at 562 nm against a blank (70% MeOH). The ability for disrupting the formation of the ferrozine–Fe2+ complex was calculated as follows:
Where: A b is the absorbance of the blank sample and A s is the absorbance of the test sample.
Statistical tests
Results were analyzed using one-way analysis of variance (ANOVA) and Duncan multiple range test at 5% level of significance to test significant differences in growth rate between treatments (AMPEP concentrations and dipping time) among the three color morphotypes on a monthly basis using SPSS v. 16. Likewise, an ANOVA in a completely randomized design was used to determine the effect of factor combinations (e.g., strain and presence–absence of AMPEP) on the average daily growth rate, phenolic content, free radical scavenging activity, and iron chelating activity. Coefficient of determination (r 2) was used to determine the relationship between temperature, salinity and daily growth rate of K. alvarezii (tambalang purple) and K. striatum (sacol green).
Results
Growth rates of three color morphotypes of K. alvarezii
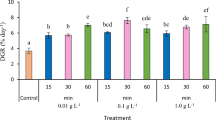
The daily growth rate (% increase in fresh weight day−1) of the three color morphotypes of K. alvarezii was generally lowest in the control that was significantly different from the rest of the treatments over the entire period of growth. The highest (7.7% ± 0) and lowest (2.4% ± 0.51) growth rates were recorded in August–September and October–November, respectively in all three color morphotypes.
Except for the K. alvarezii (purple; 0.01 g L−1; 60 min), the highest growth rates were recorded at 0.01 g L−1–30 min treatment during the 3-month study period, and this was observed in August–September. The K. alvarezii (reddish brown) responded to AMPEP favorably with higher growth rates obtained from the lower concentrations of dip, i.e., 0.01 and 0.1 g L−1 and the shorter dipping time (30 min) from August to October. However, the same morphotype required a lower concentration (0.01 g L−1) and longer dip time (60 min) in order to obtain the highest growth in October–November.
During the 3-month culture period, from August to November, the three color morphotypes showed a pattern of growth in response to the AMPEP exposure, i.e., the lower the concentration, irrespective of dip time, performed better (Fig. 1a–c). Significant differences in growth rate among the three color morphotypes of K. alvarezii were observed.
Filamentous Ulva (a green epiphyte) was consistently the dominant macro-epiphyte during the study period, followed by Hypnea and Acanthophora.
K. alvarezii (yellowish brown) in a commercial nursery
The average monthly growth rate of K. alvarezii (yellowish brown) ranged from 0.8 to 6.7% day−1 between July and January, respectively (Fig. 2). Significant differences (P < 0.05) were observed between the various months of culture. Declining growth rates were observed from February to July while increasing growth rates were observed from August to October, with a slight decrease in November, but reaching its highest growth rate in January.
Dipping of K. alvarezii and K. striatum in AMPEP
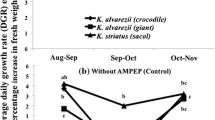
The average daily growth of K. alvarezii (tambalang purple) and K. striatum (sacol green) is shown in Figs. 3a–b. K. alvarezii showed the best growth in October–November, both with and without AMPEP dipping. Increasing growth rates were recorded from November to February when the K. alvarezii was dipped in AMPEP. However, negative growth rates were obtained when the seaweed was not dipped in AMPEP. Except for the months of October to November and November to December (with dipping), K. striatum, showed negative growth rates overall.
Amongst the treatments for each culture month, tambalang purple with AMPEP dipping sustained a positive growth. There was an interaction between the strain and AMPEP from November to December. The two strains had different responses to their exposure to AMPEP. Sacol green had less negative growth without the AMPEP dipping, but was not significantly different (P > 0.05) versus the control, whereas, tambalang purple with AMPEP dipping recorded positive growth and was statistically significant (P < 0.05) from the control.
The average (±STD) temperature and salinity readings from September to March is shown in Fig. 4. Lowest temperature (29.8°C) and salinity (29.6 ppt) were recorded in February–March while the highest temperature (31.2°C) and salinity (31.6 ppt) were observed in December–January. Little variations in both temperature and salinity readings were observed.
K. alvarezii (tambalang purple) and K. striatum (sacol green) with and without AMPEP dipping had the same trends for growth rate against seawater temperature and salinity with peaks at 30.6°C and at 30.4 ppt, respectively. High coefficient of determination (r 2) values of 0.74–0.98 were obtained between temperature–growth rate and salinity–growth rate for sacol green, and 0.73–0.98 for tambalang purple.
Phenolic content, DPPH radical scavenging assay, and ferrous iron chelating activity of K. alvarezii (tambalang purple) and K. striatum (sacol green)
The antioxidant content of K. alvarezii (tambalang purple) and K. striatum (sacol green) is shown in Fig. 5a–b. The two strains responded differently to being exposed to AMPEP before being out planted in the nursery. Decreasing phenolic content was observed from October to December, when K. alvarezii (tambalang purple) was not dipped in AMPEP, however, highest total phenolics were obtained in January–February. K. striatum (sacol green) showed the highest total phenolics in the January–February period both with AMPEP treatment and in the control with a decline in dipped plants from October to November and November to December. The changes in total antioxidant activity followed almost the same trend as the phenolic content, in both K. alvarezii (tambalang purple) and K. striatum (sacol green; Fig. 6a–b).
There were no significant differences in the Fe2+ chelating ability of K. alvarezii (tambalang purple) in the controls of either culture months; a decreased chelating ability was recorded for October to November and November to December in the treated plants and the highest chelating ability was found in samples from September–October to January–February, with a low level in February–March. K. striatum (sacol green) showed a decreased metal chelating ability in the months of November to December and December to January the highest chelating ability in January–February both with treated and control plants (Fig. 7a–b).
Discussion
Commercial extracts of various seaweeds have been used extensively for the benefit of agricultural production to promote growth, reduce damage by pests and disease and improve the quality and value of the terrestrial crops and plant products for more than 30 years and amongst others has been reported by Stirk and Van Staden (1997), Stirk et al. (2004), and reviewed extensively by Craigie (2011). However, to the knowledge of the present authors there are very few regarding the performance of applications of seaweed extract, as applied for the benefit (health and production) of other (non-related) seaweed crops—the main example of note includes: “Kelpak”—a product from the kelp E. maxima, used for enhanced production in cultivation tanks the red alga Gracilaria and the green Ulva (Robertson-Anderson et al. 2006). The results of the present study demonstrated, for the first time, the use extract from the fucoid A. nodosum, at different concentrations and for different dipping periods promoted the growth of three different color morphotypes of the red alga K. alvarezii, under commercial nursery, and open-water, field conditions. The efficiency of the Ascophyllum extract is controlled by the concentration applied. Likewise, the efficacy of the extract is probably based upon plant growth priming and elicitation compounds and trace nutrients present in the extracts (Dr. Owen Wally et al. personal communication). The response of the three, K. alvarezii color morphotypes to AMPEP varied according to the months of cultivation. The results showed that the carrageenophytes grew at the highest rates in the August–September periods, which coincided with the “rainy season” in the Philippines when salinity (28–29‰) and seawater temperature (29–30°C) were lowest.
The present study showed that dipping of the three color morphotypes of K. alvarezii for 30 min at the lower AMPEP concentrations of 0.01–0.1 g L−1 resulted in higher growth rates compared to the controls. The active compounds of the seaweed extract must be applied in small doses to be effective, as also claimed by Robertson-Anderson et al. (2006). The proliferation of young multiple shoots of the three color morphotypes of K. alvarezii and the yellowish brown K. alvarezii, grown in a commercial nursery, after 10–14 days of field growth could be attributed to the presence of growth promoters which stimulated auxin-like activity which further promoted shoot growth. Our results paralleled the results of the study of Rayorath et al. (2008b), wherein the components of the commercial A. nodosum extract modulated the concentration and localization of auxins, which could account at least in part, for the enhanced plant growth of the model plant Arabidopsis thaliana. Though it is beyond the scope of the present study to speculate on the active compounds or mode of action of AMPEP, the enhanced plant growth effects of the Ascophyllum on the color morphotypes of K. alvarezii could be correlated with auxin-like, gibberellin-like (Rayorath et al. 2008a, b), cytokinin, precursors of ethylene and betaine (Mckinnon et al. 2010) and cytokinins which are potentially involved in enhancing plant growth responses (Crouch and Van Staden 1993). Wally (in preparation, personal communication) will discuss priming and stimulation of in planta responses following application of the Ascophyllum extract.
A. nodosum extract (powder or liquid) is generally used for foliar spray or added to the soil which enhances leaf chlorophyll content (Crouch and Van Staden 1992). The dipping of K. alvarezii in AMPEP solutions in the present study was observed to enhance the pigments of each color morphotype.
One interesting observation made in the three studies was the occurrence of green filamentous Ulva which, in part, covered the thallus of K. alvarezii and K. striatum. However, the presence of this epiphyte did not hinder the robust growth of these morphotypes. Farmers have observed that excessive growth of green filamentous algae and other macro-epiphytes in the seaweed farms especially when there is an intermittent rain and sunny days, may bring severe problems to seaweed farms that lead to reduced biomass production and, more often than not, the occurrence of “ice-ice” and the epiphytic red alga Neosiphonia infestation. In the commercial nursery, it was interesting to note that the growth of K. alvarezii (yellowish brown morphotype) was almost entirely free of macro-epiphytes. The nursery site was located in an area where there were no other commercial farming set-ups; hence, the area was free from macro-epiphytes which can be thrown away indiscriminately by the farmers during the times of cultivar cleaning. Again, while not within the scope of the present study, regarding the effect of AMPEP on disease resistance, earlier studies on agricultural crops such as carrot (Jayaraj et al. 2008), cucumber (Jayaraj et al. 2011) reported that A. nodosum commercial extract enhanced disease resistance through induction of plant defense genes or proteins—while this is entirely speculation, this aspect should be studied in seaweeds as pathological studies on commercial crop seaweeds will become increasingly important since many cultivated commercial seaweeds are based on limited genetic resources, grown as mono-specific crops—a perfect situation for co-development of pest species. This is exactly what has happened in terrestrial plant production and can be entirely expected to occur in seaweed cultivation, in fact it is perhaps more surprising that, in particular, carrageenophytes have been cultivated by vegetative propagation for so long with relatively few “health” issues, until relatively recently (Critchley et al. 2004, Hurtado et al. 2006, Tisera and Naguit 2009, Solis et al. 2010, Borlongan et al. 2011).
Since the early 1990s, K. striatum has been grown more extensively than K. alvarezii because of the perception that the former is more “disease resistant”, however, earlier studies (Hurtado, unpublished data) showed that K. striatum is inferior in its growth rate, it is brittle and more easily breaks after 30 days culture, hence, farmers do not culture K. striatum beyond 30 days. K. striatum is also more generally grown in shallow, calm waters as compared to K. alvarezii which can be grown in more energetic sites and is grown in deeper waters (Omar, personal communication).
Seawater temperature and salinity were highly correlated with the growth in K. alvarezii and K. striatum, indicating a high degree of association between the two pairs of variables, hence had a positive effect on the growth.
Studies have shown that exogenous application of the commercial A. nodosum extract increased endogenous antioxidant activity in plants, such as increased amounts of non-enzymatic antioxidant compounds (i.e., α-tocopherol, ascorbate, and β-carotene), enhanced activities of antioxidant enzymes including glutathione reductase and superoxide dismutase, and elevated total phenolic content (Allen et al. 2001; Zhang and Ervin 2004; Fan et al. 2011). In the present work, no significant effect of AMPEP dipping was found in regard to total phenolics in either K. alvarezii or K. striatum, however, sacol green with AMPEP treatment showed a significant increase (P < 0.05) in total antioxidant capacity in October–November. The tambalang purple strain with AMPEP treatment produced a sustained high Fe2+ chelating ability from September–October to January–February, which coincided with the positive growth of K. alvarezii during that time. The increased free radical scavenging ability and transition metal chelating ability might be of interest from the point of view of resistance to pathogens and abiotic stresses.
The coefficient of determination (r 2) have shown that the influence of salinity on the growth of K. striatum (green) without AMPEP dipping was higher (r 2 = 98%) compared to temperature (r 2 = 94%); whereas the same seaweed dipped in AMPEP, salinity had lower influence on growth (r 2 = 74%) but showed a higher temperature influence (r 2 = 93%). On the other hand, the influence of temperature on the growth of K. alvarezii (purple) without AMPEP dipping was higher (r 2 = 98%) compared to salinity (r 2 = 95%); whereas the same seaweed dipped in AMPEP temperature has lower influence on growth (r 2 = 73%) but showed a higher salinity influence (r 2 = 76%).
The present study demonstrate the significance of AMPEP to enhance growth rate in three color morphotypes of K. alvarezii during the Philippines wet season, as further demonstrated in a commercial nursery using K. alvarezii (yellowish brown). Likewise, dipping K. alvarezii (tambalang purple) in AMPEP showed encouraging results as far as free radical scavenging and transition metal chelating abilities which are important to pathogens and abiotic stress resistance. It is however, recommended that the same study be repeated during the dry season to assess the efficacy of AMPEP treatment when the environmental conditions are considerably different in water temperature, salinity and wave action. Furthermore, it is suggested that the role of AMPEP dips to enhance disease resistance in Kappaphycus be further investigated as a possible model for future large-scale seaweed enterprises (perhaps biofuel biomass).
References
Allen VG, Pond KR, Saker KE, Fontenot JP, Bagley CP, Ivy RL, Evans RR, Brown CP, Miller MF, Montgomery JL, Dettle TM, Wester DB (2001) Tasco-Forage: III. Influence of a seaweed extract on performance, monocyte immune cell response, and carcass characteristics in feedlot-finished steers. J Anim Sci 79:1032–1040
Blunden GT, Jenkins T, Liu YW (1997) Enhanced chlorophyll levels in plants treated with seaweed extract. J Appl Phycol 8:535–543
Borlongan IAG, Tibubos KR, Yunque DAT, Hurtado AQ, Critchley AT (2011) Impact of AMPEP on the growth and occurrence of epiphytic Neosiphonia infestation on two varieties of commercially cultivated Kappaphycus alvarezii grown at different depths in the Philippines. J Appl Phycol 23:615–621
Craigie JS (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393
Critchley AT, Largo D, Wee W, Bleicher L’honneur G, Hurtado AQ, Schubert J (2004) A preliminary summary on Kappaphycus farming and the impact of epiphytes. Jap J Phycol (Supplement) 52:231–232
Crouch IJ, Van Staden J (1992) Effect of seaweed concentrate on the establishment and yield of greenhouse tomato plants. J Appl Phycol 4:291–296
Crouch IJ, Van Staden J (1993) Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Reg 13:21–29
Crouch IJ, Beckett RP, Van Staden J (1990) Effect of seaweed concentrate on the growth and mineral nutrition of nutrient stressed lettuce. J Appl Phycol 2:269–272
Dawes CJ, Lluisma AO, Trono GC (1994) Laboratory and field growth studies of commercial strains of Eucheuma denticulatum and Kappaphycus alvarezii in the Philippines. J Appl Phycol 6:21–24
Fan D, Hodges DM, Zhang JZ, Kirby CW, Ji XH, Locke SJ, Critchley AT, Prithiviraj B (2011) Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem 124:195–202
Ferreira MI (2002) The efficacy of liquid extract on the yield of canola plants. South African J Plant Soil 19:159–161
Heimler D, Isolani L, Vignolini P, Tombelli S, Romani A (2007) Polyphenol content and antioxidative activity in some species of freshly consumed salads. Agri Food Chem 55:1724–1729
Hurtado AQ, Critchley AT, Trespoey A, Bleicher-Lhonneur G (2006) Occurrence of Polysiphonia epiphytes in Kappaphycus farms at Calaguas Is. Camarines Norte, Philippines. J Appl Phycol 18:301–306
Hurtado AQ, Bleicher-Lhonneur G, Critchley AT (2008) Kappaphycus ‘cottonii’ farming (Revised Ed.). Cargill Texturizing Solutions, Cargill, Rue de Seves, Baupte, France. 26 pp
Hurtado AQ, Yunque DA, Tibubos K, Critchley AT (2009) Use of Acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J Appl Phycol 21:633–639
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, Critchley AT, Craigie JS, Norrie P, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399
Jayaraj J, Wan A, Rahman M, Punja ZK (2008) Seaweed extract reduces foliar fungal disease on carrot. Crop Prot 10:1360–1366
Jayaraj J, Norrie J, Punja ZK (2011) Commercial extract from brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J Appl Phycol 23:353–361
Kowalski B, Jager AK, Van Staden J (1999) The effect of a seaweed concentrate on the in vitro growth and acclimatization of potato plantlets. Potato Res 42:131–139
Loureiro RR, Reis RP, Critchley AT (2009) In vitro cultivation of three Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) variants (green red and brown) exposed to commercial extract of the brown alga Ascophyllum nodosum (Fucaceae, Ochrophyta). J Appl Phycol 22:101–104
Loureiro RR, Reis RP, Berrogain FD, Critchley AT (2011) Extract powder from the brown alga Ascophylum nodosum (Linnaeus) Le Jolis (AMPEP): a “vaccine-like” effect on Kappaphycus alvarezii (Doty) Doty ex P.C. Silva. J Appl Phycol (doi:10.1007/s10811-011-9735-7)
Mckinnon SL, Hiltz D, Ugarte R, Craft CA (2010) Improved methods of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts. J Appl Phycol 22:489–494
Neish IC (2008) Overview of seaweed in the world and Indonesia seaweed prospects. Paper presented at the 1st Indonesia Seaweed Forum, Oct 27–30, 2008, Makassar, Indonesia (Abstract only)
Rayorath PM, Khan W, Palanisamy R, MacKinnon SL, Stefanova R, Hankins SD, Critchley AT, Prithiviraj B (2008a) Extracts of the brown seaweed Ascophyllum nodosum induce gibberellic acid (GA3)-independent amylase activity in barley. J Plant Growth Regul 27:370–379
Rayorath PM, Narayanan JM, Farid A, Khan W, Palanisamy R, Hankins SD, Critchley AT, Prithiviraj B (2008b) Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana. J Appl Phycol 20:423–429
Robertson-Anderson DV, Leitao D, Bolton JJ, Anderson RJ, Njobeni A, Ruck K (2006) Can kelp extract (KELPAK) be useful in seaweed mariculture? J Appl Phycol 18:315–321
Singh RP, Muthy KNC, Jayaprakasha GK (2002) Studies on the antioxidant activity of pomegranate (Pinica granatum) peep and seed extracts using in vitro models. J Agri Food Chem 50:81–86
Solis JL, Draeger S, dela Cruz TEE (2010) Marine-derived fungi from Kappaphycus alvarezii and K. striatum as potential causative agents of ice-ice disease in farmed seaweed. Bot Mar 53:587–594
Stirk WA, Van Staden J (1997) Comparison of cytokinin- and auxin-like activity in some commercially used seaweed extract. J Appl Phycol 8:503–508
Stirk WA, Arthur GD, Lourens AF, Novak O, Strnad M, Van Staden J (2004) Changes in cytokinin and auxin concentrations in seaweed concentrates when stored at an elevated temperature. J Appl Phycol 16:31–39
Tisera WL, Naguit MR (2009) Ice-ice disease occurrence in seaweed farms in Bais Bay, Negros Oriental and Zamboanga del Norte. The Threshold 4:1–16
Ugarte R, Sharp G (2001) A new approach to seaweed management in Eastern Canada: the case of Ascophyllum nodosum. Cah Biol Mar 42:63–70
Ugarte R, Sharp GJ, B. Moore B (2006) Changes in the brown seaweed Ascophyllum nodosum (L.) le Jol, plant morphology and biomass produced by cutter rake harvests in southern New Brunswick, Canada. J Appl Phycol 18: 351-359
Yunque DA, Tibubos KR, Hurtado AQ, Critchley AT (2011) Optimization of culture conditions for tissue culture production of young plants of carrageenophyte Kappaphycus. J Appl Phycol 23:433–438
Zhang X, Ervin EH (2004) Cytokinin-containing seaweed and humic acid extracts associated with creeping bent grass leaf cytokinins and drought resistance. Crop Sci 44:1737–1745
Acknowledgment
The senior author is thankful to Acadian Seaplants Limited, Nova Scotia, Canada for the samples of AMPEP used in the study and the farmers of Kasanyangan Nursery Seaweed Enterprise of Zamboanga City for their assistance during sampling days.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hurtado, A.Q., Joe, M., Sanares, R.C. et al. Investigation of the application of Acadian Marine Plant Extract Powder (AMPEP) to enhance the growth, phenolic content, free radical scavenging, and iron chelating activities of Kappaphycus Doty (Solieriaceae, Gigartinales, Rhodophyta). J Appl Phycol 24, 601–611 (2012). https://doi.org/10.1007/s10811-011-9785-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-011-9785-x