Abstract
The ability of algae to change the shape of their thallus in response to the environment may be of functional and ecological importance to the alga, with many species of macroalgae exhibiting a great range of morphological variation across wave exposure gradients. However, differences in morphology detected between sheltered and exposed environments cannot determine whether such differences represent plastic responses to the local environment or whether morphology is genetically fixed. This study tested for differences in the morphology of the common kelp, Ecklonia radiata, between wave sheltered and exposed environments, and reciprocally transplanted juveniles to distinguish the nature of such differences (i.e. plastic vs fixed traits). Differences between exposure environments were consistent with known effects of exposure (i.e. a wide, thin thallus at sheltered sites and a narrow, thick thallus with a thick stipe at exposed sites). The reciprocal transplant experiment confirmed that morphological plasticity was the mechanism enabling this alga to display different patterns in morphology between exposure environments. Individuals transplanted to the exposed environment underwent a rapid and extreme response in morphology, which was not apparent in individuals transplanted to the sheltered environment that responded more slowly. These results suggest that stressors typical of sheltered environments (i.e. diffusion stress) may not be as influential (if at all) compared to stressors typical of exposed environments (i.e. breakage, dislodgement) in differentiating morphological characters between exposure environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphological variation enables a species to occupy a broad range of physical environments (Sultan 2001) and is commonly thought to enhance the survival and productivity of plants growing in physically different environments (Gerard 1987; Slatkin 1987).
Across rocky coasts, variation in wave exposure is known to have profound effects on the biology of macroalgae (Koehl 1986; Hurd 2000), with many species of algae exhibiting a great range of morphological variation across exposure gradients (e.g. Gerard and Mann 1979; Cheshire and Hallam 1989; Bäck 1993; Blanchette et al. 2002). At sheltered sites, the morphology of macroalgal fronds are often wide, thin and undulate, and at exposed sites are narrow, thick and flat with thick stipes (e.g. Cheshire and Hallam 1988; Koehl and Alberte 1988; Jackelman and Bolton 1990; Roberson and Coyer 2004). Such morphological differences are thought to enable macroalgae to inhabit wave sheltered environments without compromising their ability to efficiently photosynthesise and grow (Gerard 1982; Hurd et al. 1996) and to inhabit wave exposed environments by preventing breakage and/or dislodgement (e.g. Dudgeon and Johnson 1992; Blanchette 1997).
Much of our understanding of the relationship between kelp morphology and wave exposure is based on research done at local scales (i.e., kilometre), that compare sites of individual exposure levels for which these treatments are not replicated, though samples within treatments may be (e.g. Gerard and Mann 1979; Cousens 1982; Molloy and Bolton 1996). Differences among sites, therefore, are attributed to an effect of exposure without identifying the contribution of intrinsic spatial variation (i.e. pseudoreplication: Hurlbert 1984). Where broader-scale studies have been attempted (i.e. thousands of kilometres), these studies have either correlated measures of exposure to morphology across the sampled sites (e.g. Rice et al. 1985; Cheshire and Hallam 1989; Rice and Kenchington 1990; Fowler-Walker et al. 2005a), or have used exposure as an ad hoc explanation of large variation in morphology among sites (e.g. Ralph et al. 1998; Blanchette et al. 2002; Wernberg et al. 2003). Rarely has the relationship between wave exposure and morphology been studied among replicate levels of exposure (but see Jackelman and Bolton 1990; Kalvas and Kautsky 1993), which can be particularly insightful when testing for the existence of patterns associated with exposure.
While morphological differences associated with exposure environments have been used as evidence for a plastic response to wave exposure (Norton et al. 1982), such associations may alternatively reflect genetically fixed traits (i.e. ecotypes) that are the result of speciation in the face of strong selection by environmental factors (e.g. Chapman 1974; Serisawa et al. 2003; Roberson and Coyer 2004). We assess whether morphological variation observed in kelps is a plastic response to the local environment or whether morphology is genetically fixed; a useful distinction if we are to understand the population-biology of kelps (i.e. genetically isolated populations versus well mixed populations that respond to local conditions). Transplant experiments have previously been used to differentiate between these competing models, with the results of such experiments differing depending on the species involved and the environmental condition examined (e.g. De Paula and De Oliveira 1982; Druehl and Kemp 1982; Blanchette et al. 2002; Roberson and Coyer 2004).
The canopy-forming kelp, Ecklonia radiata (order Laminariales, family Alariaceae), characterises the rocky coast of temperate Australia (Kennelly and Underwood 1993; Goodsell et al. 2004) and inhabits environments ranging from wave exposed coastlines to sheltered embayments. Recent studies of E. radiata have demonstrated considerable variation in morphology across southern Australia (Wernberg et al. 2003; Fowler-Walker et al. 2005b), and that the magnitude of this variation may be related to differences in wave exposure (Fowler-Walker et al. 2005a). However, only a few studies have used transplant experiments to examine the effects of environmental conditions on morphological characteristics of Laminarian algae (e.g. Gerard and Mann 1979; Serisawa et al. 2002; Roberson and Coyer 2004). In the present study, we tested for differences in the morphology of E. radiata between sheltered and exposed sites, and used a reciprocal transplant experiment to examine whether morphological differences were genetically fixed or were inducible responses to the local environment.
To distinguish between models of morphologically plastic and fixed traits we tested the following predictions: (1) that the morphology of E. radiata differs between sheltered and exposed environments using replicated observations of exposure. Once natural differences in morphology between exposures were established, we then tested the prediction that if we transplant juvenile sporophytes from the sheltered to exposed side of an island (and vice versa), (2) the morphology of transplanted individuals will differ from the morphology of individuals transplanted within the native site, and (3) the morphology of transplanted individuals will not differ from the morphology of individuals transplanted within the recipient site.
Methods
Quantification of natural patterns
The morphology of E. radiata was quantified between July and August 2004 on the in-shore side (hereafter referred to as ‘sheltered’) and offshore side (hereafter referred to as ‘exposed’) of two islands: Wright Island (WI; 35°34′S, 138°36′E) and Seal Rocks (SR; 35°34′S, 138°38′E), located at Encounter Bay, South Australia (Fig. 1). Within each side of each island, 18 quadrats (1 m2) were haphazardly placed within monospecific stands of E. radiata (i.e. ≥80% of the canopy comprised E. radiata species) at 6–8 m depth. Quadrats were separated by ~10 m and positioned >1 m from the edge of a stand. Within each 1 m2 quadrat the density of mature E. radiata plants was recorded and three of these plants were haphazardly chosen and harvested as close as possible to the holdfast using a sharp knife. Only mature, stage three plants (sensu: Kirkman 1981) were sampled so that any ontogenetic effects on morphological characters were reduced. Only solitary individuals (no overlapping holdfasts) were collected, as aggregation has been shown to affect the morphology of E. radiata (Wernberg 2005). The plants were brought to the surface, drained, bagged and transported to the laboratory on ice, where they were frozen and held at −20°C until processed. Morphological measures were made on thawed specimens and characterised those previously used to describe the morphological variation in E. radiata across broad-scales (e.g. Wernberg et al. 2003; Fowler-Walker et al. 2005b). Fourteen morphological characters were measured on each thallus (cf. Table 3), eight characters as described in Fowler-Walker et al. (2005b) and six additional characters: (1) lamina thickness measured midway along the lengthwise axis, (2) lateral length measured on a mature secondary lateral protruding from the central lamina, (3) lateral width and (4) thickness measured half way along the length of a secondary lateral, (5) number of laterals counted as mature, intact secondary laterals and (6) number of corrugations per centimetre across the width of a lateral.
Wave exposure was quantified at the sheltered and exposed side of both islands using three different methods (Table 1): estimation of wave height, measurement of water velocity and calculation of an exposure index. Wave height was estimated by observing the rise and fall of 20 consecutive waves against the rocks of the island from a boat. Observations from all sites were made within 45 min of each other on the 22 July 2004. Water velocity was calculated by measuring the maximum drag force at each site using spring scales with plastic golf balls (Bell and Denny 1994). Two spring scales were deployed at each site, at the depth of specimen collection (6–8 m), between the 2 and 4 October 2004. Drag measurements were subsequently converted to water velocities using the calibration curves provided by Bell and Denny (1994). Lastly, an exposure index for each site was calculated using a modification of Baardseth’s (1970) method (see Rice and Kenchington 1990). The index is equal to the number of 9˚ sectors radiating from the site that are fully open to seaward for 7.5 km or further. In determining values of the index (0 = extreme shelter and 40 = ultimate exposure), 1:100,000 scale topographic maps were used and all rocks and headlands marked on those maps were taken into account. Three a priori planned contrasts (Day and Quinn 1989) were used to test differences in wave height and water velocity between sheltered and exposed sites at WI and SR: (1) WI sheltered vs WI exposed, (2) SR sheltered vs SR exposed and (3) WI sheltered + SR sheltered vs WI exposed + SR exposed. No tests were performed on the exposure indices as only one value was obtained per site.
Analysis of natural patterns
Multivariate tests of differences in morphology used non-parametric multivariate analysis of variance (NP-MANOVA: Anderson 2001) on untransformed data using Gower dissimilarities (Gower 1967), as recommended for variables measured on different scales (Podani 1999). NP-MANOVA enables the analysis of mixed models (random and fixed factors) in multivariate space. Tests for differences in individual characters were carried out using analysis of variance (ANOVA) using the software (GMAV5) and procedures provided by Underwood (1997). For all analyses, Island (random) and Exposure (fixed) were orthogonal to one another, and Quadrat (random) was nested within Exposure and Island.
Sampling targeted kelp occurring at similar depths (6–8 m) and densities (22–26 kelp per m2). The depth and density of each sample were quantified and assessed for differences using a two-way ANOVA, where Island (random) and Exposure (fixed) were orthogonal to one another and measurements at each quadrat (n=18) were treated as replicates. The density of E. radiata plants averaged 24.7±0.5 SE per m2 across all quadrats sampled and did not differ between sheltered and exposed sites (ANOVA: F 1,68=0.21, P=0.65) or between islands (ANOVA: F 1,68=0.08, P=0.37). Similarly, the depth at which individual quadrats were placed did not differ between sheltered and exposed sites (ANOVA: F 1,68=0.15, P=0.70) or between islands (ANOVA: F 1,68=0.08, P=0.77) and averaged 6.7±0.4 m across all quadrats sampled. Hence the density of E. radiata (at the scale of 1 m2) and the depth at which plants were taken do not offer an alternative explanation for the differences in morphology detected between exposure levels and islands.
Experimental tests of reciprocal transplants
To determine whether morphological characters of E. radiata can change to match the morphology of individuals in a new environment, or whether they maintain the morphology of their native site, a reciprocal transplant experiment was performed at WI. The transplant experiment was established on the 17 July 2004 and ran for 7 months through the summer (the period of greatest plant growth: Kirkman 1984). A total of 200 undifferentiated juveniles (defined below) were collected from both the sheltered (n=100) and exposed (n=100) side of WI. Of the 100 juveniles collected from the sheltered side, half were transplanted to the exposed side of the island and half were transplanted back to the sheltered site (=control). The same procedure was carried out on juveniles collected from the exposed site. Juveniles were defined as a single blade that showed no development of secondary laterals (i.e. Stage 1, sensu Kirkman 1984), and the average plant length was 19.3±0.4 cm (±SE). Juveniles collected at the sheltered site did not differ from those collected at the exposed site (see below).
Juveniles were collected from the reef by prying the holdfast off the substratum with a dive knife, placing them in a mesh bag and bringing them to the surface. At the surface the 200 juveniles were separated into 4 treatments (2 transplants × 2 controls, n=50 juveniles per treatment). Within each treatment 10 juveniles (each separated by 10 cm) were attached to a 1 m length of sisal rope by entwining their holdfasts into the rope, with five replicate ropes per treatment (4 treatments × 5 replicate ropes × 10 juveniles per rope=200 juveniles). All juveniles were held in flowing seawater for no longer than 18 h prior to transplantation. Individual ropes were tagged and were treated as replicates, with measurements of plant height made prior to transplantation. Replicate ropes were transplanted to either the sheltered or exposed side of WI, depending on the treatment, were placed beneath sparse canopies of E. radiata (i.e. densities <4 plants per m2) and ropes were attached to the holdfast of adult plants with cable ties. All ropes were haphazardly scattered throughout the experimental site at a similar depth as our description of natural patterns (6–8 m depth), and were separated by 1–10 m. The average depth at which transplanted ropes were attached was 7.3±0.2 m (±SE) and this did not differ between the sheltered and exposed site (ANOVA: F 1,18=0.29, P=0.60). The average length of juveniles at the start of the experiment was 19.3±0.4 cm (±SE) and did not vary among any of the treatment groups (ANOVA: F 1,16=0.69, P=0.57).
During the experiment, morphological characters that could be accurately measured underwater were sampled, which included plant length, stipe length and stipe width. This in situ sampling first occurred 3 months after the experiment was set-up (October 2004), then again in November 2004 and December 2004. Large swells prevented measurements being made in January 2005, and transplants were harvested on the 15 February 2005 once they had first reached maturity (i.e. early stage 3 plants). Harvested plants were refrigerated and transported to the laboratory where a complete set of morphological measurements was made (n=14 characters: cf. Table 5). These measurements included all characters we used to describe natural patterns (except for the number of eroded laterals and corrugations, as they were difficult to distinguish) and also included the wet weight of the stipe and holdfast to further describe morphological variation of the plant. Due to the mortality of individuals at both sheltered and exposed sites, the final sample size of each treatment (in February 2005) was: sheltered transplants (n=3 ropes), sheltered controls (n=4 ropes), exposed transplants (n=3 ropes), exposed controls (n=0 ropes).
Analysis of experimental responses
The design of the transplant experiment tested the following predictions: (1) individuals transplanted between exposures will differ in morphology to individuals transplanted within their native site (i.e. a plastic response: sheltered to exposed sites (SE) ≠ sheltered controls (SS) and exposed to sheltered sites (ES) ≠ exposed controls (EE)) and (2) individuals transplanted between exposures will not differ in morphology to individuals transplanted within the recipient site (i.e. SE = EE and ES = SS). Loss of controls from the exposed site prior to the final sampling period (February 2005) forced us to apply the experimental design on the data collected in situ (n=3 morphological characters) at the previous sampling date (December 2004) (see Fig. 4, Table 4). Data collected at the final sampling period (February 2005: n=14 morphological characters) tested the prediction that (1) individuals transplanted from sheltered to exposed environments will differ in morphology to individuals transplanted within their native site (i.e. SE ≠ SS) and (2) individuals transplanted from exposed to sheltered environments will not differ in morphology to individuals transplanted within the recipient site (i.e. ES = SS). A priori planned contrasts (Day and Quinn 1989) tested for differences in morphology between treatments in December 2004 (SE vs SS, ES vs EE, SE vs EE, and ES vs SS) and February 2005 (SE vs SS and ES vs SS).
Results
All three measures of wave exposure showed differences between sheltered and exposed sites (Table 1). Importantly, the analysis of both wave height and water velocity showed significant differences between exposed and sheltered sites (ANOVA: wave height: F 1,76=22.54, P=0.000, water velocity: F 1,4=28.51, P=0.006) that were consistent between islands (ANOVA planned contrasts: WI exposed = SR exposed > WI sheltered = SR sheltered).
Natural patterns
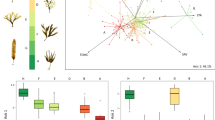
Multivariate analyses detected differences in the morphology of E. radiata between islands (WI ≠ SR) and exposure (sheltered ≠ exposed) (Fig. 2, Table 2). Significant differences were detected among quadrats within each exposure level and island, but these differences did not obscure patterns among treatments. While there was a significant interaction between island and exposure, pair wise comparisons indicated that differences between exposure levels were consistent between islands and vice versa (Table 2).
Non-metric ordination of centroid values (average of replicate plants within a quadrat) representing the morphological variation of Ecklonia radiata between sheltered sites (filled symbols) and exposed sites (unfilled symbols) for each quadrat (n = 18) within WI (triangle) and SR (circle). A stress value of 0.004 indicates an interpretable ordination of the multivariate data in two dimensions (Clarke 1993)
Notwithstanding the substantial univariate variation among quadrats within each exposure and island, each character differed between sheltered and exposed sites, except for corrugations (Fig. 3, Table 3: SNK tests). Plants at exposed sites had smaller measures of weight, surface area, lamina width, lateral length, lateral width, number of laterals and number of eroded laterals (Fig. 3a, c, g, i, j, l, m, Table 3a, c, g, i, j, l, m: SNK tests), and had greater measures of stipe width, lamina thickness and lateral thickness (Fig. 3e, h, k, Table 3e, h, k: SNK tests) compared with sheltered sites. A significant interaction between exposure and island was detected for plant length, stipe length and lamina length, such that measures were greater at exposed compared to sheltered environments at WI only, and no differences were detected at SR (Island × Exposure interaction: Fig. 3b, d, f, Table 3b, d, f: SNK tests). A significant interaction between exposure and island was also detected for lateral length; however, this interaction did not reflect inconsistent patterns between islands, but rather reflected the greater magnitude of difference between sheltered and exposed environments at SR compared to WI (Fig. 3i, Table 3i: SNK tests). Differences between islands (consistent between levels of exposure) were also detected for some morphological characters, whereby plants at WI had greater surface area, lateral width and lateral thickness, and had smaller measures of stipe width and number of laterals, compared to SR (Fig 3c, j, k, e, l, Table 3c, j, k, e, l: SNK tests).
Experimental responses
Differences in morphology were detected (December 2004) between individuals transplanted between exposures and individuals transplanted within their native site (SE ≠ SS and ES ≠ EE) for all measures of morphology (Fig. 4, Table 4). No differences in morphology were detected between individuals transplanted between exposures and individuals transplanted within the recipient site (SE = EE and ES = SS) for all measures of morphology except for stipe width, where measures of transplants to exposed environments were in fact greater than that of individuals within the recipient site (SE > EE) (Fig. 4, Table 4).
Mean measures (±standard error) of three morphological characters of E. radiata, at four sampling periods, for the transplant treatments: sheltered to exposed site (SE), exposed controls (EE), sheltered controls (SS), and exposed to sheltered site (ES). The first in situ sampling period occurred 3 months after the experiment was set-up (October 2004). December 2004 was the last sampling period where all four treatments and replicate ropes were present (see Methods), and was therefore graphed separately to clearly indicate morphological differences among the four transplant treatments
At the final sampling period (February 2005), where all 14 morphological characters were measured, differences were detected between individuals transplanted from SE and individuals transplanted within the native site (SE ≠ SS), for all characters except for weight, lamina thickness and number of laterals (Fig. 5, Table 5). Compared to controls (SS), individuals transplanted to the exposed site (SE) had greater measures of plant length, stipe length, stipe width, stipe wet weight, lamina length, lateral thickness and holdfast wet weight, and smaller measures of surface area, lamina width, lateral length and lateral width (Fig. 5b, d, e, f, g, l, n, c, h, j, k, Table 5b, d, e, f, g, l, n, c, h, j, k). These patterns in morphology were consistent with naturally occurring differences in morphology between sheltered and exposed sites that we have described. Individuals transplanted from ES adopted similar morphologies to individuals transplanted within the recipient site (ES = SS) for all 14 morphological characters (Fig. 5, Table 5).
Mean measures (±standard error) of morphological characters (taken at the final sampling period in February 2005) of E. radiata, for the treatments: SE, SS, and ES (treatment abbreviations as in Fig. 4)
The effect size of transplanting from sheltered to exposed environments (i.e. percentage difference between SE and SS) was large and rapid (e.g. stipe width: October=23.1%, November=24.5%, December=34.5%, February=35.3%, Fig. 4). However, the effect size of transplanting from exposed to sheltered environments (i.e. percentage difference between ES and EE) was comparatively small and gradual (e.g. stipe width: October=5.1%, November=10.4 %, December=17.8%, Fig. 4).
Discussion
Our combination of observational and experimental analyses revealed that different morphologies existed between exposed and sheltered localities, and that this variation was a plastic response to the local environment, and did not reflect genetically fixed traits. The two morphotypes observed in this study exhibited characteristics consistent with known physiological and mechanical responses to exposure. For example, plants at the sheltered sites adopted a thallus of larger surface area, and had longer, wider and yet thinner laterals, which may maximise surface area for nutrient uptake and light harvesting (e.g. Gerard and Mann 1979; Wheeler 1980), compared to plants at the exposed sites that adopted shorter, narrower and thicker laterals, and had thicker stipes and fewer laterals, which may decrease drag and prevent breakage (e.g. Armstrong 1989; Johnson and Koehl 1994; Blanchette et al. 2002).
A large proportion of morphological characters responded consistently to the differences in exposure independent of islands (11 of the 14 characters). However, 3 of the 14 characters responded in an inconsistent way between islands (plant length, stipe length and lamina length). Interestingly, comparisons with other studies show inconsistencies in the direction of morphological differences for similar characters, where greater levels of exposure have been associated with both longer plant length (e.g. Cheshire and Hallam 1988; Jackelman and Bolton 1990) as well as shorter plant length (Bäck 1993; e.g. Kalvas and Kautsky 1993), and with both longer stipe length (e.g. Klinger and DeWreede 1988; Molloy and Bolton 1996) as well as shorter stipe length (e.g. Chapman 1973; Cheshire and Hallam 1988). Given that the absolute values of wave exposure for any one study is unknown, and are unlikely to be similar, inconsistencies among studies may reflect a non-linear relationship between these morphological characters and an exposure gradient. For example, morphological differences between sheltered and semi-exposed sites may occur in the same direction (co-gradient), while differences between semi-exposed and exposed sites may occur in an opposing direction (counter gradient) (e.g. Cousens 1982; Cheshire and Hallam 1988; Jackelman and Bolton 1990).
Why did the morphology of E. radiata differ between the exposed and sheltered sides of islands? The results of the transplant experiment indicate that such differences were the result of a phenotypic response to the local environment. Transplanted individuals grew to differ from their controls (i.e. ES ≠ EE and SE ≠ SS), and therefore did not retain the characters of their native site (i.e. characters are not genetically fixed), but rather responded morphologically to the new environment (i.e. characters are phenotypically plastic). Transplants showed similar morphological patterns to naturally occurring patterns characteristic of exposure (i.e. exposed transplants had thicker stipes, narrower laterals and larger holdfasts than sheltered transplants). Three of the morphological characters did not differ among any of the treatments (i.e. wet weight, lamina thickness and number of laterals), which suggests that these characters had not started to differentiate in form, or that they were not affected by exposure. The general similarity between naturally occurring patterns and experimental effects not only provides strong evidence for models about exposure driven traits, but also suggests that the experimental protocol (i.e. transplants with ropes) maybe useful for continuing work on morphological variation.
Phenotypic plasticity is common in plant and animal populations, with many studies detecting chemical, physiological, developmental or morphological responses to changes in physical (e.g. Scheiner and Goodnight 1984; Dudley 1996; Relyea and Werner 2000) and biological variables (e.g. Bertness et al. 1998; Raimondi et al. 2000; Agrawal 2001). In marine algae, phenotypic plasticity has been inferred from morphological changes detected among contrasting habitats, laboratory observations (e.g. de Senerpont Domis et al. 2003) and from the one-way transplantation of adult sporophytes from one environment to another, where new blade growth has been similar to that of plants native to the environment (e.g. Norton 1969; Svendsen and Kain 1971; Gerard and Mann 1979; Druehl and Kemp 1982). More recently, transplant experiments of juveniles (both one-way and reciprocal) have been used to test whether morphological differences in marine algae are the result of a plastic response to the local environment or are genetically fixed traits (e.g. Blanchette et al. 2002; Serisawa et al. 2003; Roberson and Coyer 2004). These studies have found no change in the morphology of transplants, despite large potential gains (e.g. increased growth and survivorship) they may acquire from such a shift in morphology. However, such non-responses do not unequivocally differentiate between models of genetically fixed vs plastic traits. This is because the two competing models (1) that morphological differences are genetically fixed which prevents phenotypic modification of transplants, vs (2) that phenotypically plastic responses are canalised early in development before the transplantation of individuals, cannot be differentiated under these circumstances. In contrast, our data clearly show that the thick, narrow morphology of individuals at exposed locations, and the wide, thin morphology of individuals at sheltered locations were phenotypically plastic features that responded to local environmental conditions.
Interestingly, the results of our transplant experiment showed that individuals transplanted to the exposed site underwent large and rapid changes in morphology, which were consistent with an accommodation to high wave exposure. However, the same response was not evident for individuals transplanted to the sheltered site, where plants continued to follow the trajectory of an exposed morphology until much later in the experiment. The increase in the stipe width of individuals transplanted to the exposed site, above and beyond that of individuals transplanted within the exposed site, indicates an over-compensation response to the exposure environment, which further highlights the rapid and extreme response that plants may have to exposed conditions. Such a response was not apparent in individuals transplanted to the sheltered environment, which suggests that stressors typical of sheltered environments (i.e. diffusion stress) may not be as influential as stressors typical of exposed environments (i.e. breakage, dislodgement) in differentiating morphological characters between exposure environments, which has also been suggested by Scott et al. (2001) and Jackelman and Bolton (1990). Alternatively, exposed sites may represent particular kinds of stress (i.e. drag and breakage), while sheltered sites may represent the absence or relaxation of these stressors, as opposed to the presence of new stressors. Gerard (1987) induced an ‘exposed’ blade morphology on cultured plants subjected to wave-induced water motion, and a ‘sheltered’ blade morphology on plants where such stress was absent. We are not suggesting that sheltered environments do not affect algal morphology, but rather that stressors typical of sheltered environments, such as nutrient stress and reduced gas exchange (Topinka and Robbins 1976; Norton et al. 1982; Hanisak 1983), may affect morphology under extremely low exposure conditions that were not represented in our study.
In conclusion, variation in the morphology of E. radiata exists across southern Australia (Wernberg et al. 2003; Fowler-Walker et al. 2005b) which we now understand, at the scale of kilometres, can be driven by the external environment. This study suggests that the environmentally specific forms of E. radiata in wave exposed and wave sheltered environments, at scales of 1–10 km, are the result of a plastic response to the exposure conditions typical of the kelps native environment. The results also suggest that stressors typical of sheltered environments (i.e. diffusion stress) may not be as influential (if at all) compared to stressors typical of exposed environments (i.e. breakage, dislodgement) in differentiating morphological characters between exposure environments.
References
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Armstrong SL (1989) The behavior in flow of the morphologically variable seaweed Hedophyllum sessile (C. Ag.) Setchell. Hydrobiologia 183:115–122
Baardseth E (1970) A square-scanning, two-stage sampling method of estimating seaweed quantities, vol 20. Report of the Norwegian Institute of Seaweed Research, pp 1–6
Bäck S (1993) Morphological variation of northern Baltic Fucus vesiculosus along the exposure gradient. Annu Bot Fenn 30:275–283
Bell EC, Denny MW (1994) Quantifying ‘wave exposure’:a simple device for recording maximum velocity and results of its use at several field sites. J Exp Mar Biol Ecol 181:9–29
Bertness MD, Gaines SD, Yeh SM (1998) Making mountains out of barnacles: the dynamics of acorn barnacle hummocking. Ecology 79:1382–1394
Blanchette CA (1997) Size and survival of intertidal plants in response to wave action: a case study with Fucus gardneri. Ecology 78:1563–1578
Blanchette CA, Miner BG, Gaines SD (2002) Geographic variability in form, size and survival of Egregia menziesii around Point Conception, California. Mar Ecol Progress Ser 239:69–82
Chapman ARO (1973) Phenetic variability of stipe morphology in relation to season, exposure, and depth in the non-digitate complex of Laminaria Lamour. (Phaeophyta, Laminariales) in Nova Scotia. Phycologia 12:53–57
Chapman ARO (1974) The genetic basis of morphological differentiation in some Laminaria populations. Mar Biol 24:85–91
Cheshire AC, Hallam ND (1988) Morphology of the Southern Bull-Kelp (Durvillaea potatorum, Durvilleales, Phaeophyta) from King Island (Bass Strait, Australia). Bot Mar 31:139–148
Cheshire AC, Hallam ND (1989) Morphological differences in the Southern Bull-Kelp (Durvillaea potatorum) throughout South-Eastern Australia. Bot Mar 32:191–197
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 17:117–143
Cousens R (1982) The effect of exposure to wave action on the morphology and pigmentation of Ascophyllum nodosum (L.) Le Jolis in South-Eastern Canada. Bot Mar 25:191–195
Day RW, Quinn GP (1989) Comparisons of treatments after an analysis of variance in ecology. Ecol Monogr 59:433–463
De Paula EJ, De Oliveira EC (1982) Wave exposure and ecotypical differentiation in Sargassum cymosum (Phaeophyta—Fucales). Phycologia 21:145–153
de Senerpont Domis LN, Fama P, Bartlett AJ, Prud’homme van Reine WF, Armenta Espinosa C, Trono GC (2003) Defining taxon boundaries in members of the morphologically and genetically plastic genus Caulerpa (Caulerpales, Chlorophyta). J Phycol 39:1019–1037
Druehl LD, Kemp L (1982) Morphological and growth responses of geographically isolated Macrocystis integrifolia populations when grown in a common environment. Can J Bot 60:1409–1413
Dudgeon SR, Johnson AS (1992) Thick vs. thin: thallus morphology and tissue mechanics influence differential drag and dislodgement of two co-dominant seaweeds. J Exp Mar Biol Ecol 165:23–43
Dudley SA (1996) The response to differing selection on plant physiological traits: evidence for local adaptation. Evolution 50:103–110
Fowler-Walker MJ, Connell SD, Gillanders BM (2005a) To what extent do geographic and environmental variables correlate with kelp morphology across temperate Australia? Mar Freshw Res 56:877–887
Fowler-Walker MJ, Connell SD, Gillanders BM (2005b) Variation at local scales need not impede tests for broader scale patterns. Mar Biol 147:823–831
Gerard VA, Mann KH (1979) Growth and production of Laminaria longicruris (Phaeophyta) populations exposed to different intensities of water movement. J Phycol 15:33–41
Gerard VA (1982) In situ water motion and nutrient uptake by the giant kelp Macrocystis pyrifera. Mar Biol 69:51–54
Gerard VA (1987) Hydrodynamic streamlining of Laminaria Saccharina Lamour in response to mechanical stress. J Exp Mar Biol Ecol 107:237–244
Goodsell PJ, Fowler-Walker MJ, Gillanders BM, Connell SD (2004) Variations in the configuration of algae in subtidal forests: Implications for invertebrate assemblages. Aust Ecol 29:350–357
Gower JC (1967) A comparison of some methods of cluster analysis. Biometrics 23:623–627
Hanisak MD (1983) The nitrogen relationships of marine macroalgae. In: Carpenter EJ, Capone DG (eds) Nitrogen in the marine environment. Academic, New York, pp 699–730
Hurd CL, Harrison PJ, Druehl LD (1996) Effects of seawater velocity on inorganic nitrogen uptake by morphologically distinct forms of Macrocystis integrifolia from wave-sheltered and exposed sites. Mar Biol 126:205–214
Hurd CL (2000) Water motion, marine macroalgal physiology, and production. J Phycol 36:453–472
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Jackelman JJ, Bolton JJ (1990) Form variation and productivity of an intertidal foliose Gigartina species (Rhodophyta) in relation to wave exposure. Hydrobiologia 204/205:57–64
Johnson AS, Koehl MAR (1994) Maintenance of dynamic strain similarity and environmental stress factor in different flow habitats: thallus allometry and material properties of a giant kelp. J Environ Biol 195:381–410
Kalvas A, Kautsky L (1993) Geographic variation in Fucus vesiculosus morphology in the Baltic and North Seas. Eur J Phycol 28:85–91
Kennelly SJ, Underwood AJ (1993) Geographic consistencies of effects of experimental physical disturbance on understorey species in sublittoral kelp forests in central New South Wales. J Exp Mar Biol Ecol 168:35–58
Kirkman H (1981) The first year in the life history and the survival of the juvenile marine macrophytye, Ecklonia radiata (Turn.) J. Agardh. J Exp Mar Biol Ecol 55:243–254
Kirkman H (1984) Standing stock and production of Ecklonia radiata (A.Ag.) J. Agardh. J Exp Mar Biol Ecol 76:119–130
Klinger T, DeWreede RE (1988) Stipe rings, age, and size in populations of Laminaria setchelli Silva (Laminariales, Phaeophyta) in British Columbia, Canada. Phycologia 27:234–240
Koehl MAR (1986) Seaweeds in moving water: Form and mechanical function. In: Gavinish T (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 603–634
Koehl MAR, Alberte RS (1988) Flow, flapping, and photosynthesis of Nereocystis luetkeana: a functional comparison of undulate and flat blade morphologies. Mar Biol 99:435–444
Molloy FJ, Bolton JJ (1996) The effects of wave exposure and depth on the morphology of inshore populations of the Namibian kelp, Laminaria schinzii Foslie. Bot Mar 39:525–531
Norton TA (1969) Growth form and environment in Saccorhiza polyschides. J Mar Biol Ass U K 49:1025–1045
Norton TA, Mathieson AC, Neushul M (1982) A review of some aspects of form and function in seaweeds. Bot Mar 25:501–510
Podani J (1999) Extending Gower’s general coefficient of similarity to ordinal characters. Taxon 48:331–340
Raimondi PT, Forde SE, Delph LF, Lively CM (2000) Processes structuring communities:evidence from trait-mediated indirect effects through induced polymorphisms. Oikos 91:353–361
Ralph PJ, Morrison DA, Addison A (1998) A quantitative study of the patterns of morphological variation within Hormosira banksii (Turner) Decaisne (Fucales: Phaeophyta) in south-eastern Australia. J Exp Mar Biol Ecol 225:285–300
Relyea RA, Werner EE (2000) Morphological plasticity in four larval anurans distributed along an environmental gradient. Copeia 1:178–190
Rice EL, Kenchington TJ, Chapman ARO (1985) Intraspecific geographic-morphological variation patterns in Fucus distichus and Fucus evanescens. Mar Biol 88:207–215
Rice EL, Kenchington TJ (1990) Spatial variation patterns in the marine macroalgal Xiphophora gladiata spp. Gladiata (Phaeophyta). II. Morphological variation over large spatial scales. J Phycol 26:522–534
Roberson LM, Coyer JA (2004) Variation in blade morphology of the kelp Eisenia arborea: incipient speciation due to local water motion? Mar Ecol Prog Ser 282:115–128
Scheiner SM, Goodnight CJ (1984) The comparison of phenotypic plasticity and genetic variation in populations of the grass Danthonia spicata. Evolution 38:845–855
Scott GW, Hull SL, Hornby SE, Hardy FG, Owens NJP (2001) Phenotypic variation in Fucus spiralis (Phaeophyceae): morphology, chemical phenotype and their relationship to the environment. Eur J Phycol 36:43–50
Serisawa Y, Yokohama Y, Aruga Y, Tanaka J (2002) Growth of Ecklonia cava (Laminariales, Phaeophyta) sporophytes transplanted to a locality with different temperature conditions. Phycol Res 50:201–207
Serisawa Y, Aoki M, Hirata T, Bellgrove A, Kurashima A, Tsuchiya Y, Sato T, Ueda H, Yokohama Y (2003) Growth and survival rates of large-type sporophytes of Ecklonia cava transplanted to a growth environment with small-type sporophytes. J Appl Phycol 15:311–318
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science 236:787–792
Sultan SE (2001) Phenotypic plasticity and ecological breadth in plants. Am Zool 41:1599
Svendsen P, Kain JM (1971) The taxonomic status, distribution, and morphology of Laminaria cucullata sensu Jorde and Klavestad. Sarsia 46:1–21
Topinka JA, Robbins JV (1976) Effects of nitrate and ammonium enrichment on growth and nitrogen physiology in Fucus spiralis. Limnol Oceanogr 21:659–664
Underwood AJ (1997) Experiments in ecology. Their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge, pp 504
Wernberg T, Coleman M, Fairhead A, Miller S, Thomsen M (2003) Morphology of Ecklonia radiata (Phaeophyta: Laminarales) along its geographic distribution in south-western Australia and Australasia. Mar Biol 143:47–55
Wernberg T (2005) Holdfast aggregation in relation to morphology, age, attachment and drag for the kelp Ecklonia radiata. Aquat Bot DOI:10.1016/j.aquabot.2005.04.003
Wheeler WN (1980) Effect of boundary layer transport on the fixation of carbon by the giant kelp Macrocystis pyrifera. Mar Biol 56:103–110
Acknowledgements
This work could not have been completed without the logistical support of B. Russell, S. Hart and D. Gorman. This work was supported by a postgraduate award to M.J.F and an ARC grant to S.D.C.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.S. Johnson, Crawley
Rights and permissions
About this article
Cite this article
Fowler-Walker, M.J., Wernberg, T. & Connell, S.D. Differences in kelp morphology between wave sheltered and exposed localities: morphologically plastic or fixed traits?. Marine Biology 148, 755–767 (2006). https://doi.org/10.1007/s00227-005-0125-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0125-z