Abstract
The feasibility of the semi-continuous cultivation of Arthrospira (Spirulina) sp. and Nostoc sp. PCC 7413, using the centrate from the anaerobic digestion of wastewater from a seafood canning industry as a nutrient source, was assessed. Semi-continuous cultures were carried out in aerated tubular culture units of 250 mL by replacing a 10–20% of the volume with the centrate every 2 days. Growth, ammonium, and nitrate removal were monitored, as well as phycobiliprotein and protein production by Arthrospira sp. and released exopolysaccharides production by Nostoc sp. PCC 7413. The ammonium removal efficiency by Arthrospira sp. was 84.9 ± 1.9% in a culture maintained with an ammonium concentration of 11.3 mM. Lower ammonium concentrations (4.9 mM) could be applied to Nostoc sp. PCC 7413, reaching similar relative removal efficiency (84.1%). Taking into account the high ammonium stripping that occurs at the high pH required in Arthrospira sp. cultures, results indicate a better nutrient removal by Nostoc sp., although Arthrospira sp. is more suitable for working at high ammonium concentrations. Since all the ammonium was removed during the first 24 h for both species, much lower residence time could be supported by the cultures. High concentrations of protein (2.5 ± 0.2 mg mL−1), phycobiliprotein (allophycocyanin, 84.3 ± 10.2 mg g−1; phycocyanin, 73.7 ± 7.7 mg g−1) were obtained by Arthrospira sp. cultures. A high concentration of released exopolysaccharides (962.7 ± 26.7 mg L−1) was also obtained for Nostoc sp. PCC 7413 when cultured in the anaerobic centrate. The results indicate the possibility of the use of filamentous cyanobacteria for the treatment of industrial effluents rich in ammonium, with different possibilities of valorization of the biomass produced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgal and cyanobacterial biomass is used in highly relevant applications related to human and animal nutrition, and it is also used as raw material for the production of chemicals, biofertilizers, and biofuels (Spolaore et al. 2006). Despite the multiple applications of biomass from microalgae and cyanobacteria, the global production of this type of biomass is less than 20 kt-year−1 (Tredici et al. 2016). Microalgae and cyanobacteria biomass production involves vast quantities of nutrients, since based on the elemental composition of microalgae and cyanobacteria, 1.8 kg of CO2, 0.1 kg of nitrogen, and 0.02 kg of phosphorus are needed to produce 1 kg of biomass (Romero-Villegas et al. 2017). It is possible to use flue gas to supplement CO2 for algae cultivation; however, fertilizers are usually used for the supply of nitrogen and phosphorus, with imposes an essential limitation on production capacity, reducing the sustainability of biomass production (Collet et al. 2014). The use of fertilizers increases the production cost by around 2.0 € kg−1 because it represents up to 54% of the raw material cost (Norsker et al. 2011; Acién et al. 2013). The use of wastewater constitutes a feasible alternative to the use of fertilizers in the production of microalgae and cyanobacteria biomass (Cepoi et al. 2016; Cho et al. 2016). Many types of wastewater are rich in nutrients (carbon, nitrogen, phosphorus, potassium) that are essential to produce these phototrophic microorganisms. Therefore, wastewaters can be used for replacing the culture media prepared with fertilizers to produce biomass, which increases the sustainability of the process (Nayak et al. 2016). Moreover, the nutrients present in the wastewater can contaminate various bodies of water (Zinicovscaia 2016) if not adequately removed. Therefore, concurrently with the production of microalgae and cyanobacteria biomass using wastewater for biomass production, the wastewater is treated through the removal of the nutrients, mainly nitrogen and phosphorus (Markou and Georgakakis 2011).
The use of microalgae and cyanobacteria for the secondary treatment of different effluents has been extensively explored mainly in systems such as high-rate algal ponds (HRAP). The use of HRAP in the treatment of domestic wastewater presents economic and environmental advantages, due to the removal of nitrogen and phosphorus, nutrients present in large quantities in this type of waste (Park et al. 2011). Park and Craggs (2010) investigated the influence of the addition of CO2 on the performance of domestic wastewater treatment and algal production in two pilot-scale HRAPs; with the addition of CO2, achieving average algal productivity of 16.7 g m2 d−1, with a maximum algal productivity of 24.7 g m2 day−1. The effect of the hydraulic retention time (HRT) on the removal efficiency of 26 organic microcontaminants from urban wastewater was investigated in two HRAPs (Matamoros et al. 2015), demonstrating that biodegradation and photodegradation are the essential ways of removing these types of compounds, while volatilization and sorption occurred only in hydrophobic compounds. The cyanobacterium Leptolyngbya sp. ISTCY101 has been evaluated for the production of biomass with untreated urban wastewater (1.6 mM NH4+), reaching maximum values of biomass productivity of 2.93 ± 0.03 g m−2 day−1 (Singh and Thakur 2015). Protein production with untreated swine wastewater (4.6 mM NH4+) has been demonstrated with Phormidium sp., reaching 62% of protein content (Cañizares-Villanueva et al. 1995).
The application of microalgae and cyanobacteria for the treatment of nitrogen-rich wastewater from the aquaculture industry is of particular interest, in the framework of integrated multitrophic aquaculture (IMTA) schemes (Milhazes-Cunha and Otero 2017). The nitrogen and ammonium concentrations that are present in aquaculture effluents are in the range 1.6–17.6 mg L−1 and 0.5–5.0 mg L−1, respectively, with an N:P ratio of 9.9:16.2 (Chuntapa et al. 2003; Kamilya et al. 2006; Gao et al. 2016; Wuang et al. 2016), presenting significant differences with urban and other industrial wastewater effluents. High rates of nitrogen removal (83.8 ± 14.0%) were obtained when the cyanobacterium Spirulina platensis (now Arthrospira platensis; Komárek and Anagnostidis 2005) was co-cultivated with the black tiger shrimp (Chuntapa et al. 2003). Wuang et al. (2016) cultivated S. platensis in fish farming wastewater, and the obtained biomass was proved to be an effective biofertilizer, achieved a nitrogen removal efficiency of 80%. Growth and nutrient removal rate of S. platensis and Nostoc muscorum was evaluated in sewage from a fish culture, showing that the best nutrient removal rates were obtained with S. platensis, with 92.4, 48.6, 50.4, and 47.8% for NH4+, NO2−, NO3−, and PO43−, respectively, but no significant differences were observed between the specific growth rate of both cyanobacterial species (Kamilya et al. 2006).
Anaerobic digestion of wastewater allows the conversion of organic pollutants into a small amount of sludge and a large amount of biogas (CH4, CO2). The main advantages are the low cost of operation, small space required, significant biogas production, and low sludge production (Chowdhury et al. 2010). The use of microalgae and cyanobacteria for the tertiary treatment of the liquid phase of the effluent obtained from anaerobic digestors (centrate) in different wastewater treatment systems has also been explored intensively in recent times, mostly to produce biomass as potential raw material for biofuels, with removal efficiencies reaching 95–98% of nitrogen (Franchino et al. 2013; Ledda et al. 2015a, b, 2016). Some of the eukaryotic species that have been grown successfully on the centrate obtained from the anaerobic digestion of animal manure, sludge from domestic wastewater, and agro-waste include Neochloris oleoabundans and Scenedesmus dimorphus (Abu Hajar 2017), Chlorella vulgaris (Zuliani et al. 2016), Nannochloropsis gaditana (Ledda et al. 2015a), Scenedesmus sp. AMDD (Bjornsson et al. 2013), and a consortium of Chlorella and Scenedesmus (Raeisossadati et al. 2019). In the case of cyanobacteria, the centrate from anaerobic digestion of animal manure, activated sludge from domestic wastewater, and distillery wastewater have been used as a culture medium for Phormidium bohneri (Noüe and Bassères 1989), Synechocystis sp. PCC 6803 (Sadik 2015), and Spirulina sp. and Spirulina platensis (Chaiklahan et al. 2010; Sankaran and Premalatha 2018). Arthrospira (Spirulina) sp. has been used in the past to remove nitrogen from anaerobic effluents from digested pig waste (Olguín et al. 2001, 2003; Patnaik et al. 2001).
The high ammonium concentrations reached in the centrate from the seafood/fish canning industry, up to 868 mM or more (Chowdhury et al. 2010; Yenigün and Demirel 2013), together with the vast quantities of organic matter (soluble, colloidal and particulate), phosphorus, sulfur, metals, and solids that are present in this type of effluents, make this type of wastewater suitable for supporting microalgae and cyanobacterial growth (Contreras et al. 2000). Nevertheless, this type of ammonium-rich centrate has not been evaluated for the cultivation of microalgae and cyanobacteria. Therefore, this work aimed to assess the semi-continuous cultivation of filamentous cyanobacteria using the liquid phase of the effluent (centrate) from the anaerobic digestion of a seafood-processing factory as the nutrient source. Filamentous cyanobacteria present the additional advantage of allowing simple and low-cost harvesting since they can be easily flocculated or sieved (Chen et al. 2014); while producing biomass with a high content of protein (Habib et al. 2008), and other important secondary compounds such as polysaccharides (Otero and Vincenzini 2003) and phycobiliprotein can be obtained simultaneously.
Materials and methods
Anaerobic centrate characteristics
In this research, the centrate utilized was obtained from a 1000 m3 anaerobic mesophilic digester located in a plant of seafood/fish processing and canning on the NW of Spain (JB Ingenieros, Vigo, Spain). The digester was operated at a flow between 15 and 30 m3 day−1 (average of 25 m3 day−1), with a regular average biogas production of 600 m3 d−1. The feed was mainly composed of sewage sludge and cooking water from the fish canning industry, and occasionally dairy industry and slaughterhouse sludges. After the anaerobic digestion, a solid/liquid separation is performed. The liquid stream is sent to a sewage treatment plant and the solid is used as fertilizer. Samples of the centrate of the anaerobic digestion were taken at the outlet of the solid/liquid separation, presenting the following characteristics: NH4+ 49.33–113.22 mM, salinity 11–15‰, PO4-P 0.04–0.17 mM, COD 2090 mg L−1, pH 7.7, conductivity 12.7 mS cm−1, total solids 4450 mg L−1, and volatile solids 2150 mg L−1.
Microorganisms and culture conditions
The cyanobacterium Nostoc sp. PCC 7413 was obtained from the culture collection of the Pasteur Institute (https://webext.pasteur.fr/cyanobacteria/). The halotolerant strain of Arthrospira sp. was obtained from the culture collection of Microbiology and Parasitology Department of the Universidade de Santiago de Compostela (Spain). Cultures were acclimated to the high concentrations of ammonium by the gradual addition of increasing concentrations of anaerobic centrate for several months, reaching a maximum concentration of ammonium of 6.6 mM and 14.3 mM for Nostoc sp. PCC 7413 and Arthrospira sp., respectively. During this adaptation period, various volumetric renewal rates and renewal periods were evaluated to establish the renewal rate (RR) and renewal periodicity (RP) that allowed to obtaining better growth and better ammonia removal efficiency (RE). For Nostoc sp. PCC 7413, RR 10–20%, and RP of 24–48–72 h were evaluated, while for Arthrospira sp., the ranges evaluated were RR 10–15–20% and RP of 24–48 h. Acclimated cultures from log-phase were centrifuged to remove the culture medium and resuspended in fresh BG110 medium (Allen 1968) for Nostoc sp. PCC 7413, and diluted seawater (salinity 20‰), supplemented with NaHCO3 47.6 mM for Arthrospira sp. in order to obtain an initial pH value of 10.0, both of them supplemented with 10% (v:v) of anaerobic centrate. The control cultures were performed with standard BG11 medium (17.7 mM NaNO3, N:P 74) (Allen 1968) for Nostoc sp. PCC 7413 and standard Zarrouk medium (29.4 mM NaNO3, N:P 10.1) (Zarrouk 1966) prepared in diluted seawater (salinity 20‰) for Arthrospira sp. Cultures were initiated with a high concentration of chlorophyll-a (25 ± 0.5 mg L−1), in order to avoid the inhibitory effect of ammonium on growth (Markou et al. 2014).
Cultures were carried out in triplicate in 250 mL culture units with a diameter of 4.6 cm at 30 °C in a thermostatic bath under a circadian lighting regime 12 h light/12 h dark with an irradiance of 168 μmol photons m−2 s−1. The bioreactors were continuously aerated with air filtered with 0.2 um cellulose acetate filters (Minisart), supplemented with pulses of CO2 in order to maintain the pH at 7.8 ± 0.2 for Nostoc sp. PCC 7413 and at 9.9 ± 0.3 for Arthrospira sp. during the light cycle. Once the early stationary phase was achieved, cultures of Nostoc sp. PCC 7413 were maintained in semi-continuous mode by applying a renewal rate of 10% of the volume of the culture with the anaerobic digestate (NH4+ 49.33 mM) every other day for 16 days, reaching 90% replacement with the anaerobic digestate on the day 16. Cultures of Arthrospira sp. were maintained with a renewal rate of 10% every other day for 8 days, but in this case, the centrate presented a higher ammonium concentration (NH4+ 113.22 mM). Due to the decrease in ammonia assimilation observed in the cultures maintained with this high-ammonium centrate, a renewal rate of 20% with a centrate of lower ammonia concentration (49.33 mM) was applied from day ten. It was maintained for 14 additional days, reaching 100% replacement with the anaerobic centrate by day 14. Evaporation (less than 6% of the culture volume) was corrected daily with sterilized distilled water before proceeding to the harvesting and renewal of the culture medium.
Analysis of ammonia stripping under culture conditions
In order to evaluate the loss of ammonia due to stripping, a test in which the decrease of the NH4+/NH3 concentration in un-inoculated cultures with an initial concentration of 11.33 mM was performed, maintaining the same conditions of culture medium, pH, aeration and temperature that in the inoculated cultures. Ammonium concentration was measured daily. The non-inoculated cultures were carried out in triplicate under the culture conditions described above.
Analytical methods
Samples (5 mL) were taken daily and centrifuged at 17100 ×g for 15 min at 4 °C. For the measurement of chlorophyll-a, a modification of the Talling and Driver (1961) method was applied; in brief, the pellet was resuspended in methanol 90% (v:v) and sonicated at 20 kHz for 2 min followed by 1 h extraction at 4 °C. Cell debris was removed by centrifugation, and chlorophyll-a absorption was measured spectrophotometrically. Ammonium and nitrate were analyzed in the culture media after biomass removal, with an adaptation of the Nessler method (Krug et al. 1979) and an adaptation of the cadmium reduction method (APHA AWWA WEF 2017), respectively, with a Hanna Instruments analyzer (Hanna Instruments Inc., USA). For Nostoc sp. PCC 7413, the released carbohydrates in the culture medium were quantified spectrophotometrically in the supernatant of cultures by the phenol-sulfuric acid method using glucose as standard (Dubois et al. 1956). For Arthrospira sp. cultures, protein and phycobiliprotein were also analyzed in the pellets on day 21 (cultures maintained with a renewal rate of 20%). Protein was determined by the Lowry method (Lowry et al. 1951) modified by Herbert et al. (1971), using bovine serum albumin as standard, and phycobiliproteins were measured according to the Bennett and Bogorad method (1973) after resuspending the pellet in 6.0 mL of saline buffer (0.01 M Na3PO5–0.15 M NaCl, pH 7.0). Dry weight was measured by filtering 5 mL of biomass and washing with 5 mL HCO2NH4 0.5 M three times (Zhu and Lee 1997).
Results
Growth of Arthrospira sp. and Nostoc sp. PCC 7413 in semi-continuous cultures with anaerobic centrate
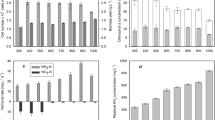
Cultures were allowed to grow for 1–2 days in the standard culture media supplemented with 10% centrate, BG110 for Nostoc sp. PCC 7413, and diluted seawater (salinity 20‰), supplemented with NaHCO3 47.6 mM for Arthrospira sp., before starting the semi-continuous operation by renewing 10% of the volume every 2 days with standard culture media or with anaerobic centrate. For Arthrospira sp., the control culture maintained in saline Zarrouk medium reached a chlorophyll-a concentration of 28.3 ± 0.2 mg L−1 at the beginning of the semi-continuous regime. This value was slightly higher than the value obtained in the cultures maintained with 10% centrate (27.6 ± 0.5 mg L−1 Fig. 1a). The application of a renewal rate of 10% every 48 h did not produce any change in the chlorophyll-a concentration of the control, which recovered the initial concentration after the first 24 h of each dilution cycle, with no further increase in chlorophyll-a concentration in the next 24 h of each 48-h renewal cycle, indicating that the cultures were probably nutrient-limited. In the cultures renewed with the anaerobic centrate, the chlorophyll-a concentration increased continuously during the first renewal cycles, stabilizing at 28.1 ± 0.4 mg L−1 (Fig. 1a), with no significant differences with the cultures maintained with the standard culture medium (Tukey test P ≤ 0.05). The increase of the renewal rate on day ten from 10 to 20% did not produce any change in the steady-state chlorophyll-a concentration either in the control cultures or in the cultures maintained with a centrate, even though the new centrate used presented a lower ammonia concentration (49.33 mM against 113.22 mM centrate used for the dilution 10%). The fact that no growth was recorded during the second 24 h of each renewal cycle regardless of the renewal rate applied excluded the existence of light limitation in the cultures. A gradual increase in the concentration of remaining ammonium in the culture medium was observed between days 6 and 10 (Fig. 1b), indicating that nitrogen was not a limiting nutrient and probably another nutrient, supposedly P could have been limiting the growth of these cultures. No symptoms of physiological stress were observed, as the cells were intact during the whole culture period.
Growth, measured as chlorophyll-a concentration, of (a) Arthrospira sp. and (c) Nostoc sp. PCC 7413, and changes in NH4+ concentration in Arthrospira sp. (b) and Nostoc sp. PCC 7413 (d). For Arthrospira sp. (a and b) arrows indicate the beginning of the semi-continuous operation with a renewal rate 10% (days 1–10, ammonium concentration in the centrate 113.22 mM) and 20% (days 10–24, ammonium concentration in the centrate 49.33 mM). Values are average ± SD (n = 3)
Likewise, for Nostoc sp. PCC 7413, no significant differences in chlorophyll-a concentration were observed between the cultures maintained with the centrate (final NH4+ concentration 4.9 mM) and the cultures maintained with the standard culture medium (Tukey test P ≤ 0.05) when a renewal rate of 10% was applied every 2 days (Fig. 1c). The values of chlorophyll-a obtained in steady state, 28.7 ± 0.4 mg L−1, were equal to those obtained for Arthrospira sp. As for Arthrospira sp., the values of chlorophyll-a on the second day after the renewal were the same as those on the first day, suggesting that the renewal could have been performed daily.
Ammonia stripping
The daily decrease of the NH4+/NH3 concentration caused by the culture conditions (aeration, pH, temperature, culture media), a process known as stripping, was analyzed in un-inoculated cultures with the culture medium supplemented with 10% of high ammonium centrate (final ammonium concentration 11.4 mM). Stripping was more pronounced in the Zarrouk medium + anaerobic centrate (pH 9.9), reaching a 28.4% (3.2 ± 0.2 mM) during the first 48 h (Fig. 2). On the contrary, and due to the lower pH of the BG110 medium, the stripping was much lower, representing a 10.7% (1.2 ± 0.1 mM) of loss (Fig. 2). No increase in nitrate concentration was observed in any condition, indicating that no significant nitrification activity was present.
Ammonium removal
In the semi-continuous culture of Arthrospira sp. with centrate, the concentration of the ammonium (NH4+/NH3) was reduced by 80.5 ± 2.8%, from 12.9 mM to values of 2–3 mM after each 48 h renewal cycle during the first four cycles (Fig. 1b). A tendency to decrease the assimilation of ammonium was observed in the last two cycles of 10% renewal (days 8–10), which was not reflected in changes in the concentration of chlorophyll-a (Fig. 1a). When a centrate of lower ammonium concentration (49.3 mM NH4+) was used, and the renewal rate was increased to 20%, achieving a final concentration of 9.86 mM NH4+ after each renewal cycle, ammonium assimilation of 89.8 ± 1.0% was observed (days 12–24), with values of NH4+/NH3 of 0.5–1.0 mM at the end of the 48 h of renewal cycles (Fig. 1b). Most of the ammonium was assimilated during the first 24 h since the NH4+/NH3 removal of the same cultures after 24 h was 64.7 ± 11.3%. Therefore, most of the ammonium decrease during the next 24 h of each renewal cycle can be mostly attributed to stripping. No nitrate concentration was detected in the cultures of both cyanobacteria maintained with anaerobic centrate.
In the cultures of Nostoc sp. PCC 7413 maintained with centrate, almost 100% of the ammonium was incorporated at the end of the 2-day renewal cycles, reducing the ammonium concentration from 4.9 to 0.4 mM in the first 24 h, during the first four cycles of renewal (Fig. 1d). Subsequently, the remaining levels of ammonium gradually increased from day 10 to 16, until reaching a concentration of 3.3 mM at the end of the 9th cycle of renewal, when 90% of the culture medium had been renewed with centrate (Fig. 1d). When observed under the microscope, these cultures of Nostoc sp. PCC 7413 showed signs of physiological stress, such as the appearance of disintegrated filaments, which were not observed in the control cultures maintained with the standard culture medium. The deficient concentrations of P in the centrate that resulted in an N:P ratio of 132.5, much higher than the N:P ratios of the culture media (10.1 in Zarrouk and 76.7 BG11), could have caused phosphorus limitation of the cultures, explaining the decreasing trend of ammonium assimilation, which may be dependent of the P provided at the beginning of the cultures.
The percentage assimilation of nitrate in the Nostoc PCC 7413 control cultures with BG11 medium (Allen 1968), prepared with fresh water with a nitrate concentration of 17.7 mM, presented average daily incorporation of 9.6 ± 5.0% for Nostoc sp. PCC 7413, while the percentage of assimilation of nitrate in the Arthrospira sp. control cultures with saline Zarrouk medium (Nitrate concentration 29.4 mM, Zarrouk 1966) prepared with diluted seawater (20‰) was slightly higher 13.1 ± 6.7%. Therefore, none of these cultures were nitrogen-limited.
Production of released exopolysaccharides by Nostoc sp. PCC 7413
Despite the renewal of the cultures every 48 h, the concentration of exopolysaccharides increased continuously in both, the control cultures and the cultures maintained with centrate, with a significantly higher concentration of released exopolysaccharides in the culture maintained with the standard culture media in comparison with the culture maintained with the anaerobic centrate (Fig. 3) (Tukey test P ≤ 0.05). The concentration of released exopolysaccharides in the cultures maintained with centrate was 962.7 ± 26.7 mg L−1 (Fig. 2). This value is 25.0% lower compared with the culture with standard BG11 medium (1282.7 ± 8.4 mg L−1).
Dry weight, protein concentration, and phycobiliprotein by a semi-continuous culture of Arthrospira sp.
Notwithstanding no differences were found in chlorophyll-a concentration between control cultures and cultures maintained with centrate (Fig. 1a), the values of dry biomass obtained were significantly different (Tukey test P ≤ 0.05). The steady-state biomass concentration of the cultures maintained with centrate with a renewal rate of 20% every 2 days achieved a mean value of 5.0 ± 0.1 mg mL−1, whereas in the standard culture medium a mean value of 4.1 ± 0.2 mg mL−1 was obtained in the harvested cultures (Table 1) (Tukey test P ≤ 0.05). This value corresponds to a 22% increase in productivity measured as dry biomass in the culture supplemented with anaerobic centrate and using ammonium as a combined nitrogen source in comparison to culture maintained in saline Zarrouk medium, despite the much higher nitrogen availability in the latter.
The protein concentration was also significantly higher in the culture maintained with centrate that achieved a protein concentration of 2.5 ± 0.2 mg mL−1, corresponding to 49.0% of the dry biomass. In contrast, only 1.7 ± 0.1 mg mL−1 of protein were achieved in the culture maintained with the standard culture medium (Turkey’s test P ≤ 0.05) (Table 1).
The phycobiliprotein pigments: allophycocyanin (APC) and phycocyanin (PC), presented lower concentration (mg g−1 biomass) in the cultures maintained with centrate compared to cultures with the standard culture medium (Table 1); however, this difference is not statistically significant (Turkey’s test P ≤ 0.05).
Discussion
The results obtained demonstrated the feasibility of maintaining a stable semi-continuous culture of the cyanobacteria Arthrospira (Spirulina) sp. and Nostoc sp. PCC 7413 using the centrate from the anaerobic digestion of a seafood/fish canning plant as a source of nutrients, achieving at the same time a high rate of ammonium removal and constituting an interesting alternative for tertiary wastewater treatment, helping to avoid the environmental impact of these nutrients on the bodies of water. In addition to having the ability to use ammonium (NH4+/NH3) as a combined nitrogen source for its metabolism, a compound that at certain concentrations is toxic, these cyanobacteria species can also be used in animal feed (Habib et al. 2008), as fertilizer and other high added-value applications such as the production of polysaccharides (Arias et al. 2020) or pigments. Another advantage of filamentous cyanobacteria is the lower cost of harvesting the biomass. The use of filamentous species constitutes a way to solve the problem of harvest associated with the species of unicellular microalgae traditionally used in wastewater treatment systems (Gerardo et al. 2015; Liu et al. 2020). Bioproducts from microalgae are promising for the future; however, the current microalgal cultivation cost is too high to allow many commercial applications. Since nutrient use may account for half of the cost of microalgal cultivation (Norsker et al. 2011; Acién et al. 2013; Craggs et al. 2013), wastewater and other liquid streams rich in nutrients could represent suitable substrates to sustain algal biomass production with an additional positive environmental impact (Hammed et al. 2016).
The results obtained in this work with the anaerobic centrate of a seafood/fish canning industry indicate the viability of the use of Arthrospira sp. and Nostoc sp. PCC 7413 in the bioremediation of this type of effluents, with the potential of becoming an alternative for the standard tertiary treatment due to its high capacity for nutrient removal. It was possible to obtain removal efficiencies of NH4+/NH3 in the range 73.4–56.5% in cultures with anaerobic centrate, at a final ammonium concentration of 4.9, and 11.9 mM for PCC 7413 and Arthrospira sp., respectively. Although the retention times used in this preliminary experiment are high, with renewals applied every 48 h, the data indicate that much lower retention times could be applied, since most of the ammonium was retrieved in the first 24 h. For the cyanobacteria, Phormidium bohneri cultured with anaerobic centrate from swine manure, an RR of 50% and an RT of 96 h were used, with a final ammonium concentration of 1.8 mM (Noüe and Bassères 1989). Wang et al. (2010) evaluated the semi-continuous cultivation of Chlorella vulgaris in anaerobic centrate of cattle manure with a final ammonium concentration of 3.9 mM. They worked with RRs of 5, 10, and 20% with an RT of 24 h; the results showed that with an RR of 20%, the microalgal biomass declined, while with an RR of 10%, the growth was stable but the ammonia removal efficiency (RE) was insufficient (58.3 ± 0.01). An RR of 5% every 24 h was selected as optimal since RE increased significantly (100.0%). In cultures of the cyanobacterium Synechocystis sp. PCC 6830 and the microalga Nannochloropsis salina with an anaerobic centrate of municipal wastewater (final ammonium concentration of 7.6 mM), two RR (25–50%) and various RT (24 to 288 h) were evaluated. The highest biomass productivity was achieved with an RR of 50%, and an RT of 48 h; however, the removal efficiency (RE) of N and P decreases significantly with this low RT, the best RE of both nutrients was achieved with an RR of 50% and an RT of 144 h (89.0 ± 1.7 and 82.8 ± 1.8, respectively) (Cai et al. 2013a, b). An RR of 10% and an RT of 24 h were applied to a consortium of Chlorella sp. and Stigeoclonium sp. and the cyanobacterium cf. Oscillatoria maintained with an anaerobic centrate of the final NH4+ concentration of 1.8 mM (Arias et al. 2017). We must emphasize that the significantly lower ammonium concentrations are used in the studies mentioned above that the ones used in our study.
Also, the values of chlorophyll-a, in Arthrospira sp., and Nostoc sp. PCC 7413 on the second day after renewal were the same as those on the first day, suggesting the presence of either-or nutrients limitation. Although a slower but significant growth of the cultures during the second 24 h of each renewal cycle would have been expected in the case of light-limited cultures, the light limitation cannot be entirely discarded. Therefore, higher levels of irradiance should be evaluated in order to determine their influence on the nutrient removal capacity of the cultures. The values of chlorophyll-a concentration obtained were similar in the control cultures and in the cultures maintained with centrate, indicating that the nutrient levels present in the centrate are enough to sustain active growth. Only in the cultures of Nostoc sp. PCC 7413, a decrease in nitrogen removal was observed in the last renewal cycles (Fig. 2b), probably related to the observed disintegrated filaments that could be indicative of physiological stress and were not observed in the control cultures maintained with the standard BG11 culture medium. The low P content of the centrate used in this experiment that resulted in high N:P ratios could have caused phosphorus limitation of the cultures, explaining the decreasing trend of ammonium assimilation, which may depend on the P provide at the beginning of the cultures. This limitation was not revealed in Arthrospira sp. cultures, probably due to the higher P concentration present initially. Therefore, the concentration of P should be monitored and corrected in this type of industrial effluents to guarantee high nitrogen removal efficiency. However, under certain conditions, the fish processing residues digestate can present high molarities of this nutrient, achieving values as high as 6–10 mM (Guerrero et al. 1999), and therefore not requiring nutritional correction. It would nevertheless be necessary to carry out additional studies to confirm the limiting role of P in these effluents.
Results described by other authors show the feasibility of the culture of microalgae and cyanobacteria in anaerobic centrates. Abu Hajar (2017) cultivated the microalga Neochloris oleoabundans in a diluted anaerobic centrate from the anaerobic digestion of animal manure with an ammonium concentration of 7.8 mM obtaining removal efficiencies (RE) of NH4+/NH3 in the range 64–78%; Uggetti et al. (2014) used the centrate from anaerobic digestion of urban wastewater for the culture of Scenedesmus sp. with an ammonium concentration in the range of 2.8–14.4 mM, and demonstrated that high concentrations of ammonium decreased the growth rate from 0.9 to 0.04 day−1. Bjornsson et al. (2013) cultivated the microalgae Scenedesmus sp. AMDD with the centrate from the anaerobic co-digestion of algal biomass and swine manure (1.5 mM NH4+) with and without CO2 addition; and obtained a maximum dry weight of 0.27 g L−1 without the addition of CO2, and 0.42 g with its addition, achieving an ammonium RE of 99.6%.
The concentration of chlorophyll-a obtained in the cultures maintained with centrate is similar to that reported by other authors with Nostoc sp. Otero and Vincenzini (2003), evaluated PCC 7413 in a similar culture system under continuous illumination, obtaining maximal chlorophyll-a values of 28.0 ± 0.5 mg L−1. In the case of Arthrospira sp., the concentration of chlorophyll-a in our cultures was significantly higher than that described by other authors in wastewater, even though we were using diluted seawater in the control cultures. Olguín et al. (1994) cultivated Arthrospira maxima in HRAPs at 30 °C, with seawater (salinity 30‰) supplemented with 2% (v:v) of centrate of pig purines with a final ammonium concentration of 1.4 mM, obtaining a maximum concentration of chlorophyll-a of 8.1 mg L−1, a value significantly lower than the concentration obtained in this work (28.3 ± 0.2 mg L−1). The lower ammonium concentration and irradiance (70 μmol photons m−2 s−1) used in those cultures and the lack of addition of CO2 may be responsible for the lower growth and removal capacity.
Arthrospira sp. presents a lower ammonium RE due to high stripping (56.5%), compared with Nostoc sp. PCC 7413 (73.4%). However, Arthrospira sp. ammonia assimilation capacity was significantly higher (1.4 times) than that obtained with Nostoc sp. PCC 7413. This characteristic, together with the high percentage of protein (50%) of the dry biomass produced in this type of wastewater, makes it interesting for the production on a larger scale. Furthermore, as reported by other authors (Abeliovich and Azov 1976; Belkin and Boussiba 1991), we confirm the high tolerance of Arthrospira sp., being an alkalophilic species, to high ammonium concentrations. However, it should be noted that PCC 7413 presented a high ammonium removal efficiency, and a daily ammonium consumption slightly lower than that of Arthrospira sp., presenting the additional advantage of its significant production of released exopolysaccharides with these residual (962.7 ± 26.7 mg L−1). This value of EPS is significantly lower than the values reported for this species in previous studies (1.8 g L−1, Otero and Vincenzini 2003). This can be caused by a higher light availability in the latter, derived from the application of continuous illumination. The lower production of exopolysaccharides in centrate cultures compared with the culture with the standard culture medium may due to the fact the ammonia can deviate the flow of carbon to the synthesis of protein in detriment to that of carbohydrates (Lawrie et al. 1976). Both the assimilation of nitrate and ammonium affect the rate of CO2 fixation and the carbon flux to a different metabolic fraction (Romero and Lara 1987). Cañizares-Villanueva et al. (1995) obtained a higher protein: carbohydrate ratio (3.9) for the filamentous cyanobacteria Phormidium sp. when cultivated in porcine wastewater with 2.3 mM NH4+, compared with the control maintained with KNO3 (1.9), despite the much higher nitrogen availability in the latter.
Very high values of dry weight, 5.0 mg mL−1, and protein concentration (2.5 mg mL−1) were obtained in the cultures of Arthrospira sp. grown with the anaerobic centrate, being higher those obtained in the standard culture media and those obtained by other researchers. Bezerra et al. (2007) obtained a maximum dry biomass concentration of 1.9 g L−1 in fed-batch cultures of A. platensis with an initial concentration of 1.7 mM NH4Cl. The lower ammonium concentration used in those cultures and the lack of addition of CO2 may be responsible for the lower dry weight. Results closer to those reported here were obtained by Delrue et al. (2017) that cultivated A. platensis in a modified Zarrouk medium with a high inoculum concentration reaching a final dry weight of 3.4 g L−1, a value similar to that obtained here for standard Zarrouk medium, 4.1 g L−1. The high biomass values obtained here may be due to the high effective irradiance available in the tubular systems (diameter 4.6 cm) and strict pH control. In fact, up to 6.0 g L−1 was achieved in Chlorella sorokiniana cultures in synthetic urine with an irradiance of 990 μmol photons m−1 s−1 (Tuantet et al. 2014).
The result obtained in this work for the protein fraction of Arthrospira sp. cultivated with centrate from the effluent from a fish canning industry is similar to those obtained by other authors with centrate from other types of effluents. In semi-continuous cultures of Arthrospira with diluted seawater supplemented with the centrate of pig purines presenting a lower concentration of ammonium (2.2 mM), the protein concentration ranged from 35 and 49%, which are similar to the value obtained with the fish canning centrate (49% w/w) in this research (Olguín et al. 2001). Only in one case with higher protein content values, 71% w/w have been reported in cultures of a mutant strain of S. maxima growing at 30 °C with constant illumination (60–70 μmol photons m−2 s−1) growing in seawater supplemented with anaerobic centrate from pig waste (2% v/v) (Olguín et al. 1994).
Conclusion
Our results indicate that the filamentous cyanobacteria Arthrospira sp. and Nostoc sp. PCC 7413 can be produced using centrate from the anaerobic digestion wastewater from a seafood/fish canning plant as the nutrient source, representing an interesting alternative for the treatment and valorization of nutrient-rich anaerobic digestion effluents. However, additional experiments are required in order to assess the feasibility of the treatment on a large scale.
References
Abeliovich A, Azov Y (1976) Toxicity of ammonia to algae in sewage oxidation ponds. Appl Environ Microbiol 31:801–806
Abu Hajar, H (2016) Sustainable cultivation of microalgae using diluted anaerobic digestate for biofuels production. Electronic Thesis or Dissertation. Retrieved from https://etd.ohiolink.edu/
Acién FG, Fernández JM, Molina-Grima E (2013) Economics of microalgae biomass production. In: Pandey J, Lee DJ, Chisti Y, Soccol C (eds) Biofuels from algae. Elsevier, Amsterdam, pp 313–325
Allen MM (1968) Simple conditions for growth of unicellular blue-green algae on plates. J Phycol 4:1–4
APHA AWWA WEF (2017) Standard method for the examination of water and wastewater, 23rd edn Washington, USA
Arias DM, Uggetti E, García-Galán MJ, García J (2017) Cultivation and selection of cyanobacteria in a closed photobioreactor used for secondary effluent and digestate treatment. Sci Total Environ 1:157–167
Arias DM, García J, Uggetti E (2020) Production of polymers by cyanobacteria grown in wastewater: current status, challenges and future perspectives. New Biotechnol 55:46–57
Belkin S, Boussiba S (1991) High internal pH conveys ammonia resistance in Spirulina platensis. Bioresour Technol 38:167–169
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Bezerra RP, Matsudo MC, Converti A, Sato S, de Carvalho JCM (2007) Influence of ammonium chloride feeding time and light intensity on the cultivation of Spirulina (Arthrospira) platensis. Biotechnol Bioeng 100:297–305
Bjornsson WJ, Nicol RW, Dickinson KE, McGinn PJ (2013) Anaerobic digestate are useful nutrient sources for microalgae cultivation: functional coupling of energy and biomass production. J Appl Phycol 25:1523–1528
Cai T, Park SY, Racharaks R, Li Y (2013a) Cultivation of Nannochloropsis salina using anaerobic digestion effluent as a nutrient source for biofuel production. Appl Energy 108:486–492
Cai T, Ge X, Park SY, Li Y (2013b) Comparison of Synechocystis sp. PCC 6803 and Nannochloropsis salina for lipid production using artificial seawater and nutrients from anaerobic digestion effluent. Bioresour Technol 144:255–260
Cañizares-Villanueva RO, Domínguez AR, Cruz MS, Ríos-Leal E (1995) Chemical composition of cyanobacteria grown in diluted, aerated swine wastewater. Bioresour Technol 51:111–116
Cepoi L, Dontu N, Şalaru V, Şalaru V (2016) Removal of organic pollutants from wastewater by cyanobacteria. In: Zinicovscaia I, Cepoi L (eds) Cyanobacteria for bioremediation of wastewater. Springer, Cham, pp 27–43
Chaiklahan R, Chirasuwan N, Siangdung W, Paithoonrangsarid K, Bunnag B (2010) Cultivation of Spirulina platensis using pig wastewater in a semi-continuous process. J Microbiol Biotechnol 20:609–614
Chen M, Li J, Zhang L, Chang S, Liu C, Wang J, Li S (2014) Auto-flotation of heterocyst enables the efficient production of renewable energy in cyanobacteria. Sci Rep 4:1–9
Cho CM, Fan Y, Li F, Hu GR (2016) Bioremediation of wastewater from edible oil refinery factory using oleaginous microalga Desmodesmus sp. S1. Int J Phytoremed 12:1195–1201
Chowdhury P, Viraraghavan T, Srinivasan A (2010) Biological treatment processes for fish processing wastewater - a review. Bioresour Technol 101:439–449
Chuntapa B, Powtongsook S, Menasveta P (2003) Water quality control using Spirulina platensis in shrimp culture tanks. Aquaculture. 220:355–366
Collet P, Lardon L, Hélias A, Bricout S, Lombaert-Valot I, Perrier B, Lépine O, Steyer JP, Bernard O (2014) Biodiesel from microalgae – life cycle assessment and recommendations for potential improvements. Renew Energy 71:525–533
Contreras EM, Giannuzi L, Zaritzky NE (2000) Growth kinetics of the filamentous microorganism Sphaerotilus natans in a model system of a food industry wastewater. Water Res 34:4455–4463
Craggs RJ, Lundquist TJ, Benemann JR (2013) Wastewater treatment and algal biofuel production. In: Borowitzka MA, Moheimani NR (eds) Algae for Biofuels and Energy. Springer, Dordrecht, pp 153–163
Delrue F, Alaux E, Moudjaoui L, Gaignard C, Fleury G, Perilhou A, Richaud P, Petitjean M, Sassi JF (2017) Optimization of Arthrospira platensis (Spirulina) growth: from laboratory scale to pilot scale. Fermentation 3:1–14
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Franchino M, Comino E, Bona F, Riggio VA (2013) Growth of three microalgae strains and nutrient removal from an agro-zootechnical digestate. Chemosphere. 92:738–744
Gao F, Li C, Yang ZH, Zeng GM, Feng LJ, Liu JZ, Liu M, Cai HW (2016) Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol Eng 92:55–61
Gerardo ML, Van Den Henden S, Vervaeren H, Coward T, Skill SC (2015) Harvesting of microalgae within a biorefinery approach: a review of the developments and case studies from pilot-plants. Algal Res 11:248–262
Guerrero L, Omil F, Méndez R, Lema JM (1999) Anaerobic hydrolysis and acidogenesis of wastewaters from food industries with high content of organic solids and protein. Water Res 33:3281–3290
Habib MAB, Parvin M, Huntington TC, Hasan MR (2008) A review of culture, production and use of Spirulina as food for humans and feeds for domestic animal and fish. FAO Fisheries and Aquaculture Circular no. 1034. FAO, Rome
Hammed AM, Prajapati SK, Simsek S, Simsek H (2016) Growth regime and environmental remediation of microalgae. Algae. 31:189–204
Herbert D, Phipps PJ, Strange RE (1971) Chemical analysis of microbial cells. In: Norris JR, Ribbons DW (eds) Methods in microbiology. Academic Press, London, pp 209–344
Kamilya D, Sarkar S, Maiti TK, Bandyopadhyay S, Mal BC (2006) Growth and nutrient removal rates of Spirulina platensis and Nostoc muscorum in fish culture effluent: a laboratory-scale study. Aquac Res 37:1594–1597
Komárek J, Anagnostidis K (2005) Süsswasserflora von Mitteleuropa. Cyanoprokaryota: 2. Teil/2nd part: Oscillatoriales, vol 19. Elsevier Spektrum, München
Krug FJ, Ruzicka J, Hansen E (1979) Determination of ammonia in low concentrations with Nessler ́s reagent by flow injection analysis. Analyst. 104:47–54
Lawrie AC, Codd GA, Stewart DP (1976) The incorporation of nitrogen into products of recent photosynthesis in Anabaena cylindrica Lemm. Arch Microbiol 107:15–24
Ledda C, Romero Villegas GI, Adani F, Acién Fernández FG, Molina Grima E (2015a) Utilization of centrate from wastewater treatment for the outdoor production of Nannochloropsis gaditana biomass at pilot-scale. Algal Res 12:17–25
Ledda C, Idà A, Allemand D, Mariani P, Adani F (2015b) Production of wild Chlorella sp. cultivated in digested and membrane-pretreated swine manure derived from a full-scale operation plant. Algal Res 12:68–73
Ledda C, Tamiazzo J, Borin M, Adani F (2016) A simplified process of swine slurry treatment by primary filtration and Haematococcus pluvialis culture to produce low cost astaxanthin. Ecol Eng 90:244–250
Liu J, Pemberton B, Lewis J, Scales PJ, Martin GJO (2020) Wastewater treatment using filamentous algae – a review. Bioresour Technol 298:1–15
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Markou G, Georgakakis D (2011) Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewater. A review. Appl Energy 88:3389–3401
Markou G, Vandamme D, Muylaert K (2014) Ammonia inhibition on Arthrospira platensis in relation to the initial biomass density and pH. Bioresour Technol 166:259–365
Matamoros V, Gutiérrez R, Ferrer R, García J, Bayona JM (2015) Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminant: a pilot-scale study. J Hazard Mater 288:34–42
Milhazes-Cunha H, Otero A (2017) Valorisation of aquaculture effluents with microalgae: the integrated multi-trophic aquaculture concept. Algal Res 24:416–424
Nayak M, Karemore A, Sen R (2016) Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res 16:216–223
Norsker NH, Barbosa MJ, Vermuë MH, Wijffels RH (2011) Microalgal production – a close look at the economics. Biotechnol Adv 29:24–47
Noüe J, Bassères A (1989) Biotreatment of anaerobically digested swine manure with microalgae. Biol Wastes 29:17–31
Olguín EJ, Hernández B, Araus A, Camacho R, González R, Ramírez ME, Galicia S, Mercado G (1994) Simultaneous high-biomass protein production and nutrient removal using Spirulina maxima in sea water supplemented with anaerobic effluents. World J Microbiol Biotechnol 10:576–578
Olguín EJ, Galicia S, Angulo-Guerrero O, Hernández E (2001) The effect of low flux and nitrogen deficiency on the chemical composition of Spirulina sp. (Arthrospira) grown on digested pig waste. Bioresour Technol 77:19–24
Olguín EJ, Galicia S, Mercado G, Pérez T (2003) Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J Appl Phycol 15:249–257
Otero A, Vincenzini M (2003) Extracellular polysaccharide synthesis by Nostoc strain as affected by N source and light intensity. J Biotechnol 102:143–152
Park JBK, Craggs RJ (2010) Wastewater treatment and algal production in high rate algal ponds with carbon dioxide addition. Water Sci Technol 61:633–639
Park JBK, Craggs RJ, Shilton AN (2011) Wastewater treatment high rate algal ponds for biofuel production. Bioresour Technol 102:35–42
Patnaik S, Sarkar R, Mitra A (2001) Alginate immobilization of Spirulina platensis for wastewater treatment. Indian J Exp Biol 39:824–826
Raeisossadati M, Vadiveloo A, Bahri PA, Parlevliet D, Moheimani NR (2019) Treating anaerobically digested piggery effluent (ADPE) using microalgae in thin layer reactor and raceway pond. J Appl Phycol 31:2311–2319
Romero JM, Lara C (1987) Photosynthetic assimilation of NO3− by intact cells of the cyanobacterium Anacystis nidulans. Plant Physiol 83:208–212
Romero-Villegas GI, Fiamengo M, Acién Fernández FG, Molina Grima E (2017) Outdoor production of microalgae biomass at pilot-scale in seawater using centrate as the nutrient source. Algal Res 25:538–548
Sadik A (2015) Microalgal growth in anaerobic digestate. M.Sc. Thesis. Istanbul Technical University
Sankaran K, Premalatha M (2018) Nutrients uptake from anaerobically digested distillery wastewater by Spirulina sp. Under xenon lamp illumination. J Water Process Eng 25:295–300
Singh J, Thakur IS (2015) Evaluation of cyanobacterial endolith Leptolyngbya sp. ISTCY101, for integrated wastewater treatment and biodiesel production: a toxicological perspective. Algal Res 11:294–303
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Review: commercial applications of microalgae. J Biosci Bioeng 2:87–96
Talling JF, Driver D (1961) Some problems in the estimation of chlorophyll-a in phytoplankton. In: Doty MS (ed) Primary Productivity Measurement, Marine, and Freshwater. U.S. Atomic Energy Commission, Washington, D.C, pp 142–146
Tredici MR, Rodolfi L, Biondi N, Bassi N, Sampietro G (2016) Techno-economic analysis of microalgal biomass productions in a 1-ha Green Wall panel (GWP®) plant. Algal Res 19:253–263
Tuantet K, Temmink H, Zeeman G, Janssen M, Wijffels RH, Buisman CJN (2014) Nutrient removal and microalgal biomass production on urine in a short light-path. Water Res 55:162–174
Uggetti E, Sialve B, Latrille E, Steyer JP (2014) Anaerobic digestate as substrate for microalgae culture: the role of ammonium concentration on the microalgae productivity. Bioresour Technol 152:437–443
Wang L, Wang Y, Chen P, Ruan R (2010) Semi-continuous cultivation of Chlorella vulgaris for treating undigested and digested dairy manures. Appl Biochem Biotechnol 162:2324–2332
Wuang SC, Khin MC, Chua PQD, Luo YD (2016) Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res 15:59–64
Yenigün O, Demirel B (2013) Ammonia inhibition in anaerobic digestion: a review. Process Biochem 48:901–911
Zarrouk C (1966) Contribution à l’étude d’une cyanophycée. Influence de divers facteurs physiques et chimiques sur la croissance et photosynthèse de Spirulina maxima Geitler. Ph.D. Thesis, University of Paris
Zhu CJ, Lee YK (1997) Determination of biomass dry weight of marine microalgae. J Appl Phycol 9:189–194
Zinicovscaia I (2016) Water Quality: A Major Global Problem. In: Zinicovscaia I, Cepoi L (eds) Cyanobacteria for bioremediation of wastewater. Springer, Cham, pp 5–16
Zuliani L, Frison N, Jelic A, Fatone F, Bolzonella D, Ballottari M (2016) Microalgae cultivation on anaerobic digestate of municipal wastewater, sewage sludge and agro-waste. Int J Mol Sci 17:1–17
Acknowledgments
Xavier Álvarez was supported by a scholarship from Secretaria de Educación Superior, Ciencia, Tecnología e Innovación (SENESCYT) of the National Government of the Republic of Ecuador. This work has been partially supported by a Grant from the Consellería de Industria, Xunta de Galicia (Programa de Consolidación y estructuración de Unidades de Investigación Competitivas, GPC2014/019). The digestates have been kindly provided by JB Ingenieros, Vigo (Pontevedra) Spain.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Álvarez, X., Otero, A. Nutrient removal from the centrate of anaerobic digestion of high ammonium industrial wastewater by a semi-continuous culture of Arthrospira sp. and Nostoc sp. PCC 7413. J Appl Phycol 32, 2785–2794 (2020). https://doi.org/10.1007/s10811-020-02175-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02175-4