Abstract
High salinity, nutrient deficiency, heavy metals, desiccation, temperature fluctuations, and ultraviolet radiations are major abiotic stress factors considered inhospitable to algal growth and development in natural and artificial environments. All these stressful conditions cause effects on algal physiology and thus biochemical functioning. For instance, long-term exposure to hyper/hypo salinity conditions inhibits cell differentiation and reduces growth. Photosynthesis is completely blocked in algae's dehydrated state, resulting in photoinhibition or photodamage. The limitation of nutrients in aquatic environments inhibits primary production via regulating phytoplankton community development and structure. Hence, in response to these stressful conditions, algae develop plenty of cellular, physiological, and morphological defences to survive and thrive. The conserved and generalized defence responses in algae include the production of secondary metabolites, desaturation of membrane lipids, activation of reactive species scavengers, and accumulation of compatible solutes. Moreover, a well-coordinated and timely response to such stresses involves signal perception and transduction mainly via phytohormones that could sustain algae growth under abiotic stress conditions. In addition, the combination of abiotic stresses and plant hormones could further elevate the biosynthesis of metabolites and enhance the ability of algae to tolerate abiotic stresses. This review aims to present different kinds of stressful conditions confronted by algae and their physiological and biochemical responses, the role of phytohormones in combatting these conditions, and, last, the future transgenic approaches for improving abiotic stress tolerance in algae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress is described as a "disparity from nominal conditions for as long as homeostasis allows" (Borowitzka 2018). Nutrient deficiency, especially nitrogen deprivation, low/high light intensity, low/high temperature, low/high salinity levels, and pH variations, are all considered physiologically stressful conditions for algae as they reduce the growth rate. The impact of these environmental factors, whether negative or positive, may significantly affect microalgal physiology and biochemical processes (Rosenberg et al. 2008). Acute variations in temperature, irradiance, salinity, or pH will disturb cellular homoeostasis by affecting cellular integrity and bio-molecular composition (Jauzein and Erdner 2013). Under salinity stress, the turgor pressure, ion distribution, and organic solutes in the cell are disrupted, resulting in reactive oxygen species (ROS), which induce cell oxidative damage (Liu and Pang 2010). Long-term exposure to hyper/hypo salinity conditions inhibits cell differentiation and decreases growth (Kumar et al. 2010).
Similarly, light intensity affects algal growth through its impact on photosynthesis (Stockenreiter et al. 2013). The growth rate of algae is maximum at saturation intensity and decreases with both rising and fall in light intensity. Microalgae undergo photoadaptation/photoacclimation, which results in a change in cell properties and increased photosynthetic activity. This will help the microalgae to survive in changed conditions of light intensity. Photoadaptation (a change in genotype that occurs over many generations in response to a change in light intensity) can occur through various processes, including changes in pigment types and quantities, growth rate, dark respiration rate, and essential fatty acid supply (Fábregas et al. 2004). Reversible phenotypic adjustments such as changes in cell volume and the number of thylakoid membranes per stack accompany morphological photoacclimation (Berner et al.1989).
Moreover, ultraviolet (UV) light (wavelengths 215–400 nm) has harmful effects on algae (Pessoa 2012). At comparable intensities, UV-B (215–380 nm) does more significant damage to the cells than UV-A (380–400 nm) (Pessoa 2012). UV-B radiation causes direct damage to DNA molecules, whereas UV-A radiation induces indirect damage by generating reactive oxygen and hydroxyl radicals. Self-shading through mat formation, migration to greater water depth (lower UV-levels), activation of anti-oxidative processes involving enzymatic and non-enzymatic strategies, or the synthesis of specific secondary metabolites such as mycosporine-like amino acids (MAAs), scytonemin, and carotenoids comprising myxoxanthophyll, β-carotene, and its derivatives (such as echinenone and zeaxanthin) are all established acclimation mechanisms in algae and cyanobacteria (Rastogi et al. 2014).
Environmental stress can cause significant damage and trigger responses that result in either acclimation or programmed cell death. Algae use various stress control and repair techniques, including modifying fatty acid saturation to change membrane fluidity, synthesising chaperonins for correct folding of denatured proteins, accumulating compatible solutes to sustain cell osmolality and controlling photosynthesis to regulate energy output and consumption. This review highlights the different kinds of abiotic stress conditions faced by algae and their physiological and biochemical responses. The role of phytohormones to combat abiotic stress and lipid production during these conditions is also discussed. In addition, transgenic approaches to cope with these stressful conditions are also considered in this review.
High salt stress
When algae are subjected to high salt stress, their photosynthetic activity is significantly reduced. This kind of inhibition appears in the photosystem II (PSII) complex. Reduced PSII activity was linked to state-2 transition in the green alga Dunaliella tertiolecta (Gilmour et al. 1984, 1985). Endo et al. (1995) confirmed this and proposed that the reduction of quantum yield of PSII electron transport in Chlamydomonas reinhardtii caused by salinity stress is connected to the state-2 transition. Lu and Vonshak (2002) demonstrated that the damage to phycobilisome and shifted the distribution of excitation energy favouring PSI in Arthrospira (Spirulina) platensis due to inhibition of the electron transport at donor and acceptor sides of PSII under salt stress.
Moreover, stress due to high salt concentration also leads to ROS generation and deficiency of different cations (such as potassium (K+), calcium (Ca2+), and manganese (Mn2+)), thus decreasing photosynthetic activity by interfering with several physiological and biochemical processes (Sudhir and Murthy 2004; Fal et al. 2022). Light-harvesting complexes (LHCs) of PSI and proteins of PSII involved in oxygen (O2) evolution are damaged by ROS at high salt concentrations in C. reinhardtii (Subramanyam et al. 2010; Neelam and Subramanyam 2013). However, to eliminate the ROS and misfolded protein production, the salt-stressed cells of C. reinhardtii, undergo upregulation of several genes such as glutathione transferase, glutaredoxin, plastid Fe superoxide dismutase 1 (SOD), thioredoxins, a GrpE family protein, heat shock protein HSP20 and heat shock factor binding proteins (Perrineau et al. 2014). In response to a spike in salt concentration, Dunaliella salina a salt-tolerant microalga, increases the Chlorophyll-a (Chl-a) content to raise the photosynthetic activity (Talebi et al. 2013).
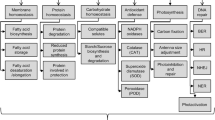
Different responses are reported in algal species to combat salinity stress, as shown in Fig. 1.
Khona et al. (2016) reported that when C. reinhardtii cells are exposed to high salt stress, they undergo a temporary stage known as “palmelloid” (Fig. 2). Palmelloid form has undergone several structural changes, including the loss of flagella, cell clustering having a minimum of two cells per cluster, increased secretion of exopolysaccharide (EPS), surrounding cells by EPS matrix, and individual cell wall thickening. The synthesis of EPS requires a lot of energy; however, the EPS matrix's protection permits to survive the stressed cells under adverse conditions. EPSs accelerate water accumulation and decrease ion influx, protecting the membrane system on palmella formation in D. salina (Wei et al. 2017).
Algal genera like Dunaliella and Chlorella have alternative strategies for dealing with salt stress. As Dunaliella does not have a cell wall, allowing the cells to rapidly alter volume during severe salinity stress by altering internal ion and glycerol content, restoring the cells’ osmotic pressure (Ben-Amotz and Avron 1980; Katz and Avron 1985; Kaçka and Dönmez 2008). On the other hand, Chlorella cells employ osmoregulation to maintain osmotic homeostasis by producing organic solutes and accumulating inorganic ions due to rigid cell walls. These solutes, also known as compatible solutes, are typically small organic compounds with a neutral charge and minimal toxicity at significant concentrations. Glycerol is an excellent example of an effective compatible solute generated by algal species during high salt stress. Glycerol accumulation in Chlamydomonas HS-5 was proportional to salt concentration, with higher salt concentration resulting in higher glycerol content (Miyasaka et al. 1998). Starch breakdown correlated closely with glycerol formation in Chlamydomonas pulsatilla, suggesting that glycerol was synthesised by the breakdown of starch (Hellebust and LIN 1989).
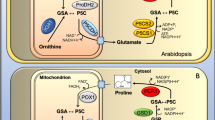
Proline is another osmoregulatory solute whose content increases linearly with increasing salt in algae (Brown and Hellebust 1978). Exogenous proline application reduces the adverse effects of high salinity by decreasing sodium (Na+) and chloride (Cl−) accumulation in C. reinhardtii (Reynoso and De Gamboa 1982). During salt stress, Picochlorum oklahomensis (Henley et al. 2004) and Picochlorum SE3 (Foflonker et al. 2016) both showed up-regulation of genes involved in proline synthesis. In contrast to Chlamydomonas sp. and D. salina, where glycerol is the primary osmolyte and starch degradation increases, proline is the primary osmolyte in Picochlorum species, and starch synthesis is increased (Xia et al. 2014; Foflonker et al. 2016). Besides proline and glycerol, trehalose also has a recognised involvement in the stability of proteins by raising the transition temperature of proteins and as an osmoregulatory molecule (Kaushik and Bhat 2003). High salt stress in Chlamydomonas (Wang et al. 2018a), Chlorella, and Scytonema increased trehalose production (del Pilar Bremauntz et al. 2011). Other polyols such as mannitol and sorbitol are also crucial in osmoregulation (Foflonker et al. 2016).
Another critical technique for dealing with salt stress is ion uptake and export via the cell membrane, which helps to maintain intracellular ion balance (Reed et al. 1981; Talebi et al. 2013). In hypersaline conditions, Dunaliella is reported to use a redox-driven sodium pump to remove Na+ ions (Katz and Pick 2001). High Na+ concentrations can interfere with the absorption of other cations, particularly K+ (Chakraborty et al. 2016). As K+ is involved in a variety of physiological activities in plants, maintaining the cytosolic K+/Na+ ratio is very critical in salty circumstances (Degl’Innocenti et al. 2009). By transporting K+ ions through membrane transport proteins, upregulation of membrane transport proteins can give resistance to high salinity in halotolerant algal species. Salt-adapted mutants of C. reinhardtii showed increased expression of numerous membrane transport proteins, as reported by Sithtisarn et al. (2017). When salt-sensitive C. reinhardtii cells were subjected to salt stress, genes for K+ ion transport were dramatically increased (Wang et al. 2018a), perhaps compensating for the disruption in K+ absorption induced by elevated Na+ ion concentrations. In addition to membrane transport proteins, the amount of two plasma membrane proteins, P150 and P60, increased significantly with increasing salt content in D. salina (Fisher et al. 1996). Furthermore, the induction of these proteins correlated with enhanced growth immediately after a severe hyperosmotic shock, indicating their role in salt acclimation.
Most green algae exhibit storage lipid buildup when subjected to salt stress. Several researches have been conducted on using high salinity conditions to improve algal lipids production. Chlamydomonas sp. JSC4 is a salt-tolerant strain obtained from a marine environment that exhibits substantial lipid accumulation when subjected to extreme salt stress. This strain was used to investigate the mechanism of lipid production in extreme salt stress (Ho et al. 2014). Under salt stress, Chlamydomonas sp. JSC4 exhibits a highly selective transition from starch synthesis to lipid synthesis (Ho et al. 2017).
Carotenoids act as antioxidants situated inside the chloroplast envelope and protect the PSII (primarily the de novo synthesis of D1 protein required to repair PSII) and LHC from the damage induced by ROS. In response to high salt stress D. salina synthesizes a high amount of carotenoids, which is exploited in industries for carotenoid production (Massyuk 1965; Borowitzka et al. 1985, 1990; Avron and Ben-Amotz 1992; Borowitzka 1995; Ye et al. 2008). At moderate levels of salt stress (0.05 M–0.15 M), C. reinhardtii and Chlorella vulgaris also show high carotenoid production (Annamalai et al. 2016).
Several studies showed that low salinity conditions also affect various physiological processes of marine algae. For instance, Wilson et al. (2004) reported the decrease in the photosynthetic efficiency (Fv/Fm) of the red alga Lithothamnion glaciale after 5-week exposure to a salinity of 3. Despite an initial reduction in photosynthetic parameters and an increase in respiration, the physiology of the Gelidium coulteri was reported to be partially recovered after a 5-week exposure to a low-salinity environment (Macler 1988). In the brown algae, Alaria esculenta, a reduction in photosynthetic efficiency was detected in the microscopic zoospores but not during adult life stages when exposed to a salinity of 20 (Fredersdorf et al. 2009). Moreover, Burdett et al. (2015) investigated the effect of low salinity on the intracellular concentration of dimethylsulphoniopropionate (DMSP), pigment composition and photosynthetic characteristics in L. glaciale. No significant difference in intracellular DMSP concentrations was observed. However, photosynthetic parameters (comprising pigment composition) exhibited a mixed response, indicating some degree of photosynthetic resilience to decreased salinity. This study shows evidence of intracellular mechanisms adopted by L. glaciale in response to reduced salinity.
Nutrient stress in algae
The limitation of nitrogen (N) and phosphorus (P) in aquatic environments inhibits primary production via regulating phytoplankton community development and structure (Harke et al. 2016). In algae, depending on which nutrient is reduced and to what degree, significant differences in biochemical composition can be found under conditions of nutrient limitation. Nitrogen and phosphorus deficiency, for example, causes lipid metabolism to switch from membrane lipid synthesis to neutral lipid storage. As a result, the overall lipid content of green algae rises, although the lipid contents in the plasma membrane decrease. Studies have shown that P-stressed cells upregulate gluconeogenesis over glycolysis transcriptionally. Trehalose concentrations rise dramatically in P-stressed cells. P-stressed cells displayed higher taurocholate levels, indicating that P-stress promotes triacylglycerol (TAG) mobilization (McLean et al. 2021). Moreover, phosphorus-containing lipids (P-lipids) of membranes are reported to get replaced with other non-P substitutes under situations of P deficiency in the diatom Thalassiosira pseudonana (Martin et al. 2011; Hunter et al. 2018). This response permits the phytoplankton to decrease its P demands in P-limited environments (Van Mooy et al. 2009).
Microalgae degrade nitrogen-containing macromolecules such as proteins, mainly when nitrogen is low. As a result of the lack of nitrogen, microalgae accumulate vast quantities of carbohydrates and fats. Current research has used nutrient limitation approaches (such as sulfur, nitrogen, and phosphate) to induce microalgae to convert protein or peptides into carbohydrates to enhance carbohydrate accumulation (Dragone et al. 2011; Harun and Danquah 2011). The carbohydrate content of the microalga C. vulgaris rose to 22.4% from the normal content of 16.0% on a dry weight basis under nutritional (nitrogen) stress, which is more acceptable in terms of the biomass required to create bioethanol (Kim et al. 2014).
Depending on the nutritional composition of the growth medium, the same algal strain might be a source of various fatty acids. Saturated fatty acids, particularly palmitic acid and total lipid content in the green alga Scenedesmus obliquus, are affected by Na, iron (Fe), cobalt (Co), and molybdenum (Mo). High potassium and magnesium trigger the production of the most polyunsaturated acids (PUFA) and oleic acid, whereas nitrogen and phosphorus trigger the least. The maximum levels of monounsaturated acids (MUFAs), particularly α-linolenic acid (ALA), are acquired when nitrogen and phosphorus deficiency retards development and results in a buildup of fatty acids that form MUFAs, particularly elaidic acid (Darki et al. 2017).
Microalgae growth and phosphate absorption are also directly related to biomass production (Solovchenko et al. 2016). Chu et al. (2013) showed that in phosphate-sufficient circumstances, the lipid yield of C. vulgaris for biodiesel generation was 58.39 mg L−1 day−1, which was more significant than in phosphate-deficient situations. Consequently, it is possible to deduce that phosphate is a crucial macronutrient for the production of microalgal lipids. However, due to a lack of light and a decline in carbon dioxide (CO2) and O2 levels in the growth medium, the phosphorus absorption rate by microalgae can achieve saturation (Chu et al. 2013).
Few experimental studies in the literature highlight the effect of high concentrations of nutrients on the physiological processes of algae. Reef et al. (2012) reported the effect of nutrient enrichment (N and P) on the growth rate, photosynthesis, nucleic acid composition, and elemental stoichiometry of three coral reef macroalgae (Caulerpa serrulata, Laurencia intricata, Sargassum polyphyllum). They observed that nutrient enrichment had positive effects on photosynthetic rates and investment in RNA. However, no correlation of growth was found with either photosynthetic rates or RNA content. Macroalgae, especially L. intricata, accumulated P to very high levels (> 0.6% of dry weight). Negative effects of P accumulation on growth were observed above 0.21%. N was not stored, but evidence of futile cycling (significant reduction in N signatures following the enrichment) was observed. The ability to store large amounts of P is probably an adaptation to the tropical oceans' patchy and low nutrient environment. Moreover, nutrient enrichment of lakes leads to eutrophication, which results in the growth of harmful and undesirable algal species. It causes changes in the physical and chemical quality of water and sediments, affecting the entire ecohydrology of lakes along with variations in diversity, composition and richness of algal species (Dubey and Dutta 2020).
Desiccation stress
Water-stressed environments lead to desiccation in certain poikilohydric plants such as algae and lichens quickly, as they cannot manage their water content actively. In the dehydrated state of algae, photosynthesis is completely blocked, and due to which, it cannot use any absorbed energy for electron excitation, which results in photoinhibition or photodamage. Allakhverdiev et al. (2008) reported different desiccation sensitive sites in the photosynthetic machinery of cyanobacteria, green algae, lichens, and mosses. These sites include the photosystems, particularly PSII with its oxygen-evolving complex, ATP-generation, and carbon assimilation processes. Due to desiccation in algae, the supply of CO2 used in carbon fixation may also decrease, resulting in the decrease of the D1 protein repair by inactivating the translation machinery (Takahashi and Murata 2008). Hence, it is not the photodamage but the loss of repair mechanism responsible for reducing photosynthesis in green algae during desiccation.
Terada et al. (2021) reported that the photosynthetic response to dehydration stress differs between two heteromorphic life-history stages of the red alga Neopyropia yezoensis f. narawaensis collected from Saga, Kyushu Island, Japan. In the microscopic sporophyte, the effective quantum yield of photosystem II dropped to zero after a 5-min of acute emersion (~ 1440-min) under 50% humidity and did not recover to initial values regardless of a following 24 h immersion in seawater. However, the macroscopic gametophyte almost recovered to initial values after the subsequent 24 h immersion in seawater. Thus, their results suggest that the photochemical efficiency in microscopic sporophytes appears to be sensitive to dehydration stress, unlike the macroscopic gametophyte.
Gasulla et al. (2013) found ultrastructural alterations in Asterochloris erici cells after 3 h of rehydration following quick (60 min) or gradual (5–6 h) desiccation. Delayed dehydration led to an increase in the number of lipid bodies (with a decrease in their size), the amount of starch deposits and electron-dense deposits in the chloroplasts (Gasulla et al. 2013). The plasma membrane remained somewhat retracted from the cell wall in the progressively dried and rehydrated cells. Rapidly dried Asterochloris cells, on the other hand, showed a clear degenerate ultrastructure after being rehydrated. The cytoplasm was highly vacuolated and filled with lipid bodies. The cytoplasm and the chloroplasts still appeared shrunken, thylakoids were swollen or fused, and numerous starch deposits were visible (Gasulla et al. 2013). It also exhibited extensive plasmolysis and cytolysis. However, even with this damage, the cells survived the dehydration treatment.
Recently, Terlova et al. (2021) found that the degree of recovery from dehydration followed by short- and long-term rehydration in the case of the green algae Tetradesmus spp. was dependent on the habitat of origin and the dehydration scenario in terrestrial, but not in aquatic species. During dehydration and rehydration, both aquatic and desert species maintained their cell ultrastructure uniformly, but staining with an amphiphilic styryl dye showed damage to the plasma membrane due to osmotically induced water loss in aquatic species. Thus, their analyses indicate that terrestrial Tetradesmus possess a vegetative desiccation tolerance phenotype, making them suitable for comparative omics studies to investigate the origins of the desiccation machinery in that group.
One method against desiccation of aeroterrestrial and aquatic green algae is to prevent dehydration through self-protection. Klebsormidium, an aeroterrestrial filamentous green algae, can form multi-layered mat-like structures on top of or interwoven with the upper millimetres of soil in natural conditions, resulting in a high degree of self-shading and reduced water loss from individual filaments within such a population. Arctic Zygnema sp. is also reported to form mats (Pichrtová et al. 2013) and provides desiccation tolerance in the field-collected Zygnema ericetorum in the Alps (Aigner et al. 2013).
Temperature stress response of algae
Like higher plants, numerous metabolic activities of algae are affected due to fluctuations in temperature. Many studies highlight the effect of temperature change on photosynthetic activity (Zheng et al. 2020), the composition and production of lipids (Calhoun et al. 2021), and many other macromolecules of algae (Zhao et al. 2020a). Zhang and Liu (2016) have reported that the activity of PS II was reduced with increasing temperature (from 25 to 37 °C) due to its structural damage, while the activity of PSI was increased and synchronized with high O2 production in the marine cyanobacterium Arthrospira (Spirulina). However, on increasing the temperature to 40 °C, the rate of photosynthetic O2 evolution was decreased, and severe reduction in PSII activity, but the rise in PSI activity was reported. Thus, photosynthetic activity of Arthrospira increased at heat stress (30–37 °C) by upregulating the PSI electron transport activity, and it was decreased at strong heat stress (40 °C) due to inhibition of PSII electron transport activity. Chlorella pyrenoidosa is cultivated largely due to its commercial importance. In C. pyrenoidosa, high temperature (38 and 41 ℃) stimulates the production of active oxygen species that damage photosynthetic machinery due to the suppression of activities of antioxidant enzymes at 41 ℃ (Ma et al. 2020).
Algae also show broad acclimations and tolerance to changing temperatures (Zheng et al. 2020), such as changes in lipids and fatty acid proportions with fluctuating temperatures. With increasing temperature, the amount of PUFA reported being reduced in Navicula, a diatom collected from Antarctica (Teoh et al. 2013) and in Nannochloropsis sp. (Hu and Gao 2006). In addition, a decrease in temperature increased the proportion of unsaturated and short-chain fatty acids in algae has also been documented in several studies (Mühling et al. 2005; Mangelsdorf et al. 2009).
A study on a high-temperature tolerant strain of Pyropia haitanensis showed that high-temperature response depends upon the length of exposure to stress. Short-term exposure caused changes in transcriptome profile simultaneous reduction in photosynthesis and utilization of energy, while long-term exposure induced an anti-oxidative response with an increase in energy utilization (Wang et al. 2018b). Tolerance to high temperature in some algal species may depend upon the biogeographical distribution that has also been reported. For instance, a difference in tolerance capacity to high temperature has been observed in two strains of the dinoflagellate Alexandrium tamarense collected from Japan and Malaysia (Kobiyama et al. 2010). Malaysian strains could survive at a higher temperature range (15- 30 ℃) than Japanese strains (0 -25℃), delineating their biogeographical boundaries.
The low-temperature stress is less explored than the high temperature in the case of microalgae (Ermilova 2020). However, some recent studies have focused on finding the effects of cold stress in C. reinhardtii (Zalutskaya et al. 2019; Li et al. 2020), a unicellular green alga, the best available model for studying the response to temperature fluctuations (Ermilova 2020). A change in the production of heat shock proteins (HSPs) as a low-temperature acclimation has also been reported in C. reinhardtii (Maikova et al. 2016). It shows variations in the expression of 3471 genes responsible for various biological processes, including cell cycle, protein synthesis, and protein kinase-based phosphorylation under cold stress (Li et al. 2020). The C. reinhardtii showed a decrease in growth due to photo-oxidative damage of several macromolecules under low temperatures (Zheng et al. 2020). A recent study by Calhoun et al. (2021) on a halotolerant microalga Scenedesmus reported an increase in the expression of genes that encode fatty acids, metabolic enzymes, and variations in the levels of amino acids under cold stress. Menegol et al. (2017) demonstrated increased ω3-fatty acids production due to low temperature in Heterochlorella luteoviridis.
CO2/pH/ocean acidification stress
The process in which a rise in atmospheric CO2 leads to a drop in the pH of the ocean surface is known as ocean acidification. CO2 uptake by the ocean changes the carbonate chemistry of seawater following a reduction in pH (Raven et al. 2005). Experiments have shown that increasing pCO2 causes decreased calcification of crustose coralline algae (Anthony et al. 2008). Burdett et al. (2012) examined the effect of low pH on the red coralline alga L. glaciale on epithelial cell morphology and DMSP/DMS(P) production. No change in DMS(P) production was observed at low pHbut cracks were observed between the cell walls of the algal skeleton. They proposed that this structural change may cause membrane damage that allows DMS(P) to leak from the cells into the water column, with subsequent implications for the cycling of DMS(P) in coralline algae habitats. Kamenos et al. (2013) observed the coralline algae survived by enhancing their rate of calcification during the day to compensate for the dissolution that happens during the night at low pH. Moreover, when the low pH change occurred at a fast rate, they observed the weakening of the calcite skeleton. The weakening of the structure decreases the potential of the alga to withstand wave energy (Ragazzola et al. 2012). Lithothamnion glaciale also show changes in the structure after incubating under increasing pCO2 (589 μatm). In the case of Chlorella ellipsoidea the carbohydrate content attained its maximum value at alkaline pH while the pigment content decreased. Moreover, vitamin E content was higher at pH 10 than at pH 6, but there was a significant reduction in vitamin C content of C. ellipsoidea both at pH 6 and pH 10 (Khalil et al. 2010).
Different macroalgae respond differently to increasing pCO2. The red alga Lomentaria articulata, Gracilaria spp and Porphyra yezoensis show an increase in the growth rate under increasing CO2 concentration (Gao et al. 1991, 1993; Kübler et al. 1999). Several macroalgae are reported to show neutral effects on the growth at elevated CO2 levels (Roleda et al. 2012). Moreover, coralline alga Arthrocardia corymbosa showed the highest growth rate at pH 8.05; however, at pH 7.65, the growth rate was lowest (Roleda et al. 2007). In the coralline alga Lithophyllum cabiochae respiration was unaltered by pCO2, but photosynthesis was decreased under elevated pCO2.whereas calcification responds differently depending on the season; net calcification decreased with increasing temperature under elevated pCO2 but increased under ambient pCO2 with rising temperature (Martin et al. 2013).
Some experimental results of ocean acidification effects on calcareous marine macroalgae are listed in Table 1. Most studies indicate a negative effect of low pH and high CO2 on calcification.
Heavy metal (HM) stress
Metals at low concentrations are essential for algal cells to carry out cellular functions as they act as cofactors for nitrogen assimilation (Fe, V and Mo in nitrogenase (Bothe et al. 2010)), DNA transcription, RNA polymerase (such as Zn (Zinc) (Sunda 2012)), and CO2 fixation (Zn in carbonic anhydrase). However, high concentrations of HMs such as Chromium (Cr), Lead (Pb), Cadmium (Cd), Arsenic (As), Mercury (Hg) in algal cells show adverse effects like blockage of cell division, reduction in photosynthesis and inhibition of various thiol group-containing enzyme activities (Monteiro et al. 2012).
Multiple studies highlight the effect of HMs on algae exist in the literature (Table 2). For instance, the effect of HMs such as copper (Cu) and Cd was carried out on the diatom Amphora coffeaeformis. These HMs drastically decreased the protein and carbohydrate content. The lipid and amino acid content were also reduced. Thus, high concentrations of these HMs affect the diatom by reducing its growth and biochemical composition (Anantharaj et al. 2011). On the other hand, Cd plays a beneficial role in diatoms; they use Cd as a catalytic metal atom in carbonic anhydrase (CA), where Zn is nearly depleted in oceans (Park et al. 2007; Xu et al. 2008). In Ulva lactuca exposure to high Cr (VI) concentration decreased cell viability and altered thallus cell morphology. When the cells were treated with 1 and 5 mM of Cr (VI), the amount of necrotic cells increased around 76.93% and 84.23%, respectively (Ünal et al. 2010).
Recently, the influence of Zn and Cu HMs on the reduction of photosynthesis pigments in cells of C. vulgaris has been shown (Kondzior and Butarewicz 2018). After 7 days of incubation, at the highest Cu concentration, C. vulgaris cells contain less carotenoid, Chl-a and Chl-b content by 60%, 63% and 58% respectively. The influence of Zn concentration shows that C. vulgaris reduced carotenoid content by 79%, while Chl-a and Chl-b were reduced by 88% in 79%, respectively, compared to the control. Hernandez (2016) reported that the primary photosynthetic processes of Zygnema were sensitive to Zn and Cd. However, in cultures treated with Zn and Cd (66 mM), the potential quantum yield (FV/FM) of PS II and relative fluorescence decline ratio (RFd) was decreased, the PS II centres still shown photosynthetic activity even after 5 h 40 min exposure to the heavy metals. Thus they concluded that Zygnema is relatively resistant to these heavy metals. Moreover, Chen et al. (2016) reported that the Hill reaction activity (HRA) of chloroplast was decreased at a high concentration of Cu (II) under both dark and illuminating conditions in C. vulgaris. Content of malondialdehyde (MDA) and activities of catalase (CAT) and SOD were also reduced at high Cu2+, accompanied with the formation of ROS, thus inhibiting the algal growth. However, Cu is an essential element at low concentrations and acts as a cofactor in several enzymes such as cytochrome c oxidase, plastocyanin, Cu/Zn superoxide dismutase, polyphenol oxidase, amino oxidase and laccase (Yruela 2005).
In Gracilaria tenuistipitata, the addition of sub-lethal concentrations of Cd2+ and Cu2+ for the short-term causes increased oxidative stress, decreasing growth and increasing lipids and proteins oxidation. However, algae respond to this oxidative stress by increasing the activity of the reactive oxygen metabolism. As Cu2+ addition has increased SOD, CAT, and ascorbate peroxide (APX) activities, while the addition of Cd2+ only induced the catalase activity. Additionally, the amount of β-carotene and lutein has increased by adding both HMs (Collén et al. 2003).
UV-A and B stress on algae
Increasing the amount of ultraviolet radiation (UVR) on the earth's surface has a significant direct negative effect on photoautotrophs like plants and algae, as they cannot escape light because it is needed for photosynthetic processes. This UVR caused morphological alterations in certain macroalgal species. In Laminaria ochroleuca, high UVR induced tissue deformation, blistering, lesions, and thickening of the meristematic tissues of the lamina (Roleda et al. 2006). However, many organisms, such as cyanobacteria and algae, have evolved a variety of strategies to minimise UVR damage, including behavioural avoidance, photoprotection, and DNA repair mechanisms. Many photosynthetic organisms have evolved coping mechanisms against such changes, including the synthesis of UV defensive compounds such as MAAs, phlorotannins, antioxidants, phenolics, HSPs, DNA repair mechanisms, and microRNA regulation. Many Arctic and Antarctic strains of Zygnema have been shown to produce sporopollenin as extracellular cell walls of zygospores in response to UV exposure. An extracellular mucilage coating in some polar Zygnema strains acts as a protective sheath. Zygnema also has macroscopic mat production to self-shade and shield cells from excessive radiation (Holzinger et al. 2009).
Effects of UV-B radiation on algae
Effects on photosynthesis
Under increased UV-B (280-315 nm), the marine microalgae Tetraselmis (Platymonas) subcordiformis and Nitzschia closterium show a decrease in growth rates, Chl-a content, and carotenoid content (Zhang et al. 2005). When measured using the Chl-a fluorescence technique, photosynthetic output in some of the Arctic and Antarctic strains of Zygnema was reduced at increased UV to photosynthetically active radiation (PAR), likely due to a reduction in PS-II efficiency under UV stress (Pichrtová et al. 2013). Overall photosynthesis decreases when vertical mixing brings deeper organisms to the surface and vice versa, as photoinhibition of deep-water algal species occurs, and upper dwellers travel deeper, where light becomes a limiting factor for them. UVR causes mild photosynthetic inhibition in eulittoral species, but substantial inhibition in sublittoral species and eulittoral species recover faster from stress than sublittoral species. Reproductive cells also are more vulnerable to increased UVR than gametophytic stages, and the impact is species-specific, as Condrus carpospores were more sensitive to increased UVR than Mastocarpus carpospores (Roleda et al. 2013).
Damage to DNA
UV-B causes DNA to modify its molecular structure. Formation of dimers and pyrimidine primidone photoproducts, which modify DNA and interfere with proper transcription and replication, as well as being misread into genetic codes, resulting in mutations and death. In D. salina, UV-B directly damaged cellular DNA, causing physiological damage and elevated death rates (Tian and Yu 2009). High UV to PAR ratio causes DNA damage in Arctic and Antarctic Zygnema strains (Pichrtová et al. 2013).
ROS production
The majority of microalgae suffer from oxidative damage by increased UV-B levels. Higher amounts of thiobarbituric acid reactive substance (TBARS) and hydrogen peroxide (H2O2) have been reported in D. salina in response to increased UV-B levels, but no such change in controls, indicating acclimation to reduce UV or solar radiation levels (Tian and Yu 2009). ROS generation and oxidative stress are also seen in Zygnema strains from the Arctic and Antarctic (Pichrtová et al. 2013).
Suppression of antioxidant system
Non-enzymatic antioxidants such as carotenoids and glutathione (GSH) were reported to decrease under increased UV-B in T. subcordiformis and N. closterium. In both species enzymatic antioxidants like SOD and CAT were also reduced as UV-B radiation levels increased. UV irradiation has different adverse effects depending on the species. For N. closterium, increased UV-B is more harmful than for P. subcordiformis (Zhang et al. 2005).
Adaptations against harmful UV radiations in algae
DNA repair
Algae primarily use photoenzymatic repair in which DNA photolyase monomerizes cyclobutane dimers in the presence of UV-A or visible light to repair damaged DNA. Nucleotide excision repair is enrolled to recognise damaged DNA strands, then excision and resynthesis of the damaged portions by DNA polymerase enzyme. Damaged DNA is repaired by the recombinational repair mechanism, bypassed by the replication mechanism (Karentz et al. 1991). In certain arctic and Antarctic Zygnema strains, photodamage to DNA activates multiple DNA repair pathways (Pichrtová et al. 2013).
Adaptations for photosynthesis
Short-term acclimation, such as fluorescence or heat dissipation via the xanthophyll cycle, or energy redistribution between the two photosystems, are made to protect the photosynthetic system against excessive UVR. To protect macroalgal species from ROS, defensive mechanisms such as antioxidant enzymes, carotenoid production inside cellular membranes, and the creation of water-soluble reductants occur in the cytosol during long-term UVR exposure (Dunlap and Chalker 1986). UVR damages PS-II directly, resulting in ROS generation, which slows protein synthesis, particularly of PS-II D1 proteins, which are necessary to replace damaged D1 proteins (Nishiyama et al. 2004). Photodamage caused by high UVR is controlled by the steady-state oxidation–reduction of the main quinone acceptor (QA). Seaweeds in the sublittoral zones are particularly vulnerable to UVR because they cannot downregulate photosynthesis via photoprotection (Hanelt and Nultsch 2003).
Production of mycosporine- like amino acids
MAAs are chemical compounds with a low molecular weight that absorb the most light in the UV range of 310-365 nm. They are called MAAs because they're similar to mycosporines found in terrestrial fungi. Some of the MAAs that have been identified in algal species are included in the table below (Table 3). They are essentially sunscreen chemicals that protect organisms from damaging UV radiation and act as antioxidants that scavenge toxic ROS.
The percentage of MAAs reported being greatest in red algae and with the most MAAs kinds and numbers (Sun et al. 2021). MAAs in macroalgae have been found in the arctic to tropical species. MAAs levels have been observed to be decreasing in species that grow at greater depths and in species that live at higher latitudes. The distribution of MAAs in macroalgae is mainly based on three patterns: Initially high MAAs content with no further growth in their amount after light treatment, increased MAAs content with increasing light treatment but no initial MAAs or generation after light treatment (Sinha et al. 2001).
Antioxidant production
Increased UV radiation favoured a rise in Chl-a in case of D. salina at first, although carotenoid content did not appear to alter. Many green microalgae, such as Chlamydomonas nivalis (Bidigare et al. 1993), Chlorella sp. (Sayed and Hegazy 1992), Chlamydomonas nivalis (Remias et al. 2010), can accumulate secondary carotenoids under stress. Some Arctic and Antarctic strains of Zygnema also exhibit higher quantities of secondary carotenoids, but no secondary carotenoids or MAAs have been found in Zygnematophyceae (Remias et al. 2012).
Haematococcus pluvialis accumulates astaxanthin in cytoplasmic globules. β-carotene synthesised in the chloroplast is first transported to the cytoplasm for oxygenation, where the keto group is added to C4 and C4', as well as hydroxyl groups to C3 and C3'. This is done using the products of crto/bkt, and crt R-b, which are nuclear genes that encode phytoene desaturase. Nuclear factor X regulates the expression of these genes. Three distinct bkt genes have been identified, each of which is up-regulated at different amounts in response to stress. The presence of numerous bkt genes has been shown to be critical for H. pluvialis' production of significant quantities of astaxanthin under stressful conditions (Huang et al. 2006). When green algae, such as H. pluvialis, are stressed, they produce secondary ketocarotenoids such as canthaxanthin and astaxanthin. Astaxanthin storage is a cost-effective way to store energy and carbon in less demanding environments. Furthermore, the high quantity of astaxanthin prevents oxidative stress generated by various stressors, including UV-B radiation, making it a multifunctional stress response (Lemoine and Schoefs 2010).
Production of phenolics
The production of phenolics in several arctic and Antarctic Zygnema species represents a stress adaptation. Because phenolics are stored in electron-dense particles and vacuoles at the cell's perimeter, they operate like UVR screens, safeguarding its internal organelles. After UV exposure, Antarctic strains exhibit the largest quantities of phenolics and have high variable fluorescence by maximum fluorescence (FV/FM) ratio, indicating that phenolics preserve photosynthetic machinery. Zygnemopis decussata with high phenolic content has a high FV/FM ratio and the best photosynthetic rates (Figueroa et al. 2009). Phenolics, as phlorotannins, are widely found in brown algae (Abdala-Díaz et al. 2006), can serve as cell wall components (Schoenwaelder 2002), deter herbivores (Targett and Arnold 1998), and protect from UVR (Pavia et al. 1997). Several studies have found that the release of phlorotannin in response to UV exposure varies depending on the species, season, and stage of thalli formation. After two weeks of UV-B treatment, the concentration of phlorotannin in Ascophyllum nodosum (Pavia et al. 1997) increased by 30%. However, no similar effect of UV-B on the concentration of phlorotannin was found in another investigation of Fucus gardneri juveniles and embryos (Henry and Van Alstyne 2004). Lack of phlorotannin accumulation in Fucus vesiculosus under UV-B exposure is linked to lack of overexpression of genes Pks-III and other genes involved in phlorotannin modifications such as ast6 and vbpo, implying that this metabolism is not activated and only constitutive accumulation of phlorotannins occurs during the development of F. vesiculosus rather than inducible processes (Creis et al. 2015). Several Chlorophyceae and Rhodophyceae have been shown to have phlorotannins, which operate as UVR absorbers.
MicroRNA regulation
Because most of the UV adaptation in algae has focused on physiological control and linked protein-coding genes, there have been few publications on connected protein non-coding genes. Heat stress caused Cre-miRNA to be downregulated, responsible for heat shock adaptation. Using qPCR and bioinformatics computing, the function of Cre-miR914 and its target gene was determined under UV stress. Cre-miR914 was downregulated, resulting in increased expression of its target gene, ribosomal protein L18 (RPL18), promoting UV-B adaptation in Chlamydomonas (Fig. 3). So, overexpression of Cre-miR914 lowered UV-B tolerance in algae, but overexpression of RLP18 increased UV-B tolerance and lowered ROS and MDA levels (Wang et al. 2019a).
Mechanical stress
Macroalgae experience considerable mechanical stress in the form of hydrodynamic movements (acceleration, drag, and lift). Studies as early as 1932 (Delf 1932; Koehl 1982, 1986) have shown that although the seaweeds are dependent upon ambient seawater flow for the transport of nutrients and are generally adapted to hydrodynamic stress, occasional wave actions could be powerful enough to shear the thallus (“pruning”), especially in wave-swept rocky shores. In general, benthic macroalgae are more susceptible to hydrodynamic stresses than planktonic species. Among diatoms, studies have shown that nonfilamentous species have higher resistance than filamentous ones against mechanical stress (Biggs and Thomsen 1995).
Various adaptations against mechanical stress have been documented in marine algae, including the cell wall composition of long-chain polysaccharides (Janot and Martone 2016) and the ability to secrete viscous mucilage. Recently discovered porous helical microstructure of mineralized coralline alga Jania sp. might also play roles in mechanical stress resistance as an adaptation (Bianco‐Stein et al. 2020). A morphological convergence in the form of cellulosic secondary cell-wall in coralline algae at wave-swept habitats has also been described recently (Martone et al. 2019). Phylogenetic analysis of character evolution of uncalcified joints (genicula) in coralline red algae indicated three independent origins as a bending strategy to cope with the hydrostatic force (Janot and Martone 2018). Benthic seaweeds at turbulent coasts like Chondracanthus exasperates tend to have softer, extensible and flexible tissues that act as a shock absorber and stipes that can be twisted and bent (Koehl 2000). The red seaweed Mastocarpus papillatus has been shown that its stipe had similar thickness throughout (to avoid a single point prone for shear), and its thallus had a streamlined shape to flow along with waves (Carrington 1990). Mechanical forces were also reported to constrain the size of intertidal seaweeds in wave-swept habitats (Gaylord et al. 1994). Such physiological adaptations are expected to have ramifications in the form of trade-offs with other physiological processes, including photosynthesis, in the so-called ‘form-function hypothesis’ (Dudgeon et al. 1995). Morphological switch from one thallus morphology to another in response to stress is a well-known adaptation in marine algae. For example, the brown seaweed Ecklonia radiata have shown that the species had wide, thin thallus at sheltered habitats while narrow, thick thallus with thick stipes at exposed habitats (Fowler-Walker et al. 2006). Reciprocal transplantation of algae from one habitat to another also confirmed this morphological plasticity in response to environmental constraints. Similar morphological plasticity in response to exposure gradients has also been reported for Laminaria longicruris (Gerard and Mann 1979) Durvillaea potatorum (Cheshire and Hallam 1989), F. vesiculosus (Bäck 1993) and Egregia menziesii (Blanchette et al. 2002). A recent study approached physiological adaptation to the wind-swept habitat in a more integrative fashion, combining biomechanics with seasonality and herbivory in kelp E. menziesii (Burnett and Koehl 2019). They suggest correlated physiological strategies with season and herbivory, such as higher growth rate (leading to more softer tissues) during summer months when the wave action is comparatively weaker, while lower growth rate (leading to hardened tissue) during winter when wave action is stronger.
Self-pruning of algal thalli has been suggested as a possible adaptation for helping the alga to reduce the size and reducing the risk of dislodgement from substratum (Black 1976; Demes et al. 2013). However, a recent study suggested this conjecture is flawed because on a long run, unpruned individuals of kelp E. menziesii survived better than the pruned individuals (Burnett and Koehl 2020). Yet another recent study revealed that the attachment strength of isomorphic red alga Chondrus verrucosus differs with its life-history stages. The tetrasporophyte stage of this alga tends to have weaker stipe-holdfast junctions rendering them more susceptible to thallus dislodgement compared with the gametophyte stage (Bellgrove and Aoki 2020).
There have been a few experimental studies to assess the hydrodynamic effects on microalgae. The green microalga Dunaliella sp. has been shown to be sensitive to increasing specific bubble rates in tubular airlift photobioreactor, that can be minimized by the addition of carboxymethylcellulose and agar (Silva et al. 1987). Photosynthetic rate, growth rate and cellular morphology of green cyanobacterium A. platensis also has been shown to be affected with the hydrodynamic flow rate (Mitsuhashi et al. 1994). Higher rates of mechanical agitation also have been shown to induce cell damage in the chrysophyte Ochromonas malhamensis (Yang and Wang 1992) and the red microalga Porphyridium cruentum (Camacho et al. 2000).
On the other hand, studies conducted on several freshwater red algae have shown that no preferential advantage was apparent for one morphological form over the other for the hydrodynamic stress resistance, perhaps indicative of the adaptations that these various species have been going through for a very long period (Sheath and Handbook 1988). Such algae of various morphological forms, including tufts, mucilaginous filament, nonmucilaginous erect filament and so on, might have evolved through independent mechanisms for hydrodynamic resistance. Studies of the red seaweeds Chondrus crispus and Mastocarpus stellatus also concluded that no clear relationships exist between algal morphology and its responses to environmental variation (Dudgeon et al. 1995). However, algal thallus morphology should not be assessed for its adaptations from hydrodynamic stress in isolation, as most of the algal communities exist in nature as large canopies consisting of multi-species communities. Studies of the red alga C. crispus have shown that although bushy morphology in isolated thalli increases the drag- seemingly a counter-intuitive to resist the wave-action, this morphology significantly reduce the drag when in canopy (Johnson 2001). A recent study contextualized the adaptation strategies of windswept kelps within the broader evolutionary framework of avoidance-tolerance spectra. The study concluded that kelps are either strong and tenacious (tolerance-dominant) or streamlined (avoidance-dominant), but not both concurrently, thus indicating a trade-off between these two strategies (Starko and Martone 2016).
Algal hormones involved in response to stress
A well-coordinated and timely response to various abiotic stresses in plants involves signal perception and transduction mainly via plant hormones, that is, phytohormones. Phytohormones comprise a wide array of signaling compounds present in minute quantities in cells, playing crucial roles in minimizing environmental stresses by facilitating growth, developmental processes and coordinating various signal transduction pathways during stress responses. The main classes of phytohormones include (1) classical phytohormones such as auxin (AUX), abscisic acid (ABA), brassinosteroids (BRs), ethylene (ET), gibberellins (GAs), and cytokinin (CK); (2) molecular phytohormones, for example, jasmonic acid (JA), salicylic acid (SA), and nitric oxide (NO); and (3) newly discovered karrikins (KARs), and strigolactones (SLs) (Smith and Li 2014; Pandey et al. 2016). In different phylogenetic groups of algae, all known plant hormones are found. Generally, phytohormones of various algal groups are poorly understood because of the extreme diversity of this group and difficulties in working methodologies due to their small size. So here in this review, we tried to summarize the published data elucidating the role of phytohormones in algae in combating various abiotic stress conditions.
Nitrogen stress
Certain hormones such as GA3, triacontanol (TRIA), kinetin (K), and zeatin (Z) promote cell elongation, cell division, growth and photosynthesis under nitrogen deficiency (Park et al. 2013; Babu et al. 2017; Renuka et al. 2017). Indole-3-acetic acid (IAA), GA, K, 1- TRIA improved biomass in nitrogen-deficient C. reinhardtii (Park et al. 2013). This treatment with several coupled hormones increased the FV/FM and relative electron transport rate (rETR) of certain algae under nitrogen limitation conditions. Thus, the combination of several phytohormones may enhance algal growth and lipid production under abiotic stress more efficiently than one phytohormone alone by regulating oxidative stress and photosynthetic rate. By keeping in view, zeatin and kinetin enhanced algal growth and lipid production in A. obliquus (Renuka et al. 2017) under nitrogen stress. Babu et al. (2017) reported that under nitrogen limitation, the low doses of IAA and diethyl aminoethyl hexanoate (DAH) treatments in C. sorokiniana showed the highest enhancement in biomass productivity over the control. Yu et al. (2018) reported that the combination of the phytohormones such as naphthylacetic acid (NAA) and indolebutyric acid (IBA) has positive effects on growth and lipid production in Scenedesmus sp. SDEC-8 and C. sorokiniana SDEC-18 under nitrogen starvation. Salama et al. (2014) demonstrated that the biomass was increased by 1.9- and 2.5-fold, and the PUFA content was enhanced by up to 56% and 59% at 10 − 5 M by the application of IAA and DAH, respectively. The treatment with these coupled hormones induced antioxidant enzyme activities, which protect cells from damage caused by abiotic stresses, and significantly upregulated the levels of RuBisCO and ACCase under N limitation.
ROS
Fulvic Acid (FA) appeared to promote microalgal lipid biosynthesis significantly by regulating cellular ROS levels. The application of FA, melatonin (MT), butylated hydroxyanisole (BHA), and butylated hydroxytoluene (BHT) reduced high light intensity and nutrient deficiency stress resulting in increased biomass and enhancement of various pigments such as astaxanthin, β- carotene and lipids in H. pluvialis (Ding et al. 2018a; b; 2019; Zhao et al. 2018b; 2019a; b). These studies indicated that the application of phytohormones could sustain or induce cell growth and metabolite accumulation in microalgae under abiotic stress conditions, mainly by regulating oxidative stress.
HM stress
In response to HM stress in microalgae, the application of several hormones such as Z, K, IBA, GA, JA, IAA, NAA and phenylacetic acid (PAA) are involved in the induction of cellular growth and division and augmentation of photosynthetic activity (Bajguz 2011; Piotrowska-Niczyporuk et al. 2012) and can alleviate stress symptoms by preventing HM biosorption. Cr stress is alleviated by K in Nostoc muscorum. Here K application significantly improved the dry weight and carotenoid production of the cyanobacterium N. muscorum (Tiwari et al. 2018). The possible reason for this change is that K triggers photosynthesis and antioxidant enzyme activities while depressing respiration and oxidative stress in algae. The endogenous levels of auxins (IAA) and CKs (transzeatin, tZ) were reduced in the green alga, C. vulgaris (Bajguz 2011) and A. obliquus (Piotrowska-Niczyporuk et al. 2017) when subjected to the higher concentration (100 μM) of Pb. In this case, the endogenous application of auxin and CKs mitigated toxicity, promoted growth and development, and regulated HM sorption in C. vulgaris (Piotrowska-Niczyporuk et al. 2012) and A. obliquus (Piotrowska-Niczyporuk et al. 2018a). Exogenous CKs were found to protect proteins and scavenge various components of photosynthetic apparatus (Chls, carotenoids, xanthophylls) and significantly reduce damaging effects of HMs on green algae, C. vulgaris (Piotrowska-Niczyporuk et al. 2012), and A. obliquus (Piotrowska-Niczyporuk et al. 2018b). CKs alleviated HM toxicity by inhibiting ROS formation in C. vulgaris when challenged by Cd, Cu, or Pb (Piotrowska-Niczyporuk et al. 2012).
Not many reports are available regarding the biological functions of GAs in algae; however, their presence has been confirmed in multiple microalgae strains (Stirk et al. 2013). GA3 showed the positive effect on growth, protein contents, Chl-a and b, carotenoids, and monosaccharides in C. vulgaris exposed to HMs (Falkowska et al. 2011; Piotrowska-Niczyporuk et al. 2012). Here in this study, GA3 activated defence responses and decreased oxidative damages by promoting the production of thiol compounds which could bind to HM ions (Bajguz 2002) in cells of C. vulgaris (Falkowska et al. 2011; Piotrowska-Niczyporuk et al. 2012). These results indicate that GA3 can help algae to withstand the toxic concentrations of Cd and Pb based upon the efficiency of cellular division in C. vulgaris (Falkowska et al. 2011). Exogenous presence of JA exacerbated Cd, Cu, or Pb toxicity accompanied by an increase in metal biosorption, lipid peroxidation and H2O2 level in C. vulgaris. In response to JA treatment under HM exposure, several indicators of cell health and their ability to deal with HM, like cell number, Chl levels, carotenoids, monosaccharides, soluble proteins, ascorbate and GSH content, and antioxidant enzyme activity, were considerably reduced (Piotrowska-Niczyporuk et al. 2012). These findings concluded that high amounts of JA accelerated the senescence program and algal cell death (Czerpak et al. 2006).
Heat stress
BRs enhanced the level of ABA in C. vulgaris in response to short term (3 h) heat stress (30–40 °C) (Bajguz 2009). Exogenous BL partially overcomes the inhibitory effect of HMs on C. vulgaris, reducing the accumulation of HMs in the cells and increasing ABA, IAA and zeatin content (Bajguz 2011). Endogenous level of BRs increases in response to salt and low temperature (15 °C) stress in several species of algae such as Chlorococcum ellipsoideum, Gyoerffyana humicola, Nautococcus mamillatus, Acutodesmus acuminatus, Protococcus viridis and C. vulgaris. The response of algal cultures was observed within 30 min of the salt shock.
In macroalgae, studies regarding the role of phytohormones are limited. Application of ACC (the ethylene precursor 1-aminocylopropane1-carboxylic acid) repressed gametophytes and enhanced tolerance to oxidative stress in Pyropia yezoensis (Uji et al. 2016). Gene expression profiles of small heat shock proteins in P. yezoensis showed that exogenous application of ACC could significantly increase the expression levels of small heat shock proteins (Uji et al. 2019) against heat stress. Wang et al. (2019b) showed that SA and jasmonic acid (JA) could promote the growth of algae and enhance the resistance of P. haitanensis against temperature stress. Transcriptome data showed that the phytohormones GA and ABA played essential roles in responding to temperature stress in P. yezoensis (Sun et al. 2014; Wang et al. 2017).
Plant hormones are also associated with signal transduction pathways, including pathways that use NO, Ca2+, mitogen-activated protein kinase (MAPK) and specifically stress hormones (ABA, SA and GA), which form a complex signalling network connected to growth, metabolic, and stress tolerance in higher plants (Peleg and Blumwald 2011; Lu et al. 2014; de Zelicourt et al. 2016; Wani et al. 2016; Raja et al. 2017). These stress hormones may promote cell growth by improving photosynthetic activity by increasing Chl content (Lu and Xu 2015). NO, and Ca2+ are cellular second messengers that mediate oxidative stress and metabolite synthesis in algae under abiotic stress conditions (Zhang et al. 2017; Kováčik and Dresler 2018). Figure 4 shows the signal transduction pathway of phytohormones that includes the NO and Ca2+ as second messengers during abiotic stress conditions.
Abiotic stress to stimulate lipid production
Algae are widely studied due to their ability to accumulate high content of lipids and bioactive compounds under various abiotic stress conditions such as temperature, nutrient deprivation, UV-radiation, salinity, pH, phytohormones and HMs (Paliwal et al. 2017; Dong et al. 2019). Microalgae have attracted particular interest due to their high-value applications in nutraceuticals, pharmaceuticals, biofuel production, and capacity to accumulate a large amount of lipids, high growth rate, economical and environmentally friendly (mitigating fossil CO2 pollution) (Yu et al. 2012; Barati et al. 2019). During abiotic stresses, algae modulate their metabolites in their physiology and biochemistry to survive through adaptation (Roleda et al. 2013; Bermejo et al. 2018). Upon exposure to numerous abiotic stresses, algae influenced their intracellular concentration of lipids, antioxidant enzymes, carotenes, and other metabolites (Rothschild and Mancinelli 2001; Forján et al. 2015). However, under prolonged stress conditions, the algal growth ceases and leads to programmed cell death (PCD) or apoptosis (Zuppini et al. 2010; Markou and Nerantzis 2013; Bermejo et al. 2018; Barati et al. 2019).
Microalgae are a potential biofuel producing source as they are not dependent on fertile land, can begrown in wastewater, and offer higher productivities. However, large-scale production of lipids is facing a cost-related bottleneck because of low biomass production, high water footprint, high nutrient input, low cell density, and harvesting issues. Currently, several strategies have been explored to overcome the challenges and achieve maximum growth and large-scale production of lipids (Kim et al. 2016; Wang et al. 2016; Babu et al. 2017). Recently, advanced strategies have been employed to overcome this kind of problem, which are a combination of two different stresses; two-stage cultivation; co-culturing with other organisms; and the addition of phytohormones (ABA, IAA, CKs, GAs, and ET), salts, and flue gases (CO2, NO×, SO×) (Lu and Xu 2015; Li et al. 2017; Salama et al. 2018). The two-stage cultivation strategy has been extensively used, in which cells are firstly grown in nutrient-sufficient conditions (for higher biomass), and then stress conditions are induced, which stimulates the accumulations of higher amounts of proteins, carbohydrates, lipids, fatty acids and other bioactive compounds (Fig. 5) (Chen et al. 2011; Aziz et al. 2020).
Microalgal lipids are categorized into structural lipids such as PUFAs, and storage lipids like non-polar saturated fatty acids (SFAs) and MUFAs. Storage lipids (PUFAs), primarily stored in the form of TAGs, are transesterified to produce biofuel (Thompson Jr 1996). Moreover, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are the most valuable fatty acids in the microalgae, which make them suitable for high biofuel production (Gimpel et al. 2015). Nutrient starvation (primarily nitrogen and phosphate) and salinity have been well documented to stimulate TAG accumulation in microalgae (Li et al. 2008; Yeesang and Cheirsilp 2011; Paliwal et al. 2017; Shi et al. 2017; Wase et al. 2017). The algal lipid production under varying abiotic stress factors is summarised below (Table 4).
Transgenic approaches for stress resistance in algae
Significant breakthroughs in genetic modification of green microalgae have been made over the years (Mayfield and Golden 2015), as evidenced by the synthesis of omega-3 fatty acids, carotenoids, biofuels, and better photosynthetic growth (Gimpel et al. 2015). Following the development of advanced automated sequencing technology over the last two decades, genomics has become a potent tool. Bioinformatics is used to assemble DNA sequences and evaluate gene structure and expression. In the case of microalgae, whole-genome sequencing of strains with commercial potential will allow scientists to apply other omics technologies to gain a better knowledge of high-value cell components, lipid metabolism, and overall cell activity under stress and non-stress conditions. The genomes of Botryococcus braunii Showa, Botryococcus braunii UTEX 572, C. reinhardtii, Chlorella sp. NC64A, Coccomyxa sp. C-169, D. salina, Micromonas pusilla, N. oceanica and Ostreococcus tauri were recently sequenced.
The understanding of mechanisms for the adaptation of microalgae to extreme environments has been contributed by comparative genome analysis. Microalgae genomes can be altered in the nuclear, chloroplast, or mitochondrial genomes (Specht et al. 2010). The majority of enzymes involved in secondary metabolism are encoded in the nuclear genome, although others are directed to the chloroplast to accomplish their function (Heydarizadeh et al. 2013). In these circumstances, the nuclear or plastid genomes might be modified to confer a particular metabolic function (Johanningmeier and Fischer 2010). The major ways for delivering DNA to microalgae include electroporation, shaking with glass beads, and particle gun bombardment (biolistic), with the letter proving to be the only effective way for chloroplast transformation thus far (Purton et al. 2013).
The pursuit of stress-tolerant lines has led to an unprecedented rise in algae genetic modification for peptides, enhanced photosynthesis, and key metabolic routes, including the production of lucrative dietary supplements, pharmaceuticals, and hydrocarbons (Gangl et al. 2015). Moreover, genetic sequence omics have assisted in efficient algal modification. Despite enormous accomplishments, only a few algae species continue to display strong and stable expressions of foreign proteins. There are several microalgal gene silencing methods, including oppressive histone H3 lysine alteration, DNA cytosine modification, RNA interference, and miRNA gene regulatory systems to eliminate highly unstable external transcription proteins. Chlamydomonas has been shown to have effective but simple nuclear gene targeting mechanisms (Zorin et al. 2009). Various promoters, and 5`UTR modifications, such as the 16S rRNA promoters and the atpA 5`UTR, were shown to allow sufficient heterogeneous genome editing and efficient transgenic protein production within the C. reinhardtii plastid (Tissot-Lecuelle et al. 2014). Codon enhanced gene regulation hemH and lba throughout the plastid of C. reinhardtii for optimum bio-hydrogen production is evidence of genetic manipulation in microalgae (Wu et al. 2010). Furthermore, chemically synthesized promoters were created to induce high levels of nuclear gene expression in Chlamydomonas (Scranton et al. 2016). Moreover, increased targeted genomic alteration in C. reinhardtii has been described using zinc finger nuclease enzymes, activator effectors, and the recently discovered clustered, regularly interspersed short palindromic repeat (CRISPR) pathway (Jiang et al. 2014).
Although most genetic engineering strategies are aimed at enhancing the production of high-value metabolites (such as antioxidant pigments and PUFAs) and biofuel molecules (such as hydrogen and TAG), a few attempts have been made toward developing resilient strains for applications such as HM mitigation and CO2 sequestration. A moth lentil d1-pyrroline-5-carboxylate synthetase (P5CS) allele, for instance, was already found to be primarily expressed in Chlamydomonas transformants used to have 80 times better free-Pro levels, exponential growth at detrimental Cd concentration levels, and remarkable binding at four-fold higher Cd densities than wild-type cell types. The findings suggest that free-Proasan antioxidant plays a function in Cd-stressed cells, with greater GSH levels promoting more excellent phytochelatin production and Cd sequestration. Speedy genomic manipulation of such algae was being utilized to increase HM susceptibility and binding selectivity for contaminated water bodies and sediments. Changing both the large and small subunits of Rubisco (rbcS and rbcL) as possibilities for enhancing net CO2 uptake (photosynthesis) and sequestration through growth promotion received a lot of interest (Whitney et al. 2011). Chlamydomonas is a significant host genetic manipulator in this regard since it can cause alterations in both the rbcS and rbcL genes. Hybrid Rubiscos, for instance, have indeed been created by combining plant’s small (rbcS) subunits with algae large (rbcL) subunits by transforming a C. reinhardtii mutant deficient in the rbcS gene. Despite an increase in CO2/O2 binding of 3–11 per cent, the transgenic enzyme retains high Vmax ratios and enzymatically efficient Rubisco. Genetically modified strains, on either hand, are deficient in plastid pyrenoids and also have restricted photosynthesis. Continued research to build and evaluate better Rubiscos, according to (Whitney et al. 2011), would mainly rely on algae in vitro experiments, especially Chlamydomonas.
Conclusion
Algae are considered a source of many bioactive compounds with attractive properties, including pigments, carbohydrates, lipids, proteins, and vitamins. They are studied as encouraging feedstocks to satisfy future sustainable energy demands as they have a high ability to absorb CO2, no need for arable land, and year-round cultivation that make them attractive for commercial exploitation. However, the growth and development of algae may be inhibited by adverse stresses such as high salinity, nutrient deficiency, HMs, low or excessive water, temperature fluctuations, and UVRs. These stressful conditions cause a variety of metabolic, physiological, biochemical and molecular alterations, subsequently causing oxidative damage and ultimately declining cell growth and biomass. It has been reported that the algal species respond to these stressful conditions by modifying their metabolites. Nitrogen and phosphorus deficiency, for example, causes lipid metabolism to switch from membrane lipid synthesis to neutral lipid storage. Salinity stress causes the accumulation of various osmoregulatory solutes (like glycerol, proline, trehalose), antioxidants, and lipids. The increase in temperature is considered the most tuning factor for polyunsaturated fatty acid production, whereas a decrease in temperature increases the composition of unsaturated and short-chain fatty acids. In recent years, phytohormones emerged as a topic of intense focus in microalgae research. They could sustain the growth of microalgae under abiotic stress conditions. Moreover, it is widely studied that the abiotic stress conditions (such as a two-stage cultivation strategy) can be used to produce lipids and high-value by-products in microalgae.
Recent advancement in our understanding of the molecular mechanisms underlying the responses of algae to abiotic stresses emphasizes their multilevel nature that involves multiple processes such as sensing, signaling, transcription, transcript processing, translation and post-translational protein alterations. However, there is considerable uncovered ground in understanding how algae signal each other to start the cascade of pathways that will improve survival. Moreover, comparative genome analysis has contributed to understanding mechanisms for the adaptation of microalgae to extreme environments. Further, studies integrated with next-generation sequencing strategies and directed experimental evolution approaches will continue to raise and deepen our knowledge of how algae respond and adapt to stressful conditions.
References
Abdala-Díaz R, Cabello-Pasini A, Pérez-Rodríguez E, Álvarez RC, Figueroa F (2006) Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar Biol 148:459–465
Aigner S, Remias D, Karsten U, Holzinger A (2013) Unusual phenolic compounds contribute to ecophysiological performance in the purple-colored green alga Zygogonium ericetorum (Zygnematophyceae, Streptophyta) from a high-alpine habitat. J Appl Phycol 49:648–660
Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98:541–550
Álvarez-Gómez F, Korbee N, Figueroa FL (2019) Effects of UV radiation on photosynthesis, antioxidant capacity and the accumulation of bioactive compounds in Gracilariopsis longissima, Hydropuntia cornea and Halopithys incurva (Rhodophyta). J Appl Phycol 55:1258–1273
Anantharaj K, Govindasamy C, Natanamurugaraj G, Jeyachandran S (2011) Effect of heavy metals on marine diatom Amphora coffeaeformis (Agardh. Kutz). Glob J Environ Res 5:112–117
Andosch A, Höftberger M, Lütz C, Lütz-Meindl U (2015) Subcellular sequestration and impact of heavy metals on the ultrastructure and physiology of the multicellular freshwater alga Desmidium swartzii. Int J Mol Sci 16:10389–10410
Annamalai J, Shanmugam J, Nallamuthu T (2016) Salt stress enhancing the production of phytochemicals in Chlorella vulgaris and Chlamydomonas reinhardtii. J Algal Biomass Util 7:37–44
Anthony KR, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O (2008) Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci 105:17442–17446
Avron M, Ben-Amotz A (1992) Dunaliella: Physiology, biochemistry and biotechnology. CRC Press, Boca Raton, pp 240
Aziz MMA, Kassim KA, Shokravi Z, Jakarni FM, Liu HY, Zaini N, Tan LS, Islam AS, Shokravi H (2020) Two-stage cultivation strategy for simultaneous increases in growth rate and lipid content of microalgae: A review. Renew Sust Energ Rev 119:109621
Babu AG, Wu X, Kabra AN, Kim D-P (2017) Cultivation of an indigenous Chlorella sorokiniana with phytohormones for biomass and lipid production under N-limitation. Algal Res 23:178–185
Bäck S (1993) Morphological variation of northern Baltic Fucus vesiculosus along the exposure gradient. Annu Bot Fenn 30:275–283
Bajguz A (2002) Brassinosteroids and lead as stimulators of phytochelatins synthesis in Chlorella vulgaris. J Appl Phycol 159:321–324
Bajguz A (2009) Brassinosteroid enhanced the level of abscisic acid in Chlorella vulgaris subjected to short-term heat stress. J Appl Phycol 166:882–886
Bajguz A (2011) Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch Environ Contam Toxicol 60:406–416
Barati B, Gan S-Y, Lim P-E, Beardall J, Phang S-M (2019) Green algal molecular responses to temperature stress. Acta Physiol Plant 41:26
Bellgrove A, Aoki MN (2020) Attachment strength differs amongst life-history stages of an intertidal, isomorphic red alga. Phycol Res 68:144–151
Ben-Amotz A, Avron M (1980) Glycerol and b-carotene metabolism in the halotolerant algae Dunaliella: A model system for bisolar energy conversion. Trends Biochem Sci 6:297–299
Bermejo E, Ruiz-Domínguez MC, Cuaresma M, Vaquero I, Ramos-Merchante A, Vega JM, Vílchez C, Garbayo I (2018) Production of lutein, and polyunsaturated fatty acids by the acidophilic eukaryotic microalga Coccomyxa onubensis under abiotic stress by salt or ultraviolet light. J Biosci Bioeng 125:669–675
Berner T, Dubinsky Z, Wyman K, Falkowski PG (1989) Photoadaptation and the “package” effect in Dunaliella tertiolecta (Chlorophyceae). J Phycol 25:70–78
Bianco-Stein N, Polishchuk I, Seiden G, Villanova J, Rack A, Zaslansky P, Pokroy B (2020) Helical microstructures of the mineralized coralline red algae determine their mechanical properties. Adv Sci 7:2000108
Bidigare RR, Ondrusek ME, Kennicutt MC, Iturriaga R, Harvey HR, Hoham RW, Macko SA (1993) Evidence a photoprotective for secondary carotenoids of snow algae. J Phycol 29:427–434
Biggs BJ, Thomsen HA (1995) Disturbance of stream periphyton by perturbations in shear stress: time to structural failure and differences in community resistance. J Phycol 31:233–241
Black R (1976) The effects of grazing by the limpet, Acmaea insessa, on the kelp, Egregia laevigata, in the intertidal zone. Ecology 57:265–277
Blanchette C, Miner B, Gaines S (2002) Geographic variability in form, size and survival of Egregia menziesii around Point Conception, California. Mar Ecol Prog Ser 239:69–82
Borowitzka MA (1995) Microalgae as sources of pharmaceuticals and other biologically active compounds. J Appl Phycol 7:3–15
Borowitzka MA (2018) The ‘stress’ concept in microalgal biology—homeostasis, acclimation and adaptation. J Appl Phycol 30:2815–2825
Borowitzka LJ, Moulton TP, Borowitzka MA (1985) Salinity and the commercial production of beta-carotene from Dunaliella salina. In: Barclay WJ, McIntosh R (eds) Algal Biomass: An Interdisciplinary Perspective. J. Cramer Verlag, Verduz, pp 217–222
Borowitzka MA, Borowitzka LJ, Kessly D (1990) Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J Appl Phycol 2:111–119
Bothe H, Schmitz O, Yates MG, Newton WE (2010) Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol Molec Biol Rev 74:529–551
Brown LM, Hellebust JA (1978) Sorbitol and proline as intracellular osmotic solutes in the green alga Stichococcus bacillaris. Can J Bot 56:676–679
Burdett HL, Aloisio E, Calosi P, Findlay HS, Widdicombe S, Hatton AD, Kamenos NA (2012) The effect of chronic and acute low pH on the intracellular DMSP production and epithelial cell morphology of red coralline algae. Mar Biol Res 8:756–763
Burdett HL, Hatton AD, Kamenos NA (2015) Effects of reduced salinity on the photosynthetic characteristics and intracellular DMSP concentrations of the red coralline alga Lithothamnion glaciale. Mar Biol 162:1077–1085
Burnett NP, Koehl M (2019) Mechanical properties of the wave-swept kelp Egregia menziesii change with season, growth rate and herbivore wounds. J Exp Biol 222:jeb190595
Burnett NP, Koehl M (2020) Thallus pruning does not enhance survival or growth of a wave-swept kelp. Mar Biol 167:1–12
Calhoun S, Bell TAS, Dahlin LR, Kunde Y, LaButti K, Louie KB, Kuftin A, Treen D, Dilworth D, Mihaltcheva S (2021) A multi-omic characterization of temperature stress in a halotolerant Scenedesmus strain for algal biotechnology. Commun Biol 4:1–15
Camacho FGa, Gomez AC, Sobczuk TM, Grima EM (2000) Effects of mechanical and hydrodynamic stress in agitated, sparged cultures of Porphyridium cruentum. Process Biochem 35:1045–1050
Carfagna S, Lanza N, Salbitani G, Basile A, Sorbo S, Vona V (2013) Physiological and morphological responses of lead or cadmium exposed Chlorella sorokiniana 211–8K (Chlorophyceae). Springerplus 2:1–7
Carrington E (1990) Drag and dislodgment of an intertidal macroalga: consequences of morphological variation in Mastocarpus papillatus Kützing. J Exp Mar Biol Ecol 139:185–200
Chakraborty K, Bose J, Shabala L, Shabala S (2016) Difference in root K+ retention ability and reduced sensitivity of K+-permeable channels to reactive oxygen species confer differential salt tolerance in three Brassica species. J Exp Bot 67:4611–4625
Chandra R, Ghosh UK (2019) Effects of various abiotic factors on biomass growth and lipid yield of Chlorella minutissima for sustainable biodiesel production. Environ Sci Pollut Res 26:3848–3861
Chen M, Tang H, Ma H, Holland TC, Ng KS, Salley SO (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour Technol 102:1649–1655
Chen Z, Song S, Wen Y, Zou Y, Liu H (2016) Toxicity of Cu (II) to the green alga Chlorella vulgaris: a perspective of photosynthesis and oxidant stress. Environ Sci Pollut Res 23:17910–17918
Cheshire AC, Hallam ND (1989) Morphological differences in the southern bull kelp (Durvillaea potatorum) throughout South-Eastern Australia. Bot Mar 32:191–197
Chetouhi C, Masseret E, Satta CT, Balliau T, Laabir M, Jean N (2020) Intraspecific variability in membrane proteome, cell growth, and morphometry of the invasive marine neurotoxic dinoflagellate Alexandrium pacificum grown in metal-contaminated conditions. Sci Total Environ 715:136834
Cho K, Cho D-H, Heo J, Kim U, Lee YJ, Choi D-Y, Kim H-S (2019) Nitrogen modulation under chemostat cultivation mode induces biomass and lipid production by Chlorella vulgaris and reduces antenna pigment accumulation. Bioresour Technol 281:118–125
Chokshi K, Pancha I, Trivedi K, George B, Maurya R, Ghosh A, Mishra S (2015) Biofuel potential of the newly isolated microalgae Acutodesmus dimorphus under temperature induced oxidative stress conditions. Bioresour Technol 180:162–171
Chokshi K, Pancha I, Ghosh A, Mishra S (2017) Nitrogen starvation-induced cellular crosstalk of ROS-scavenging antioxidants and phytohormone enhanced the biofuel potential of green microalga Acutodesmus dimorphus. Biotechnol Biofuels 10(1):60
Chu F-F, Chu P-N, Cai P-J, Li W-W, Lam PK, Zeng RJ (2013) Phosphorus plays an important role in enhancing biodiesel productivity of Chlorella vulgaris under nitrogen deficiency. Bioresour Technol 134:341–346
Chu F-J, Wan T-J, Pai T-Y, Lin H-W, Liu S-H, Huang C-F (2020) Use of magnetic fields and nitrate concentration to optimize the growth and lipid yield of Nannochloropsis oculata. J Environ Manage 253:109680
Collén J, Pinto E, Pedersen M, Colepicolo P (2003) Induction of oxidative stress in the red macroalga Gracilaria tenuistipitata by pollutant metals. Arch Environ Contam Toxicol 45:337–342
Creis E, Delage L, Charton S, Goulitquer S, Leblanc C, Potin P, Ar Gall E (2015) Constitutive or inducible protective mechanisms against UV-B radiation in the brown alga Fucus vesiculosus? A study of gene expression and phlorotannin content responses. PLoS ONE 10:e0128003
Czerpak R, Piotrowska A, Szulecka K (2006) Jasmonic acid affects changes in the growth and some components content in alga Chlorella vulgaris. Acta Physiol Plant 28:195–203
D’Agostino PM, Javalkote VS, Mazmouz R, Pickford R, Puranik PR, Neilan BA (2016) Comparative profiling and discovery of novel glycosylated mycosporine-like amino acids in two strains of the cyanobacterium Scytonema cf. crispum. Appl Environ Microbiol 82:5951–5959
Darki BZ, Seyfabadi J, Fayazi S (2017) Effect of nutrients on total lipid content and fatty acids profile of Scenedesmus obliquus. Braz Arch Biol Technol 60:1–12
de Zelicourt A, Colcombet J, Hirt H (2016) The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci 21:677–685
Degl’Innocenti E, Hafsi C, Guidi L, Navari-Izzo F (2009) The effect of salinity on photosynthetic activity in potassium-deficient barley species. J Plant Physiol 166:1968–1981
del Pilar Bremauntz M, Torres-Bustillos LG, Duran-Paramo E, Fernández-Linares L (2011) Trehalose and sucrose osmolytes accumulated by algae as potential raw material for bioethanol. Nat Res 2:173
Delf EM (1932) Experiments with the stipes of Fucus and Laminaria. J Exp Biol 9:300–313
Demes KW, Harley CD, Anderson LM, Carrington E (2013) Shifts in morphological and mechanical traits compensate for performance costs of reproduction in a wave-swept seaweed. J Ecol 101:963–970
Diaz-Pulido G, Anthony KR, Kline DI, Dove S, Hoegh-Guldberg O (2012) Interactions between ocean acidification and warming on the mortality and dissolution of coralline algae. J Phycol 48:32–39
Ding W, Zhao P, Peng J, Zhao Y, Xu J-W, Li T, Reiter RJ, Ma H, Yu X (2018a) Melatonin enhances astaxanthin accumulation in the green microalga Haematococcus pluvialis by mechanisms possibly related to abiotic stress tolerance. Algal Res 33:256–265
Ding W, Zhao Y, Xu J-W, Zhao P, Li T, Ma H, Reiter RJ, Yu X (2018b) Melatonin: a multifunctional molecule that triggers defense responses against high light and nitrogen starvation stress in Haematococcus pluvialis. J Agric Food Chem 66:7701–7711
Ding W, Li Q, Han B, Zhao Y, Geng S, Ning D, Ma T, Yu X (2019) Comparative physiological and metabolomic analyses of the hyper-accumulation of astaxanthin and lipids in Haematococcus pluvialis upon treatment with butylated hydroxyanisole. Bioresour Technol 292:122002
Dong X, Zhao Y, Li T, Huang L, Zhao P, Xu J-W, Ma H, Yu X (2019) Enhancement of lipid production and nutrient removal of Monoraphidium sp. FXY-10 by combined melatonin and molasses wastewater treatment. J Taiwan Inst Chem Eng 99:123–131
Dragone G, Fernandes BD, Abreu AP, Vicente AA, Teixeira JA (2011) Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy 88:3331–3335
Dubey D, Dutta V (2020) Nutrient enrichment in lake ecosystem and its effects on algae and macrophytes. In: Shukla V, Kumar N (eds) Environmental concerns and sustainable development. Springer, Singapore, pp 81–126
Dudgeon SR, Kübler JE, Vadas RL, Davison IR (1995) Physiological responses to environmental variation in intertidal red algae: does thallus morphology matter? Mar Ecol Prog Ser 117:193–206
Dunlap W, Chalker B (1986) Identification and quantitation of near-UV absorbing compounds (S-320) in a hermatypic scleractinian. Coral Reefs 5:155–159
Endo T, Schreiber U, Asada K (1995) Suppression of quantum yield of photosystem II by hyperosmotic stress in Chlamydomonas reinhardtii. Plant Cell Physiol 36:1253–1258
Ermilova E (2020) Cold stress response: an overview in Chlamydomonas. Front Plant Sci 11:1364
Fábregas J, Maseda A, Domínguez A, Otero A (2004) The cell composition of Nannochloropsis sp. changes under different irradiances in semicontinuous culture. World J Microbiol Biotechnol 20:31–35
Fal S, Aasfar A, Rabie R, Smouni A, Arroussi HE (2022) Salt induced oxidative stress alters physiological, biochemical and metabolomic responses of green microalga Chlamydomonas reinhardtii. Heliyon 8(1):e08811
Falkowska M, Pietryczuk A, Piotrowska A, Bajguz A, Grygoruk A, Czerpak R (2011) The effect of gibberellic acid (GA3) on growth, metal biosorption and metabolism of the green algae Chlorella vulgaris (Chlorophyceae) Beijerinck exposed to cadmium and lead stress. Pol J Environ Stud 20:53–59
Figueroa FL, Korbee N, Carrillo P, Medina-Sánchez JM, Mayte M, Bonomi J, Sanchez-Castillo PM (2009) The effects of UV radiation on photosynthesis estimated as chlorophyll fluorescence in Zygnemopsis decussata (Chlorophyta) growing in a high mountain lake (Sierra Nevada, Southern Spain). J Limnol 68:206
Filová A, Fargašová A, Molnárová M (2021) Cu, Ni, and Zn effects on basic physiological and stress parameters of Raphidocelis subcapitata algae. Environ Sci Pollut Res 28:58426–58441
Fisher M, Gokhman I, Pick U, Zamir A (1996) A salt-resistant plasma membrane carbonic anhydrase is induced by salt in Dunaliella salina. J Biol Chem 271:17718–17723
Foflonker F, Ananyev G, Qiu H, Morrison A, Palenik B, Dismukes GC, Bhattacharya D (2016) The unexpected extremophile: tolerance to fluctuating salinity in the green alga Picochlorum. Algal Res 16:465–472
Forján E, Navarro F, Cuaresma M, Vaquero I, Ruíz-Domínguez MC, Gojkovic Ž, Vázquez M, Márquez M, Mogedas B, Bermejo E (2015) Microalgae: fast-growth sustainable green factories. Crit Rev Environ Sci Technol 45:1705–1755
Fowler-Walker MJ, Wernberg T, Connell SD (2006) Differences in kelp morphology between wave sheltered and exposed localities: morphologically plastic or fixed traits? Mar Biol 148:755–767
Fredersdorf J, Müller R, Becker S, Wiencke C, Bischof K (2009) Interactive effects of radiation, temperature and salinity on different life history stages of the Arctic kelp Alaria esculenta (Phaeophyceae). Oecologia 160:483–492
Fuentes-Tristan S, Parra-Saldivar R, Iqbal HM, Carrillo-Nieves D (2019) Bioinspired biomolecules: Mycosporine-like amino acids and scytonemin from Lyngbya sp. with UV-protection potentialities. J Photochem Photobiol B Biol 201:111684
Gangl D, Zedler JA, Rajakumar PD, Martinez EMR, Riseley A, Włodarczyk A, Purton S, Sakuragi Y, Howe CJ, Jensen PE (2015) Biotechnological exploitation of microalgae. J Exp Bot 66:6975–6990
Gao K, Zheng Y (2010) Combined effects of ocean acidification and solar UV radiation on photosynthesis, growth, pigmentation and calcification of the coralline alga Corallina sessilis (Rhodophyta). Glob Chang Bio 16:2388–2398
Gao K, Aruga Y, Asada K, Ishihara T, Akano T, Kiyohara M (1991) Enhanced growth of the red alga Porphyra yezoensis Ueda in high CO2 concentrations. J Appl Phycol 3:355–362
Gao K, Aruga Y, Asada K, Kiyohara M (1993) Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis. J Appl Phycol 5:563–571
Gasulla F, Jain R, Barreno E, Guera A, Balbuena TS, Thelen JJ, Oliver MJ (2013) The response of Asterochloris erici (Ahmadjian) Skaloud et Peksa to desiccation: a proteomic approach. Plant Cell Environ 36:1363–1378
Gautam S, Pandey LK, Vinayak V, Arya A (2017) Morphological and physiological alterations in the diatom Gomphonema pseudoaugur due to heavy metal stress. Ecol Indic 72:67–76
Gaylord B, Blanchette CA, Denny MW (1994) Mechanical consequences of size in wave-swept algae. Ecol Monogr 64:287–313
Geraldes V, Jacinavicius FR, Genuário DB, Pinto E (2020) Identification and distribution of mycosporine-like amino acids in Brazilian cyanobacteria using ultrahigh-performance liquid chromatography with diode array detection coupled to quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 34:e8634
Gerard VA, Mann KH (1979) Growth and production of Laminaria longicruris (Phaeophyta) populations exposed to different intensities of water movement. J Phycol 15:33–41
Gilmour DJ, Mf H, Boney AD (1984) The effect of osmotic and ionic stress on the primary processes of photosynthesis in Dunaliella tertiolecta. J Exp Bot 35:18–27
Gilmour D, Hipkins M, Webber A, Baker N, Boney A (1985) The effect of ionic stress on photosynthesis in Dunaliella tertiolecta. Planta 163:250–256
Gimpel JA, Henríquez V, Mayfield SP (2015) In metabolic engineering of eukaryotic microalgae: potential and challenges come with great diversity. Front Microbiol 6:1376
Gissi F, Adams MS, King CK, Jolley DF (2015) A robust bioassay to assess the toxicity of metals to the Antarctic marine microalga Phaeocystis antarctica. Environ Toxicol Chem 34:1578–1587
Hanelt D, Nultsch W (2003) Photoinhibition in seaweeds. In: Heldmaier G, Werner D (eds) Environmental signal processing and adaptation. Springer, Berlin, pp 141–167
Harke MJ, Steffen MM, Gobler CJ, Otten TG, Wilhelm SW, Wood SA, Paerl HW (2016) A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54:4–20
Harun R, Danquah MK (2011) Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem 46:304–309
Hellebust J, LIN YH (1989) Regulation of glycerol and starch metabolism in Chlamydomonas pulsatilla in response to changes in salinity. Plant Cell Environ 12:621–627
Henley WJ, Hironaka JL, Guillou L, Buchheim MA, Buchheim JA, Fawley MW, Fawley KP (2004) Phylogenetic analysis of the ‘Nannochloris-like’algae and diagnoses of Picochlorum oklahomensis gen. et sp. nov.(Trebouxiophyceae, Chlorophyta). Phycologia 43:641–652
Henry BE, Van Alstyne KL (2004) Effects of UV radiation on growth and phlorotannins in Fucus gardneri (Phaeophyceae) juveniles and embryos. J Phycol 40:527–533
Hernandez HPV (2016) Effects of heavy metals ions on primary photosynthetic processes in Antarctic filamentous alga Zygnema sp. Czech Polar Rep 6:180–185
Heydarizadeh P, Poirier I, Loizeau D, Ulmann L, Mimouni V, Schoefs B, Bertrand M (2013) Plastids of marine phytoplankton produce bioactive pigments and lipids. Mar Drugs 11:3425–3471
Ho S-H, Nakanishi A, Ye X, Chang J-S, Hara K, Hasunuma T, Kondo A (2014) Optimizing biodiesel production in marine Chlamydomonas sp. JSC4 through metabolic profiling and an innovative salinity-gradient strategy. Biotechnol Biofuels 7:1–16
Ho S-H, Nakanishi A, Kato Y, Yamasaki H, Chang J-S, Misawa N, Hirose Y, Minagawa J, Hasunuma T, Kondo A (2017) Dynamic metabolic profiling together with transcription analysis reveals salinity-induced starch-to-lipid biosynthesis in alga Chlamydomonas sp. JSC4. Sci Rep 7:1–11
Hofmann LC, Yildiz G, Hanelt D, Bischof K (2012) Physiological responses of the calcifying rhodophyte, Corallina officinalis (L.), to future CO2 levels. Mar Biol 159:783–792
Holzinger A, Roleda MY, Lütz C (2009) The vegetative arctic freshwater green alga Zygnema is insensitive to experimental UV exposure. Micron 40:831–838
Hoyer K, Karsten U, Wiencke C (2002) Induction of sunscreen compounds in Antarctic macroalgae by different radiation conditions. Mar Biol 141:619–627
Hu H, Gao K (2006) Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol Lett 28:987–992
Huang J-C, Chen F, Sandmann G (2006) Stress-related differential expression of multiple β-carotene ketolase genes in the unicellular green alga Haematococcus pluvialis. J Biotechnol 122:176–185
Hunter JE, Brandsma J, Dymond MK, Koster G, Moore CM, Postle AD, Mills RA,Attard GS (2018) Lipidomics of Thalassiosira pseudonana under phosphorus stress reveal underlying phospholipid substitution dynamics and novel diglycosylceramide substitutes. Appl Environ Microbiol 84(6):e02034–17
Janot K, Martone PT (2016) Convergence of joint mechanics in independently evolving, articulated coralline algae. J Exp Biol 219:383–391
Janot KG, Martone PT (2018) Bending strategies of convergently evolved, articulated coralline algae. J Phycol 54:305–316
Jauzein C, Erdner DL (2013) Stress-related responses in Alexandrium tamarense cells exposed to environmental changes. J Eukaryot Microbiol 60:526–538
Jiang W, Brueggeman AJ, Horken KM, Plucinak TM, Weeks DP (2014) Successful transient expression of Cas9 and single guide RNA genes in Chlamydomonas reinhardtii. Eukaryot Cell 13:1465–1469
Johanningmeier U, Fischer D (2010) Perspective for the use of genetic transformants in order to enhance the synthesis of the desired metabolites: engineering chloroplasts of microalgae for the production of bioactive compounds. In: Giardi MT, Rea G, Berra B (eds) Bio-farms for nutraceuticals. Springer, Boston, pp 144–151
Johnson AS (2001) Drag, drafting, and mechanical interactions in canopies of the red alga Chondrus crispus. Biol Bull 201:126–135
Kaçka A, Dönmez G (2008) Isolation of Dunaliella spp. from a hypersaline lake and their ability to accumulate glycerol. Bioresour Technol 99:8348–8352
Kageyama H, Waditee-Sirisattha R (2019) Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: Molecular and cellular mechanisms in the protection of skin-aging. Mar Drugs 17:222
Kamenos NA, Burdett HL, Aloisio E, Findlay HS, Martin S, Longbone C, Dunn J, Widdicombe S, Calosi P (2013) Coralline algal structure is more sensitive to rate, rather than the magnitude, of ocean acidification. Glob Change Biol 19:3621–3628
Karentz D, Cleaver JE, Mitchell DL (1991) Cell survival characteristics and molecular responses of antarctic phytoplankton to ultraviolet-B radiation. J Phycol 27:326–341
Katz A, Avron M (1985) Determination of intracellular osmotic volume and sodium concentration in Dunaliella. Plant Physiol 78:817–820
Katz A, Pick U (2001) Plasma membrane electron transport coupled to Na+ extrusion in the halotolerant alga Dunaliella. Biochim Biophys Acta -Bioenergetics 1504:423–431
Kaushik JK, Bhat R (2003) Why is trehalose an exceptional protein stabilizer?: An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J Biol Chem 278:26458–26465
Khalil ZI, Asker MM, El-Sayed S, Kobbia IA (2010) Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J Microbiol Biotechnol 26:1225–1231
Khona DK, Shirolikar SM, Gawde KK, Hom E, Deodhar MA, D’Souza JS (2016) Characterization of salt stress-induced palmelloids in the green alga, Chlamydomonas reinhardtii. Algal Res 16:434–448
Kim KH, Choi IS, Kim HM, Wi SG, Bae H-J (2014) Bioethanol production from the nutrient stress-induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour Technol 153:47–54
Kim B-H, Ramanan R, Kang Z, Cho D-H, Oh H-M, Kim H-S (2016) Chlorella sorokiniana HS1, a novel freshwater green algal strain, grows and hyperaccumulates lipid droplets in seawater salinity. Biomass Bioenergy 85:300–305
Kim HS, Kim M, Park WK, Chang YK (2020) Enhanced lipid production of Chlorella sp. HS2using serial optimization and heat shock. J Microbiol Biotechnol 30:136–145
Kobiyama A, Tanaka S, Kaneko Y, Lim P-T, Ogata T (2010) Temperature tolerance and expression of heat shock protein 70 in the toxic dinoflagellate Alexandrium tamarense (Dinophyceae). Harmful Algae 9:180–185
Koehl M (1982) Mechanical design of spicule-reinforced connective tissue: stiffness. J Exp Biol 98:239–267
Koehl MAR (1986) Seaweeds in moving water: form and mechanical function. In: Givnich TJ (ed) On the economy of plant form and function. Cambridge University Press, New York, pp 603–634
Koehl M (2000) Mechanical design and hydrodynamics of blade-like algae: Chondracanthus exaspera. In: Spatz HC, Speck T (eds) Third International Plant Biomechanics Conference. Thieme Verlag, Stuttgart, pp 295–308
Kondzior P, Butarewicz A (2018) Effect of heavy metals (Cu and Zn) on the content of photosynthetic pigments in the cells of algae Chlorella vulgaris. J Ecol Eng 19(3):18–28
Kováčik J, Dresler S (2018) Calcium availability but not its content modulates metal toxicity in Scenedesmus quadricauda. Ecotoxicol Environ Saf 147:664–669
Kübler JE, Johnston AM, Raven JA (1999) The effects of reduced and elevated CO2 and O2 on the seaweed Lomentaria articulata. Plant Cell Environ 22:1303–1310
Kuffner IB, Andersson AJ, Jokiel PL, Ku‘ulei SR, Mackenzie FT, (2008) Decreased abundance of crustose coralline algae due to ocean acidification. Nat Geosci 1:114–117
Kumar M, Kumari P, Gupta V, Reddy C, Jha B (2010) Biochemical responses of red alga Gracilaria corticata (Gracilariales, Rhodophyta) to salinity induced oxidative stress. J Exp Mar Biol Ecol 391:27–34
Lemoine Y, Schoefs B (2010) Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth Res 106:155–177
Li Z, Guo M (2018) Healthy efficacy of Nostoc commune Vaucher. Oncotarget 9:14669
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Li D, Zhao Y, Ding W, Zhao P, Xu J-W, Li T, Ma H, Yu X (2017) A strategy for promoting lipid production in green microalgae Monoraphidium sp. QLY-1 by combined melatonin and photoinduction. Bioresour Technol 235:104–112
Li X, Li X, Han B, Zhao Y, Li T, Zhao P, Yu X (2019) Improvement in lipid production in Monoraphidium sp. QLY-1 by combining fulvic acid treatment and salinity stress. Bioresour Technol 294:122179
Li L, Peng H, Tan S, Zhou J, Fang Z, Hu Z, Gao L, Li T, Zhang W, Chen L (2020) Effects of early cold stress on gene expression in Chlamydomonas reinhardtii. Genomics 112:1128–1138
Liu F, Pang SJ (2010) Stress tolerance and antioxidant enzymatic activities in the metabolisms of the reactive oxygen species in two intertidal red algae Grateloupia turuturu and Palmaria palmata. J Exp Mar Bio Ecol 382:82–87
Liu J, Qiu W, Xia D (2018) Brassinosteroid improves lipid productivity and stress tolerance of Chlorella cells induced by high temperature. J Appl Phycol 30:253–260
Llewellyn CA, Airs RL (2010) Distribution and abundance of MAAs in 33 species of microalgae across 13 classes. Mar Drugs 8:1273–1291
Lu C, Vonshak A (2002) Effects of salinity stress on photosystem II function in cyanobacterial Spirulina platensis cells. Physiol Plant 114:405–413
Lu Y, Xu J (2015) Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends Plant Sci 20:273–282
Lu Y, Tarkowská D, Turečková V, Luo T, Xin Y, Li J, Wang Q, Jiao N, Strnad M, Xu J (2014) Antagonistic roles of abscisic acid and cytokinin during response to nitrogen depletion in oleaginous microalga Nannochloropsis oceanica expand the evolutionary breadth of phytohormone function. Plant J 80:52–68
Ma X, Liu J, Liu B, Chen T, Yang B, Chen F (2016) Physiological and biochemical changes reveal stress-associated photosynthetic carbon partitioning into triacylglycerol in the oleaginous marine alga Nannochloropsis oculata. Algal Res 16:28–35
Ma D, Li Y, Fu H (2020) Effect of high temperature on the balance between photosynthetic light absorption and energy utilization in Chlorella pyrenoidosa (Chlorophyceae). J Oceanol Limnol 38:186–194
Macler BA (1988) Salinity effects on photosynthesis, carbon allocation, and nitrogen assimilation in the red alga, Gelidium coulteri. Plant Physiol 88:690–694
Maikova A, Zalutskaya Z, Lapina T, Ermilova E (2016) The HSP70 chaperone machines of Chlamydomonas are induced by cold stress. J Plant Physiol 204:85–91
Mangelsdorf K, Finsel E, Liebner S, Wagner D (2009) Temperature adaptation of microbial communities in different horizons of Siberian permafrost-affected soils from the Lena Delta. Geochemistry 69:169–182
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542
Martin S, Gattuso JP (2009) Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Glob Change Biol 15:2089–2100
Martin P, Van Mooy BAS, Heithoff A, Dyhrman ST (2011) Phosphorus supply drives rapid turnover of membrane phospholipids in the diatom Thalassiosira pseudonana. ISME J 5:1057–1060
Martin S, Cohu S, Vignot C, Zimmerman G, Gattuso JP (2013) One-year experiment on the physiological response of the Mediterranean crustose coralline alga, Lithophyllum cabiochae, to elevated pCO2 and temperature. Ecol Evol 3:676–693
Martone PT, Janot K, Fujita M, Wasteneys G, Ruel K, Joseleau J-P, Estevez JM (2019) Cellulose-rich secondary walls in wave-swept red macroalgae fortify flexible tissues. Planta 250:1867–1879
Massyuk NP (1965) Effect of Na, Mg, Cl and SO4 ions on growth, reproduction and carotene production in Dunaliella salina Teod. Ukr Bot Z 22:3–11
Mayfield S, Golden SS (2015) Photosynthetic bio-manufacturing: food, fuel, and medicine for the 21st century. Springer, Berlin
McLean C, Haley ST, Swarr GJ, Soule MCK, Dyrhman ST, Kujawinski EB (2021) Harmful algal bloom-forming organism responds to nutrient stress distinctly from model phytoplankton. bioRxiv
Menegol T, Diprat AB, Rodrigues E, Rech R (2017) Effect of temperature and nitrogen concentration on biomass composition of Heterochlorella luteoviridis. J Food Sci Technol 37:28–37
Meng Y, Chen H-y, Liu J, Zhang C-Y (2020) Melatonin facilitates the coordination of cell growth and lipid accumulation in nitrogen-stressed Chlamydomonas reinhardtii for biodiesel production. Algal Res 46:101786
Mitsuhashi S, Fujimoto M, Muramatsu H, Tanishita K (1994) Effect of simple shear flow on photosynthesis rate and morphology of micro algae. Acta Astronaut 33:179–187
Miyasaka H, Ohnishi Y, Akano T, Fukatsu K, Mizoguchi T, Yagi K, Maeda I, Ikuta Y, Matsumoto H, Shioji N (1998) Excretion of glycerol by the marine Chlamydomonas sp. strain W-80 in high CO2 cultures. J Ferment Bioeng 85:122–124
Moha-León JD, Pérez-Legaspi IA, Ortega-Clemente LA, Rubio-Franchini I, Ríos-Leal E (2019) Improving the lipid content of Nannochloropsis oculata by a mutation-selection program using UV radiation and quizalofop. J Appl Phycol 31:191–199
Monteiro CM, Castro PM, Malcata FX (2012) Metal uptake by microalgae: underlying mechanisms and practical applications. Biotechnol Prog 28:299–311
Mori K, Tanaka Y, Morikawa M, Toyama T (2020) Enhanced lipid productivity of Chlamydomonas reinhardtii with combination of NaCl and CaCl2 stresses. Bioprocess Biosyst Eng 43:971–980
Mühling M, Belay A, Whitton BA (2005) Variation in fatty acid composition of Arthrospira (Spirulina) strains. J Appl Phycol 17:137–146
Nazifi E, Wada N, Yamaba M, Asano T, Nishiuchi T, Matsugo S, Sakamoto T (2013) Glycosylated porphyra-334 and palythine-threonine from the terrestrial cyanobacterium Nostoc commune. Mar Drugs 11:3124–3154
Nazifi E, Wada N, Asano T, Nishiuchi T, Iwamuro Y, Chinaka S, Sakamoto T (2015) Characterization of the chemical diversity of glycosylated mycosporine-like amino acids in the terrestrial cyanobacterium Nostoc commune. J Photochem Photobiol B 142:154–168
Neelam S, Subramanyam R (2013) Alteration of photochemistry and protein degradation of photosystem II from Chlamydomonas reinhardtii under high salt grown cells. J Photochem Photobiol B 124:63–70
Ngoennet S, Nishikawa Y, Hibino T, Waditee-Sirisattha R, Kageyama H (2018) A method for the isolation and characterization of mycosporine-like amino acids from cyanobacteria. Methods Protoc 1:46
Nishiyama Y, Allakhverdiev SI, Yamamoto H, Hayashi H, Murata N (2004) Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry 43:11321–11330
Ouyang H, Kong X, He W, Qin N, He Q, Wang Y, Wang R, Xu F (2012) Effects of five heavy metals at sub-lethal concentrations on the growth and photosynthesis of Chlorella vulgaris. Chin Sci Bull 57:3363–3370
Paliwal C, Mitra M, Bhayani K, Bharadwaj SV, Ghosh T, Dubey S, Mishra S (2017) Abiotic stresses as tools for metabolites in microalgae. Bioresour Technol 244:1216–1226
Pancha I, Chokshi K, Maurya R, Trivedi K, Patidar SK, Ghosh A, Mishra S (2015) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348
Pandey GK, Pandey A, Prasad M, Böhmer M (2016) Abiotic stress signaling in plants: functional genomic intervention. Front Plant Sci 7:681
Park H, Song B, Morel FM (2007) Diversity of the cadmium-containing carbonic anhydrase in marine diatoms and natural waters. Environ Microbiol 9:403–413
Park W-K, Yoo G, Moon M, Kim CW, Choi Y-E, Yang J-W (2013) Phytohormone supplementation significantly increases growth of Chlamydomonas reinhardtii cultivated for biodiesel production. Appl Biochem Biotech 171:1128–1142
Pavia H, Cervin G, Lindgren A, Åberg P (1997) Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 157:139–146
Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14:290–295
Perrineau MM, Zelzion E, Gross J, Price DC, Boyd J, Bhattacharya D (2014) Evolution of salt tolerance in a laboratory reared population of Chlamydomonas reinhardtii. Environ Microbiol 16:1755–1766
Pessoa MF (2012) Harmful effects of UV radiation in algae and aquatic macrophytes-a review. Emir J Food Agric 24:510–526
Pham T-L, Dao T-S, Bui HN, Pham TKN, Ngo TTH, Bui HM (2020) Lipid production combined with removal and bioaccumulation of Pb by Scenedesmus sp. green alga. Pol J Environ Stud 29:1785–1791
Pichrtová M, Remias D, Lewis LA, Holzinger A (2013) Changes in phenolic compounds and cellular ultrastructure of Arctic and Antarctic strains of Zygnema (Zygnematophyceae, Streptophyta) after exposure to experimentally enhanced UV to PAR ratio. Microb Ecol 65:68–83
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Żyłkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka-Szelewa E (2017) Response and the detoxification strategies of green alga Acutodesmus obliquus (Chlorophyceae) under lead stress. Environ Exp Bot 144:25–36
Piotrowska-Niczyporuk A, Bajguz A, Kotowska U, Bralska M, Talarek-Karwel M (2018a) Growth, metabolite profile, oxidative status, and phytohormone levels in the green alga Acutodesmus obliquus exposed to exogenous auxins and cytokinins. J Plant Growth Regul 37:1159–1174
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka-Szelewa E, Bralska M (2018b) Exogenously applied auxins and cytokinins ameliorate lead toxicity by inducing antioxidant defence system in green alga Acutodesmus obliquus. Plant Physiol Biochem 132:535–546
Piotrowska-Niczyporuk A, Bajguz A, Kotowska U, Zambrzycka-Szelewa E, Sienkiewicz A (2020) Auxins and cytokinins regulate phytohormone homeostasis and thiol-mediated detoxification in the green alga Acutodesmus obliquus exposed to lead stress. Sci Rep 10:1–14
Purton S, Szaub J, Wannathong T, Young R, Economou C (2013) Genetic engineering of algal chloroplasts: progress and prospects. Russ J Plant Physiol 60:491–499
Ragazzola F, Foster LC, Form A, Anderson PS, Hansteen TH, Fietzke J (2012) Ocean acidification weakens the structural integrity of coralline algae. Glob Change Biol 18:2804–2812
Raja V, Majeed U, Kang H, Andrabi KI, John R (2017) Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ Exp Bot 137:142–157
Rastogi RP, Sinha RP, Moh SH, Lee TK, Kottuparambil S, Kim Y-J, Rhee J-S, Choi E-M, Brown MT, Häder D-P (2014) Ultraviolet radiation and cyanobacteria. J Photochem Photobiol B 141:154–169
Raven J, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P, Riebesell U, Shepherd J, Turley C, Watson A (2005) Ocean acidification due to increasing atmospheric carbon dioxide. Policy Document 12/05, The Royal Society, pp 68
Reed RH, Collins JC, Russell G (1981) The effects of salinity upon ion content and ion transport of the marine red alga Porphyra purpurea (Roth) C. Ag J Exp Bot 32:347–367
Reef R, Pandolfi JM, Lovelock CE (2012) The effect of nutrient enrichment on the growth, nucleic acid concentrations, and elemental stoichiometry of coral reef macroalgae. Ecol Evol 2:1985–1995
Remias D, Karsten U, Lütz C, Leya T (2010) Physiological and morphological processes in the Alpine snow alga Chloromonas nivalis (Chlorophyceae) during cyst formation. Protoplasma 243:73–86
Remias D, Schwaiger S, Aigner S, Leya T, Stuppner H, Lütz C (2012) Characterization of an UV-and VIS-absorbing, purpurogallin-derived secondary pigment new to algae and highly abundant in Mesotaenium berggrenii (Zygnematophyceae, Chlorophyta), an extremophyte living on glaciers. FEMS Microbiol Ecol 79:638–648
Renuka N, Guldhe A, Singh P, Ansari FA, Rawat I, Bux F (2017) Evaluating the potential of cytokinins for biomass and lipid enhancement in microalga Acutodesmus obliquus under nitrogen stress. Energy Convers Manag 140:14–23
Renuka N, Guldhe A, Singh P, Bux F (2018) Combined effect of exogenous phytohormones on biomass and lipid production in Acutodesmus obliquus under nitrogen limitation. Energy Convers Manag 168:522–528
Reynoso G, De Gamboa B (1982) Salt tolerance in the freshwater algae Chlamydomonas reinhardii: Effect of proline and taurine. Comp Biochem Physiol A 73:95–99
Roleda MY, Hanelt D, Wiencke C (2006) Exposure to ultraviolet radiation delays photosynthetic recovery in Arctic kelp zoospores. Photosynth Res 88:311–322
Roleda MY, Wiencke C, Hanelt D, Bischof K (2007) Sensitivity of the early life stages of macroalgae from the northern hemisphere to ultraviolet radiation. Photochem Photobiol 83:851–862
Roleda MY, Morris JN, McGraw CM, Hurd CL (2012) Ocean acidification and seaweed reproduction: increased CO2 ameliorates the negative effect of lowered pH on meiospore germination in the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae). Glob Change Biol 18:854–864
Roleda MY, Slocombe SP, Leakey RJ, Day JG, Bell EM, Stanley MS (2013) Effects of temperature and nutrient regimes on biomass and lipid production by six oleaginous microalgae in batch culture employing a two-phase cultivation strategy. Bioresour Technol 129:439–449
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19:430–436
Rothschild LJ, Mancinelli RL (2001) Life in extreme environments. Nature 409:1092–1101
Salama E-S, Kabra AN, Ji M-K, Kim JR, Min B, Jeon B-H (2014) Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour Technol 172:97–103
Salama E-S, Hwang J-H, El-Dalatony MM, Kurade MB, Kabra AN, Abou-Shanab RA, Kim K-H, Yang I-S, Govindwar SP, Kim S (2018) Enhancement of microalgal growth and biocomponent-based transformations for improved biofuel recovery: a review. Bioresour Technol 258:365–375
Sayed O, Hegazy A (1992) Inhibition of secondary carotenoid biosynthesis during degreening of Chlorella fusca (Chlorococcales, Chlorophyta) and implications for growth and survival. Cryptogam Algol 13:181–186
Schoenwaelder ME (2002) The occurrence and cellular significance of physodes in brown algae. Phycologia 41:125–139
Scranton MA, Ostrand JT, Georgianna DR, Lofgren SM, Li D, Ellis RC, Carruthers DN, Dräger A, Masica DL, Mayfield SP (2016) Synthetic promoters capable of driving robust nuclear gene expression in the green alga Chlamydomonas reinhardtii. Algal Res 15:135–142
Sheath RG, Handbook JA (1988) Mechanical adaptations to flow in freshwater red algae. J Phycol 24:107–111
Shi K, Gao Z, Shi T-Q, Song P, Ren L-J, Huang H, Ji X-J (2017) Reactive oxygen species-mediated cellular stress response and lipid accumulation in oleaginous microorganisms: the state of the art and future perspectives. Front Microbiol 8:793
Silva HJ, Cortifas T, Ertola RJ (1987) Effect of hydrodynamic stress on Dunaliella growth. J Chem Technol Biotechnol 40:41–49
Sinha RP, Klisch M, Helbling EW, Häder D-P (2001) Induction of mycosporine-like amino acids (MAAs) in cyanobacteria by solar ultraviolet-B radiation. J Photochem Photobiol B 60:129–135
Sithtisarn S, Yokthongwattana K, Mahong B, Roytrakul S, Paemanee A, Phaonakrop N, Yokthongwattana C (2017) Comparative proteomic analysis of Chlamydomonas reinhardtii control and a salinity-tolerant strain revealed a differential protein expression pattern. Planta 246:843–856
Smith SM, Li J (2014) Signalling and responses to strigolactones and karrikins. Current Opin Plant Biol 21:23–29
Solovchenko A, Verschoor AM, Jablonowski ND, Nedbal L (2016) Phosphorus from wastewater to crops: An alternative path involving microalgae. Biotech Adv 34:550–564
Song X, Zhao Y, Han B, Li T, Zhao P, Xu J-W, Yu X (2020) Strigolactone mediates jasmonic acid-induced lipid production in microalga Monoraphidium sp. QLY-1 under nitrogen deficiency conditions. Bioresour Technol 306:123107
Soru S, Malavasi V, Caboni P, Concas A, Cao G (2019) Behavior of the extremophile green alga Coccomyxa melkonianii SCCA 048 in terms of lipids production and morphology at different pH values. Extremophiles 23:79–89
Specht E, Miyake-Stoner S, Mayfield S (2010) Micro-algae come of age as a platform for recombinant protein production. Biotechnol Lett 32:1373–1383
Starko S, Martone PT (2016) Evidence of an evolutionary-developmental trade-off between drag avoidance and tolerance strategies in wave-swept intertidal kelps (Laminariales, Phaeophyceae). J Phycol 52:54–63
Stirk WA, Ördög V, Novák O, Rolčík J, Strnad M, Bálint P, van Staden J (2013) Auxin and cytokinin relationships in 24 microalgal strains. J Phycol 49:459–467
Stockenreiter M, Haupt F, Graber AK, Seppälä J, Spilling K, Tamminen T, Stibor H (2013) Functional group richness: implications of biodiversity for light use and lipid yield in microalgae. J Phycol 49:838–847
Subramanyam R, Jolley C, Thangaraj B, Nellaepalli S, Webber AN, Fromme P (2010) Structural and functional changes of PSI-LHCI supercomplexes of Chlamydomonas reinhardtii cells grown under high salt conditions. Planta 231:913–922
Sudhir P, Murthy S (2004) Effects of salt stress on basic processes of photosynthesis. Photosynthetica 42:481–486
Sun Y, Han X, Hu Z, Cheng T, Tang Q, Wang H, Han X (2021) Extraction, isolation and characterization of mycosporine-like amino acids from four species of red macroalgae. Mar Drugs 19:615
Sun X, Wang W, Shen J, Xu N (2014) Transcriptome sequencing of the marine microalga, Chlorella pyrenoidosa (Chlorophyta), and analysis of carbonic anhydrase expression under salt stress. Bot Mar 57:403–412
Sunda W (2012) Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front Microbiol 3:204
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13:178–182
Talebi AF, Tabatabaei M, Mohtashami SK, Tohidfar M, Moradi F (2013) Comparative salt stress study on intracellular ion concentration in marine and salt-adapted freshwater strains of microalgae. Not Sci Biol 5:309–315
Targett NM, Arnold TM (1998) Minireview—predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J Phycol 34:195–205
Teoh M-L, Phang S-M, Chu W-L (2013) Response of Antarctic, temperate, and tropical microalgae to temperature stress. J Appl Phycol 25:285–297
Terada R, Nishihara GN, Arimura K, Watanabe Y, Mine T, Morikawa T (2021) Photosynthetic response of a cultivated red alga, Neopyropia yezoensis f. narawaensis (= Pyropia yezoensis f. narawaensis; Bangiales, Rhodophyta) to dehydration stress differs with between two heteromorphic life-history stages. Algal Res 55:102262
Terlova EF, Holzinger A, Lewis LA (2021) Terrestrial green algae show higher tolerance to dehydration than do their aquatic sister-species. Microb Ecol 82:770–782
Thompson GA Jr (1996) Lipids and membrane function in green algae. Biochim Biophys Acta -Lipids and Lipid Metabolism 1302:17–45
Tian J, Yu J (2009) Changes in ultrastructure and responses of antioxidant systems of algae (Dunaliella salina) during acclimation to enhanced ultraviolet-B radiation. J Photochem Photobiol B 97:152–160
Tissot-Lecuelle G, Purton S, Dubald M, Goldschmidt-Clermont M (2014) Synthesis of recombinant products in the chloroplast. In: Theg SM, Wollman F-A (eds) Plastid biology. Springer, NY, pp 517–557
Tiwari S, Patel A, Prasad SM (2018) Kinetin alleviates chromium toxicity on growth and PS II photochemistry in Nostoc muscorum by regulating antioxidant system. Ecotoxicol Environ Saf 161:296–304
Uji T, Matsuda R, Takechi K, Takano H, Mizuta H, Takio S (2016) Ethylene regulation of sexual reproduction in the marine red alga Pyropia yezoensis (Rhodophyta). J Appl Phycol 28:3501–3509
Uji T, Gondaira Y, Fukuda S, Mizuta H, Saga N (2019) Characterization and expression profiles of small heat shock proteins in the marine red alga Pyropia yezoensis. Cell Stress Chaperones 24:223–233
Ünal D, Işik NO, Sukatar A (2010) Effects of chromium VI stress on green alga Ulva lactuca (L.). Turk J Biol 34:119–124
Van Mooy BAS, Fredricks HF, Pedler BE, Dyhrman ST, Karl DM, Koblížek M, Lomas MW, Mincer TJ, Moore LR, Moutin T, Rappé MS, Webb EA (2009) Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 458:69–72
Wang Y, He B, Sun Z, Chen Y-F (2016) Chemically enhanced lipid production from microalgae under low sub-optimal temperature. Algal Res 16:20–27
Wang T, Tian X, Liu T, Wang Z, Guan W, Guo M, Chu J, Zhuang Y (2017) A two-stage fed-batch heterotrophic culture of Chlorella protothecoides that combined nitrogen depletion with hyperosmotic stress strategy enhanced lipid yield and productivity. Process Biochem 60:74–83
Wang N, Qian Z, Luo M, Fan S, Zhang X, Zhang L (2018a) Identification of salt stress responding genes using transcriptome analysis in green alga Chlamydomonas reinhardtii. Int J Mol Sci 19:3359
Wang W, Teng F, Lin Y, Ji D, Xu Y, Chen C, Xie C (2018b) Transcriptomic study to understand thermal adaptation in a high temperature-tolerant strain of Pyropia haitanensis. PLoS ONE 13:e0195842
Wang B, Zhang F, Hu J, Gao X, Bian P, Liu Y, Wang G (2019a) Cre-miR914-regulated RPL18 is involved with UV-B adaptation in Chlamydomonas reinhardtii. J Plant Physiol 232:151–159
Wang Q, Li X, Tang L, Fei Y, Pan Y, Sun L (2019b) Based electroanalytical devices for in situ determination of free 3-indoleacetic acid and salicylic acid in living Pyropia haitanensis thallus under various environmental stresses. J Appl Phycol 32:485–497
Wang S, Li Q, Huang S, Zhao W, Zheng Z (2021) Single and combined effects of microplastics and lead on the freshwater algae Microcystis aeruginosa. Ecotoxicol Environ Saf 208:111664
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4:162–176
Wase N, Tu B, Allen JW, Black PN, DiRusso CC (2017) Identification and metabolite profiling of chemical activators of lipid accumulation in green algae. Plant Physiol 174:2146–2165
Wei S, Bian Y, Zhao Q, Chen S, Mao J, Song C, Cheng K, Xiao Z, Zhang C, Ma W (2017) Salinity-induced palmella formation mechanism in halotolerant algae Dunaliella salina revealed by quantitative proteomics and phosphoproteomics. Front Plant Sci 8:810
Whitney SM, Houtz RL, Alonso H (2011) Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme. Rubisco Plant Physiol 155:27–35
Wilson S, Blake C, Berges JA, Maggs CA (2004) Environmental tolerances of free-living coralline algae (maerl): implications for European marine conservation. Biol Conserv 120:279–289
Wu S, Huang R, Xu L, Yan G, Wang Q (2010) Improved hydrogen production with expression of hemH and lba genes in chloroplast of Chlamydomonas reinhardtii. J Biotechnol 146:120–125
Xia B-B, Wang S-H, Duan J-B, Bai L-H (2014) The relationship of glycerol and glycolysis metabolism patway under hyperosmotic stress in Dunaliella salina. Cent Eur J Biol 9:901–908
Xu Y, Feng L, Jeffrey PD, Shi Y, Morel FM (2008) Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature 452:56–61
Yang JD, Wang NS (1992) Cell inactivation in the presence of sparging and mechanical agitation. Biotechnol Bioeng 40:806–816
Ye Z-W, Jiang J-G, Wu G-H (2008) Biosynthesis and regulation of carotenoids in Dunaliella: progresses and prospects. Biotechnol Adv 26:352–360
Yeesang C, Cheirsilp B (2011) Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour Technol 102:3034–3040
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17:145–156
Yu X, Zhao P, He C, Li J, Tang X, Zhou J, Huang Z (2012) Isolation of a novel strain of Monoraphidium sp. and characterization of its potential application as biodiesel feedstock. Bioresour Technol 121:256–262
Yu Z, Song M, Pei H, Jiang L, Hou Q, Nie C, Zhang L (2017) The effects of combined agricultural phytohormones on the growth, carbon partitioning and cell morphology of two screened algae. Bioresour Technol 239:87–96
Yu Z, Pei H, Jiang L, Hou Q, Nie C, Zhang L (2018) Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresour Technol 247:904–914
Yuan W, Ma Y, Wei W, Liu W, Ding Y, Balamurugan S (2019) Sequential treatment with bicarbonate and low-temperature to potentiate both biomass and lipid productivity in Nannochloropsis oceanica. J Chem Technol Biotechnol 94:3413–3419
Zalutskaya ZM, Skryabina U, Ermilova E (2019) Generation of hydrogen peroxide and transcriptional regulation of antioxidant enzyme expression in Chlamydomonas reinhardtii under hypothermia. Russ J Plant Physiol 66:223–230
Zhang L, Liu J (2016) Effects of heat stress on photosynthetic electron transport in a marine cyanobacterium Arthrospira sp. J Appl Phycol 28:757–763
Zhang PY, Yu J, Tang XX (2005) UV-B radiation suppresses the growth and antioxidant systems of two marine microalgae, Platymonas subcordiformis (Wille) Hazen and Nitzschia closterium (Ehrenb.) W. Sm. J Integr Plant Biol 47:683–691
Zhang X, Tang X, Wang M, Zhang W, Zhou B, Wang Y (2017) ROS and calcium signaling mediated pathways involved in stress responses of the marine microalgae Dunaliella salina to enhanced UV-B radiation. J Photochem Photobiol B 173:360–367
Zhang L, Zhang C, Liu J, Yang N (2020) A strategy for stimulating astaxanthin and lipid production in Haematococcus pluvialis by exogenous glycerol application under low light. Algal Res 46:101779
Zhao Y, Li D, Xu J-W, Zhao P, Li T, Ma H, Yu X (2018a) Melatonin enhances lipid production in Monoraphidium sp. QLY-1 under nitrogen deficiency conditions via a multi-level mechanism. Bioresour Technol 259:46–53
Zhao Y, Yue C, Ding W, Li T, Xu J-W, Zhao P, Ma H, Yu X (2018b) Butylated hydroxytoluene induces astaxanthin and lipid production in Haematococcus pluvialis under high-light and nitrogen-deficiency conditions. Bioresour Technol 266:315–321
Zhao Y, Song X, Yu L, Han B, Li T, Yu X (2019a) Influence of cadmium stress on the lipid production and cadmium bioresorption by Monoraphidium sp. QLY-1. Energy Convers Manag 188:76–85
Zhao Y, Xing H, Li X, Geng S, Ning D, Ma T, Yu X (2019b) Physiological and metabolomics analyses reveal the roles of fulvic acid in enhancing the production of astaxanthin and lipids in Haematococcus pluvialis under abiotic stress conditions. J Agric Food Chem 67:12599–12609
Zhao T, Han X, Cao H (2020a) Effect of temperature on biological macromolecules of three microalgae and application of FT-IR for evaluating microalgal lipid characterization. ACS Omega 5:33262–33268
Zhao Y, Song X, Zhong D-B, Yu L, Yu X (2020b) γ-Aminobutyric acid (GABA) regulates lipid production and cadmium uptake by Monoraphidium sp. QLY-1 under cadmium stress. Bioresour Technol 297:122500
Zheng Y, Xue C, Chen H, He C, Wang Q (2020) Low-temperature adaptation of the snow alga Chlamydomonas nivalis is associated with the photosynthetic system regulatory process. Front Microbiol 11:1233
Zorin B, Lu Y, Sizova I, Hegemann P (2009) Nuclear gene targeting in Chlamydomonas as exemplified by disruption of the PHOT gene. Gene 432:91–96
Zuppini A, Gerotto C, Baldan B (2010) Programmed cell death and adaptation: two different types of abiotic stress response in a unicellular chlorophyte. Plant Cell Physiol 51:884–895
Acknowledgements
The study is supported by DST-SERB Core Research Grant (CRG/20l9/005499). MK likes to thank the University Grants Commission (UGC), New Delhi, India, for the financial support for Ph.D. KCS gratefully acknowledges the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for the financial support towards Ph.D.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, M., Saini, K.C., Ojah, H. et al. Abiotic stress in algae: response, signaling and transgenic approaches. J Appl Phycol 34, 1843–1869 (2022). https://doi.org/10.1007/s10811-022-02746-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02746-7