Abstract
The cyanobacterium Arthrospira platensis plays a unique role in the food industry and is a promising and valuable natural source of bioactive compounds. The culture density of A. platensis should be further increased to improve biomass production and productivity, resulting in high conversion efficiency and reducing the cost of production. In this work we utilize a series of methods to increase the biomass yield from 2.26 to 21.57 g L−1. By screening live algae filaments and removing dead algal mass via filtration before cultivation, the biomass production increased from 2.26 to 2.77 g L−1. Using response surface monitoring methodology to optimize the light intensity and initial culture density further improved biomass production to 5.97 g L−1. We also evaluated the feasibility of fed-batch and turbidostatic cultivation for enhancing biomass production of A. platensis GMPA7. The results showed that fed-batch cultivation can increase the biomass production to 15.56 g L−1. Finally, turbidostatic cultivation can further improve the biomass production to 21.57 g L−1, which is a more than eightfold increase compared to the starting culture. Therefore, the turbidostatic cultivation strategy can be further exploited for large-scale and long-term cultivation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arthrospira platensis is a spiral, unbranched, multicellular filamentous cyanobacterium widely found in tropical alkaline lakes (Soni et al. 2017; Ting et al. 2018). It is commercially important due to several nutritional qualities, such as its high protein content (60–70% of dry weight is proteins) and low fat. Furthermore, it contains essential amino acids, unsaturated fatty acids, vitamins, minerals, and pigments such as phycocyanin, β-carotene, and chlorophyll a (Vonshak 1997; Zhang et al. 2015).
These compounds in A. platensis contribute to antiviral, anticancer, antioxidant, anti-inflammatory, and other biological activities (Soheili and Khosravi-Darani 2011; de la Jara et al. 2018). For instance, Remziye Aysun et al. (2013) reported that A. platensis could protect against hepatotoxicity induced by CCl4. In addition, because its cell wall consists of polysaccharides, it had high digestibility and absorption rate (Hernández-Corona et al. 2002). Therefore, A. platensis is widely used as a nutritional supplement (Azcarate et al. 2018; Lucas et al. 2018; Muys et al. 2018). A. platensis is also used in cosmetics (Xiu-Ping et al. 2013), medicines (Gorban et al. 2003), and wastewater treatment (Zhai et al. 2017; Álvarez and Otero 2020).
Previous studies on cultivation methods of A. platensis have mainly focused on mass production in open ponds (Vonshak and Richmond 1988; Belay 1997; Grobbelaar 2012). However, there are many problems with this culture system which need further optimization. Several studies have indicated that the growth of A. platensis highly dependent on the cultivation strategy (Xie et al. 2013; Manirafasha et al. 2018). Chen et al. (2013) used batch cultivation with optimum light intensity and initial nitrate concentration to reach a biomass of 10.0 g L−1. Moreover, Manirafasha et al. (2018) utilized the fed-batch strategy to increase biomass to 13.37 g L−1. However, it could cause a reduction of biomass productivity for long-term cultivation (Xie et al. 2014, 2015). Therefore, it is necessary to develop a more effective strategy to overcome the drawbacks of fed-batch cultivation.
In this study, we use A. platensis GMPA7 as a model strain and optimize its cultivation conditions by using response surface monitoring methodology. Moreover, we show the feasibility of using the turbidostatic strategy to maintain a high level of biomass productivity. We demonstrate that this strategy is a promising culture method for long-term cultivation, which improves biomass productivity and eliminates side effects of irradiance attenuation caused by high cell density.
Methods
Microalgal strain and preculture conditions
The Arthrospira platensis GMPA7 was obtained from the Institute of Pharmaceutical Biotechnology and Engineering, Fuzhou University, Fujian, China. The medium (Rajasekaran et al. 2016) used in culture experiments consisted of (per liter) 16.8 g NaHCO3, 0.625 g K2HPO4, 2.5 g NaNO3, 1 g K2SO4, 1 g NaCl, 0.1 g MgSO4, 0.04 g CaCl4·2H2O, 0.01 g FeSO4·7H2O, 0.08 g Na2EDTA·2H2O, and 1.0 mL of trace element solution. The trace element solution consisted of (per liter) 2.86 g H3BO3, 1.81 g MnCl4·4H2O, 0.222 g ZnSO4·4H2O, 0.0177 g Na2MoO4, and 0.079 g CuSO4·5H2O. The microalgae were inoculated in 500-mL flasks containing 150-mL medium and the initial culture density measured at 680 nm was 0.5. The conditions of the incubator shaker used as a photobioreactor were set as follows: temperature at 30 °C, light intensity at 90 μmol photons m−2 s−1 (continuous light) and rotational speed at 150 rpm.
Screening of living algae filament
Arthrospira filaments were separated with 20 mesh, 100 mesh, and 300 mesh screens. The groups were categorized as follows: (a) unscreened; (b) 20–100 mesh filtered by 20 mesh screen and collected by 100 mesh screen; and (c) 100–300 mesh filtered by 100 mesh screen and collected by 300 mesh screen. The algae collected by 20 mesh screen were dead microalgae aggregations and therefore discarded. The morphology of microalgae collected by different treatments was observed using an optical microscope (Eclipss Ts2R; Nikon Co., Japan).
Cultivation of Arthrospira platensis GMPA7 under different pH
The algae collected by 100–300 mesh were cultivated in medium with pH of 8.0, 9.0, 10.0, and 11.0 for 10 days, respectively. The pH of the culture was adjusted with 1 M HCl or 1 M NaOH every day. Other culture conditions were the same as those of preculture. Samples were collected every 24 h to determine the biomass production and productivity. All experiments were performed in triplicate.
Biomass optimization using response surface methodology
The Box–Behnken design model was established to increase biomass production. In consideration of the effect of cell density and liquid depth on irradiance (Ooms et al. 2016; Martínez et al. 2018), light intensity (X1), initial culture density (X2), and volume (X3) were selected as variables for response surface methodology experiments. All variables were set at 3 levels (− 1, 0, 1), with X1 (90, 135, and 180 μmol photons m−2 s−1), X2 (0.3, 0.5, and 0.7 OD680nm), and X3 (100, 150, and 200 mL). The mean values of the triplicate experiments were fitted by nonlinear quadratic model:
where Y is the response value; X1, X2, and X3 are independent variables; β0 represents the intercept; β1 to β3, β4 to β6, and β7 to β9 are the linear, interaction, and quadratic coefficients, respectively. Analysis of variance (ANOVA) was performed to verify the significance of the model with Design Expert 8.0.6 (Stat-Ease, Inc., USA).
Fed-batch cultivation of Arthrospira platensis GMPA7
Based on the optimum culture conditions obtained from response surface methodology experiments, the fed-batch strategy was carried out, adding concentrated total nutrient medium stock (nitrate concentration 50 g L−1) for feeding culture. The optimum culture conditions for the maximum biomass were determined to be light intensity of 169 μmol photons m−2 s−1, initial OD680nm of 0.52, and volume of 164 mL (500 mL conical flask). When the nitrate content was exhausted, the concentrated total nutrient medium stock was added into the flasks to attain a nitrate concentration of 0.417 g L−1 and distilled water was added to maintain the total volume of 164 mL (the volume of the culture medium decreased continuously due to evaporation). The feeding time intervals were set at 24 h. Liquid samples were collected before and after medium feeding to determine the biomass production and residual nitrate concentration.
Turbidostatic cultivation of Arthrospira platensis GMPA7
The turbidostatic cultivation was carried out in the optimum culture conditions. When the biomass concentration exceeded 4.5 g L−1, fresh medium was added into the flasks to attain a cell density of 4.5 g L−1. The cell concentration was adjusted every 24 h. Samples were collected before and after dilutions to determine the biomass production and residual nitrate concentration.
Determination of biomass production and nitrate concentration
The biomass production was determined by establishing the calibration between dry cell weight and OD680 (Manirafasha et al. 2018) as follows:
The nitrate concentration was measured according to Ho et al. (2013), and calculated using the following equation:
Results
Screen living algae filaments in the culture of Arthrospira platensis GMPA7
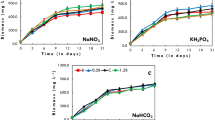
In this study, screens of 20, 100, and 300 mesh were applied to screen living algae filaments. The morphology and biomass level of A. platensis GMPA7 under different screening conditions are shown in Fig. 1. It can be seen that live algae appeared to be green filaments, while dead algae were yellow-green aggregations (Fig. 1a). The 100–300 mesh screen method achieved the separation of live and dead algae (Fig. 1c), while the 20–00 mesh screen method could not (Fig. 1b). The biomass production of A. platensis separated by the screen method of 100–300 mesh was 2.77 g L−1 (Fig. 1d), which was 22% higher than the control. This might be due to the fact that dead cells caused agglomerations of algal filaments (Fig. 1a), which hindered the absorption of nutrients and inhibited the growth of A. platensis. As a result, the screening operation was beneficial to increase the biomass production of A. platensis GMPA7.
Morphology and biomass of Arthrospira platensis GMPA7 under different screening methods. (a) unscreened; (b) 20–100 mesh; (c) 100–300 mesh; (d) biomass level of Arthrospira platensis GMPA7 separated by different treatments. The results are expressed as the mean ± standard deviation (SD) from three independent experiments. Significant differences were determined by using one-way ANOVA (* p < 0.05 and ** p < 0.01 compared with unscreened group biomass production; # p < 0.05 and ## p < 0.01 compared with unscreened group biomass productivity)
Effect of pH on the cultivation of Arthrospira platensis GMPA7
As shown in Fig. 2, compared with the control group, the biomass production increased significantly when the pH was maintained between 8.0 and 10.0. In addition, biomass production and biomass productivity increased gradually with increasing pH. Further increase in the pH to 11.0 led to a sharp reduction of biomass production and productivity. Thus, medium pH of 10.0 was the optimum pH for the growth of A. platensis, with the maximal biomass production of 4.99 g L−1 and biomass productivity of 0.50 g L−1 d−1.
Optimization of the biomass production for Arthrospira platensis GMPA7 using response surface methodology
Model fitting and variance analysis
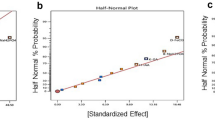
An experiment of 17 runs was carried out based on the Box–Behnken design. The factors, levels, and results of the runs are listed in Table 1, and ANOVA results are presented in Table 2. The response surface 3D graphs are displayed in Fig. 3. A quadratic polynomial equation was established by regression fitting to evaluate the relationship between biomass production of A. platensis GMPA7 and variables as shown below:
ANOVA revealed that the regression model was significant (p < 0.001), and the lack of fit was non-significant (p > 0.05). The determination coefficient (R2) was 0.991.
Effect of the variables on the biomass production
According to the regression coefficient (β) (Table 2), X22 presented a major effect, which was followed by X32, X1, X12, X3, X1X2, and X1X3. Light intensity and volume showed a highly significant (p < 0.01) positive effect on biomass, while quadratic terms of light intensity, initial culture density, and volume showed highly significant (p < 0.01) negative effect. Meanwhile, the interaction of light intensity and initial culture density presented highly significant (p < 0.01) effect on biomass, while the interaction of light intensity and volume was significant (p < 0.05). These results showed that the interactions between cell density and light intensity, as well as between liquid depth and light intensity, both significantly affected the growth of A. platensis. Higher cell density and liquid depth led to the irradiance attenuation and resulted in a decrease of the growth rate of A. platensis (Soni et al. 2017). However, lower cell density and liquid depth caused photoinhibition as the light intensity received by A. platensis exceeding light saturation point (Benedetti et al. 2018).
Model verification
Based on the analysis of the regression equation and response surface plots, the optimum culture conditions for the maximum biomass were determined to be light intensity of 169.32 μmol photons m−2 s−1, initial culture density of 0.52 (OD680nm), and volume of 163.65 mL. For reasons of ease of execution, the optimum parameters were modified to be light intensity of 169 μmol photons m−2 s−1, initial culture density of 0.52 (OD680nm), and volume of 164 mL. All the experiments under the optimum conditions were carried out in triplicate, and the results were 5.97 ± 0.13 g L−1, which were close to the prediction (6.00 g L−1). The biomass production of A. platensis in the optimum conditions was increased by 20% compared with that before response surface optimization. In addition, the biomass productivity was increased from 0.50 g L−1 day−1 to 1.00 g L−1 day−1.
Improvement of the biomass production of Arthrospira platensis GMPA7 using fed-batch and turbidostatic cultivation
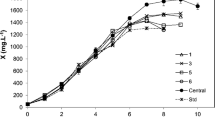
As shown in Fig. 4, nitrogen depletion led to the reduction of biomass under batch cultivation. In order to further increase the biomass, A. platensis needs sufficient nutrients during the cultivation process. Thus, fed-batch cultivation was performed and the nitrate concentration was used as a monitoring indicator in the process (Fig. 4b). The results revealed that the biomass production was significantly enhanced by the fed-batch cultivation. The maximum biomass production in this process was 15.56 g L−1, which was 161% higher than that in the batch cultivation (Fig. 4a). Thence, using only nitrate feeding would cause deprivation of other nutrients for the long-term cultivation, resulting in inhibition of cell growth and photosynthesis. Based on the results above, fed-batch with medium feeding is shown to be an effective method to improve biomass production of A. platensis GMPA7.
Although the feasibility of fed-batch operation was demonstrated regarding enhancement of the biomass production, the growth rate was lower than that of the batch cultivation with prolonging cultivation time (Fig. 4c). In order to achieve high biomass production and high biomass productivity at the same time, turbidostatic cultivation with continuously controlled cell density was performed to maintain high biomass productivity. As shown in Fig. 4, the biomass concentration in culture broth was continuously adjusted to 4.5 g L−1 with fresh medium during turbidostatic cultivation. Fig. 4 showed that the cell growth rate during the turbidostatic cultivation remained constant and was significantly higher than that during the fed-batch cultivation. Moreover, as shown in Table 3, the biomass production (21.57 g L−1) and biomass productivity (1.81 g L−1 d−1) in turbidostatic cultivation were 39% and 155% higher, respectively, than those in fed-batch cultivation. These results were significantly better than those obtained from related studies (Table 3). Thus, the turbidostatic cultivation is indeed a more effective strategy than the fed-batch cultivation.
Discussion
Arthrospira platensis contains a variety of nutrients and biologically active compounds, and it is easily digested and absorbed by the human body. It has broad application prospects in food (Mozafari et al. 2013), medicine (Gorban et al. 2003), environmental protection (Nithya et al. 2019), health care (Luo 2003), cosmetics (Xiu-Ping et al. 2013), etc. Therefore, it is commercially desirable to improve the biomass production and productivity, which can result in high conversion efficiency and is essential to reduce the cost of production.
Previous studies indicate that dead microalgae generated during the stationary phase of cultivation can cause inconvenience for the collection of living algae filaments (Levert and Xia 2001; Behl 2013). Therefore, this study used a sieve to separate living algae cells from dead ones. Living algal cells with the strongest growth vigor are sorted out through a 100–300 mesh screen for the subsequent optimization of cultivation conditions.
Among the factors that affect the cultivation of A. platensis, pH is one of the most critical ones. Changing the pH of the medium will affect the existence of bicarbonate in the medium, the availability of nutrients, photosynthesis, and biological mechanisms of microalgae (Hodaifa et al. 2009; Khalil et al. 2010; Chen et al. 2016). Therefore, it is essential to determine the optimum pH for the growth of A. platensis. In this study, the optimum pH for A. platensis growth is 10.0, the maximum biomass of A. platensis at this pH is 4.99 g L−1, and the maximum productivity is 0.50 g L−1 d−1. This result is comparable to that reported by Gupta et al. (2018). A possible explanation for this is that higher pH can affect the availability of carbon and damages cell membrane process, both of which may hinder photosynthesis (Ismaiel et al. 2016). In another study, Shi et al. 2016 discover that as pH increases, the growth rate of A. platensis first increases and then decreases, where pH from 8.0~10.5 is suitable for growth, while 9.5~10.0 is the optimum pH for growth. Ismaiel et al. (2016) also found that the suitable pH for A. platensis growth was 8.5~9.5, and the optimum pH was 9.0. Our results in this study are consistent with these studies.
In the phototrophic cultivation system cell density and liquid depth can affect the amount of light received by the algae and thus indirectly affect the cell growth of microalgae (Ooms et al. 2016; Martínez et al. 2018). Several physicochemical (Jiménez et al. 2003) (e.g., pH, dissolved oxygen concentration, temperature, conductivity, and irradiance) and biological (e.g., biomass concentration and yield) variables were studied. The prediction model of algal yield was obtained. In this work, the interactions and the best combination of light intensity, initial culture density, and volume were studied using response surface methodology. As a result, the best combination was found to be: light intensity of 169 μmol photons m−2 s−1, initial culture density of 0.52 (OD680 nm), and the liquid volume of 164 mL. Using this optimum combination, the biomass yield of A. platensis was 5.97 ± 0.13 g L−1 and the productivity was 1.00 g L−1 day−1, which are consistent with the predicted values. Related researches show that higher cell density and liquid depth can lead to irradiance attenuation and result in a decrease of the growth rate of A. platensis (Soni et al. 2017). However, lower cell density and liquid depth can cause the light intensity received by A. platensis to exceed light saturation point and result in photoinhibition (Benedetti et al. 2018). Therefore, it is necessary to control the ratios between the light intensity, the initial culture density, and the liquid volume, and obtain an optimum combination through the response surface test, and eventually achieve a higher biomass production.
In order to further increase the biomass of A. platensis, this study adopted batch culture and constant turbidity culture. Previous studies suggested fed-batch cultivation could prolong cell growth phase and enhance biomass production via controlling the nutrient content (Xie et al. 2013; Li et al. 2018). In this study, fed-batch culture was used to obtain a biomass of 15.56 g L−1 and a productivity of 0.71 g L−1 day−1. Xie et al. (2015) also indicated that fed-batch strategy could increase biomass production by 40%. Several researches have demonstrated that C, N, P, and S elements were essential macronutrients necessary for healthy growth of microalgae, and nutrients depletion would inhibit the growth rate and rate of photosynthetic CO2 fixation (Markou and Georgakakis 2012; Procházková et al. 2014; Li et al. 2018). However, studies also showed that higher cell density would lead to the irradiance attenuation, which would then inhibit the growth of microalgae and photosynthesis (Xie et al. 2014; Soni et al. 2017). Therefore, a new strategy which can effectively increases biomass production while maintaining a high productivity is needed. In this study, the constant turbidity culture strategy was used to obtain a biomass of 21.57 g L−1 and a productivity of 1.81 g L−1 d−1. Similarly, Xie et al. (2014) reported that the biomass productivity obtained from a repeated fed-batch strategy was increased from 0.88 to 1.04 g L−1 day−1. Hsieh and Wu (2009) also reported that using the semi-continuous cultivation could maintain high biomass productivity. In this work, we found that the fed batch strategy could increase the biomass production significantly, but the growth rate decreased during long-term cultivation. In contrast, the turbidostatic cultivation had the advantage of increasing biomass production and maintaining higher growth rate for a long time. Therefore, the turbidostatic cultivation strategy may be a better candidate for large-scale cultivation of A. platensis GMPA7.
Conclusion
In this work we show that the biomass production and productivity of A. platensis GMPA7 can be increased by screening living algae filaments and maintaining a pH of 10.0 in media. Based on the results of response surface methodology analysis, the optimum culture conditions are determined as follows: light intensity of 169 μmol photons m−2 s−1, initial culture density of 0.52 (OD680nm), and volume of 164 mL. While the fed-batch cultivation can significantly improve biomass production, the turbidostatic cultivation is demonstrated to be a more effective strategy to further improve cell growth, with the highest biomass production and productivity achieved at 21.57 g L−1 and 1.81 g L−1day−1, respectively. These results are better compared to most of the previous reports.
References
Álvarez X, Otero A (2020) Nutrient removal from the centrate of anaerobic digestion of high ammonium industrial wastewater by a semi-continuous culture of Arthrospira sp. and Nostoc sp. PCC 7413. J Appl Phycol 32:2785–2794
Azcarate SM, Camiña JM, Savio M (2018) Nutritional analysis of Spirulina dietary supplements: Optimization procedure of ultrasound-assisted digestion for multielemental determination. Food Chem 15:257–295
Behl Y (2013) Laboratory scale studies of Cyanobacteria, Synechococcus BG0011. MSc Thesis, University of Florida
Belay A (1997) Mass culture of Spirulina outdoors - The Earthrise Farms experience. In: Vonshak A (ed) Spirulina platensis (Arthrospira): Physiology, cell-biology and biochemistry. Taylor & Francis, London, pp 131-158
Benedetti M, Vecchi V, Barera S, Dall’Osto L (2018) Biomass from microalgae: the potential of domestication towards sustainable biofactories. Microb Cell Fact 17:172
Chen CY, Kao PC, Tsai CJ, Lee DJ, Chang JS (2013) Engineering strategies for simultaneous enhancement of C-phycocyanin production and CO2 fixation with Spirulina platensis. Bioresour Technol 145:307–312
Chen CY, Kao PC, Tan CH, Show PL, Cheah WY, Lee WL, Ling TC, Chang JS (2016) Using an innovative pH-stat CO2 feeding strategy to enhance cell growth and C-phycocyanin production from Spirulina platensis. Biochem Eng J 112:78–85
de la Jara A, Ruano-Rodriguez C, Polifrone M, Assunçao P, Brito-Casillas Y, Wägner AM, Serra-Majem L (2018) Impact of dietary Arthrospira (Spirulina) biomass consumption on human health: main health targets and systematic review. J Appl Phycol 30:2403–2423
Gorban EN, Kuprash LP, Gorban NE (2003) Spirulina: perspectives of the application in medicine. Likars'ka sprava / Ministerstvo okhorony zdorov'ia Ukrainy 7:100–110
Grobbelaar JU (2012) Microalgae mass culture: the constraints of scaling-up. J Appl Phycol 24:315–318
Gupta A, Mohan D, Saxena RK, Singh S (2018) Phototrophic cultivation of NaCl-tolerant mutant of Spirulina platensis for enhanced C-phycocyanin production under optimized culture conditions and its dynamic modeling. J Phycol 54:44–55
Hernández-Corona A, Nieves I, Meckes M, Chamorro G, Barron BL (2002) Antiviral activity of Spirulina maxima against Herpes simplex virus type 2. Antiviral Res 56:279–285
Ho SH, Kondo A, Hasunuma T, Chang JS (2013) Engineering strategies for improving the CO2 fixation and carbohydrate productivity of Scenedesmus obliquus CNW-N used for bioethanol fermentation. Bioresour Technol 143:163–171
Hodaifa G, Martinez ME, Sanchez S (2009) Influence of pH on the culture of Scenedesmus obliquus in olive-mill wastewater. Biotechnol Bioproc Eng 14:854–860
Hsieh CH, Wu WT (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 99:3921–3926
Ismaiel MMS, Elayouty YM, Pierceynormore M, Ismaiel MMS, Elayouty YM, Pierceynormore M (2016) Role of pH on antioxidants production by Spirulina (Arthrospira) platensis. Braz J Microbiol 47:298–304
Jiménez C, Cossio BR, Niell FX (2003) Relationship between physicochemical variables and productivity in open ponds for the production of Spirulina: a predictive model of algal yield. Aquaculture 221:331–345
Khalil ZI, Asker MMS, El-Sayed S, Kobbia IA (2010) Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J Microbiol Biotechnol 26:1225–1231
Levert JM, Xia J (2001) Modeling the growth curve for Spirulina (Arthrospira) maxima, a versatile microalga for producing uniformly labelled compounds with stable isotopes. J Appl Phycol 13:359–367
Li X, Li W, Zhai J, Wei H (2018) Effect of nitrogen limitation on biochemical composition and photosynthetic performance for fed-batch mixotrophic cultivation of microalga Spirulina platensis. Bioresour Technol 263:555–561
Lucas BF, Morais MG, Santos TD, Costa JAV (2018) Spirulina for snack enrichment: Nutritional, physical and sensory evaluations. LWT Food Sci Technol 90:270–276
Luo GH (2003) Application of HACCP in Processing of Spirulina Health Food. Food Sci 24(8):70–73
Manirafasha E, Murwanashyaka T, Ndikubwimana T, Ahmed NR, Liu J, Lu Y, Zeng X, Ling X, Jing K (2018) Enhancement of cell growth and phycocyanin production in Arthrospira (Spirulina) platensis by metabolic stress and nitrate fed-batch. Bioresour Technol 255:293–301
Markou G, Georgakakis D (2012) Effects of phosphorus concentration and light intensity on the biomass composition of Spirulina platensis. World J Microbiol Biotechnol 28:2661–2670
Martínez C, Mairet F, Bernard O (2018) Theory of turbid microalgae cultures. J Theor Biol 456:190–200
Mozafari MR, Khosravi-Darani K, Shahbazizadeh S, Hosseini SM (2013) Spirulina platensis: Food and Function. Curr Nutr Food Sci 9:189
Muys M, Sui Y, Schwaiger B, Lesueur C, Vandenheuvel D, Vermeir P, Vlaeminck SE (2018) High variability in nutritional value and safety of commercially available Chlorella and Spirulina biomass indicates the need for smart production strategies. Bioresour Technol 275:247–257
Nithya K, Sathish A, Pradeep K, Kiran BS (2019) Algal biomass waste residues of Spirulina platensis for chromium adsorption and modeling studies. J Env Chem Eng 7:103273
Ooms MD, Dinh CT, Sargent EH, Sinton D (2016) Photon management for augmented photosynthesis. Nature Commun 7:12699
Procházková G, Brányiková I, Zachleder V, Brányik T (2014) Effect of nutrient supply status on biomass composition of eukaryotic green microalgae. J Appl Phycol 26:1359–1377
Rajasekaran C, Ajeesh CPM, Balaji S, Shalini M, Kalaivani T (2016) Effect of modified Zarrouk’s medium on growth of different Spirulina strains. Walailak J Sci Technol 13:67–75
Remziye Aysun K, Sait P, Ahmet Ç, Nuray B, Saadet Demirörs S (2013) Protective effect of Spirulina platensis enriched in phenolic compounds against hepatotoxicity induced by CCl4. Food Chem 141:1972–1979
Shi WQ, Li SD, Li GR, Wang WH, Chen QX, Li YQ, Ling XW (2016) Investigation of main factors affecting the growth rate of Spirulina. Optik 127:6688–6694
Soheili M, Khosravi-Darani K (2011) The potential health benefits of algae and micro algae in medicine: a review on Spirulina platensis. Curr Nutr Food Sci 7:279–285
Soni RA, Sudhakar K, Rana RS (2017) Spirulina – From growth to nutritional product: a review. Trends Food Sci Technol 69:157–171
Ting Z, Jingjing W, Hongli Z, Xiaodan W, Yunpu W, Mingzhi L, Shuyu X, Leipeng C, Roger R, Yuhuan L (2018) Characterization of additional zinc ions on the growth, biochemical composition and photosynthetic performance from Spirulina platensis. Bioresour Technol 269:285–291
Vonshak A (1997) Use of Spirulina biomass. In: Vonshak A (ed) Spirulina platensis (Arthrospira): Physiology, cell-biology and biochemistry. Taylor & Francis, London, pp 205–212
Vonshak A, Richmond A (1988) Mass production of the blue-green alga Spirulina: an overview. Biomass 15:233–247
Xie Y, Ho SH, Chen CN, Chen CY, Ng IS, Jing KJ, Chang JS, Lu Y (2013) Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: effects of nitrate concentration, light intensity and fed-batch operation. Bioresour Technol 144:435–444
Xie YP, Ho SH, Chen CY, Chen CNN, Liu CC, Ng IS, Jing KJ, Yang SC, Chen CH, Chang JS (2014) Simultaneous enhancement of CO2 fixation and lutein production with thermo-tolerant Desmodesmus sp. F51 using a repeated fed-batch cultivation strategy. Biochem Eng J 86:33–40
Xie Y, Jin Y, Zeng X, Chen J, Lu Y, Jing K (2015) Fed-batch strategy for enhancing cell growth and C-phycocyanin production of Arthrospira (Spirulina) platensis under phototrophic cultivation. Bioresour Technol 180:281–287
Xiu-Ping P, Zhong-Dong HE, Zhi-Gang C, Xin C, Xiao-Gang C, Dong-Long L (2013) Application of Spirulina polysaccharide in moisturizing lotion. China Surfactant Detergent & Cosmetics 43(1):65–69
Zeng X, Danquah MK, Zhang S, Xia Z, Wu M, Xiao DC, Ng IS, Jing K, Lu Y (2012) Autotrophic cultivation of Spirulina platensis for CO2 fixation and phycocyanin production. Chem Eng J 183:192–197
Zhai J, Li X, Li W, Rahaman MH, Wei H (2017) Optimization of biomass production and nutrients removal by Spirulina platensis from municipal wastewater. Ecol Eng 108:83–92
Zhang X, Zhang F, Luo G, Yang S, Wang D (2015) Extraction and separation of phycocyanin from Spirulina using aqueous two-phase systems of ionic liquid and salt. J Food Nutr Res 3:15–19
Funding
This work was supported by Natural Science Foundation of Fujian Province of China (2020 J01491), Natural Science Foundation of Fujian Province of China (No. 2017 J01854), Regional Marine Economic Innovation and Development Demonstration Project of Fujian (No. 2012FJ14), Regional Marine Economic Innovation and Development Demonstration Project of Fujian (No. 2014FJ20), and Fuzhou University Testing Fund of precious apparatus (No. 2020 T019).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, L., Zhong, X., Zheng, Y. et al. Enhancement of biomass production and productivity of Arthrospira platensis GMPA7 using response surface monitoring methodology and turbidostatic cultivation strategy. J Appl Phycol 33, 755–763 (2021). https://doi.org/10.1007/s10811-020-02360-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02360-5