Abstract
The toxic effects of Cu (II) on the freshwater green algae Chlorella vulgaris and its chloroplast were investigated by detecting the responses of photosynthesis and oxidant stress. The results showed that Cu (II) arrested the growth of C. vulgaris and presented in a concentration- and time-dependent trend and the SRichards 2 model fitted the inhibition curve best. The chlorophyll fluorescence parameters, including qP, Y (II), ETR, F v /F m , and F v /F 0, were stimulated at low concentration of Cu (II) but declined at high concentration, indicating the photosystem II (PSII) of C. vulgaris was destroyed by Cu (II). The chloroplasts were extracted, and the Hill reaction activity (HRA) of chloroplast was significantly decreased with the increasing Cu (II) concentration under both illuminating and dark condition, and faster decline speed was observed under dark condition. Activities of superoxide dismutase (SOD) and catalase (CAT) and malondialdehyde (MDA) content were also significantly decreased at high concentration Cu (II), companied with a large number of reactive oxygen species (ROS) production. All these results indicated a severe oxidative stress on algal cells occurred as well as the effect on photosynthesis, thus inhibiting the growth of algae, which providing sights to evaluate the phytotoxicity of Cu (II).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution is a global problem that has gained considerable attention because of the great toxicity to organisms and cannot be biologically degraded (Luo et al. 2010, Shen et al. 2013, Wen et al. 2015). Once released into the environment, these heavy metals may bring about deleterious effects on sensitive microorganisms. As a consequence, the effect on microorganisms such as algae will extend to the whole ecosystem. Therefore, the potential impact of these heavy metals on the ecosystem must be evaluated as closely as that of other hazardous chemicals. Photoautotrophic microalgae are the primary producers in the ecosystem. They are on the lowest level of the nutrient pyramid and constitute the foundation of the entire food web in an aquatic ecosystem. Consequently, toxicity of heavy metals to these organisms is of great importance (Couderchet and Vernet 2003, Ma et al. 2002, Nie et al. 2008, Nyström et al. 2002).
Copper is an essential micro element for numerous physiological processes at low concentrations, but the beneficial range for the growth and development of plants is particularly narrow thus becomes toxic at high concentration (Gaetke and Chow 2003). Due to the multiple oxidation state (Cu (I) and Cu (II)) existence in vivo, copper plays an important role in photosynthetic electron transport, where the transfer of electrons takes place through plastocyanin, the most abundant of Cu-protein in green tissue (Yamasaki et al. 2008), which gets reduced and oxidized as the electron is transferred from the cytochrome b6f complex to the photosystem I (PSI) reaction center. On the other hand, Cu (II) can also combine with the chlorophyll molecule instead of Mg2+; these altered chlorophylls are unsuitable for photosynthesis because of their less stable singlet excited state and lower tendency to bind axial ligands (Küpper et al. 2006). The excited energy from these altered chlorophylls may be accidentally transferred to oxygen resulting in the production of singlet oxygen, one of the reactive oxygen species (ROS), which causes oxidative damage (Pinto et al. 2003). Potential participation of Cu (II) in Fenton reaction would also result in ROS production (Halliwell and Gutteridge, 1984).
In addition, high concentration of Cu (II) inhibits photosynthesis including the mainly of the light reactions thus damaging the photosynthetic organisms (Kupper and Kroneck 2005). During photosynthesis, photosystem II (PSII) is essential to the regulation because it catalyzes the oxidation of water into oxygen (oxygen-evolving complex-Hill reaction) and supports electron transport (Geiken et al. 1998). Chlorophyll fluorescence parameters can be used as indicators of stress affecting photochemical pathway of utilization of absorbed light energy based on chlorophyll fluorescence techniques (Branquinho et al. 1997, Krause and Weis, 1991). The induced chlorophyll fluorescence technique and Hill reaction activity (HRA) assessment can be the earlier detection of the effect on the primary processes of photosynthesis. However, due to the complex plant system, it is difficult to exactly and directly assess the phytotoxicity of Cu (II). As we know, chloroplast is an organelle-specialized subunits in plant and algal cells, and its main role is to conduct photosynthesis, as well as fatty acid synthesis, much amino acid synthesis, and the immune response in plants (Shtaida et al. 2015; Caplan et al. 2015). Thus, it is in urgently need of the effect of Cu (II) on intact chloroplast in vitro. To better evaluate its effect on photosynthesis and the phytotoxicity accurately, knowledge about the effects of Cu (II) on chloroplast in vivo and in vitro is therefore of great importance and calls for combined in vivo and in vitro studies. Therefore, in the present study, freshwater algae Chlorella vulgaris was chosen as a representative green microalga to evaluate the effect of Cu (II) on the chlorophyll fluorescence characteristics, responses of antioxidant system and HRA, and level of ROS of isolated chloroplast and whole algal cell, in order to detect the toxicity of Cu (II) to microalgae and provide possible mechanisms from the perspective of oxidative damage and photosynthesis.
Material and methods
Algal growth inhibition assay
The freshwater microalgae C. vulgaris were used as a test organism, and the initial stock organisms were obtained from the Institute of Hydrobiology, Chinese Academy of Sciences (Wuhan, China). Before the assay, the algae were cultivated as described previously (Wen et al. 2010) with minor modification. Briefly, the algae were incubated in HB-4 medium at 25 ± 1.0 °C in an incubator under continuous illumination at 3000–4000 lx with a 12-h light and 12-h dark cycle. In each incubator, the cultures were shaken five times per day to ensure optimal growth. The algae were periodically inoculated into fresh media to keep the cells in the logarithmic growth phase and to prepare them for subsequent bioassays (Liu and Xiong 2009). The sensitivity of the algae was tested using copper dichloride (CuCl2). C. vulgaris were then exposed to a series of concentrations of CuCl2 (0.0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 10.0, and 12.0 μM) for 72 h. Three replicates were performed for each treatment and the control. After 72-h treatment, the optical density at 680 nm (OD680) of algae was monitored with a Shimadzu UV-2401 spectrophotometer. The algal growth inhibition rate was used as one of the indices to evaluate the copper chloride toxicity. The inhibition of algal growth was calculated by normalizing the data to the results of the control cultures. In addition, to detect the intuitive effect of Cu (II) on C. vulgaris, the electron microscopy analysis was performed modified with previous study (Qian et al. 2008) using transmission electron microscopy (TEM) (JEM-1230, JEOL, Japan).
Chlorophyll fluorescence characteristics
Chlorophyll fluorescence characteristics were identified in a 96-well plate using a Maxi-PAM chlorophyll fluorometer (LI6400–40). The algal suspension of C. vulgaris (200 μl) was pipetted into each well and then incubated in the dark for 30 min. The maximum effective quantum yield of photosystem II (PSII) was calculated as F v /F m = (F m – F 0) / F m . F v is the difference between F m and F 0, which are the maximum and minimum fluorescence of the dark-adapted stage of PSII, respectively. The potential activity of PSII (F v /F 0), the yield of photochemical quantum (Y (II)), photochemical quenching coefficient (qP), non-photochemical quenching coefficient (qN), and the rate of photosynthetic electron transport (ETR) are obtained automatically (Maxwell and Johnson 2000).
Extraction of chloroplast and determination of Hill reaction activity
Extraction of chloroplast was performed with enzyme solution (0.5 M mannitol, 0.2 % bovine serum albumin (BSA), 50 mM 2-(N-morpholino) ethanesulfonic acid (MES) (pH 5.8), 2 % cellulase, 2 % macerozyme) after the previous studies (Braun and Aach 1975, Nishimura et al. 1982). In detail, it was done by taking a certain amount of C. vulgaris and spinning in microfuge at 5000g for 5 min, then removing supernatant and adding 10-ml enzyme solution. The final density of C. vulgaris was about 4.5 × 105/ml. The solution was shaken in dark at 37 °C, 60–70 rpm for 24 h. Then, the filtrate obtained from filtering with 400-mesh cell strainer was spun in microfuge at 120g for 5 min. The supernatant was removed, and the pellet was washed with distilled water, after which the pellet was kept suspended in buffer solution (0.33 M mannitol, 5 mM CaCl2, 2 mM EDTA, 10 mM NaCl, 50 mM Tricine, pH 7.5 ). Syringe was used to break it down, and chloroplast was obtained after being spun in microfuge at 1000g for 1 min. Samples are stored at 4 °C until analysis. The HRA was detected with 2,6-dichlorophenol indophenol (DCPIP) (Wu et al. 2015). In detail, 2 ml 20 μM DCPIP was added to 0.1-ml chlorophyll solution (final concentration, 10 μg chl/ml), and after reaction under light (4000–5000 lx) for 10 min, the absorbance at 590 nm was read. Reaction of chlorophyll solution with buffer solution was set as control. The HRA was defined according to the difference of DCPIP between the control and treated groups.
Determination of reactive oxygen species production
The production of ROS was measured using the cell-permeable indicator 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) (Knauert and Knauer 2008). For the quantitative determination of ROS, an aliquot of 200 μl of the suspended algal solution was transferred to a 96-well microplate. The 2,7-dichlorodihydrofluorescein (DCF) fluorescence was measured using a SpectraMax M5 multi-label microplate reader, with excitation and emission filters of 485 and 530 nm, respectively. The production of ROS was defined as absolute fluorescent units of DCF by 105 algae cells. H2O2 and ·OH production were determined with Amplex red agent and Coumarin-3-carboxylic acid (3-CCA), respectively, according previous studies (Manevich et al. 1997, Mohanty et al. 1997). In addition, the ROS production in the chloroplast of algae was also determined after the extraction chloroplast described above.
Analysis of antioxidant response
The algal suspension of C. vulgaris (35 ml) after 72-h exposure was spun in microfuge at 10,000g for 15 min at 4 °C, after which the supernatant was discarded. The pellet was washed twice with phosphate buffered saline (PBS) (50 mM, pH 7.4) buffer and then grinded with 5-ml buffer and quartz powder on the ice. The grinding solution was spun in microfuge at 10,000g for 5 min at 4 °C, and the supernatant was used for enzyme activity assays. The total soluble protein (TSP) was determined with Coomassie blue dye (G-250) binding method (Bradford 1976). In detail, 3 ml G-250 was added to 1-ml algal sample, and the absorbance at 595 nm was detected. TSP contents are obtained by referring to the standard curve.
The determination of superoxide dismutase (SOD) was performed with the nitro blue tetrazolium (NBT) (Choo et al., 2004). One unit of SOD activity was defined as the amount of enzyme that caused a 50 % decrease of the SOD-inhibited NBT reduction. The content of malondialdehyde (MDA), product of lipid hydroperoxide, is determined with thiobarbituric acid (TBA) (Fraga et al. 1988). Briefly, 3 ml 0.5 % TBA (contain 20 % trichloroacetic acid, TCA) and samples were reacting in boiling water for 30 min and cooling down at room temperature, after which the absorbance at 600, 532, and 450 nm respectively is read. The content of MDA can be formulated mathematically as follows:
Where OD450, OD532, and OD600 are the optical density at specific wavelengths, and C (MDA) is the content of malondialdehyde.
Statistical analysis
The data were analyzed using Origin 8.0 software (OriginLab, Northampton, MA, USA) according to the methods provided by the manufacturer in the test kit. Comparisons were made using one-way analyses of variance (ANOVA), followed by a multiple comparison test of means (Tukey test). The differences were considered statistically significant when p was less than 0.05.
Results and discussion
Toxicity of Cu (II) to C. vulgaris
The effects of Cu (II) on the inhibition ratio of C. vulgaris were depicted in Fig. 1. As shown in Fig. 1, after 24-h exposure, the treatments exhibited an increasing inhibition rate as the Cu (II) concentration increased, showing 10.10, 23.18, 29.63, 33.20, 35.30, and 36.74 %, respectively. The greatest inhibition rate was less than 40 % and changed little when Cu (II) concentration was larger than 5 μM. Significant increasing inhibition rates occurred when C. vulgaris exposed to another 24-h and 48-h treatments, exhibiting more than 70 and 80 % inhibition rate at high Cu (II) concentration, and the EC50 was 3.02 and 3.16 μM, respectively. In addition, to best fit the effect of Cu (II) on the growth of C. vulgaris, three different fitting models were compared. The parameters of SRichards 2 model, Slogistic 1 model, and SGompertz model have been shown in Table 1. As it shown, the SRichards 2 model fitted the growth effect curve best, where R 2 was 1.000 and 0.999 for 48-h and 72-h exposure, respectively.
As one of essential micro nutrition elements, the promoting role of Cu (II) may be reversed as the concentration going up. Previous studies have revealed that at high concentration, Cu (II) has deleterious effects on C. vulgaris including the ROS production and chlorophyll synthesis (Mehta and Gaur 1999, Qian et al. 2009). In this study, the effect of Cu (II) on the growth of C. vulgaris exhibited a concentration-dependent manner as Fig.2 depicted. As for the control group (Fig 2(a)), algal cell was presented in a healthy state, but when the concentration of Cu (II) increased (Fig. 2(b)–(f), 1–5 μM), plasmolysis occurred in a concentration-dependent manner, indicating that alga cells suffered under Cu (II) stresses.
On the whole, the stress on C. vulgaris increased with the increasing concentration of Cu (II) and the inhibition presented in a concentration- and time-dependent trend. Therefore, to evaluate the toxicity of Cu (II) and further impacts to C. vulgaris, we choose Cu (II) concentrations of 1, 2, 3, 4, and 5 μM.
Effect of Cu (II) on chlorophyll fluorescence
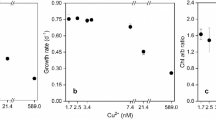
Algae cannot grow without photosynthesis; once the photosynthetic system is affected by contaminants, the growth of algae will be affected inevitably (Bernal et al. 2006). The chlorophyll fluorescence reflects the absorption, distribution, and utilization efficiency of energy by cells (Cid Blanco et al. 1995, Pätsikkä et al. 1998, 2002). The fluorescence kinetic analysis can be used as a tool to identify changes in photosynthetic light reactions, because environmental stress can reduce the ability of plants to metabolize normally, resulting to an imbalance between the absorption of light energy by chlorophyll and the use of energy in photosynthesis. The effect of Cu (II) on the chlorophyll fluorescence was depicted in Table 2 and Fig 3. At low Cu (II) concentration, the minimum fluorescence (F 0) value and maximum fluorescence (F m) value of dark-adapted samples had a slight increase comparing the control, as well as the photosynthetic quantum efficiency of photosystem II (PSII) in dark-adapted state (measured as F v /F m ) and the potential activity of PSII (measured as F v / F 0, which represents the potential of energy, absorbed by antenna pigment, transferring to PSII). These slight increases may due to the fact that Cu is an essential trace element in plants that low dose of Cu (II) ions may exhibit a promoting effect on plants. As the concentration of Cu (II) increased, the F 0 and F m values had a significant decreasing trend towards Cu (II) concentration at 4 and 5 μM. Additionally, both the F v /F m value and the F v /F 0 value, were maximal at 1 μM Cu (II), followed a significant decreasing trend towards Cu (II) concentration of 3 μM, and almost zero at 4 and 5 μM (Table 2). The negative effect of high-dose Cu (II) on the F v /F m and F v /F 0 values may due to Cu (II) harmful effects on the oxidation transport chain in PSII or PSII primary electron acceptor, indicating that the photo-damage of PSII under Cu (II) stress may be accompanied with an increase in energy dissipation as heat and increasing of photo-damage to the photosynthetic apparatus (Huang et al. 2013).
As for the other photochemical and non-photochemical quenching coefficients shown in Fig. 4, the photochemical activity of PSII in actinic light (qP) was optimal at around 1 μM Cu (II), but a sharp decrease occurred at high concentration of Cu (II) (Fig 4a). The non-photochemical quenching (qN, measured as 1 − F v ′ / F v ), a parameter related to the capacity of dissipating excess photon energy in the form of thermal, had a significant decreasing trend towards Cu (II) concentration of 3 μM, almost zero at 4 and 5 μM (Fig 4b). As shown in Fig. 4c, low concentrations (1 and 2 μM) of Cu (II) did not significantly affect the yield of photochemical quantum (Y (II)), the parameter defined as actual photochemical efficiency of PSII in the light and measured as (Fm′ − F)/Fm′ (Bilger and Björkman, 1990), and there is a slight boost. This is probably because Cu is one kind of essential trace elements. The value of Y (II) had significant decline under the concentration of 3 μM, to almost zero at the concentration more than 4 μM. The electron transport rate (ETR) increased with the increasing light intensity from 0 to 600 μmol m−2 s−1, then decreased and fluctuated (Fig 4d).
The parameter qP, an index to indicate the ratio of the opened reaction center in PSII, reflects the reducing state of primary electron acceptor (Q A ). The greater qP, the more energetic electron transfer (Genty et al. 1989). Thus, the decreasing trend of qP under high concentration Cu (II) suggested that a larger percentage of the PSII reaction centers was closed. These results showed that the balance between excitation rate and electron transfer rate had changed, thus leading to a more reduced state of the PSII reaction center and more luminous energy dissipated by heat or other way (Efeoğlu et al. 2009).
Chloroplast extraction and Hill reaction activity of isolated chloroplast
The extraction of chloroplasts in C. vulgaris was performed with enzyme solution based on previous studies (Braun and Aach 1975, Nishimura et al. 1982). The extraction results were depicted in Fig. 5. The photo in Fig 5a was pictured by phase contrast microscope, and the photo in Fig 5b was taken by UV fluorescence microscope with fluorescence-whitening agent Brightner 28. From the aspect of luster and the integrity of dual membrane structure, the chloroplast extracted with enzyme solution belonged to class A according to the classification (Hall 1972).
The Hill reaction is known as the first step of photosynthesis, splitting of water to donate two electrons to the reaction center and can be used to evaluate photosynthetic capability (Ye et al. 2009). Followed the extraction, the Hill reaction activity (HRA) of isolated chloroplasts under illuminating and dark condition in different concentrations was determined and compared. In the results, after exposure to Cu (II) for 2 h, the HRA significantly decreased with the increasing Cu (II) concentration under both illuminating and dark conditions (Fig 6). In detail, the HRA declined more than 50 % at the Cu concentration of 20 μM under the light condition, which occurred to 10-μM treatment under the dark condition. The largest decrease amplitude of HRA under the illuminating and dark conditions was 60 and 83 %, respectively. It suggested that Cu (II) inhibited the photochemical activity of isolated chloroplast, as well as the photosynthetic ability of C. vulgaris was disturbed with increasing concentration of Cu (II) and may due to the damage of thylakoids in the chloroplast. In fact, the effects of Cu (II) on the photosynthesis of algae have been frequently reported (Shioi et al. 1978, Singh and Singh, 1987). The Hill reaction was inhibited, which leads to the generation of singlet oxygen and oxidative damage of photosynthesis apparatus (thylakoids). In addition, the speed of HRA decline with or without light condition exhibited a different manner, in which HRA decreased faster without light than that with light, indicating that light condition plays an important role in the effect of Cu (II) on the HRA of chloroplasts in C. vulgaris.
Cu (II)-induced ROS production of whole algal cell and isolated chloroplast
Environmental stresses can provoke an oxidative burden by overproducing reactive oxygen species (ROS), which is highly reactive to cellular constituents, thus fatally disturbing plant metabolism and influencing the growth of plants. Excessive ROS production may be produced when organisms are exposed to external stress, leading to oxidative stress and cause lipid peroxidation and resulting in random target attack and toxic effects (Finkel and Holbrook, 2000, Wen et al. 2011). In this study, the level of ROS of the whole algal cell and isolated chloroplast was determined at Cu (II) stress for 2 h. In the whole algal cell, as Fig. 7 depicted, ROS increased with the increasing Cu (II) stress and remained stable when the concentration was larger than 5 μM (Fig 7a). As for the H2O2 production, Cu treatments at low concentration did not show significantly effect, but as Cu (II) increased, the effects showed up and the production of H2O2 increased accordingly (Fig 7b). In addition, the ·OH production of C. vulgaris under Cu (II) stress exhibited similar trend (Fig 7c). Similar phenomenon took place in the ROS production of isolated chloroplast in C. vulgaris under Cu (II) stress (Fig. 8).
ROS, as the chemically reactive molecules containing oxygen, are formed as a natural byproduct of the normal metabolism of oxygen and play important roles in cell signaling and homeostasis (Devasagayam et al. 2004). However, environmental stress, such as UV or heat can increase the ROS levels dramatically (Devasagayam et al. 2004); therefore, ROS can be triggered by external contaminants like heavy metals, and their harmful effects have long been recognized (Halliwell and Gutteridge, 1999). These results obtained in present study confirmed that Cu (II) induced the algae to produce reactive oxygen species, providing a sight to explain the mechanism of Cu (II) toxicity to plants.
Activity of superoxide dismutase, catalase, and lipid peroxidation
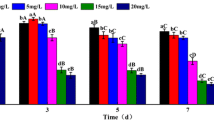
Under non-stress condition, SOD can prevent cells from oxidative damage by eliminating O2·− (Halliwell and Gutteridge, 1999). As it shown in Fig. 9, under Cu (II) stress, SOD activity in C. vulgaris first increased, maximal at 2 μM, then decreased with the increasing Cu (II) stress. SOD activity of the treated group was significantly higher than that of the control (Fig 9a). It indicated that as Cu (II) stress intensity is raised, the level of SOD activity might be firstly increased as a defense mechanism against Cu (II)-induced O2·− , then decreased as a result of enzyme inactivation. Similar phenomenon occurred in the effect on CAT activities (Fig 9c).
As Cu (II) stress intensity is raised, antioxidant system cannot remove ROS completely to prevent cells from oxidative damage, such as lipid peroxidation (Xu et al. 2013, Wen et al. 2009). MDA, the product of lipid peroxidation, reflects the degree of lipid hydroperoxide and cell damage (Box et al. 2008). With the increasing Cu (II) stress, MDA content firstly increased then decreased. The contents of the treated group (2, 3, and 4 μM) were significantly higher than those of the control, but lower at 5 μM. It indicated that Cu (II) caused lipid hydro peroxide damage to algae (Fig 9b).
Conclusion
Our experiments showed that high Cu (II) stress (larger than 3 μM) caused PSII damage, leading to weak capacity of photosynthetic and self-protection. However, low Cu (II) stress (less than 2 μM) stimulated PSII starting the self-protection mechanism thus enhancing actual photochemical efficiency of PSII in the light (Y(II)),the photochemical activity of PSII in actinic light (qP) and electron transport rate (ETR). Similar trends of ROS production in the whole algal cell and isolated chloroplast were observed. As Cu (II) stress intensity was raised, the level of antioxidant response might be firstly increased as a defense mechanism against Cu (II)-induced ROS, then decreased as a result of enzyme inactivation. Low Cu (II) stress (less than 1 μM) did not cause lipid peroxidation; high Cu (II) stress (larger than 2 μM) caused lipid peroxidation obviously. Cu (II) could reduce HRA of isolated chloroplast significantly at Cu (II) stress (larger than 20 μM) for 2 h. The change of the level of ROS and HRA presents a good negative correlation, suggesting ROS might damage the chloroplast to conduct photosynthesis. Results obtained in the present study may provide sights to evaluate the mechanism of heavy metal phytotoxicity, and the extraction of chloroplast may also offer the direct evidence of deleterious effects to plant caused by external contaminants.
References
Bernal M, Ramiro MV, Cases R, Picorel R, Yruela I (2006) Excess copper effect on growth, chloroplast ultrastructure, oxygen-evolution activity and chlorophyll fluorescence in Glycine max cell suspensions. Physiol Plant 127:312–325
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Box A, Sureda A, Terrados J, Pons A, Deudero S (2008) Antioxidant response and caulerpenyne production of the alien Caulerpa taxifolia (Vahl) epiphytized by the invasive algae Lophocladia lallemandii (Montagne). J Exp Mar Biol Ecol 364:24–28
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochemistry 72:248–253
Branquinho C, Brown DH, Catarino F (1997) The cellular location of Cu in lichens and its effects on membrane integrity and chlorophyll fluorescence. Environ Exp Bot 38:165–179
Braun E, Aach H (1975) Enzymatic degradation of the cell wall of Chlorella. Planta 126:181–185
Caplan JF, Kumar AS, Park E, Padmanabhan MS, Hoban K, Modla S, Czymmek K, Dinesh-Kumar SP (2015) Chloroplast stromules function during innate immunity. Dev Cell 34:45–57
Choo K-S, Snoeijs P, Pedersén M (2004) Oxidative stress tolerance in the filamentous green algae Cladophora glomerata and Enteromorpha ahlneriana. J Exp Mar Biol Ecol 298:111–123
Cid Blanco Á, Herrero López C, Torres E, Abalde Alonso JE (1995) Copper toxicity on the marine microalga Phaeodactylum tricornutum: effects on photosynthesis and related parameters. Aquat Toxicol 31:165–174
Couderchet M, Vernet G (2003) Pigments as biomarkers of exposure to the vineyard herbicide flazasulfuron in freshwater algae. Ecotoxicol Environ Saf 55:271–277
Devasagayam T, Tilak J, Boloor K, Sane KS, Ghaskadbi SS, Lele R (2004) Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India 52:794–804
Efeoğlu B, Ekmekci Y, Cicek N (2009) Physiological responses of three maize cultivars to drought stress and recovery. S Afr J Bot 75:34–42
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Fraga CG, Leibovitz BE, Tappel AL (1988) Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med 4:155–161
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189:147–163
Geiken B, Masojidek J, Rizzuto M, Pompili M, Giardi M (1998) Incorporation of [35S] methionine in higher plants reveals that stimulation of the D1 reaction centre II protein turnover accompanies tolerance to heavy metal stress. Plant Cell Environ 21:1265–1273
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta Gen Subj 990:87–92
Hall D (1972) Nomenclature for isolated chloroplasts. Nature 235:125–126
Halliwell B, Gutteridge J (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219:1
Halliwell B, Gutteridge JM (1999) Free radicals in biology and medicine, 3. Oxford university press, Oxford
Huang CJ, Zhao SY, Wang LC, Anjum SA, Chen M, Zhou H, Zou C (2013) Alteration in chlorophyll fluorescence, lipid peroxidation and antioxidant enzymes activities in hybrid ramie (Boehmeria nivea L.) under drought stress. Aust J Crop Sci 7:594
Küpper H, Küpper FC, Spiller M (2006) [Heavy metal]-chlorophylls formed in vivo during heavy metal stress and degradation products formed during digestion, extraction and storage of plant material, Chlorophylls and Bacteriochlorophylls. Springer, pp. 67–77
Knauert S, Knauer K (2008) The role of reactive oxygen species in copper toxicity to two freshwater green algae. J Phycol 44:311–319
Krause G, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Biol 42:313–349
Kupper H, Kroneck PM (2005) Heavy metal uptake by plants and cyanobacteria. Met Ions Biol Syst 44:97
Liu HJ, Xiong MY (2009) Comparative toxicity of racemic metolachlor and S-metolachlor to Chlorella pyrenoidosa. Aquat Toxicol 93:100–106
Luo L, Zhang SZ, Christie P (2010) New insights into the influence of heavy metals on phenanthrene sorption in soils. Environ Sci Technol 44:7846–7851
Ma JY, Zheng RQ, Xu LG, Wang SF (2002) Differential sensitivity of two green algae, Scenedesmus obliqnus and Chlorella pyrenoidosa, to 12 pesticides. Ecotoxicol Environ Saf 52:57–61
Manevich Y, Held KD, Biaglow JE (1997) Coumarin-3-carboxylic acid as a detector for hydroxyl radicals generated chemically and by gamma radiation. Radiat Res 148:580–591
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Mehta SK, Gaur JP (1999) Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol 143:253–259
Mohanty J, Jaffe JS, Schulman ES, Raible DG (1997) A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Methods 202:133–141
Nie XP, Wang X, Chen JF, Zitko V, An TC (2008) Response of the freshwater alga Chlorella vulgaris to trichloroisocyanuric acid and ciprofloxacin. Environ Toxicol Chem 27:168–173
Nishimura M, Douce R, Akazawa T (1982) Isolation and characterization of metabolically competent mitochondria from spinach leaf protoplasts. Plant Physiol 69:916–920
Nyström B, Becker-Van Slooten K, Bérard A, Grandjean D, Druart J-C, Leboulanger C (2002) Toxic effects of Irgarol 1051 on phytoplankton and macrophytes in Lake Geneva. Water Res 36:2020–2028
Pätsikkä E, Aro E-M, Tyystjärvi E (1998) Increase in the quantum yield of photoinhibition contributes to copper toxicity in vivo. Plant Physiol 117:619–627
Pätsikkä E, Kairavuo M, Šeršen F, Aro E-M, Tyystjärvi E (2002) Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol 129:1359–1367
Pinto E, Sigaud-kutner T, Leitao MA, Okamoto OK, Morse D, Colepicolo P (2003) Heavy metal-induced oxidative stress in algae. J Phycol 39:1008–1018
Qian HF, Chen W, Sheng GD, Xu XY, Liu WP, Fu ZW (2008) Effects of glufosinate on antioxidant enzymes, subcellular structure, and gene expression in the unicellular green alga Chlorella vulgaris. Aquat Toxicol 88:301–307
Qian HF, Li JJ, Sun LW, Chen W, Sheng GD, Liu WP, Fu ZW (2009) Combined effect of copper and cadmium on Chlorella vulgaris growth and photosynthesis-related gene transcription. Aquat Toxicol 94:56–61
Shen CS, Chen H, Wu SS, Wen YZ, Li LN, Jiang Z, Li MC, Liu WP (2013) Highly efficient detoxification of Cr (VI) by chitosan-Fe(III) complex: process and mechanism studies. J Hazard Mater 244:689–697
Shioi Y, Tamai H, Sasa T (1978) Inhibition of photosystem II in the green alga Ankistrodesmus falcatus by copper. Physiol Plant 44:434–438
Shtaida N, Khozn-Goldberg I, Boussiba S (2015) The role of pyruvate hub enzymes in supplying carbon precursors for fatty acid synthesis in photosynthetic microalgae. Photosynth Res 125:407–422
Singh DP, Singh SP (1987) Action of heavy metals on Hill activity and O2 evolution in Anacystis nidulans. Plant Physiol 83:12–14
Wen YZ, Yuan YL, Shen CS, Liu HJ, Liu WP (2009) Spectroscopic investigations of the chiral interactions between lipase and the herbicide dichlorprop. Chirality 21:396–401
Wen YZ, Yuan YL, Chen H, Xu DM, Lin KD, Liu WP (2010) Effect of chitosan on the enantioselective bioavailability of the herbicide dichlorprop to Chlorella pyrenoidosa. Environ Sci Technol 44:4981–4987
Wen YZ, Chen H, Shen CS, Zhao MR, Liu WP (2011) Enantioselectivity tuning of chiral herbicide dichlorprop by copper: roles of reaction oxygen species. Environ Sci Technol 45:4778–4784
Wen YZ, Ma JQ, Chen J, Shen CS, Li H, Liu WP (2015) Carbonaceous sulfur-containing chitosan-Fe (III): a novel adsorbent for efficient removal of copper (II) from water. Chem Eng J 259:372–380
Wu X, Wu H, Ye JY, Zhong B (2015) Study on the release routes of allelochemicals from Pistia stratiotes Linn., and its anticyanobacteria mechanisms on Microcystis aeruginosa. Environ Sci Pollut R 22:18994–19001
Xu DM, Li CD, Wen YZ, Liu WP (2013) Antioxidant defense system responses and DNA damage of earthworms exposed to Perfluorooctane sulfonate (PFOS). Environ Pollut 174:131–127
Yamasaki H, Pilon M, Shikanai T (2008) How do plants respond to copper deficiency? Plant Signal Behav 3:231–232
Ye J, Zhang Q, Zhang AP, Wen YZ, Liu WP (2009) Enantioselective effects of chiral herbicide diclofop acid on rice Xiushui 63 seedlings. B Environ Contam Toxicol 83:85–91
Acknowledgments
This manuscript was supported by the National Natural Science Foundation of China (Nos. 21377111 and 21377115).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Thomas Braunbeck
Rights and permissions
About this article
Cite this article
Chen, Z., Song, S., Wen, Y. et al. Toxicity of Cu (II) to the green alga Chlorella vulgaris: a perspective of photosynthesis and oxidant stress. Environ Sci Pollut Res 23, 17910–17918 (2016). https://doi.org/10.1007/s11356-016-6997-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6997-2