Abstract
Arthrospira platensis has been photoautotrophically cultivated for the production of high quality biomass. It contains satisfactory contents of protein, pigments, and fatty acids. The use of organic compounds as a carbon source has been studied, aiming to improve the biomass productivity in a mixotrophic process. In this study, A. platensis was cultivated by fed-batch process with the addition of sodium acetate, evaluating the effect of different concentrations and feeding time of this organic carbon source by the use of response surface methodology. Addition of sodium acetate was shown to be suitable for increasing the maximum cell concentration. The optimum condition was estimated by the model to be achieved with 387 mg L−1d−1 of sodium acetate for 6.5 days. Employing this condition, average Xm of 1769 ± 71 mg L−1 was achieved. This Xm value had an increase of almost 39 % in comparison with the standard cultivation, without acetate addition (1275 mg L−1).
Similar content being viewed by others
Introduction
Photosynthetic microorganisms are potential sources of currently underexplored chemicals, and even drugs, with potentially high commercial value (Chen and Zhang 1997). Particularly, Arthrospira platensis is widely studied due to favorable properties of this biomass such as high digestibility, low content of nucleic acid, high protein content, and the presence of essential amino acids and fatty acids (Cohen 1997).
Arthrospira platensis can be autotrophically cultivated in a medium containing high levels of carbonate and bicarbonate as carbon sources (Matsudo et al. 2012). These are inexpensive carbon sources that are beneficial for ensuring adequate pH conditions. However, this cyanobacterium can also be cultivated under mixotrophic conditions, with the possibility of obtaining higher biomass productivity (Ogbonna and Tanaka 2000). The simultaneous occurrence of photoautotrophic and heterotrophic metabolism has been observed in several microalgae such as Chlorella sorokiniana (Qiao et al. 2012), Micractinium pusillum (Bouarab et al. 2004), Phaeodactylum tricornutum (Cerón-Garcia et al. 2000), Crypthecodinium cohnii (de Swaaf et al. 2003), and Chlorella vulgaris (Menzyanova and Bozhkov 2003), as well as in cyanobacteria such as Synechococcus (Ihlenfeldt and Gibson 1997) and Arthrospira platensis (Vonshak et al. 2000; Chen et al. 2006).
Arthrospira platensis is capable of growing heterotrophically with glucose under aerobic-dark conditions, in which photosynthetic activity and oxidative assimilation of glucose can independently operate mixotrophically under light conditions (Marquez et al. 1993). Mixotrophic culture possesses many advantages, including increased productivity of the biomass, ability to work at high cell concentrations, and ease of maintaining optimal growth conditions (Chen et al. 1996).
In a mixotrophic cultivation of Spirulina platensis with glucose, Zhang et al. (1999) observed that biomass production may be inhibited by high concentrations of organic carbon substrate. Chen et al. (2006), adding sodium acetate in the cultivation of Spirulina platensis, also verified that there was a limiting concentration of this organic carbon source for avoiding growth inhibition (4 g L−1, which is the upper limit of concentration).
It is important to note that the addition of an organic carbon source has usually been performed by batch process. In this sense, taking into account the possibility of growth inhibition by the organic carbon source, the amount of this substrate added for a satisfactory cultivation might be further optimized if its addition is performed throughout the cultivation time. The fed-batch process could be an important strategy for managing the addition of organic carbon sources, such as sodium acetate, when aiming to increase A. platensis biomass production (Carvalho et al. 2013).
The aim of this paper is to evaluate the effect of feeding sodium acetate in the cultivation of Arthrospira platensis, adopting different concentrations of this organic carbon and feeding times. This kind of feeding regime is commonly applied for the use of glucose in heterotrophic or mixotrophic cultivation of photosynthetic microorganisms, but not for adding sodium acetate. Moreover, RSM (Response Surface Methodology) was applied not only for verifying the possibility of optimization, but also to evaluate the effect and relationship of these two independent variables (amount of sodium acetate added per day and feeding time).
Response Surface Methodology has been successfully applied to evaluate the simultaneous use of urea and potassium nitrate for cultivating Arthrospira platensis (Vieira et al. 2012), or to assess the influence of feeding time (ammonium chloride) and light intensity to cultivate the same cyanobacterium (Bezerra et al. 2008).
Material and methods
Cultivation conditions
Arthrospira platensis UTEX 1926, obtained from the Culture Collection of the University of Texas (Austin, TX, USA), was maintained and cultivated in Schlösser culture medium (Schlösser 1982), containing the following nutrients (g L−1): NaHCO3, 13.61; Na2CO3, 4.03; NaCl, 1.00; K2SO4, 1.00; NaNO3, 2.50; K2HPO4, 0.50; MgSO4·7H2O, 0.20; CaCl2·2H2O, 0.04. All nutrients were dissolved in distilled water containing (per liter): 6 mL of metal solution (750 mg Na2EDTA, 97 mg FeCl3·6H2O, 41 mg MnCl2·4H2O, 5.0 mg ZnCl2, 2 mg CoCl2.·6H2O, 4.0 mg Na2MoO4·2H2O) and 1 mL of micronutrient solution (50.0 mg Na2EDTA, 618 mg H3BO3, 19.6 mg CuSO4·5H2O, 44.0 mg ZnSO4·7H2O, 20.0 mg CoCl2·6H2O, 12.6 mg MnCl2·4H2O, 12.6 mg Na2MoO4·2H2O).
In the experimental runs, however, sodium acetate (in the form of concentrated solution) was added with concentrations in accordance with Table 1. Standard cultivations, without sodium acetate addition, were carried out for data comparison.
Cultivations were carried out in a rotary shaker at 120 rpm, 29 °C (Sanchez-Luna et al. 2007), light intensity of 72 μmol photons m−2 s−1 (Sánchez-Luna et al. 2004; Sanchez-Luna et al. 2007; Matsudo et al. 2009), and initial pH of 9.5 (Sanchez-Luna et al. 2007), in 500-mL Erlenmeyer flasks containing 200 mL of Schlösser culture medium. Initial cell concentration was approximately 50 mg L−1, expressed as dry weight (Carvalho et al. 2004). Cultivations were ended when stabilization of cell concentration was observed.
Analytical techniques
Cell concentration (X) was periodically determined by comparing the optical density of the culture at 560 nm with previously prepared calibration curves (optical density versus dry weight biomass concentration) (Leduy and Therien 1977). pH was determined by a potentiometer (Mettler Toledo, Barueri-SP, Brazil).
At the end of each cultivation, total carbonate was determined in accordance with Pierce and Haenisch (1948). In this method, the samples are alkalinized with sodium hydroxide in order to convert all the bicarbonate into carbonate. The dissociated carbonate is titrated with hydrochloric acid by two steps: 1) neutralization with phenolphthalein as indicator; 2) carbonic acid production with methyl orange as indicator. Since the first step of the titration (phenolphthalein endpoint) will neutralize not only the excess of hydroxide added, but also 50 % of carbonate in the sample, the total carbonate concentration (g Na2CO3 L−1) is calculated from the volume of HCl used in the second step of titration multiplied by two.
Continuous light intensity, expressed in μmol photons m−2 s−1, was measured by a light intensity meter (Li-Cor, Lincoln, NE, USA).
The specific growth rate (μ) was calculated by the method of LeDuy and Zajic (1973). This method is a geometric approach considering that three neighboring data points rest on a circumference, and the tangent in the middle data point corresponds to the instantaneous growth rate. This instantaneous growth rate divided by the cell concentration corresponding to the same point results in the specific growth rate.
Experimental design and results analysis
Experimental runs were done in accordance with an experimental design called “star-planning” (Box et al. 1978), with two factors in five levels of independent variables. This method was performed to evaluate the influence of two independent variables, daily addition of sodium acetate and feeding time, on the selected response variable: maximum cell concentration (Xm). The central point was threefold repeated for checking the reproducibility of the results.
The general equation:
was proposed to estimate the selected response variable (yi), specifically y1 = Xm, as a result of varying the codified values (x1 and x2) of the independent variables, daily addition of sodium acetate and feeding time. In the equation, i and j represent the dependent and the independent variables, respectively, j’ indicates their interactions; ai is the intercept, bij are the linear coefficients, cijare the quadratic coefficients, and dijj’ are the interaction coefficients. Significance levels <0.10 were considered for the regression analysis (Bezerra et al. 2008) with the statistical package S-PLUS 2000, and for the analyses of variance (ANOVA), an error of 5 % at most (p < 0.05).
Results and discussion
Cell growth
Table 1 shows the results of Xm and total carbonate concentration at the end of each cultivation.
It is evident that the standard cultivation, without any organic carbon addition, had the weakest performance, with Xm value of 1275 mg L−1 (Table 1). Among the runs within the experimental design, the lowest values for Xm were obtained in runs 5 (x1 = 0; x2 = −1.414) and 6 (x1 = −1.414; x2 = 0) with a value of 1426 mg L−1 for both. The low addition of sodium acetate (run 6) and the short feeding time (run 5) are the most probable reason for the limited cell growth. These results are in accordance with those obtained by (Chen et al. 1996), which showed that at low acetate concentrations (1 g L−1), values of specific growth rate and cell concentration were only slightly higher than those of the control. Rym et al. (2010), evaluating mixotrophic growth of A. platensis with glucose, also achieved maximum biomass concentration 2.3 times superior than that obtained in photoautrotrophic condition.
Runs 1 and 3 were performed with a daily addition of sodium acetate of 100 mg L−1 but with different feeding times (4 and 8 days, respectively) and obtained Xm values very similar to each other, 1532 and 1507 mg L−1, respectively. These data suggest that when adding 100 mg L−1, feeding times longer than that employed in these runs (1 and 3) might not further increase biomass concentration. In the same way, in run 7 (x1 = 0, x2 = 1.414), the same value of daily addition of sodium acetate as the central point (300 mg L−1) was maintained, but for a longer feeding time, and the Xm obtained was very similar (1735 mg L−1), indicating that also for this sodium acetate addition, extending the feeding time does not increase the biomass concentration. These results show that there is a limit to the addition of acetate as a carbon source to increase the Xm.
In the same way, increasing the daily addition of sodium acetate to 500 mg L−1 day−1 (run 2, for 4 days, and run 4 for 8 days) or 582.8 mg L−1 day−1 (run 8, for 6 days), did not increase the maximum cell concentration, which also indicated that the daily addition of sodium acetate adopted for the central point was probably near the optimum value.
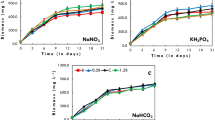
It is possible to observe in Fig. 1 that besides standard cultivation, runs 1, 3, 5, and 6 had the biomass concentration stabilized earlier than the central point cultivations and, consequently, had lower values of maximum cell concentration. The increase in pH values is one factor that can explain this phenomenon, since high pH conditions may be harmful for inorganic carbon uptake by the microorganism (Azov 1982). In fact, Fig. 2 shows that pH values above 11.0 were obtained for these runs, in contrast to central point cultivations, in which it took one or more additional days to reach these pH values.
Biomass concentration (X) versus time for runs 1, 3, 5, 6, central point (9 – 11), and standard cultivation (see Table 1)
pH versus time for runs 1, 3, 5, 6, central point (9–11), and standard cultivation (see Table 1)
It is possible to infer that addition of sodium acetate delayed the pH increase, thereby maintaining the pH at the optimum value for a longer time. Also indicated in Table 1 is the standard run, without sodium acetate addition; this showed a final total carbonate concentration lower than the cultivations with the addition of this organic carbon source. The addition of organic carbon source might allow the preservation of bicarbonate ions in the culture medium, which could justify the maintenance of pH values for a longer time.
The increase in the pH of the medium may be explained by the consumption of bicarbonate, which is the preferable form for assimilation by the microorganism (Miller and Colman 1980). Therefore, the ideal pH value is one that assures the displacement of the bicarbonate-carbonate equilibrium to form bicarbonate. This information supports the importance of acetate in delaying the pH increase, because above pH 11 carbonate is the predominant form and bicarbonate is almost absent.
Qiao et al. (2012) observed that Chlorella sorokiniana exhibited a lag phase when adapting for the uptake of acetate added in the cultivation. Nevertheless, it is possible to observe in Fig. 1 that there was not an adaptation period when Arthrospira platensis was provided acetate.
Specific growth rate
Table 1 shows the values of maximum specific growth rate (μmax) for each run. These values are very similar (ranging from 0.93 to 1.04 day−1) and were always obtained in the beginning of the cultivation (day 1). These μmax values were higher than that obtained in photoautotrophic cultures (Matsudo et al. 2009) most probably as a result of the synergistic effect of photosynthesis and acetate consumption. In addition, these μmax values are also higher than those obtained by Chen et al. (1996), using acetate (0.52 day−1) and glucose (0.62 day−1) as carbon source in Spirulina platensis mixotrophic growth, likely due to the lower light intensity (4 klux = 48 μmol photons m−2 s−1 ) used by these authors. According to Chojnacka and Noworyta (2004), specific growth rate of Spirulina platensis in the mixotrophic growth increases with increasing light, and the light intensity of 72 μmol photons m−2 s−1, employed in the present study, may have propitiated a higher value of μmax.
Figure 3 shows the μ values throughout the cultivation time for some runs. On the 7th day, it is possible to observe that for runs 5, 6, and standard (without sodium acetate addition) μ values are equal to or almost 0 (zero), whereas central point cultivations (runs 9, 10, and 11) still show μ values near 0.1 day−1. It may explain the difference in the maximum cell concentration for different runs; i.e., runs in which μ values decreased to 0 (zero) faster had lower Xm values. In fact, mixotrophic cultures require less light for growth (Vonshak et al. 2000) and, therefore, organic carbon source might be crucial for maintaining cell growth in the final stage of the cultivation, when shadowing effect takes place.
Specific growth rate versus time for runs 5, 6, central point (9–11), and standard cultivation (see Table 1)
Analysis of multivariable regression
The analysis of multivariate regression with the obtained data presented in Table 1 was performed in order to verify the influence of the daily addition of sodium acetate and feeding time. In this sense, the general equation (Eq. 1) was employed to estimate the selected variables as a response to the variations of codified variables x1 (daily addition of sodium acetate, mg L−1 day−1) and x2 (feeding time, days).
For improving the regression fit for Xm, the coefficient of x1/x2 interaction was neglected in the adjustment of the mathematical model, resulting in:
This adjusted model resulted in a R2 (adjusted determination coefficient) of 0.79; i.e., it explained 79 % of the variability in the maximum cell concentration. This value may be considered satisfactory for the purpose of this study, being equivalent to values found in other studies in the field of bioprocess (Cruz-Martinez et al. In press; Fratelli et al. 2005; Bezerra et al. 2008; Vieira et al. 2012). Additionally, p value of 0.007 generated by the analysis of variance indicates that the regression is statistically significant.
It is possible to observe that Xm was a quadratic function of both the daily addition of sodium acetate and the feeding time, and the independent variables influenced Xm separately. The negative values of quadratic coefficients point out that the values of x1 and x2 responsible for the highest value of Xm are included inside the area given by the response surface methodology analysis (Fig. 4) and are very close to those considered as the central point of the experimental design.
By Eq. 2, it is possible to calculate the optimal conditions for maximizing Xm : x1 = 0.43 (real value = 387 mg L−1d−1) and x2 = 0.26 (real value = 6.5 days). Confirmation runs (12 – 14, Table 1) were performed under the conditions estimated to optimize Xm. In these runs, it was possible to obtain an Xm mean value of 1769 ± 71 mg L−1, which is only 4.1 % lower than the value estimated by the mathematical model for Xm (1845 mg L−1). This information confirms the suitability of multivariate regression aiming to optimize the experimental conditions for the maximization of biomass production, which can also be observed in Fig. 5. This figure shows the linear relationship between estimated and the experimental values of Xm at the end of each run.
Rym et al. (2010) satisfactorily developed a model showing a relationship between biomass concentration and organic carbon source (glucose), although they couldn’t achieve the optimization of the mixotrophic cultivation.
These results in the present study indicate that A. platensis could successfully use acetate as a carbon source and cell concentration increased almost 39 % in comparison with the standard cultivation, without acetate addition.
Experimental results are in agreement with those reported by Chen et al. (1996), who observed that the intermittent addition of 2.0 g L−1 acetate in mixotrophic culture of Spirulina platensis resulted in higher biomass concentration (1.81 g L−1). These values were similar to those obtained in runs 9, 10, and 11 of the present study (central point in the experimental design) which resulted in Xm values of 1774 mg L−1, 1845 mg L−1, and 1830 mg L−1, respectively (average Xm = 1816 ± 37 mg L−1). These values are relatively higher than most of the other runs.
Other organic carbon sources for A. platensis mixotrophic culture have been studied. Coca et al. (2014) analyzed the growth of S. platensis in cultures supplemented with beet vinasse. (2 g L−1 of vinasse) and obtained high biomass concentration of 3.3 g L−1. Although the use of vinasse has provided high cell concentration, the cell productivity (122 ± 5 mg L−1day−1) was lower than that obtained in the present study, with acetate (Table 1; 252 ± 10 mg L−1day−1).
In the confirmation runs, Xm was obtained on the 7th day of cultivation, resulting in a cell productivity mean value of 252 ± 10 mg L−1day−1. Considering that this cultivation was carried out in Erlenmeyer flasks, this is a very promising result if compared with cultivations in 5 L mini-tanks (Bezerra et al. 2008; Matsudo et al. 2009), in which the best results of cell productivity were 126 mg L−1day−1 (Bezerra et al. 2008), and 241 mg L−1day−1 (Matsudo et al. 2009), by photoautotrophic cultivation.
Conclusion
The influence of daily addition of sodium acetate and feeding time on the mixotrophic cultivation of Arthrospira platensis was evaluated. The addition of organic substrate, providing both carbon and energy for the microorganism's metabolism, resulted in a higher biomass concentration in comparison with standard cultivation, which was performed without sodium acetate addition. The process was optimized using surface response methodology and the optimum condition for Xm was shown to be 387 mg L−1day−1 of sodium acetate for 6.5 days. Under this condition, Xm of 1769 mg.L−1 was achieved, a value only 4.1 % lower than that estimated by the mathematical model (1845 m g L−1).
References
Azov Y (1982) Effect of pH on inorganic carbon uptake in algal cultures. Appl Environ Microbiol 43:1300–1306
Bezerra RP, Matsudo MC, Converti A, Sato S, Carvalho JCM (2008) Influence of ammonium chloride feeding time and light intensity on the cultivation of Spirulina (Arthrospira) platensis. Biotechnol Bioeng 100:297–305. doi:10.1002/bit.21771
Bouarab L, Dauta A, Loudiki M (2004) Heterotrophic and mixotrophic growth of Micractinium pusillum Fresenius in the presence of acetate and glucose: effect of light and acetate gradient concentration. Water Res 38:2706–2712. doi:10.1016/j.watres.2004.03.021
Box GE, Hunter WG, Hunter JS (1978) Statistics for experiments. Wiley, New York
Carvalho JCM, Francisco FR, Almeida KA, Sato S, Converti A (2004) Cultivation of Arthrospira (Spirulina) platensis (cyanophyceae) by fed-batch addition of ammonium chloride at exponentially increasing feeding rates. J Phycol 40:589–597. doi:10.1111/j.1529-8817.2004.03167.x
Carvalho JCM, Bezerra RP, Matsudo MC, Sato S (2013) Cultivation of Arthrospira (Spirulina) platensis by fed-batch process. In: Lee J (ed) Adv. Biofuels bioprod. Springer New York, New York, pp 781–805
Cerón Garcia MC, Sevilla JMF, Acién Fernandez FG, Molina Grima E, Garcia Camacho F (2000) Mixotrophic growth of Phaeodactylum tricornutum on glycerol: growth rate and fatty acid profile. J Appl Phycol 12:239–248
Chen F, Zhang Y (1997) High cell desity mixotrophic culture of Spirulina platensis on glucose for phycocyanin production using a fed-batch system. Enzym Microb Technol 20:221–224. doi:10.1016/0141–0229
Chen F, Zhang Y, Guo S (1996) Growth and phycocyanin formation of Spirulina platensis in photoheterotrophic culture. Biotechnol Lett 18:603–608
Chen T, Zheng W, Yang F, Bai Y, Wong Y-S (2006) Mixotrophic culture of high selenium-enriched Spirulina platensis on acetate and the enhanced production of photosynthetic pigments. Enzym Microbiol Technol 39:103–107. doi:10.1016/j.enzmictec.2005.10.001
Coca M, Barrocal VM, Lucas S, Gonzalez-Benito G, Garcia-Cubero MT (2014) Protein production in Spirulina platensis biomass using beet vinasse-supplemented culture media. Food Bioprod Process. doi:10.1016/j.fbp.2014.03.012
Cohen Z (1997) The chemicals of Spirulina. In: Vonshak A (ed) Spirulina platensis physiol. cell-biology biotechnol. Taylor & Francis Ltd., London, pp 175–204
Cruz-Martinez LC, Jesus CKC, Matsudo MC, et al. Growth and composition of Arthrospira (Spirulina) platensis in tubular photobioreactor using ammonium nitrate as the nitrogen source in a fed-batch process. Brazilian J. Chem. Eng. In press.
De Swaaf ME, Pronk JT, Sijtsma L (2003) Fed-batch cultivation of the docosahexaenoic-acid-producing marine alga Crypthecodinium cohnii on ethanol. Appl Microbiol Biotechnol 61:40–43. doi:10.1007/s00253-002-1118-1
Fratelli F, Siquini T, Prado S, Higashi HG, Converti A, de Carvalho JC (2005) Effect of medium composition on the production of tetanus toxin by Clostridium tetani. Biotechnol Prog 21:756–761
Ihlenfeldt MJA, Gibson J (1997) Acetate uptake by the unicellular cyanobacteria Synechococcus and Aphanocapsa. Arch Microbiol 113:231–241
Leduy A, Therien N (1977) An improved method for optical density measurement of the semimicroscopic blue algae Spirulina maxima. Biotechnol Bioeng 19:1219–1224
Leduy A, Zajic JE (1973) A geometrical approach for differentiation of an experimental function at a point applied to growth and product formation. Biotechnol Bioeng 25:805–810
Marquez FJ, Sasaki K, Kakizono T, Nishio N, Nagai S (1993) Growth characteristics of Spirulina platensis in mixotrophic and heterotrophic conditions. J Ferment Bioeng 76:408–410
Matsudo M, Bezerra R, Sato S, Perego P, Converti A, Carvalho JCM (2009) Repeated fed-batch cultivation of Arthrospira (Spirulina) platensis using urea as nitrogen source. Biochem Eng J 43:52–57. doi:10.1016/j.bej.2008.08.009
Matsudo MC, Bezerra RP, Sato S, Converti A, Carvalho JCM (2012) Photosynthetic efficiency and rate of CO2 assimilation by Arthrospira (Spirulina) platensis continuously cultivated in a tubular photobioreactor. Biotechnol J 7:1412–1417. doi:10.1002/biot.201200177
Menzyanova NG, Bozhkov AI (2003) Influence of ethanol on metabolism of algae. Growth dynamics, content of nucleic acids, proteins, and lipids in Chlorella vulgaris Beijer and Spirulina platensis (nordst.) geitl. Cells. Int J Algae 5:64–73
Miller A, Colman B (1980) Evidence for HCO3- transport by the blue-green alga (cyanobacterium) Coccochloris peniocystis. Plant Physiol 65:397–402
Ogbonna JC, Tanaka H (2000) Light requirement and photosynthetic cell cultivation – development of processes for efficient light utilization in photobioreactors. J Appl Phycol 12:207–218
Pierce WC, Haenisch EL (1948) Quantitative analysis, 3rd edn. Wiley, New York
Qiao H, Wang G, Liu K, Gu W (2012) Short-term effects of acetate and microaerobic conditions on photosynthesis and respiration in Chlorella sorokiniana gxnn 01 (chlorophyta)1. J Phycol 48:992–1001. doi:10.1111/j.1529-8817.2012.01189.x
Rym BD, Nejeh G, Lamia T, Ali Y, Rafika C, Khemissa G, Jihene A, Hela O, Hatem BO (2010) Modeling growth and photosynthetic response in Arthrospira platensis as function of light intensity and glucose concentration using factorial design. J Appl Phycol 22:745–752. doi:10.1007/s10811-010-9515-9
Sánchez-Luna L, Converti A, Tonini G (2004) Continuous and pulse feedings of urea as a nitrogen source in fed-batch cultivation of Spirulina platensis. Aquac Eng 31:237–245. doi:10.1016/j.aquaeng.2004.04.003
Sanchez-Luna LD, Bezerra RP, Matsudo MC, Sato S, Converti A, Carvalho JCM (2007) Influence of pH, temperature, and urea molar flowrate on A rthrospira platensis fed-batch cultivation: a kinetic and thermodynamic approach. Biotechnol Bioeng 96:702–711. doi:10.1002/bit
Schlösser UG (1982) Sammlung von algenkulturen. Ber Dtsch Bot Ges 95:181–276
Vieira DCM, Matsudo MC, Sato S, Converti A, Carvalho JCM (2012) Simultaneous use of urea and potassium nitrate for Arthrospira (Spirulina) platensis cultivation. Biotechnol J 7:649–655
Vonshak A, Cheung SM, Chen F (2000) Mixotrophic growth modifies the response of Spirulina (Arthrospira) platensis (Cyanobacteria) cells to light. J Phycol 36:675–679
Zhang XW, Zhang Y, Chen F (1999) Application of mathematical models to the determination of optimal glucose concentration and light intensity for mixotrophic culture of Spirulina platensis. Process Biochem 34:477–481
Acknowledgement
The authors acknowledge the financial supports of CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsudo, M.C., Moraes, F.A., Bezerra, R.P. et al. Use of acetate in fed-batch mixotrophic cultivation of Arthrospira platensis . Ann Microbiol 65, 1721–1728 (2015). https://doi.org/10.1007/s13213-014-1011-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-014-1011-z