Abstract
The genus Dictyota (Dictyotales, Phaeophyceae) comprises parenchymatous algae occurring predominantly in tropical to warm-temperate waters and has gathered attention due to its diverse secondary metabolites with antibiofouling and pharmaceutical potential, its oil content, and its potential as animal feed. This has resulted in an increase in economic potential during the last decade. In this review, we summarise the recent knowledge on the genus and concentrate on the applications and the economic potential of Dictyota. In addition, the review summarises the taxonomy, anatomy, cytology, genetic data, life history, chemical composition, nutritional value and ecological and economic importance of Dictyota species. Currently, around 100 species are recognised together with the morphologically similar and closely related genera Dilophus, Canistrocarpus and Rugulopteryx (tribus Dictyoteae). The thallus is characterised by one or more lens-shaped apical cells that divide into cortical and medullary cell layers. Species typically grow in rocky intertidal pools and subtidal areas. Dictyota is consumed locally in the Caribbean, Malayan-Indonesian and Hawaiian regions. Extracts of Dictyota which contain active compounds, such as diterpenes and phlorotannins, have been attributed antimicrobial, health and wellness promoting effects which render them promising candidates for the design of functional foods, phytomedicinal products, and cosmetics. The high fraction of lipids and fatty acids has propelled emerging applications in the biofuel industry and as a feedstock species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Identity
Nomenclature and taxonomy
Valid scientific name
Jean Vincent Félix Lamouroux (1809) described the genus Dictyota but only much later, Dictyota dichotoma (Hudson) Lamouroux was selected as the lectotype of the genus. The name Dictyota is derived from the Greek word ‘Διχτυον’ meaning ‘net’ or ‘network’, which refers to the aspect of medulla and cortical cells when observed under a microscope.
Nomenclatural synonyms
Heterotypic synonyms: Dichophyllium Kützing 1843; Dilophus J. Agardh, 1882; Glossophora J. Agardh, 1882; Pachydictyon J. Agardh, 1894; Bicrista Kuntze 1898; Glossophorella M. Nizamuddin & A.C.Campbell, 1995.
Vernacular names
Although vernacular names do exist, e.g. Divided Net Weed, Brown Fan Weed, Brown Forkweed (Bunker et al. 2010), these are not commonly used. Most authors refer to ‘Dictyota’, using the latin name of the taxon.
Taxonomy
Dictyota is one of the 19 genera of the order Dictyotales. Together with the Sphacelariales, Syringodermatales and Onslowiales, the Dictyotales are member of the SSDO-clade, which diverged early from the rest of the Phaeophyceae (Bittner et al. 2008, Phillips et al. 2008). The Dictyotaceae, the only family in the Dictyotales, is subdivided in two tribes, Dictyoteae and Zonarieae. The Dictyoteae are characterised by the presence of a single lens-shaped apical cell and thereby stand out from the Zonarieae which have a row or cluster of apical cells. The definition of the Dictyoteae and its genera has evolved considerably over time.
In the nineteenth century, the concept of Dictyota was gradually narrowed down from a broadly defined genus that initially included all known representatives of the Dictyotales, Cutleriales and the odd red alga, to a very narrowly defined genus differentiated by related genera on the number of medulla and cortex layers. J. Agardh (1882, 1894) was largely responsible for a very narrow concept of Dictyota. He segregated Dilophus, Glossophora and Pachydictyon from Dictyota restricting the latter to include species with a single-layered medulla and cortex, while Dilophus and Pachydictyon are characterised by a multilayered medulla or cortex, respectively. Last, Glossophora was chiefly characterised by the presence of multiple surface proliferations. Late in the twentieth century Glossophorella was described (Nizamuddin and Campbell, 1995) to accommodate a species from the Arabian Sea similarly characterised by surface proliferations but a variable number of cortex and medulla layers. The distinction among these genera has been the subject of considerable debate, as some species are particularly hard to assign to one or another genus (Setchell and Gardner 1925, Taylor 1945, Dawson 1950). Following observations that the number of cell layers can be manipulated in culture in several Dictyota or Dilophus species, Hörnig et al. (1992a, b) merged Dilophus in Dictyota, abandoning the narrow genus concept proposed by J.Agardh. Using DNA sequence data, De Clerck et al. (2006) demonstrated that also Glossophora, Glossophorella and Pachydictyon are embedded in Dictyota, resulting in synonymisation of these genera with Dictyota. The phylogenetic analyses also led to the exclusion of certain species from Dictyota and the description of Canistrocarpus and Rugulopteryx. The latter are distinguished by a combination of characters related to male reproductive structures (e.g. paraphyses, sterile cells surrounding the antheridia) and meiospores (e.g. the number of stalk cells supporting the sporangium). The phylogenetic analyses by De Clerck et al. (2006) were inconclusive regarding the status of Dilophus, since the generitype (Dil. gunnianus) was missing from the dataset. More recently, however, Küpper et al. (2019) presented a phylogeny of Dictyota and related genera that included Dilophus gunnianus as well as Dilophus fastigiatus. Both species were resolved as a clade separate from Dictyota proper. These results argue in favor of a genus Dilophus, distinct from Dictyota which most likely includes the Australian species characterised by a multilayered medulla and sporangia borne on 2 stalk cells.
Morphology/anatomy
External morphology
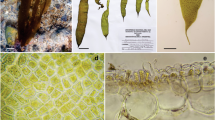
Dictyota is a parenchymatous alga, with flat, ribbon-like axes, which grow from well-defined apical meristems that differentiate into an outer cortical layer and an inner medullary layer. Thalli branch via dichotomous primary branching by longitudinal cell divisions of the apical meristem cells (Gaillard et al., 1986) (Fig. 1a) or by adventitious branching after re-differentiation of cortical cells (Gaillard and L’Hardy-Halos, 1990) or other cortical differentiations such as male paraphyses and dedifferentiated reproductive cells (Hwang et al., 2005). Dichotomous branching may range from isotomous (equal branches) to anisotomous (unequal branches) (Fig. 1b, c), a process that is controlled by light, nutrients and apparently also the base of the thallus (Gaillard and L’Hardy-Halos, 1977, 1979, 1980, 1984). Branches may curve back forming ‘recurved’ branches (Canistrocarpus cervicornis Kützing f. pseudohamata (Cribb) De Clerck & Coppejans). Unequal branches may differentiate as falcate branchlets (Dictyota hamifera Setchell), which likely serve as additional attachment structures in exposed habitats (De Clerck, 2003) (Fig. 1d). Cervicorn branching results from anisotomous dichotomous branching combined with spirally twisted axes (Castinocarpus cervicornis Kützing) (Fig. 1d). The shape of the apical meristems can be truncate, rounded or acute (Fig. 1d). Branching patterns and apical morphologies may show in some species a large degree of morphological plasticity, most notably in Dictyota dichotoma (Hudson) Lamouroux (Schnetter and Hörnig 1987; Tronholm et al., 2010). Adventitious branching is typically induced by grazing or loss of the apical meristem activity where they take over the function of the main axis (Tanaka et al. 2016), but may also be a common phenomenon in some species. Cortical cells from the margins may dedifferentiate into meristematic cells and grow small teeth (Dictyota ciliolata Sonder ex Kützing) or cortex from the center of the thalli may develop adventitious branches or ‘ligulae’ (for example Dictyota kunthii (C.Agardh) Greville). Apart from the occurrence of above mentioned outgrowths and fertile structures (discussed below), thalli develop tufts of hair 20–50 μm in diameter. Hair formation is under control of ecological factors such as blue light (Müller and Clauss, 1976).
Morphology and anatomy of Dictyota. a Apical meristem with a transverse lens-shaped apical cell which has recently divided longitudinally resulting in two lens-shaped apical cells and consequently a dichotomous branching. b Isotomous vs anisotomous dichotomous branching. c Cross section of thalli of different species showing variations in cortex and medullary layer. d Shape of apical segments (upper) and specialised branching patterns. b, c and d are modified from De Clerck (2003)
Attachment of the thallus is accomplished by means of multicellular, uniseriate, branching, hyaline rhizoids that may be differentiated terminally into fixing disks that firmly adhere the algae to the substratum (Gaillard et al., 1986). Depending on the species they may have a single point of attachment or have several points of attachment, resulting in a large variety of growth forms ranging from fully erect to creeping thalli firmly attached to the substrate (De Clerck, 2003). A few species attach to the substrate via terete stoloniferous holdfasts (e.g. D. spiralis, D. stolonifera). Living thalli range from straw colour to dark brown or sometimes greenish, depending on the species (Fig. 2). In situ, many species show a yellowish to deep blue-green iridescence, which is lost upon removal from the water (for example D. friabilis, Fig. 2b).
Anatomy and cytology
Plants are parenchymatous with apical meristems dividing into a three-dimensional multicellular structure (Gaillard and L’Hardy-Halos, 1984; Katsaros and Galatis, 1985). The apical meristem contains a lens-shaped apical cell dividing transversely with a discoid subapical cell as result (Fig. 1a). This subapical cell will divide twice parallel to the thallus surface, resulting in a primary medullary cell and two surrounding primary cortical cells. While the cortical cell layers undergo further cell divisions perpendicular to the surface that keep track of cell expansion of the segments, the medullary cell layer divides less frequently and almost exclusively longitudinally. The large medullary cells are hyaline and highly vacuolated with a central nucleus often surrounded by a cluster of lipid vesicles or physodes and sparse chloroplasts. The small cortical cells contain many physodes and chloroplasts without pyrenoids. In both cases, the nuclei are positioned centrally. The size differences of cortical and medullary cells have proven to be a useful taxonomic character (Weber-Peukert, 1985). In some species, the medulla may be multilayered in the entire thallus or only at the base or margins (Fig. 1c). A multilayered cortex is also present is some species. Cell walls are characterised by large pitfields containing many plasmodesmata ensuring symplastic continuity (Terauchi et al., 2012).
Genetic data
Chromosomes and nuclear content
In Dictyota, haploid chromosome numbers generally range from 9 to 32 (Lewis, 1996; Hörnig et al., 1992a). Both Cole (1967) and Lewis (1996) indicated the basic chromosome number of the Dictyotales to be 8 or 12, while Hörnig et al. (1992a) suggested a basic chromosome number of 4. In several cases multiple chromosome numbers have been reported for the same species. For example, both haploid chromosome numbers of n = 16 (Mottier, 1900; Williams, 1904a, b; Giraud, 1956) and n = 32 (Yabu, 1958; Kumagae and Inoh, 1960) have been described for Dictyota dichotoma, tempting authors to conclude the occurrence of polyploidy (Lewis, 1996). However, these observations can most likely be attributed to misidentifications, given the current knowledge on the distribution of D. dichotoma (Tronholm et al., 2008; Tronholm et al., 2010).
Next to chromosome numbers, several studies have determined the DNA content of algal nuclei by means of DAPI microspectrophotometry. Kapraun (2005) indicated a haploid genome size of 1.1 pg for Dictyota dichotoma, while Ribera Siguan et al. (2011) reported nuclear DNA contents ranging from 0.7–0.9 pg for 5 Dictyota species, with a nuclear content of 0.9 pg in the narrow growth form of Dictyota dichotoma (var. intricata). By comparing these values to nuclear DNA contents obtained for other taxa within the Dictyotales, the authors concluded that Dictyota species displayed a more narrow range of haploid nuclear genome sizes. This narrower range may represent a synapomorphy, although the authors acknowledge that more species should be examined to make more conclusive statements.
Molecular data
Given the high morphological plasticity of Dictyota, molecular techniques are indispensable for species identification. For example, Tronholm et al. (2010) adopted a multigene approach, using up to 6 markers derived from chloroplast, mitochondrial, and nuclear DNA, to resolve the taxonomic positions of members of the genus Dictyota in Europe. The most common molecular markers used include psbA, rbcL and cox1.
At the moment no complete Dictyota genome is available, although a whole genome sequence of D. dichotoma is expected as an outcome of ongoing research initiatives. Transcriptomic data (Bogaert et al. 2017a) and a complete mitochondrial genome (length 31,617 bp) are available for D. dichotoma (Oudot-Le Secq et al., 2006).
Distribution, ecology and metabolism
Distribution
At the genus level, Dictyota is characterised by a near cosmopolitan distribution, only lacking representatives in polar seas. Dictyota is essentially a tropical to warm-temperate genus, with only a handful of species adapted to colder waters (e.g. D. decumbens from Macquarie Island; Ricker 1987 and D. falklandica from the Falkland Islands and Tierra del Fuego; Küpper et al. 2019) (Fig. 3). D. dichotoma grows up to southern Norway, where it is a summer species and its biomass disappears in winter. Distribution ranges are inadequately characterised for many species still, but from detailed studies in Europe and the Atlantic Ocean, a pattern emerges of species with predominantly regional distributions confined to realms or provinces (sensu Spalding et al. 2007), but some species definitely have large ranges spanning more than one ocean basin (Tronholm et al. 2010, 2012, 2012). At least one species, D. cyanoloma has been shown to be introduced, probably from southern Australia to the Mediterranean Sea (Steen et al. 2017).
Ecology
Growing in upper subtidal areas and intertidal rock pools, Dictyota species occupy a broad range of ecological niches worldwide (Tronholm et al., 2010). While the genus is distributed widely, abundance data are mainly available for the northern Atlantic region, with particular focus on coral reef communities in the northwest Atlantic and island communities in the northeast Atlantic archipelagos (Table 1). These abundances have repeatedly been shown to vary seasonally and are influenced by a combination of both abiotic and biotic factors (Lirman, 2001; Thacker et al., 2001; Diaz-Pulido and Garzón-Ferreira, 2002; Tronholm et al., 2008; Gauna et al., 2013). Dictyota species may reach considerable abundances at their seasonal peak (Table 1) and have been reported to bloom in the Florida Keys (Beach and Walters 2000) and as an invasive in the Strait of Gibraltar (Rugulopteryx okamurae, formerly known as D. okamurae) where it dominates subtidal vegetations (> 90% coverage) at 10–20-m depth since 2016, causing large supralittoral wrack deposits (García-Gómez et al. 2020).
Seasonality
Seasonality in abundance and reproduction has been observed for several Dictyota species in temperate regions. In the case of D. dichotoma, these seasonal patterns seem to be largely regulated by temperature and therefore vary along the latitudinal range of the species. Specifically, southern populations of this warm temperate species tend to be present throughout most of the year but virtually disappear during the warmest months, surviving this period as microscopic stages (Tronholm et al., 2008; Steen et al., 2019). In contrast, northern populations show high abundance and fertility during summer but are absent in winter, when sea surface temperatures are lowest (Steen et al., 2019). Next to temperature, both daylength and solar radiation have also been shown to play an important role in the seasonality of Dictyota species (Ferrari et al., 2012; Gauna et al., 2013). Several studies examined the seasonal dynamics of algal assemblages, including Dictyota, indicating the existence of seasonal trends in occurrence, abundance, size, and fertility within the genus Dictyota (Diaz-Pulido and Garzón-Ferreira, 2002; Quan-Young et al., 2004; Peña and Bárbara, 2010). Besides phenological characters, the chemical compositions of the biomass is also influenced as seasonal variation in protein and fatty acid content has been reported (see the “Protein and amino acids” and “Biodiesel” sections).
Abiotic interactions
Important environmental factors affecting Dictyota species include light, temperature, nutrient availability, pH and water motion (Cronin and Hay 1996; Renken et al., 2010; Dailer et al., 2012; Ho and Carpenter, 2017). Among these, the effects of nutrient availability and light conditions have received considerable attention and will therefore be discussed here in more detail.
Light
Several studies examined the effects of the quality and quantity of irradiance on members of the genus Dictyota. Regarding light quality, Kuhlenkamp et al. (2001) demonstrated a strong reduction in growth rate when germlings of D. dichotoma were exposed to UV radiation (UVR). In accordance with these findings, Dictyota ciliolata showed reduced growth and survival, as well as a decrease in the production of secondary metabolites after prolonged exposure to surface levels of UVR (Cronin and Hay 1996). However, Flores-Moya et al. (1999) found that the recovery of photosynthesis after high solar radiation was impaired in the absence of UV-B in D. dichotoma, suggesting that this type of radiation may have beneficial effects on photoprotective processes.
Next to these studies, which focus mainly on the effects of UV radiation, extensive efforts have been made in elucidating the response of Dictyota species to different irradiance levels. In general, species of the genus Dictyota tolerate a wide range of light intensities, being able to maintain high photosynthetic rates even at low irradiances (Dawes and Kovach, 1992; Peckol and Ramus, 1992). This allows them to obtain high abundances in certain deep-water habitats, such as the deep-water seaweed assemblages of Bermuda, where they constitute one of the most dominant groups (Searles and Schneider, 1987). A wide bathymetric distribution has also been observed for temperate species, such as D. dichotoma, which may be linked to changes in photosynthetic pigment concentration with depth (Perez-Bermudez et al., 1981). In addition to low-light tolerance, Dictyota species possess several mechanisms aimed at protecting the photosynthetic apparatus from damage induced by excessive irradiance levels, allowing them to withstand strong light exposure. Mostly studied in the species D. dichotoma, examples of these mechanisms include dynamic photoinhibition (Nultsch et al., 1987), the reversible conversion of violaxanthin to antheraxanthin and eventually zeaxanthin in the xanthophyll cycle (Uhrmacher et al., 1995), and chromatophore displacement (Hanelt and Nultsch, 1991).
Nutrients
The effects of nutrients on Dictyota species have mainly been studied in tropical ecosystems, where they were predominantly evaluated in the context of community studies. In general, species of Dictyota are considered opportunistic macroalgae, owing to their ability to rapidly take up nutrients upon supply (i.e. surge uptake) (Aisha et al., 1995; Raikar and Wafar, 2006; Clausing and Fong, 2016, den Haan et al., 2016). Extra nutrients, in turn, may provide several fitness-enhancing effects, such as increases in growth rate, photosynthetic capacity and saturation irradiance (Dailer et al., 2012). Moreover, the capability for rapid uptake after episodic nutrient inputs can be largely maintained in the dark, which may represent an ecological advantage in nutrient-poor waters (Raikar and Wafar, 2006). However, the effects of nutrient enrichment have been shown to interact with other variables including herbivory (Sotka and Hay, 2009), environmental context (Clausing and Fong, 2016), and dissolved inorganic carbon (DIC) concentration (Martins et al., 2016), emphasising the need to consider multiple factors in order to draw realistic inferences.
Biotic interactions
Owing to their significant abundance in various intertidal and shallow subtidal habitats (Table 1), Dictyota species constitute a food source for a wide range of marine organisms, including sea turtles, sea urchins, amphipods and certain fish species (Azzurro et al., 2007; Carrión-Cortez et al. 2010; McCarty and Sotka, 2013; Moreno-Sánches et al. 2014; Cabanillas-Terán et al. 2016). In the specific case of the amphipod Ampithoe longimana, several studies indicated local adaptation of feeding preferences, with populations that are naturally coexisting with Dictyota showing higher feeding preference for and greater fitness when feeding on Dictyota, compared to populations that are not (Sotka and Hay, 2002; Sotka et al., 2003; McCarty and Sotka, 2013). These differences in fitness are shown to be heritable and persist even after rearing multiple generations in the laboratory (Sotka and Hay, 2002; Sotka et al., 2003). Overall, these findings indicate that Dictyota may play an important role in the genetic differentiation of its associated marine herbivores.
Next to its role as a food source, Dictyota provides substrate and shelter to numerous marine organisms. A study conducted in the Abrolhos Bank, Brazil, identified 9 higher taxa of marine invertebrates associated with the genus Dictyota, with further analyses of the most abundant taxa resulting in 64 families and 120 species (Cunha et al., 2013). The most abundant families belonged to the polychaetes, isopods, gastropods, and amphipods. For certain amphipod species, including A. longimana, Dictyota’s value as a protective refuge also plays a role in its prevalence as a food source, indicating these interactions to be driven by a combination of the need for qualitative food and effective shelter (Duffy and Hay 1991; Lasley-Rasher et al., 2011). Next to its association with macrofaunal communities, Dictyota species have been shown to harbour a wide diversity of dinoflagellate species as well as diatoms (Irola-Sansores et al., 2018; Park et al., 2018; Boisnoir et al., 2019). Finally, they can also play a role as shelter for larger species. For example, decorator crabs selectively use Dictyota menstrualis as camouflage on their backs in order to reduce their susceptibility to predation, while juvenile parrotfishes have been shown to use Dictyota patches as an effective recruitment microhabitat when there is a lack of coral cover (Stachowicz and Hay, 1999; Paddack and Sponaugle, 2008).
While beneficial interactions between Dictyota species and their associated biota are plentiful, this genus is also known to exert negative effects on various marine organisms. One of the most studied examples of such effects includes the competitive interactions between Dictyota and corals. Over the past decades, numerous reefs in the Atlantic and Pacific have undergone a marked phase-shift from coral-dominated communities to communities dominated by macroalgae (Done, 1992; Hughes, 1994; Edmunds, 2002). Especially in the Caribbean region, Florida and Fiji, the genus Dictyota constitutes an important component of the reef flora (Shulman and Robertson, 1996; Lapointe et al. 1997; Lirman and Biber, 2000; Ferrari et al., 2012; Bonaldo and Hay, 2014). Dictyota species have repeatedly been shown to reduce survival and recruitment of coral larvae, and to cause bleaching, reduced photosynthetic efficiency, and death when in direct contact with adult coral tissue (Kuffner et al., 2006; Rasher and Hay, 2010; Paul et al., 2011; Shearer et al., 2012; Olsen et al., 2015). These effects can, at least partly, be linked to the production of allelopathic secondary metabolites that are deployed at the seaweed surface (Longo and Hay, 2017). In addition, algae of the genus Dictyota have been linked to changes in coral-associated bacterial communities, expanding their impact to the holobiont level (Barott et al., 2012; Morrow et al. 2011).
Life history
Life cycle
The life cycle of Dictyota was one of the first cases where the alternation of generations was demonstrated to be accompanied by a change in ploidy (Svedelius, 1927; Haig, 1984). This discovery supported the theory of Strasburger (1894) which linked alternation of generations with ploidy change. Dictyota is characterised by an isomorphic life cycle (Fig. 4a), in which the gametophyte phase and the sporophyte phase are morphologically similar (Williams, 1897, 1904a, 1904b; Hoyt, 1910). This morphological similarity is also reflected in nutritional quality and chemical defense against predators in both sporophytes and gametophytes (Cronin and Hay 2009). Gametophytes produce motile gametes that, upon fertilisation, produce a diploid sporophyte that will bear sporangia undergoing a reduction division with each sporangium producing a tetrad of 4 haploid spores (Williams, 1904a; Hoyt, 1910) (Fig. 4b). Spores grow into both male and female gametophytes in about equal amounts (Hoyt, 1910).
Life cycle and reproduction in Dictyota. a Life cycle of Dictyota. Bold black arrows denote haploid stages, white arrows denote diploid stage. Both sporophytes and gametophytes may reproduce asexually (see text for details). Modified from Bogaert et al. (2013). b Schematic drawing of cross section of sexual reproductive organs of sporophyte (left), male gametophyte (middle) and female gametophyte (right). Modified from De Clerck (2003)
The life cycle is relatively simple. Unlike Fucus—which shows both dioecious and monoecious species—all studied species of Dictyota are dioecious (Phillips et al., 1990; Phillips, 1992; De Clerck, 2003). While for example the life cycle of Ectocarpus—a model system for life cycle research in brown algae—shows a very complex life cycle and possible deviations from the classic alternations of ploidy (Müller, 1972; Bothwell et al., 2010), no deviations from this alternation of haploid gametophytes to diploid sporophytes have been described for Dictyota. Unfertilised eggs invariably die after 2–3 (aberrant) cell divisions (Williams, 1904b).
Reproduction
Reproduction in Dictyota has been relatively well-studied compared to other seaweed genera. Male gametophytes develop antheridia that are organised in ellipse or puzzle-shaped white sori (Williams, 1904b; Phillips et al., 1990; Phillips, 1992; Phillips and Clayton, 1993). The antheridia are surrounded by sterile elongated cells, ‘paraphyses’, which persist after release of the gametes. Paraphyses are unicellular, contrary to Canistrocarpus where paraphyses are multicellular. Gametogenesis is constant for all species and occurs by the swelling of a group of cortical cells which undergo a mitotic cell division parallel to the surface to form an antheridial stalk cell and an antheridial initial. The antheridial initial divides frequently to produce 16–26 tiers consisting each of 16 loculi, containing a single spermatozoid each. Atypical for heterokont algae, Dictyota spermatozoids bear only the anterior flagellum, the posterior flagellum has been completely reduced.
Female gametophytes develop oogonia that are similarly organised in ellipsoid sori, except for a single species (D. robusta) where the oogonia are not organised in groups. Like the male sori, they develop after outward expansion of the cortex, followed by a cell division parallel to the surface, producing a stalk cell and an oogonium (Williams, 1904b; Foster et al., 1972). Central oogonia are generally larger than the peripheral ones. Each oogonium only produces a single egg cell.
Spores are formed by diploid sporophytes in unilocular sporangia. A cortical cell expands outwardly after which a mitotic division form the tetraspore mother cell and a stalk cell (Williams, 1904a; Phillips, 1992). The tetraspore mother cell finally undergoes a meiotic cell division shortly prior to release resulting in four cruciately arranged meiospores. In most species, sporangia can be easily discerned from oogonia by their scattered arrangement throughout the thallus, but some species possess sporangia that are organised in sori as well, potentially confounding discrimination of female gametophytes and sporophytes (Foster et al., 1972). Gametangia and sporangia may differentiate all over the thallus with a minimal distance of a couple of centimeters from actively growing meristems.
Gametes are released with both a diurnal and lunar periodicity (Williams, 1905). Gametes are typically released about 20–30 min in the early morning after first light (Kumke, 1973; Phillips et al., 1990). The male gametes show chemotaxis and are attracted by the release of the oxylipin n-butyl-cyclohepta-2,5-diene (dictyotene) (Muller et al., 1981; Phillips et al., 1990). In the presence of male gametes, oogonia become fertilised after which they elongate in about 90 s (Bogaert et al., 2017a). The elongation results into a ‘rugby’ ball-shaped cell with two poles of which one eventually will develop into the rhizoid tip and the other into the apical cell (Bogaert et al., 2017a). The shape change is accompanied by the immediate production of an adhesive layer in which a dense halo of male superfluous gametes may be embedded (Bogaert et al., 2017b). After attachment to the substrate, the cell will undergo an asymmetric cell division with a thallus and a rhizoid cell as a result (Bogaert et al., 2017a). Similar to Fucus zygotes, Dictyota uses the light direction to determine which one of the poles will develop into the rhizoid side, a process which involves the phytohormone auxin (Bogaert et al., 2019).
Besides a diurnal periodicity, gametophytes of Dictyota are the best studied brown algae with a clear lunar periodicity (Tessmar-Raible et al., 2011). D. dichotoma releases its gametes with two peaks a month (bilunar) (Williams, 1905; Hoyt, 1907, 1927; Müller, 1962). Under controlled conditions, the periodicity can be induced and controlled by illuminating the algae during one night every 28 days, mimicking the full moon (Müller, 1962). The release periodicity of most other reported species is lunar instead of bilunar: D. menstrualis, D. diemensis and D. gunniana gametes are released only once a month (Hoyt, 1927; Phillips et al., 1990; Phillips, 1992), while species like D. fastigiata Sonder or D. binghamiae apparently do not show a lunar periodicity (Foster et al., 1972; Phillips, 1992).
Apart from sexual reproduction, Dictyota is able to propagate asexually (Fig. 4a). This mode of reproduction may contribute to the large biomasses often observed in warmer regions (Herren et al., 2006). Many adventitious branches or ligulae in species such as D. kunthii are likely adaptations for vegetative propagation. In Dictyota, the loss of the apical meristem, typically induces a fast proliferation of new adventitious branching (Tanaka et al., 2016). Adventitious branches have a tendency to break off easily, which may explain how Dictyota can withstand high predation pressures. Similarly, in a Korean Dictyota species, some populations tend to propagate by the formation of in situ germlings. These germlings form by the apomictic development of the spore mother cell into a multicellular propagule (Hwang et al., 2005).
Chemical composition
The water content of Dictyota species fluctuates around 86.4–99.0% (McDermid and Stuercke, 2003; Taylor et al., 2003; Mcdermid et al., 2007; Tabarsa et al., 2012) (Table 2). It is not known whether the chemical content is species-specific because considerable variation has been observed between samples from the same species taken at different localities and during different seasons (Taylor et al., 2003; Gosch et al., 2015).
Inorganic elements
Like most seaweeds, Dictyota is rich in minerals and has an ash content of around 17.2–30.1% of dry weight (DW) (Table 2). Species may therefore be useful as mineral supplements. Dictyota species appear significantly enriched in iron compared to other screened species from the same locality with concentrations higher than 29,774 μg g−1 DW (Tabarsa et al., 2012; Billah et al., 2017), but values 2 to 3 orders of magnitude lower have also been reported (McDermid and Stuercke, 2003; Deyab et al., 2017). Varying amounts of heavy metals have been observed in Dictyota (Table 2). Especially copper and chromium might be relevant (Chakraborty et al., 2014). While Dictyota was reported to be enriched in iodine (EL-Naggar, 2009), relatively moderate quantities are reported in other studies (Grimm, 1952; Solimabi and Das 1977).
Carbohydrates
The reported total carbohydrate fraction varies between 10.8 and 54.2% of DW (Table 2). Total soluble carbohydrates (fucans, laminarans and monosaccharides) are reported to range from 5.9 to 26.7% in the field (McDermid and Stuercke, 2003; Martins et al., 2018). These can be artificially increased to ~ 50% of DW by addition of nitrogen and CO2 in a bioreactor setup in D. menstrualis (Martins et al., 2016). The crude fraction of fibers ranges between 10.2 and 14.1% of DW (Tabarsa et al., 2012; Mwalugha et al., 2015). The cellulose microfibrils in the cell wall of brown algae are embedded in a matrix of polysaccharides comprising alginates and fucans (Abdel-Fattah et al., 1978), which are estimated to comprise respectively 7.4–22.9% and 22.2% of DW (Garc a-Rios et al., 2012; Deyab et al., 2017). Storage polysaccharides are laminarans and mannitol (0.4–7.8% of DW) (Table 2). The fucoidan fractions have been particularly well-studied due to their antiviral, ROS scavenging and anti-coagulant properties (García-Ríos et al., 2012; Rabanal et al. 2014). Despite the lower content in fucans and alginates compared to, for example, Fucales, polysaccharides of Dictyotales are of particular interest due to the presence of some O-acetyl groups (García-Ríos et al., 2012). More than 60 subfractions of sulfated heteropolysaccharides can be obtained, all differing in their relative proportions of residues of D-arabinose, D-fucose, D-galactose, D-glucose, D-mannose, D-rhamnose, D-uronic acid and xylose (Hussein et al., 1979; Rabanal et al., 2014).
Protein and amino acids
As a fast-growing alga, especially in warm temperate to tropical environments, Dictyota may be of interest as a food, feed or protein source. Protein levels are highly variable, with estimates varying between 1.7 and 27.6% of DW depending on the local conditions, seasonality and nitrogen availability. It should be noted that many of the protein estimates are obtained using a traditional nitrogen-to-protein factor of 6.25, which is unsuitable for seaweeds and has been estimated as 4.55 ± 0.38 for D. menstrualis and 18 other tropical seaweeds by Lourenço et al. (2002) while Angell et al. (2016) have suggested a mean value of 5. In a bioreactor setup, the protein content can be increased by addition of nitrogen irrespective of CO2 addition (Martins et al., 2016).
Lipid and fatty acids
Estimates of total lipid content varies from 0.5 to 20.2% of DW (McDermid and Stuercke, 2003; Deyab et al., 2017). In multispecies comparisons, Dictyota is often among the brown algae displaying the highest fatty acids fractions (Burkholder et al., 1971; Montgomery and Gerking, 1980; McDermid and Stuercke, 2003; Gosch et al., 2012; Machado et al., 2014; Martins et al., 2018) and rendering a relatively high caloric content ranging from 2424 to 3500 cal g−1 (Montgomery and Gerking, 1980; McDermid and Stuercke, 2003; Pillans et al., 2004). Consequently, species of Dictyota are seen as a suitable feedstock species because the total fatty acid content is reported to exceed that of any other seaweed (Gosch et al., 2015). Furthermore, Dictyota contains large amounts of ω3 fatty acids with a low ω6/ω3 ratio ranging from 0.3 to 3.9 on a DW basis (Table 2). Dictyota is therefore appealing for its use in bio-oil industry and as a healthy food source (Tabarsa et al., 2012; Martins et al., 2016, 2018). Lipid content (Gosch et al., 2012; Radulovich et al., 2015; Deyab et al., 2017) and total fatty acids content (Gosch et al., 2012) can be highly variable in Dictyota and can be modulated depending on nitrogen and CO2 contents (Martins et al., 2016). Fatty acids spectra have been characterised for different Dictyota species (Heiba et al., 1997; Chakraborty and Santra, 2008; Gosch et al., 2012, 2015; Tabarsa et al., 2012; Martins et al., 2016, 2018) and may show a high content in PUFAs in especially D. bartayresiana (Gosch et al., 2012, 2015; Machado et al., 2014) and Dictyota sp. (Gosch et al., 2012) (Table 2).
Besides fatty acids, Dictyota possesses phenolics and phlorotannins that are included in small vesicles (physodes), which play a role in cell wall crosslinking (Deniaud-Bouët et al., 2017) and potentially antiherbivore defense (Targett et al., 1995). The phenolic content is of pharmaceutical interest. Dictyota species, however, contain relatively low contents of phenolics (0.01–1.34% of DW) compared to other brown algae (Targett et al., 1992, 1995; Targett and Arnold, 1998; Chkhikvishvili and Ramazanov, 2000). Bioflavonoids such as rutin, quercetin and kaempferol have been detected in relatively high percentages (Al-Saif et al. 2014).
Like all brown algae, Dictyota is characterised by the presence of oxylipins, C11-carbohydrates that are derived from fatty acids (Pohnert and Boland, 2002). Both adult thalli (Schnitzler et al., 2001; Wiesemeier et al., 2008) and female gametes (Phillips et al., 1990) release a blend of oxylipins. The blend of female gametes contains the pheromone, dictyotene, that attracts sperm cells (Maier and Muller, 1986). Oxylipins also function as grazing deterrent, protecting the zygotes and adult thalli (Hay et al., 1998).
Pigments
Four kinds of pigments are present in the fucoxanthin-chlorophyll a/c protein assemblies (FCPA) that perform the energy transfer that supports photosynthesis in D. dichotoma chloroplasts: Chl a, Chl c, fucoxanthin and violaxanthin in a molar ratio of 13:3:10:1 (Mimuro et al., 1990). However, water-depth effects modulate the ratios, with the ratio of chl c/chl a decreasing in light exposed samples (Perez-Bermudez et al., 1981). The carotenoid composition of adult thalli is 69% fucoxanthin, 19% violaxanthin and 12% β-carotene (Katoh et al., 1989). Violaxanthin can be interconverted to antheraxanthin or zeaxanthin in response to photoinhibition (Uhrmacher et al., 1995). Dictyota undergoes a fast recovery after photoinhibition, which suggests photoinhibition is an adaptation to strong light exposure (Nultsch et al., 1987).
Vitamins
Dictyota contains large quantities (24.5–42.8 μg g−1 fresh weight) of vitamin E, also known as α-tocopherol (Jayasree et al. 1985; de Sousa et al. 2008). The algae also contain vitamin A (4.7 μg g−1 fresh weight) and β-carotene (0.8 μg g−1 fresh weight) (de Sousa et al. 2008), but overall little is known about the vitamin content in Dictyota.
Secondary metabolites
Dictyota is a rich source of secondary metabolites which are under increasing interest due to their bioactivity. Several phenols (Zouaoui and Ghalem, 2017), sterols (Bouzidi et al., 2008), fatty acids (Gosch et al., 2015) and polysaccharides (Rabanal et al., 2014) display significant bioactivity. The main class of secondary metabolites of interest, however, are diterpenes. Diterpenes are often specific to certain species of Dictyota (Teixeira and Kelecom, 1988). The rich complement of secondary metabolites is thought to enable the survival in environments with large pressure from herbivores and pathogens and the diversity between species or even populations has been suggested to result from different evolutionary pressures in different localities (de Paula et al., 2011). Dictyota regulates the release of diterpenes after wounding which influences susceptibility to grazers (Wiesemeier et al., 2008). Up until now, more than 230 diterpenes have been isolated from Dictyota species (recently reviewed by Chen et al. 2018).
The diterpenes have been grouped in three categories depending on the first formal cyclisation of the precursor.
-
Group I diterpenes are derived by the first cyclisation between C1 and C10 of the geranyl-geraniol precursor (Teixeira and Kelecom, 1988). They contain mainly the prenylated-guaiane diterpenes. But also some prenylated-germacrane diterpenes (Sun and Fenical, 1979; Konig et al., 1991), and an prenylated-epi-elemane diterpene (Wright et al., 1993) and two prenylated-cadiane diterpenes (Kolesnikova et al., 2006) have been isolated.
-
Group II is the largest group and consists of the diterpenes that have resulted from a cyclisation of the geranyl-geraniol precursor between C1 and C11 (Teixeira and Kelecom, 1988). Based on their diterpene skeletons, they are subdivided into the dolebellane, dolestane and secodolestane diterpenes (Chen et al., 2018). One dictyoxetane diterpene was isolated (Sullivan et al., 1986).
-
Group III diterpenes are derived from a cyclisation between C2 and C10 or by ring contraction of the prenylated-germacrane. They are subdivided in the xenicane, crenulidane, dichotomane and crenulane diterpenes (Chen et al., 2018).
Utilisation
At present no published reports of cultivation of Dictyota are available and harvesting of the genus has not been industrialised as is the case for species like Porphyra, Undaria or Saccharina. Thalli are generally collected from natural populations (Kaliaperumal and Chennubhotla 2017). In Hawaii D. acutiloba is often grown in what are known as “algal gardens” (Pereira 2016).
Food for human consumption
Documented use of Dictyota appears restricted to the Caribbean, Malayan-Indonesian and Hawaiian regions where some species are either eaten raw, cooked in coconut milk, pickled or ground as flour (Pereira 2016, Brandham et al. 2002). Alginates extracted from Dictyota are also being used in various food products (Ravi et al. 2019).
Health and wellness applications
There is considerable interest in the pharmaceutical application of secondary metabolites of Dictyota (and other Dictyotales). There is a large body of literature describing the effects of diverse extracts containing secondary metabolites of Dictyota against many diseases. Because of the increasing need for bioactive molecules due to side effects or antibiotic resistance, many of the above mentioned isolated phenolics, fucans, laminarans and especially diterpenes are being tested for a diverse range of bioactivities, ranging from antioxidant to anticancer activity. While it is clear that different diterpenes may have different bioactivity, the choice of study species by the different labs is largely driven by the local availability.
Neuroprotective effect
Alzheimer’s disease is a chronic neurological disorder that has taken epidemic proportions in developed countries. Increasing the acetylcholine concentrations by inhibition of the enzyme butyrylcholinesterase (BuChE) is considered as an effective treatment against the disease (Mehta et al., 2012). Methanolic extracts of Dictyota have been shown to inhibit this enzyme and therefore are expected to have a neuroprotective effect (Stirk et al., 2007; Suganthy et al., 2010). In addition, two dolastane diterpenes present in at least several species have an inhibitory effect on Na+K+-ATPase, which is involved in the physiology of Alzheimer’s disease and a diverse range of other pathologies (Garcia et al., 2009).
Anticoagulant and antihemolytic activity
Dysfunction in coagulation and platelet aggregation may lead to diverse cardiovascular pathologies. Therefore, there is an interest in phytochemicals with an antihemostatic effect for drug design. The most available and used is heparin. Heterofucans of Dictyota (and C. cervicornis) are of interest because of their strong anticoagulant activity (Garcia et al., 2009; Costa et al., 2010). Interestingly the purified heterofucans have a higher anticoagulant activity than heparin (Albuquerque et al., 2004). Besides heterofucans, some diterpenes have also been attributed an anticoagulative effect (Moura et al. 2011; 2014). Secodolastane diterpenes from C. cervicornis where attributed an inhibitory effect against the clotting and proteolytic effects of crude snake venom of Lachesis muta, a South-American pit viper (Domingos et al., 2011). The same diterpenes did also inhibit the hemolytic effect of phospholipase A2 (a component in the venom) but could not inhibit the hemolytic effect of the crude venom (Domingos et al., 2011). Similarly, the coagulation activity and hemolytic effects of the venom of Lonomia obliqua, a toxic caterpillar, can be inhibited by extracts from Dictyota species (Domingos et al. 2009). The activities can be specific to particular diterpenes because both C. cervicornis and D. pfaffii extracts do inhibit the coagulation activity but only extracts from D. pfaffii inhibit also the hemolytic activity (Domingos et al. 2009). In vivo studies have shown that CH2Cl2/MeOH extracts from D. pulchella induce vasorelaxation in rats (Queiroz et al., 2011).
Anti-inflammatory activity
CH2Cl2 fractions of methanolic extracts or specific isolated diterpenes from Dictyota have been shown to be efficient inhibitors of nitric oxide and PGE2 generation in macrophages, suggesting these contain interesting compounds for anti-inflammatory drug discovery (Lee et al., 2008; Yoon et al., 2009; Cheng et al., 2014; Zhao et al., 2015). Anti-inflammatory effects are not confined to diterpenes, because also heterofucans may provide candidates as anti-inflammatory or antinociceptive molecules (Albuquerque et al., 2013).
Antimelanogenesis
Excess production of melanin in the skin may cause hyperpigmentation phenomena such as freckles and aging spots. A diterpene of D. coriacea was shown to exhibit antimelanogenesis effects without showing cell toxicity (Ko et al., 2013).
Anticancer activity
Extracts from Dictyota have been demonstrated to show a particularly strong cytotoxic effect on cancer cell lines and to have a relatively high antiproliferative effect compared to many other algae (Zubia et al., 2009; Guedes et al., 2013; Miranda-Delgado et al., 2018; Kosanić et al., 2019), where they can induce apoptosis of the cancer cells (Gomes et al., 2015). Different molecules in the extracts have been attributed this anticancer activity. In hexane extracts, fucosterol and two diterpenes have been identified with cytotoxic activities (Caamal-Fuentes et al., 2014). Both sulfated laminarans and fucans of Dictyota are being explored for their anticancer activity and show synergistic effects with X-radiation (Abdel-Fattah et al., 1978; Usoltseva et al., 2018; Yousefi et al., 2018; Malyarenko et al., 2019). Additionally, the polyphenol fraction was attributed an antiproliferative effect on pancreatic cancer cells (Aravindan et al., 2013). The relatively weak selectivity of the cytotoxic effect may be seen as a disadvantage (Harada and Kamei, 1997), but may be due to the diverse nature of the effect. Potential application of fucan-coated silver nanoparticles are being studied in cancer therapy (Fernandes-Negreiros et al., 2017). Next to coating of nanoparticles with its fucans, Dictyota may also be used for the environmentally friendly synthesis of anti-ruthenium nanoparticles and delivery to tumors is being explored (Yacoob et al., 2017).
Antiviral activity
Dolabelladienetriol, a dolabellane diterpene isolated from D. pfaffii, was shown to have activity against herpes simplex virus Type-1 (HSV-1), where it inhibits the replication by inhibiting reverse transcriptase in a non-competitive manner (Barbosa et al., 2004; Abrantes et al. 2010; Cirne-Santos et al. 2008). The potential drug showed low cytotoxicity in preclinical tests (Cirne-Santos et al., 2008; Abrantes et al. 2010; Miceli et al. 2012) and has shown to be non-toxic in experimental animals (Garrido et al., 2011; Garrido et al. 2017). Experiments on mice showed crude extracts from C. cervicornis are potential treatments of HSV-1 cutaneous lesions (Barros et al. 2017). The effect has been attributed to two dolastane diterpenes which represent promising anti-HSV-1 molecules (Vallim et al. 2010). The effect against HSV-1 is specific to some diterpenes, because for example isopachydictyolal from D. dichotoma and 4-α-acetyldictyodial from D. linearis did not show such a strong effect (Siamopoulou et al. 2004). CH2Cl2/MeOH extracts and isolated diterpenes from different species of Dictyota were shown to exhibit anti-HIV-1 and anti-ZIKV (Zika virus) effect, where they inhibit replication of the virus in cell cultures (Pereira et al. 2004; Abrantes et al. 2010; Barros et al. 2017; Cirne-Santos et al. 2019). The effect in D. pfaffii has been attributed to dolabelladienol A and dolabelladienol B, which showed to be even more effective against HIV-1 than dolabelladienetriol and non-cytotoxic against the tested cell lines (Pardo-Vargas et al. 2014). In D. menstrualis the anti-HIV-1 effect was attributed to two dichotomane diterpenes (Pereira et al. 2005), while in D. friabilis the effect has been attributed to dolabelladienetriol (Stephens et al. 2017). In D. plectens 2 xenicane diterpenes were found to exhibit a similar effect, while another xenicane from D. plectens was effective against H5N1 (Zhao et al., 2015). Also, antiviral activities (against HSV-1 and CVB3) of the galactofucan rich subfractions of fucoidans have been demonstrated (Rabanal et al. 2014).
Antifungal activity
Methanolic extracts of Dictyota are also of interest for their antifungal activity against Candida albicans (ATCC 10231, ATCC 14053), Aspergillus niger MTCC109 (Stirk et al., 2007; Solomon and Santhi 2008; Manzo et al., 2009; Zouaoui and Ghalem, 2017; Kosanić et al., 2019), and other fungi (Reichelt & Borowitzka 1984). One xenicane has been shown to have a mild antifungal activity against Candida albicans ATCC14053 (Manzo et al., 2009), however, diterpenes with an antifungal activity appear to be much more common in other Dictyotales species like Dictyopteris (de Paula et al. 2011).
Antibacterial activity
Although extracts of Dictyota spp. have shown in vitro antibiotic activity (Reichelt & Borowitzka 1984) they have not led to new antibiotics. Beta-lactam antibiotics comprise almost half of the total fraction of the global antibiotic usage. Their effectivity is under threat due to prolonged usage and the evolution of beta-lactamases that hydrolyse the antibiotics and render bacterial strains resistant. Dictyota methanolic extracts were shown to effectively inhibit beta-lactamases and therefore may be a source of new beta-lactamase inhibitors (Houchi et al., 2019). Several compounds from Dictyota have been attributed an antibacterial activity (see Chen et al., (2018) for a review).
Antileishmanial and antitrypanosomal activity
Different diterpenes, including dolabelladienetriol, from Dictyota showed interesting activity against Leishmania amazonensis and Trypanosoma cruzi responsible for respectively leishmaniasis and trypanosomiasis (Chagas disease) (León-Deniz et al., 2009; Soares et al., 2012; Alançia et al., 2014; Lira et al., 2016; Chiboub et al., 2019), an activity that is not shared by extracts from other marine algae and vertebrates (Bianco et al., 2013).
Antioxidant activity
Although similar antioxidant activities are not confined to Dictyota and related species, several multispecies comparisons have demonstrated that especially Dictyota (and Dictyotales) extracts have high antioxidant activity compared to other tested seaweeds (Martins et al., 2013; Tariq et al., 2015). Polysaccharide content is at least a part of the explanation of this high activity (Camara et al., 2011; Tariq et al., 2015). Also, the phenolic content of Dictyota is of interest for their antioxidant activities (Zouaoui and Ghalem, 2017; Miranda-delgado et al., 2018); however, the high antioxidant effect does not always correlate with the total phenolic content in the extract (Tariq et al., 2011; Martins et al., 2013; Van et al., 2013; Tariq et al., 2015; Chale-Dzul et al., 2017). Due to its high antioxidant potential and anti-inflammatory effects the extracts of D. coriacea are among the most chemopreventive ones among 30 seaweeds with an estimated chemoprevention index (the ratio between the cytotoxicity and the quinone reductase activity) of 4.36 using Hepa1c17 cells (Lee et al., 2008).
Biodiesel
While other macroalgae have low prospect for biodiesel production because of their low lipid content, the high lipid fraction in D. bartayresiana and Dictyota sp. (10.8–11.9% of DW) (Gosch et al., 2012) of up to 16.1% and 20.2% of DW in D. acutiloba and D. sandvicensis, respectively, suggests they may be comparable to or exceeding that of several microalgal species such as Tetraselmis, Rhodomonas and Scenedesmus and strains of Skeletonema and Isochrysis (Gosch et al., 2012; Kumari et al., 2013). However, also lower estimates (0.5–4.2% of DW) have been reported (Chakraborty and Santra, 2008; Deyab et al., 2017; Mwalugha et al., 2015; Tabarsa et al., 2012). Consequently, bioreactor-based cultures have been explored which modulate the lipid and fatty acids content of Dictyota (Martins et al., 2016) and seasonal and spatial variation of lipid and TFA contents has been monitored (Gosch et al., 2012, 2015). A protocol for nano-catalyzed biodiesel production from the lipids of D. dichotoma has been described (Khan et al., 2017).
Biofouling
Several studies have explored the effect of Dictyota extracts against biofouling (Barbosa et al., 2007; Ktari et al. 2010; Murugan and Begum, 2010; Othmani et al., 2013; Bakar et al., 2019). Larvae of invertebrates show mortality and abnormal development in response to application of diterpenes (Schmitt et al., 1998). Diterpenes of Canistrocarpus and Dictyota were shown to inhibit byssal threads of mussels (Bianco et al., 2009; Siless et al., 2018). Furthermore, coral mortality was induced in the presence of Dictyota species (Kuffner et al., 2006). Several other studies demonstrated the antibacterial activity of a range of diterpenes (reviewed by Chen et al., 2018) and sterols (Bakar et al., 2019), which can consequently inhibit bacterial biofilm formation (Viano et al., 2009; Othmani et al., 2013).
Animal feed
Traditionally dried and ground Dictyota is utilised as supplements to cattle, poultry and fish feed (Kaliaperumal and Chennubhotla, 2017). Recently, it was established that the addition of supplements of D. bartayresiana may reduce in vitro methane production of ruminants with 92.2%, and therefore offers a promising alternative for mitigating enteric CH4 emissions (Machado et al., 2014).
Conclusions
Dictyota is characterised by a thin parenchymatous thallus structure with a meristem region developing in a thallus with cortical and medullary cell layer(s) depending on the species. Its synchronously released and dividing zygotes are under increasing academic interest. Dictyota species often show a large degree of morphological plasticity. The life cycle is an isomorphic alternation of a haploid and diploid phase. As a near cosmopolitan genus, Dictyota only lacks from the polar seas. Dictyota and its taxonomically and morphologically closely associated dictyotalean sister genera can seasonally emerge as dominant species in the sublittoral and eulittoral rock pools and may form algal blooms under certain conditions. Traditionally, its commercial value has been closely connected to local uses and traditions such as food, feed or cosmetics. In more recent times, propelled by the increased interest in its secondary metabolites such as phenols, sterols, fatty acids, polysaccharides and especially diterpenes, the genus has received increased attention. Especially the demonstrated antibiofouling and pharmaceutical potential (Alzheimer’s disease, anticoagulant, anti-inflammatory, anticancer, antioxidant, antibiotic and antiviral activity) of their extracts and isolated compounds stand out. Recently, some new potential applications as biofuel source and cattle feed supplement, respectively due to its reported high lipid content and its antimethanogenic effect, have emerged warranting further exploration.
References
Abdel-Fattah AF, Hussein MMD, Fouad ST (1978) Carbohydrates of the brown seaweed Dictyota dichotoma. Phytochemistry 17:741–743
Abrantes JL, Barbosa J, Cavalcanti D, Pereira RC, Frederico Fontes CL, Teixeira VL, Moreno TML, Paixão ICP (2010) The effects of the diterpenes isolated from the Brazilian brown algae Dictyota pfaffii and Dictyota menstrualis against the herpes simplex type-1 replicative cycle. Planta Med 76:339–344
Agardh J.G. (1882) Till algernes systematik. Nya bidrag. (Andra afdelningen). Acta Univ Lund 17: 134 pp.
Agardh JG (1894) Analecta algologica. Continuatio I Acta Univ Lund 29:144 pp
Al-Saif SSA, Abdel-Raouf N, El-Wazanani HA, Aref IA (2014) Antibacterial substances from marine algae isolated from Jeddah coast of Red Sea, Saudi Arabia. Saudi J Biol Sci 21:57–64
Alançia ASD, Dos Anjos KFL, De Vasconcelos Reis TN, Higino TMM, Brelaz-De-Castro MCA, Bianco ÉM, de Figueiredo RCBQ (2014) The in vitro biological activity of the Brazilian brown seaweed Dictyota mertensii against Leishmania amazonensis. Molecules 19:14052–14065
Aisha KA, Shabana EF, El-Abyad MS, Kobbia IA, Schanz F (1995) Pulse feeding with nitrate and phosphate in relation to tissue composition and nutrient uptake by some macroalgae from the Red Sea at Ghardaqa (Egypt). J Basic Microbiol 35:135–145
Albuquerque IRL, Cordeiro SL, Gomes DL, Dreyfuss JL, Filgueira LGA, Leit EL, Nader HB, Rocha HAO (2013) Evaluation of anti-nociceptive and anti-inflammatory activities of a heterofucan from Dictyota menstrualis. Mar Drugs 11:2722–2740
Albuquerque IRL, Queiroz KCS, Alves LG, Santos EA, Leite EL, Rocha HAO (2004) Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz J Med Biol Res 37:167–171
Angell AR, Mata L, de Nys R, Paul NA (2016) The protein content of seaweeds: a universal nitrogen-to-protein conversion factor of five. J Appl Phycol 28:511–524
Aravindan S, Delma CR, Thirugnanasambandan SS, Herman TS, Aravindan N (2013) Anti-pancreatic cancer deliverables from sea: first-hand evidence on the efficacy, molecular targets and mode of action for multifarious polyphenols from five different brown algae. PLoS One 8:0061977
Azzurro E, Fanelli E, Mostarda E, Catra M, Andaloro F (2007) Resource partitioning among early colonizing Siganus luridus and native herbivorous fish in the Mediterranean: an integrated study based on gut-content analysis and stable isotope signatures. J Mar Biol Assoc UK 87:991–998
Barbosa JP, Pereira RC, Abrantes JL, Cirne Dos Santos CC, Rebello MA, De Palmer Paixão Frugulhetti IC, Teixeira VL (2004) In vitro antiviral diterpenes from the Brazilian brown alga Dictyota pfaffii. Planta Med 70:856–860
Barbosa JP, Fleury BG, da Gama BAP, Teixeira VL, Pereira RC (2007) Natural products as antifoulants in the Brazilian brown alga Dictyota pfaffii (Phaeophyta, Dictyotales). Biochem Syst Ecol 35:549–553
Barott KL, Rodriguez-Mueller B, Youle M, Marhaver KL, Vermeij MJA, Smith JE, Rohwer FL (2012) Microbial to reef scale interactions between the reef-building coral Montastraea annularis and benthic algae. Proc R Soc B 279:1655–1664
Barros CDS, Garrido V, Melchiades V, Gomes R, Gomes MWL, Teixeira VL, de Palmer Paixão ICN (2017) Therapeutic efficacy in BALB/C mice of extract from marine alga Canistrocarpus cervicornis (Phaeophyceae) against herpes simplex virus type 1. J Appl Phycol 29:769–773
Beach KS, Walters LJ (2000). Dictyota bloom in Florida Keys National Marine Sanctuary: fragments and fouling. AAUS 20th Symposium Proceedings 8: 61-63
Beach K, Walters L, Borgeas H, Smith C, Coyer J, Vroom P (2003) The impact of Dictyota spp. on Halimeda populations of Conch Reef, Florida Keys. J Exp Mar Biol Ecol 297:141–159
Bianco ÉM, De Oliveira SQ, Rigotto C, Tonini ML, Da Rosa GT, Bittencourt F, Gouvêa LP, Aresi C, De Almeida MTR, Moritz MIG, Martins CDL, Scherner F, Carraro JL, Horta PA, Reginatto FH, Steindel M, Simões CMO, Schenkel EP (2013) Anti-infective potential of marine invertebrates and seaweeds from the Brazilian coast. Molecules 18:5761–5778
Bianco ÉM, Rogers R, Teixeira VL, Pereira RC (2009) Antifoulant diterpenes produced by the brown seaweed Canistrocarpus cervicornis. J Appl Phycol 21:341–346
Billah MM, Mustafa Kamal AH, Idris MH, Ismail J (2017) Mangrove macroalgae as biomonitors of heavy metal contamination in a tropical estuary, Malaysia. Water Air Soil Pollut 228:347
Bittner L, Payri CE, Couloux A, Cruaud C, de Reviers B, Rousseau F (2008) Molecular phylogeny of the Dictyotales and their position within the Phaeophyceae, based on nuclear, plastid and mitochondrial DNA sequence data. Mol Phylogenet Evol 49:211–226
Bogaert KA, Arun A, Coelho SM, De Clerck O (2013) Brown algae as a model for plant organogenesis. Methods Mol Biol 959:97–125
Bogaert KA, Beeckman T, De Clerck O (2017a) Two-step cell polarization in algal zygotes. Nat Plants 3:16221
Bogaert KA, Beeckman T, De Clerck O (2017b) Egg activation-triggered shape change in the Dictyota dichotoma (Phaeophyceae) zygote is actin–myosin and secretion dependent. Ann Bot 120:529–538
Bogaert KA, Blommaert L, Ljung K, Beeckman T, De Clerck O (2019) Auxin function in the brown alga Dictyota dichotoma. Plant Physiol 179:280–299
Boisnoir A, Pascal PY, Cordonnier S, Lemée R (2019) Spatio-temporal dynamics and biotic substrate preferences of benthic dinoflagellates in the Lesser Antilles, Caribbean Sea. Harmful Algae 81:18–29
Bothwell JH, Marie D, Peters AF, Cock JM, Coelho SM (2010) Role of endoreduplication and apomeiosis during parthenogenetic reproduction in the model brown alga Ectocarpus. New Phytol 188:111–121
Bonaldo RM, Hay ME (2014) Seaweed-coral interactions: variance in seaweed allelopathy, coral susceptibility, and potential effects on coral resilience. PLoS One 9:30–34
Bouzidi N, Daghbouche Y, El Hattab M, Aliche Z, Culioli G, Piovetti L, Garrigues S, de la Guardia M (2008) Determination of total sterols in brown algae by Fourier transform infrared spectroscopy. Anal Chim Acta 616:185–189
Bunker FSTPD, Brodie JA, Maggs CA, Bunker AR (2010) Seasearch guide to seaweeds of Britain and Ireland. pp. [1]-224, many colour photographs. Ross-on-Wye: Marine Conservation Society
Burkholder PD, Burkholder LM, Almodovar LR (1971) Nutritive constituents of some Caribbean marine algae. Bot Mar 14:132–135
Caamal-Fuentes E, Moo-Puc R, Freile-Pelegrín Y, Robledo D (2014) Cytotoxic and antiproliferative constituents from Dictyota ciliolata, Padina sanctae-crucis and Turbinaria tricostata. Pharm Biol 52:1244–1248
Cabanillas-Terán N, Loor-Andrade P, Rodríguez-Barreras R, Cortés J (2016) Trophic ecology of sea urchins in coral-rocky reef systems, Ecuador. PeerJ 4:e1578
Camara RBG, Costa LS, Fidelis GP, Nobre LTDB, Dantas-Santos N, Cordeiro SL, Costa MSSP, Alves LG, Rocha HAO (2011) Heterofucans from the brown seaweed Canistrocarpus cervicornis with anticoagulant and antioxidant activities. Mar Drugs 9:124–138
Carrión-Cortez JA, Zárate P, Seminoff JA (2010) Feeding ecology of the green sea turtle (Chelonia mydas) in the Galapagos Islands. J Mar Biol Assoc UK 90:1005–1013
Chakraborty S, Bhattacharya T, Singh G, Maity JP (2014) Benthic macroalgae as biological indicators of heavy metal pollution in the marine environments: a biomonitoring approach for pollution assessment. Ecotoxicol Environ Saf 100:61–68
Chakraborty S, Santra SC (2008) Biochemical composition of eight benthic algae collected from Sunderban. Indian J Mar Sci 37:329–332
Chale-Dzul J, Freile-Pelegrín Y, Robledo D, Moo-Puc R (2017) Protective effect of fucoidans from tropical seaweeds against oxidative stress in HepG2 cells. J Appl Phycol 29:2229–2238
Chen J, Li H, Zhao Z, Xia X, Li B, Zhang J, Yan X (2018) Diterpenes from the marine algae of the genus Dictyota. Mar Drugs 16:159
Cheng S, Zhao M, Sun Z, Yuan W, Zhang S, Xiang Z, Cai Y, Dong J, Huang K, Yan P (2014) Diterpenes from a Chinese collection of the brown alga Dictyota plectens. J Nat Prod 77:2685–2693
Chiboub O, Sifaoui I, Lorenzo-Morales J, Abderrabba M, Mejri M, Fernández JJ, Piñero JE, Díaz-Marrero AR (2019) Spiralyde A, an antikinetoplastid dolabellane from the brown alga Dictyota spiralis. Mar Drugs 17:192
Chkhikvishvili ID, Ramazanov ZM (2000) Phenolic substances of brown algae and their antioxidant activity. Appl Biochem Microbiol 36:289–291
Cirne-Santos CC, Souza TML, Teixeira VL, Fontes CFL, Rebello MA, Castello-Branco LRR, Abreu CM, Tanuri A, Frugulhetti ICPP, Bou-Habib DC (2008) The dolabellane diterpene dolabelladienetriol is a typical noncompetitive inhibitor of HIV-1 reverse transcriptase enzyme. Antivir Res 77:64–71
Cirne-Santos CC, Barros CDS, Gomes MWL, Gomes R, Cavalcanti DN, Obando JMC, Ramos CJB, Villaça RC, Teixeira VL, Paixão ICND (2019) In vitro antiviral activity against Zika virus from a natural product of the Brazilian brown seaweed Dictyota menstrualis. Nat Prod Commun 14:1–7
Clausing RJ, Fong P (2016) Environmental variability drives rapid and dramatic changes in nutrient limitation of tropical macroalgae with different ecological strategies. Coral Reefs 35:669–680
Cole K (1967) Chromosome numbers in the Phaeophyceae. Can J Genet Cytol 9:519–530
Costa LS, Fidelis GP, Cordeiro SL, Oliveira RM, Sabry DA, Câmara RBG, Nobre LTDB, Costa MSSP, Almeida-Lima J, Farias EHC, Leite EL, Rocha HAO (2010) Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed Pharmacother 64:21–28
Cronin G, Hay ME (1996) Induction of seaweed chemical defenses by amphipod grazing. Ecology 77:2287–2301
Cunha TJ, Güth AZ, Bromberg S, Sumida PYG (2013) Macrofauna associated with the brown algae Dictyota spp. (Phaeophyceae, Dictyotaceae) in the Sebastião Gomes Reef and Abrolhos Archipelago, Bahia, Brazil. Cont Shelf Res 70:140–149
Dailer ML, Smith JE, Smith CM (2012) Responses of bloom forming and non-bloom forming macroalgae to nutrient enrichment in Hawai, USA. Harmful Algae 17:111–125
Dawes CJ, Kovach CW (1992) Ecology of the algae of a Florida Key. II. Effects of irradiance, salinity and desiccation on intertidal and subtidal populations of seven macroalgae. Bull Mar Sci 50:165–170
Dawson EY (1950) Notes on some Pacific Mexican Dictyotaceae. Bull Torrey Bot Club 77:83–93
De Clerck O (2003) The genus Dictyota (Dictyotales, Phaeophyta) in the Indian ocean. Opera Bot Belg 47:1–205
De Clerck O, Leliaert F, Verbruggen H, Lane CE, De Paula JC, Payo DA, Coppejans E (2006) A revised classification of the Dictyoteae (Dictyotales, Phaeophyceae) based on rbcL and 26S ribosomal DNA sequence analyses. J Phycol 42:1271–1288
de Sousa MB, Pires KMDS, De Alencar DB, Sampaio AH, Saker-Sampaio S (2008) Alfa, betacaroteno E alfatocoferol em algas marinhas in natura. Ciência Tecnol Aliment 28:953–958
Den Haan J, Huisman J, Brocke HJ, Goehlich H, Latijnhouwers KRW, Van Heeringen S, Honcoop SA, Bleyenberg TE, Schouten S, Cerli C, Hoitinga L (2016) Nitrogen and phosphorus uptake rates of different species from a coral reef community after a nutrient pulse. Sci Rep 6:28821
Deniaud-Bouët E, Hardouin K, Potin P, Kloareg B, Hervé C (2017) A review about brown algal cell walls and fucose-containing sulfated polysaccharides: cell wall context, biomedical properties and key research challenges. Carbohydr Polym 175:395–408
Deyab MA, El-Katony TM, El-Adl MF, Ward FM (2017) Temporal variation in chemical composition of Dictyota dichotoma (Hudson) J.V. Lamouroux (Dictyotales, Phaeophyceae) from Red Sea coast, Egypt. J Coast Life Med 5:149–155
Diaz-Pulido G, Garzón-Ferreira J (2002) Seasonality in algal assemblages on upwelling-influenced coral reefs in the Colombian Caribbean. Bot Mar 45:284–292
Done TJ (1992) Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247:121–132
Domingos TFS, Vallim MA, Carvalho C, Sanchez EF, Teixeira VL, Fuly AL (2011) Anti-snake venom effect of secodolastane diterpenes isolated from Brazilian marine brown alga Canistrocarpus cervicornis against Lachesis muta venom. Brazilian J Pharmacogn 21:234–238
Domingos TFS, Carvalho C, de Moura LA, Teixeira VL, Pereira RC, Bianco EM, Ferreira WJ, Ramos CJB, de Miranda ALP, Melo PA, Guimarães JA, Fuly AL (2009) Antilonomic effects of brazilian brown seaweed extracts. Nat Prod Commun 4:1075–1078
Duffy JE, Hay ME (1991) Food and shelter as determinants of food choice by an herbivorous marine amphipod. Ecology 72:1286–1298
Edmunds PJ (2002) Long-term dynamics of coral reefs in St. John, US Virgin Islands. Coral Reefs 21:357–367
El-Naggar M (2009) Distribution of iodine in Egyptian marine algae. Mar Sci 6:151–160
Fernandes-Negreiros M, Araújo Machado R, Bezerra F, Nunes Melo M, Alves M, Alves Filgueira L, Morgano M, Trindade E, Costa L, Rocha H (2017) Antibacterial, antiproliferative, and immunomodulatory activity of silver nanoparticles synthesized with fucans from the alga Dictyota mertensii. Nanomaterials 8:6
Ferrari R, Gonzalez-Rivero M, Ortiz JC, Mumby PJ (2012) Interaction of herbivory andseasonality on the dynamics of Caribbean macroalgae. Coral Reefs 31:683–692
Flores-Moya A, Hanelt D, Figueroa FL, Altamirano M, Viñegla B, Salles S (1999) Involvement of solar UV-B radiation in recovery of inhibited photosynthesis in the brown alga Dictyota dichotoma (Hudson) Lamouroux. J Photochem Photobiol B 49:129–135
Foster M, Neushul M, Chi EY (1972) Growth and reproduction of Dictyota binghamiae J. G. Agardh. Bot Mar 15:96–101
Gaillard J, Hardy-Halos MT, Pellegrini L (1986) Morphogenèse du Dictyota dichotoma (Huds.) Lamouroux (Phaeophyta). II. Ontogenèse du thalle et cytologic ultrastructurale des différents types de cellules. Phycologia 25:340–357
Gaillard J, L’Hardy-Halos MT (1990) Morphogenèse du Dictyota dichotoma (Dictyotales, Phaeophyta); III: Ontogenèse et croissance des frondes adventives. Phycologia 29:39–53
Gaillard J, L’Hardy-Halos MT (1977) A propos de la morphogenèse du Dictyota dichotoma (Huds.) Lamouroux (Phéophycée, Dictyotale); phénomènes corrélatifs mis en évidence sur les troncons apicaux isolés expérimentalement. Rev Algol 12:101–110
Gaillard J, L’Hardy-Halos MT (1980) Croissance et ramification chez le Dictyota dichotoma (Hudson) Lamouroux (Phéophycée, Dictyotales). Phycologia 19:159–167
Gaillard J, L’Hardy-Halos MT (1984) Morphogenesis of Dictyota dichotoma (Huds.) Lamouroux (Phaeophyceae, Dictyotales). Ann des Sci Nat Bot Biol Végétale 6:111–133
Gaillard J, L’Hardy-Halos MT (1979) Corrélations de croissance chez le Dictyota dichotoma (Huds.) Lamouroux (Phéophycée, Dictyotale); contrôles mutuels de l’apex et de la base du thalle au cours du développement. Rev Algol 14:149–162
García-Ríos V, Ríos-Leal E, Robledo D, Freile-Pelegrin Y (2012) Polysaccharides composition from tropical brown seaweeds. Phycol Res 60:305–315
Garcia DG, Bianco EM, dos de Batisa Santos MC, Peireira RC, de Faria MVC, Teixeira VL, Burth P (2009) Inhibition of mammal Na+K+ATPase by diterpenes extracted from the brazilian alga Dictyota cervicornis. Phyther Res 23:943–947
García-Gómez JC, Sempere-Valverde J, González AR, Martínez-Chacón M, Olaya-Ponzone L, Sánchez-Moyano E, Ostalé-Valriberas E, Megina C (2020) From exotic to invasive in record time: the extreme impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the strait of Gibraltar. Sci Total Environ 704:135408
Garrido V, Barros C, Melchiades VA, Fonseca RR, Pinheiro S, Ocampo P, Teixeira VL, Cavalcanti DN, Giongo V, Ratcliffe NA, Teixeira G (2017) Subchronic toxicity and anti-HSV-1 activity in experimental animal of dolabelladienetriol from the seaweed, Dictyota pfaffii. Regul Toxicol Pharmacol 86:193–198
Garrido V, Teixeira GAPB, Teixeira VL, Ocampo P, Ferreira WJ, Cavalcanti DN, Campos SMN, de Pedruzzi MMB, Olaya P, dos Santos CCC, Giogo V (2011) Evaluation of the acute toxicity of dolabelladienotriol, a potential antiviral from the brown alga Dictyota pfaffii, in BALB/c mice. Brazilian J Pharmacogn 21:209–215
Gauna MC, Cáceres EJ, Parodi ER (2013) Temporal variations of vegetative features, sex ratios and reproductive phenology in a Dictyota dichotoma (Dictyotales, Phaeophyceae) population of Argentina. Helgol Mar Res 67:721–732
Giraud G (1956) Recherches sur l’action de substances mitoclasiques sur quelques algues marines. Rev Gen Bot 63:202–236
Gomes D, Telles C, Costa M, Almeida-Lima J, Costa L, Keesen T, Rocha H (2015) Methanolic extracts from brown seaweeds Dictyota cilliolata and Dictyota menstrualis induce apoptosis in human cervical adenocarcinoma HeLa cells. Molecules 20:6573–6591
Gosch BJ, Magnusson M, Paul NA, de Nys R (2012) Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. GCB Bioenergy 4:919–930
Gosch BJ, Paul NA, de Nys R, Magnusson M (2015) Spatial, seasonal, and within-plant variation in total fatty acid content and composition in the brown seaweeds Dictyota bartayresii and Dictyopteris australis (Dictyotales, Phaeophyceae). J Appl Phycol 27:1607–1622
Grimm MR (1952) Iodine content of some marine algae. Pacific Sci VI:318–323
Guedes ÉAC, da Silva TG, Aguiar JS, de Barros LD, Pinotti LM, Sant’Ana AEG (2013) Cytotoxic activity of marine algae against cancerous cells. Braz J Pharmacogn 23:668–673
Haig D (1984) Coleochaete and the origin of sporophytes. Am J Bot 102:417–422
Hanelt D, Nultsch W (1991) The role of chromatophore arrangement in protecting the chromatophores of the brown alga Dictyota dichotoma against photodamage. J Plant Physiol 138:470–475
Harada H, Kamei Y (1997) Selective cytotoxicity of marine algae extracts to several human leukemic cell lines. Cytotechnology 25:213–219
Heiba HI, Al-Easa HS, Rizk AFM (1997) Fatty acid composition of twelve algae from the coastal zones of Qatar. Plant Foods Hum Nutr 51:27–34
Herren LW, Walters LJ, Beach KS (2006) Fragment generation, survival, and attachment of Dictyota spp. at Conch Reef in the Florida Keys, USA. Coral Reefs 25:287–295
Ho M, Carpenter RC (2017) Differential growth responses to water flow and reduced pH in tropical marine macroalgae. J Exp Mar Biol Ecol 491:58–65
Hörnig I, Schnetter R, Prud'homme van Reine WF (1992a) The genus Dictyota (Phaeophyceae) in the North Atlantic. I. A new generic concept and new species. With contributions from Coppejans E., Aschenbach-Wege K., Over J.M. Nova Hedwigia 54:45–62
Hörnig I, Schnetter R, Prud'homme van Reine WF (1992b) The genus Dictyota (Phaeophyceae) in the North Atlantic. II. Key to the species. Nova Hedwigia 54:397–402
Houchi S, Mahdadi R, Khenchouche A, Song J, Zhang W (2019) Microbial pathogenesis investigation of common chemical components and inhibitory effect on GES-type β-lactamase (GES22) in methanolic extracts of Algerian seaweeds. Microb Pathog 126:56–62
Hoyt W (1910) Alternation of generations and sexuality in Dictyota dichotoma. Bot Gaz 49:55–57
Hoyt WD (1927) The periodic fruiting of Dictyota and its relation to the environment. Am J Bot 14:592–619
Hoyt WD (1907) Periodicity in the production of the sexual cells of Dictyota dichotoma. Bot Gaz 43:383–392
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551
Hussein MM-D, Fouad ST, Abdel-Fattah AF (1979) Structural features of a sulphated, fucose-containing polysaccharide from the brown seaweed Dictyota dichotoma. Carbohydr Res 72:177–181
Hwang IK, Kim HS, Lee WJ (2005) Polymorphism in the brown alga Dictyota dichotoma (Dictyotales, Phaeophyceae) from Korea. Mar Biol 147:999–1015
Irola-Sansores ED, Delgado-Pech B, García-Mendoza E, Núñez-Vázquez EJ, Olivos-Ortiz A, Almazán-Becerril A (2018) Population dynamics of benthic-epiphytic dinoflagellates on two macroalgae from coral reef systems of the northern Mexican Caribbean. Front Mar Sci 5:487
Jayasree V, Solimabi, Kamat SY (1985) Distribution of tocopherol (vitamin E) in marine algae from Goa, west coast of India. Indian J Mar Sci 14:228–229
Kaliaperumal N, Chennubhotla V (2017) Studies on value added products from Indian marine algae - a review. Seaweed Res Util 39:1–9
Kapraun DR (2005) Nuclear DNA content estimates in multicellular green, red, and brown algae: phylogenetic considerations. Ann Bot 95:7–44
Katoh T, Mimuro M, Takaichi S (1989) Light-harvesting particles isolated from a brown alga, Dictyota dichotoma. Biochim Biophys Acta Bioenerg 976:233–240
Katsaros C, Galatis B (1985) Ultrastructural studies on thallus development in Dictyota dichotoma (Phaeophyta, Dictyotales). Br Phycol J 20:263–276
Khan AM, Obaid M, Shah MR (2017) Nanocatalyzed biodiesel synthesis from the oily contents of marine brown alga Dictyota dichotoma. Int J Green Energy 14:925–933
Ko RK, Kang M-C, Kim SS, Oh TH, Kim G-O, Hyun C-G, Hyun JW, Lee NH (2013) Anti-melanogenesis constituents from the seaweed Dictyota coriacea. Nat Prod Commun 8:427–428
Kolesnikova SA, Kalinovsky AI, Fedorov SN, Shubina LK, Stonik VA (2006) Diterpenes from the far-eastern brown alga Dictyota dichotoma. Phytochemistry 67:2115–2119
Konig GM, Wright AD, Sticher O (1991) Diterpenes from the brown alga Dictyota divaricata. Phytochemistry 30:3679–3682
Kosanić M, Ranković B, Stanojković T (2019) Brown macroalgae from the Adriatic Sea as a promising source of bioactive nutrients. J Food Meas Charact 13:330–338
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117
Ktari L, Ismail-Ben Ali A, Ben Redjem Y, Langar H, El Bour M (2010) Antifouling activity and chemical investigation of the brown alga Dictyota fasciola (Dictyotales) from Tunisian coast. Cah Biol Mar 51:109–115
Kumagae N, Inoh S (1960) Morphogenesis in Dictyotales. II. On the meiosis of tetraspore mother-cell in Dictyota dichotoma (Hudson) Lamouroux and Padina japonica Yamada. La Kromosomo 46:1521–1530
Kumari P, Bijo AJ, Mantri VA, Reddy CRK, Jha B (2013) Fatty acid profiling of tropical marine macroalgae: an analysis from chemotaxonomic and nutritional perspectives. Phytochemistry 86:44–56
Kumke J (1973) Beiträge zur Periodizität der Oogon-Entleerung bei Dictyota dichotoma (Phaeophyta). Z Pflanzenphysiol 70:191–210
Kuntze O (1898) Revisio generum plantarum vascularium atque cellularium multarum secundum leges nomenclaturae internationales cum enumeratione plantarum exoticarum in itinere mundi collectarum. (Vol. 1). A. Felix
Küpper FC, Peters AF, Kytinou E, Asensi AO, Macaya EC, Asensi AO, Vieira C, Macaya EC, De Clerck O (2019) Dictyota falklandica sp. nov. (Dictyotales, Phaeophyceae) from the Falkland Islands and southernmost South America. Phycologia 58:640–647
Kützing FT (1843) Phycologia generalis oder Anatomie, Physiologie und Systemkunde der Tange. Brockhaus pp 458
Kuhlenkamp R, Franklin LA, Lüning K (2001) Effect of solar ultraviolet radiation on growth in the marine macroalga Dictyota dichotoma (Phaeophyceae) at Helgoland and its ecological consequences. Helgol Mar Res 55:77–86
Lapointe BE (1997) Nutrient thresholds for bottom-up control of macroalgal blooms on coral reefs in Jamaica and southeast Florida. Limnol Oceanogr 42:1119–1131
Lasley-Rasher RS, Rasher DB, Marion ZH, Taylor RB, Hay ME (2011) Predation constrains host choice for a marine mesograzer. Mar Ecol Prog Ser 434:91–99
Lee SB, Lee JY, Song D-G, Pan C-H, Nho CW, Kim MC, Lee EH, Jung SH, Kim H-S, Kim YS, Um BH (2008) Cancer chemopreventive effects of Korean seaweed extracts. Food Sci Biotechnol 17:613–622
León-Deniz LV, Dumonteil E, Moo-Puc R, Freile-Pelegrin Y (2009) Antitrypanosomal in vitro activity of tropical marine algae extracts. Pharm Biol 47:864–871
Lewis RJ (1996) Chromosomes of the brown algae. Phycologia 35:19–40
Lira MLF, Lopes R, Gomes AP, Barcellos G, Verícimo M, Osako K, Ortiz-Ramirez FA, Ramos CJB, Cavalcanti DN, Teixeira VL, Amaral V (2016) Anti-leishmanial activity of Brazilian green, brown, and red algae. J Appl Phycol 28:591–598
Lirman D, Biber P (2000) Seasonal dynamics of macroalgal communities of the Northern Florida Reef Tract. Bot Mar 43:305–314
Lirman D (2001) Competition between macroalgae and corals: effects of herbivore exclusion and increased algal biomass on coral survivorship and growth. Coral Reefs 19:392–399
Longo GO, Hay ME (2017) Seaweed allelopathy to corals: are active compounds on, or in, seaweeds? Coral Reefs 36:247–253
Lourenço SO, Barbarino E, De-Paula JC, Pereira LODS, Lanfer Marquez UM (2002) Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol Res 50:233–241
Machado L, Magnusson M, Paul NA, De Nys R, Tomkins N (2014) Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS One 9:e85289
Malyarenko OS, Usoltseva RV, Zvyagintseva TN, Ermakova SP (2019) Laminaran from brown alga Dictyota dichotoma and its sulfated derivative as radioprotectors and radiosensitizers in melanoma therapy. Carbohydr Polym 206:539–547
Manzo E, Ciavatta ML, Bakkas S, Villani G, Varcamonti M, Zanfardino A, Gavagnin M (2009) Diterpene content of the alga Dictyota ciliolata from a Moroccan lagoon. Phytochem Lett 2:211–215
Martins AP, Yokoya NS, Colepicolo P (2016) Biochemical modulation by carbon and nitrogen addition in cultures of Dictyota menstrualis (Dictyotales, Phaeophyceae) to generate oil-based bioproducts. Mar Biotechnol 18:314–326
Martins AP, Zambotti-villela L, Yokoya NS, Colepicolo P (2018) Biotechnological potential of benthic marine algae collected along the Brazilian coast. Algal Res 33:316–327
Martins CDL, Ramlov F, Nocchi Carneiro NP, Gestinari LM, dos Santos BF, Bento LM, Lhullier C, Gouvea L, Bastos E, Horta PA, Soares HR (2013) Antioxidant properties and total phenolic contents of some tropical seaweeds of the Brazilian coast. J Appl Phycol 25:1179–1187
McCarty AT, Sotka EE (2013) Geographic variation in feeding preference of a generalist herbivore: the importance of seaweed chemical defenses. Oecologia 172:1071–1083
McDermid KJ, Stuercke B (2003) Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol 15:513–524
McDermid KJ, Stuercke B, Balazs G (2007) Nutritional composition of marine plants in the diet of the green sea turtle (Chelonia mydas) in the Hawaiian islands. Bull Mar Sci 81:55–71
Mehta M, Adem A, Sabbagh M (2012) New acetylcholinesterase inhibitors for Alzheimer's disease. Int J Alzheimer’s 2012:728983
Miceli LA, de Souza AMT, Rodrigues CR, Paixão ICNP, Teixeira VL, Castro HC (2012) HIV-1 reverse transcriptase: a potential target for marine products. Brazilian J Pharmacogn 22:881–888
Mimuro M, Katoh T, Kawai H (1990) Spatial arrangement of pigments and their interaction in the fucoxanthin-chlorophyll ac protein assembly (FCPA) isolated from the brown alga Dictyota dichotoma. Analysis by means of polarized spectroscopy. Biochim Biophys Acta Bioenerg 1015:450–456
Miranda-delgado A, Montoya MJ, Paz-araos M, Mellado M, Villena J, Arancibia P, Madrid A, Jara-gutiérrez C (2018) Antioxidant and anti-cancer activities of brown and red seaweed extracts from Chilean coasts. Lat Am J Aquat Res 46:301–313
Montgomery WL, Gerking SD (1980) Marine macroalgae as foods for fishes: an evaluation of potential food quality. Environ Biol Fish 5:143–153
Moreno-Sánchez XG, Abitia-Cárdenas LA, Riosmena-Rodríguez R, Cabrera-Huerta M, Gutiérrez-Sánchez FJ (2014) Diet of the yellowtail surgeonfish Prionurus punctatus (Gill, 1862) on the rocky reef of Los Frailes, Baja California Sur, México. Cah Biol Mar 55:1–8
Morrow KM, Paul VJ, Liles MR, Chadwick NE (2011) Allelochemicals produced by Caribbean macroalgae and cyanobacteria have species-specific effects on reef coral microorganisms. Coral Reefs 30:309–320
Mottier DM (1900) Nuclear and cell division in Dictyota dichotoma. Ann Bot 14:163–192
Moura LD, Bianco ÉM, Pereira RC, Teixeira VL, Fuly AL (2011) Anticoagulation and antiplatelet effects of a dolastane diterpene isolated from the marine brown alga Canistrocarpus cervicornis. J Thromb Thrombolysis 31:235–240
Moura LD, de Almeida ACM, Domingos TFS, Ortiz-Ramirez F, Cavalcanti DN, Teixeira VL, Fuly AL (2014) Antiplatelet and anticoagulant effects of diterpenes isolated from the marine alga, Dictyota menstrualis. Mar Drugs 12:2471–2484
Müller D (1962) Über jahres- und lunarperiodische Erscheinungen bei einigen Braunalgen. Bot Mar 4:140–155
Muller D, Gassman G, Boland W, Marner F, Jaenicke L (1981) Dictyota dichotoma (Phaeophyceae): identification of the sperm attractant. Science 212:1040–1041
Müller DG (1972) Studies on reproduction in Ectocarpus siliculosus. Bull Soc Bot Fr 119:87–97
Müller S, Clauss H (1976) Aspects of photomorphogenesis in the brown alga Dictyota dichotoma. Z Pflanzenphysiol 78:461–465
Murugan A, Begum MS (2010) Antifouling and antipredatory activity of natural products of the seaweeds Dictyota dichotoma and Chaetomorpha linoides. Nat Prod Res 26:975–978
Mwalugha HM, Wakibia JG, Kenji GM, Mwasaru MA (2015) Chemical composition of common seaweeds from the Kenya Coast. J Food Res 4:2
Nizamuddin M, Campbell AC (1995) Glossophorella a new genus of the Family Dictyotaceae (Dictyotales-Phaeophyta) and its ecology from the coast of the Sultanate of Oman. Pak J Bot 27:257–262
Nultsch W, Pfau J, Materna-Weide M (1987) Fluence and wavelength dependence of photoinhibition in the brown alga Dictyota dichotoma. Mar Ecol Prog Ser 41:93–97
Olsen K, Paul VJ, Ross C (2015) Direct effects of elevated temperature, reduced pH, and the presence of macroalgae (Dictyota spp.) on larvae of the Caribbean coral Porites astreoides. Bull Mar Sci 91:255–270
Othmani A, Bouzidi N, Viano Y, Alliche Z, Seridi H, Blache Y, El Hattab M, Briand JF, Culioli G (2013) Anti-microfouling properties of compounds isolated from several Mediterranean Dictyota spp. J Appl Phycol 26:1573–1584
Oudot-Le Secq M-P, Loiseaux-de Goër S, Stam WT, Olsen JL (2006) Complete mitochondrial genomes of the three brown algae (Heterokonta: Phaeophyceae) Dictyota dichotoma, Fucus vesiculosus and Desmarestia viridis. Curr Genet 49:47–58
Paddack MJ, Sponaugle S (2008) Recruitment and habitat selection of newly settled Sparisoma viride to reefs with low coral cover. Mar Ecol Prog Ser 369:205–212
Pardo-Vargas A, Oliveira IDB, Stephens PRS, Cirne-Santos CC, Paixão ICNDP, Ramos FA, Jiménez C, Rodríguez J, Resende JALC, Teixeira VL, Castellanos L (2014) Dolabelladienols A-C, new diterpenes isolated from Brazilian brown alga Dictyota pfaffii. Mar Drugs 12:4247–4259
Park JS, Lobban CS, Lee KW (2018) Diatoms associated with seaweeds from Moen Island in Chuuk lagoon, Micronesia. Phytotaxa 351:101–140
Paul VJ, Kuffner IB, Walters LJ, Ritson-Williams R, Beach KS, Becerro MA (2011) Chemically mediated interactions between macroalgae Dictyota spp. and multiple life-history stages of the coral Porites astreoides. Mar Ecol Prog Ser 426:161–170
de Paula JC, Vallim MA, Teixeira VL (2011) What are and where are the bioactive terpenoids metabolites from Dictyotaceae (Phaeophyceae). Braz J Pharmacogn 21:216–228
Peckol P, Ramus J (1992) Photosynthetic performance of deep-water macroalgae (Phaeophyta, Dictyotales) off Bermuda. Hydrobiologia 231:93–98
Peña V, Bárbara I (2010) Seasonal patterns in the maërl community of shallow European Atlantic beds and their use as a baseline for monitoring studies. Eur J Phycol 45:327–342
Pereira HS, Leão-Ferreira LR, Moussatché N, Teixeira VL, Cavalcanti DN, Costa LJ, Diaz R, Frugulhetti ICPP (2004) Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV-1). Antivir Res 64:69–76
Pereira, HDS, Leão-Ferreira LR, Moussatché N, Teixeira VL, Cavalcanti DN, Da Costa LJ, Diaz R, De Palmer Paixão Frugulhetti IC (2005) Effects of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis on HIV-1 reverse transcriptase. Planta Med 71:1019–1024
Perez-Bermudez P, Garcia-Carrascosa M, Cornejo MJ, Segura J (1981) Water-depth effects in photosynthetic pigment content of the benthic algae Dictyota dichotoma and Udotea petiolata. Aquat Bot 11:373–377
Phillips J (1992) Taxonomy and reproduction in Australian species of Dilophus (Dictyotales, Phaeophyta). Aust Syst Bot 5:657
Phillips J, Clayton M (1993) Comparative flagellar morphology of spermatozoids of the Dictyotales (Phaeophyceae). Eur J Phycol 28:123–127
Phillips JA, Clayton MN, Maier I, Boland W, Müller DG (1990) Sexual reproduction in Dictyota diemensis (Dictyotales, Phaeophyta). Phycologia 29:367–379
Phillips N, Burrowes R, Rousseau F, De Reviers B, Saunders GW (2008) Resolving evolutionary relationships among the brown algae using chloroplast and nuclear genes. J Phycol 44:394–405
Pillans RD, Franklin CE, Tibbetts IR (2004) Food choice in Siganus fuscescens: influence of macrophyte nutrient content and availability. J Fish Biol 64:297–309
Quan-Young LI, Diaz-Martin MA, Espinoza-Avalos MA (2004) Floristics, cover, and phenology of marine macroalgae from Bajo Pepito, Isla Mujeres, Mexican Caribbean. Bull Mar Sci 75:11–25
Queiroz TM, Machado NT, Furtado FF, Oliveira-Filho AA, Alustau MC, Figueiredo CS, Miranda GEC, Barbosa-Filho JM, Braga VA, Medeiros IA (2011) Vasorelaxation, induced by Dictyota pulchella (Dictyotaceae), a brown alga, is mediated via inhibition of calcium influx in rats. Mar Drugs 9:2075–2088
Rabanal M, Ponce NMA, Navarro DA, Gómez RM, Stortz CA (2014) The system of fucoidans from the brown seaweed Dictyota dichotoma: chemical analysis and antiviral activity. Carbohydr Polym 101:804–811
Radulovich R, Umanzor S, Cabrera R, Mata R (2015) Tropical seaweeds for human food, their cultivation and its effect on biodiversity enrichment. Aquaculture 436:40–46
Rasher DB, Hay ME (2010) Chemically rich seaweeds poison corals when not controlled by herbivores. Proc Natl Acad Sci U S A 107:9683–9688
Raikar V, Wafar M (2006) Surge ammonium uptake in macroalgae from a coral atoll. J Exp Mar Biol Ecol 339:236–240
Ravi A, Vijayanand S, Ramya G, Shyamala A, Rajeshkannan V, Aisverya S, Sudha PN, Hemapriya J (2019) Alginates: current uses and future perspective. In: Ahmed S (ed) Alginates: applications in the biomedical and food industries. Wiley & Sons, Hoboken, pp 283–312
Reichelt JL, Borowitzka MA (1984) Antibiotics from algae: results of a large scale screening programme. Hydrobiologia 116:158–168
Renken H, Mumby PJ, Matsikis I, Edwards HJ (2010) Effects of physical environmental conditions on the patch dynamics of Dictyota pulchella and Lobophora variegata on Caribbean coral reefs. Mar Ecol Prog Ser 403:63–74
Ribera Siguan MA, Gomez Garreta A, Salvador Soler N, Rull Lluch J, Kapraun DF (2011) Nuclear content estimates suggest a synapomorphy between Dictyota and six other genera of the Dictyotales (Phaeophyceae). Cryptogam Algol 32:205–219
Ricker RW (1987) Taxonomy and biogeography of Macquarie Island seaweeds. British Museum (Natural History), London pp. 344
Sangil C, Martins GM, Hernández JC, Alves F, Neto AI, Ribeiro C, León-Cisneros K, Canning-Clode J, Rosas-Alquicira E, Carlos Mendoza JC, Titley I, Wallenstein F, Couto RP, Kaufmann M (2018) Shallow subtidal macroalgae in the North-eastern Atlantic archipelagos (Macaronesian region): a spatial approach to community structure. Eur J Phycol 53:83–98
Searles RB, Schneider CW (1987) Observations on the deep-water flora of Bermuda. In: Ragan MA, Bird CJ (eds) Twelfth International Seaweed Symposium. W. Junk, Dordrechtpp, pp 261–266
Schmitt TM, Lindquist N, Hay ME (1998) Seaweed secondary metabolites as antifoulants: effects of Dictyota spp. diterpenes on survivorship, settlement, and development of marine invertebrate larvae. Chemoecology 8:125–131
Schnetter R, Hörnig I, Weber-peukert G (1987) Taxonomy of some North-Atlantic Dictyota species (Phaeophyta). Hydrobiologia 151:193–197
Shearer TL, Rasher DB, Snell TW, Hay ME (2012) Gene expression patterns of the coral Acropora millepora in response to contact with macroalgae. Coral Reefs 31:1177–1192
Shulman MJ, Robertson DR (1996) Changes in the coral reefs of San Blas, Caribbean Panama: 1983 to 1990. Coral Reefs 15:231–236
Setchell WA, Gardner NL (1925) The marine algae of the Pacific coast of North America. Univ Calif Publ Bot 8:383–898
Siamopoulou P, Bimplakis A, Iliopoulou D, Vagias C, Cos P, Vanden Berghe D, Roussis V (2004) Diterpenes from the brown algae Dictyota dichotoma and Dictyota linearis. Phytochemistry 65:2025–2030
Siless GE, García M, Pérez M, Blustein G, Palermo JA (2018) Large-scale purification of pachydictyol A from the brown alga Dictyota dichotoma obtained from algal wash and evaluation of its antifouling activity against the freshwater mollusk Limnoperna fortunei. J Appl Phycol 30:629–636
Stachowicz JJ, Hay ME (1999) Reducing predation through chemically mediated camouflage: indirect effects of plant defenses on herbivores. Ecology 80:495–509
Soares DC, Calegari-Silva TC, Lopes UG, Teixeira VL, de Palmer Paixão ICN, Cirne-Santos C, Bou-Habib DC, Saraiva EM (2012) Dolabelladienetriol, a compound from Dictyota pfaffii algae, inhibits the infection by Leishmania amazonensis. PLoS Negl Trop Dis 6:1–12
Solimabi, Das B (1977) Distribution of iodin in marine algae of Goa region. Indian J Mar Sci 6:6–7
Sotka EE, Hay ME (2002) Geographic variation among herbivore populations in tolerance for a chemically rich seaweed. Ecology 83:2721–2735
Sotka EE, Wares JP, Hay ME (2003) Geographic and genetic variation in feeding preference for chemically defended seaweeds. Evolution 57:2262–2276
Sotka EE, Hay ME (2009) Effects of herbivores, nutrient enrichment, and their interactions on macroalgal proliferation and coral growth. Coral Reefs 28:555–568
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson MAX et al (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57:573–583
Steen F, Aragay J, Zuljevic A, Verbruggen H, Mancuso FP, Bunker F, Vitales D, Gómez Garreta A, De Clerck O (2017) Tracing the introduction history of the brown seaweed Dictyota cyanoloma (Phaeophyceae, Dictyotales) in Europe. Eur J Phycol:1–12
Steen F, Verlaque M, D’hondt S,Vieira C, Clerck O (2019). Population structure and geographically structured reproductive strategies of the haplodiplontic seaweed Dictyota dichotoma. bioRxiv, 595587
Strasburger E (1894) The periodic reduction of the number of the chromosomes in the life. History of living organisms. Ann Bot 8:281–316
Stephens PRS, Cirne-Santos CC, Barros CDS, Teixeira VL, Carneiro LAD, Amorim L dos SC, Ocampo JSP, Castello-Branco LRR, de Palmer Paixão ICN (2017) Diterpene from marine brown alga Dictyota friabilis as a potential microbicide against HIV-1 in tissue explants. J Appl Phycol 29: 775–780
Stirk WA, Reinecke DL, Van Staden J (2007) Seasonal variation in antifungal, antibacterial and acetylcholinesterase activity in seven South African seaweeds. J Appl Phycol 19:271–276
Solomon RJ, Santhi VS (2008) Purification of bioactive natural product against human microbial pathogens from marine seaweed Dictyota acutiloba J. Ag. World J Microbiol Biotechnol 24:1747–1752
Suganthy N, Karutha Pandian S, Pandima Devi K (2010) Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar Marine Biosphere Reserve): cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci Lett 468:216–219
Sullivan BW, Albizati KF, Faulkner DJ (1986) The diterpenes of Dictyota dichotoma from the Indian ocean. J Organomet Chem 51:2736–2742
Sun HH, Fenical W (1979) Hydroxydilophol, a new monocyclic diterpenoid from the brown alga Dictyota masonii. J Organomet Chem 44:1354–1356
Svedelius N (1927) Alternation of generations in relation to reduction division. Bot Gaz 83:362–384
Tabarsa M, Rezaei M, Ramezanpour Z, Robert Waaland J, Rabiei R (2012) Fatty acids, amino acids, mineral contents, and proximate composition of some brown seaweeds. J Phycol 48:285–292
Tanaka A, Hoshino Y, Nagasato C, Motomura T (2016) Branch regeneration induced by sever damage in the brown alga Dictyota dichotoma (Dictyotales, Phaeophyceae). Protoplasma 254:1341–1351
Targett NM, Arnold TM (1998) Predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J Phycol 34:195–205
Targett NM, Boettcher AA, Targett TE, Vrolijk NH (1995) Tropical marine herbivore assimilation of phenolic-rich plants. Oecologia 103:170–179
Targett NM, Coen LD, Boettcher AA, Tanner CE (1992) International association for ecology biogeographic comparisons of marine algal polyphenolics: evidence against a latitudinal trend. Oecologia 89:464–470
Tariq A, Ara J, Sultana V, Ehteshamul-Haque S, Athar M (2011) Antioxidant potential of seaweeds occurring at Karachi coast of Pakistan. J Appl Bot Food Qual 84:207–212
Tariq A, Athar M, Ara J, Sultana V, Ehteshamul-Haque S, Ahmad M (2015) Biochemical evaluation of antioxidant activity in extracts and polysaccharide fractions of seaweeds. Glob J Environ Sci Manag 1:47–62
Taylor WR (1945) Pacific marine algae of the Allan Hancock expeditions to the Galapagos Islands. Allan Hancock Pac Expedit 12:1–528
Taylor RB, Lindquist N, Kubanek J, Hay ME (2003) Intraspecific variation in palatability and defensive chemistry of brown seaweeds: effects on herbivore fitness. Oecologia 136:412–423
Teixeira VL, Kelecom A (1988) A chemotaxonomic study of diterpenes from marine brown algae of the genus Dictyota. Sci Total Environ 75:271–283
Terauchi M, Nagasato C, Kajimura N, Mineyuki Y, Okuda K, Katsaros C, Motomura T (2012) Ultrastructural study of plasmodesmata in the brown alga Dictyota dichotoma (Dictyotales, Phaeophyceae). Planta 236:1013–1026
Tessmar-Raible K, Raible F, Arboleda E (2011) Another place, another timer: marine species and the rhythms of life. BioEssays 33:165–172
Thacker RW, Ginsburg DW, Paul VJ (2001) Effects of herbivore exclusion and nutrient enrichment on coral reef macroalgae and cyanobacteria. Coral Reefs 19:318–329
Tronholm A, Sanson M, Afonso-Carrillo J, De Clerck O (2008) Distinctive morphological features, life-cycle phases and seasonal variations in subtropical populations of Dictyota dichotoma (Dictyotales, Phaeophyceae). Bot Mar 51:132–144
Tronholm A, Sanson M, Alfonso-Carillo J, Verbruggen H, De Clerck O (2010) Niche partitioning and the coexistence of two cryptic Dictyota (Dictyotales, Phaeophyceae) species from the Canary Islands. J Phycol 46:1057–1087
Tronholm A, Leliaert F, Sansón M, Afonso-Carrillo J, Tyberghein L, Verbruggen H, De Clerck O (2012) Contrasting geographical distributions as a result of thermal tolerance and long-distance dispersal in two allegedly widespread tropical brown algae. PLoS ONE 7 (1):e30813
Uhrmacher S, Hanelt D, Nultsch W (1995) Zeaxanthin content and the degree of photoinhibition are linearly correlated in the brown alga Dictyota dichotoma. Mar Biol 123:159–165
Usoltseva RV, Shevchenko NM, Malyarenko OS, Ishina IA, Ivannikova SI, Ermakova SP (2018) Structure and anticancer activity of native and modified polysaccharides from brown alga Dictyota dichotoma. Carbohydr Polym 180:21–28
Vallim MA, Barbosa JE, Cavalcanti DN, De-Paula JC, Galvao da Silva VAG, Teixeira VL, Paixao ICNP (2010) In vitro antiviral activity of diterpenes isolated from the Brazilian brown alga Canistrocarpus cervicornis. J Med Plant Res 4:2379–2382
Van TTT, Hieu VMN, Vi TNH, Ly BM, Thuy TTT (2013) Antioxidant activities and total phenolic content of macroalgae from central coast of Vietnam. Asian J Chem 25:6639–6642
Viano Y, Bonhomme D, Camps M, Briand JF, Ortalo-Magné A, Blache Y, Piovetti L, Culioli G (2009) Diterpenoids from the Mediterranean brown alga Dictyota sp. evaluated as antifouling substances against a marine bacterial biofilm. J Nat Prod 72:1299–1304
Weber-Peukert (1985) Ontogenetische, autökologische und taxonomische Untersuchungen an ausgewählten Arten der Gattung Dictyota (Dictyotales, Phaeophyceae). I. Cytologische Daten als differenzierungsmerkmale. Nova Hedwigia 42:123–149
Williams JL (1897) The antherozoids of Dictyota and Taonia. Ann Bot 11:545–553
Williams J (1904a) Studies in the Dictyotaceae. I. The cytology of the tetrasporangium and the germinating tetraspore. Ann Bot 18:141–160
Williams JL (1904b) Studies in the Dictyotaceae: II. The cytology of the gametophyte generation. Ann Bot 18:183–204
Williams LJ (1905) Studies in the Dictyotaceae. III. The periodicity of the sexual cells in Dictyota dichotoma. Ann Bot 19:531–560
Wright AD, Knig GM, Sticher O (1993) New and highly oxidised hydroazulenoid diterpenes from the tropical marine brown alga Dictyota volubilis. Tetrahedron 49:571–580
Yabu H (1958) On the nuclear division in tetrasporangia of Dictyopteris divaricata (Okamura) Okamura and Dictyota dichotoma Lamour. Bull Fac Fish Hokkaido Univ 8:290–296
Yacoob M, Ali S, Anuradha V, Abishek R, Yogananth N, Sheeba H (2017) In vitro anticancer activity of green synthesis ruthenium nanoparticle from Dictyota dichotoma marine algae. NanoWorld J 3:66–71
Yoon W, Ham YM, Kim K, Park S, Lee NH, Hyun G, Lee WJ (2009) Anti-inflammatory activity of brown alga Dictyota dichotoma in murine macrophage RAW 264.7 cells. J Med Plant Res 3:1–8
Yousefi MK, Hashtroudi MS, Moradi AM, Ghasempour AR (2018) In vitro investigating of anticancer activity of fucuxanthin from marine brown seaweed species. Glob J Environ Sci Manag 4:81–90
Zhao M, Cheng S, Yuan W, Dong J, Huang K, Sun Z, Yan P (2015) Further new xenicanes from a Chinese collection of the brown alga Dictyota plectens. Chem Pharm Bull 63:1081–1086
Zouaoui B, Ghalem BR (2017) The phenolic contents and antimicrobial activities of some marine algae from the Mediterranean Sea (Algeria). Russ J Mar Biol 43:491–495
Zubia M, Fabre MS, Kerjean V, Lann KL, Stiger-Pouvreau V, Fauchon M, Deslandes E (2009) Antioxidant and antitumoural activities of some phaeophyta from Brittany coasts. Food Chem 116:693–701
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bogaert, K.A., Delva, S. & De Clerck, O. Concise review of the genus Dictyota J.V. Lamouroux. J Appl Phycol 32, 1521–1543 (2020). https://doi.org/10.1007/s10811-020-02121-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02121-4