Abstract
Primates show various behavioral responses to resource seasonality, including changes in diet and habitat use. These responses may be particularly important for species living in large groups, owing to strong competition for resources. We investigated seasonality in diet and habitat use in wild mandrills (Mandrillus sphinx), which form some of the largest primate groups, in Moukalaba–Doudou National Park, Gabon. We used a fallen fruit census to measure fruit availability and camera trapping to measure visit frequency by mandrill groups on 11 line transects from January 2012 to November 2013, and collected mandrill feces for 25 months in 2009–2013 to assess their diets. Fruit availability varied seasonally, with a peak in December–February, and a scarce period in March–August. Relative volumes of fruit skin, pulp, and intact seeds in fecal remains varied with fruit availability, whereas feces contained as large a proportion of crushed seeds in the fruit-scarce season as in the fruit-peak season. The relative volumes of woody tissue (e.g., bark and roots) and the number of food types increased in the fruit-scarce season compared to in the fruit-peak season. Camera trapping revealed seasonality in habitat use. In fruit-rich seasons, mandrill visits were highly biased toward transects where fruit species that appeared in the majority of feces in a group were abundant. In contrast, in fruit-scarce seasons, visit frequencies were distributed more uniformly and the relationship with fruit availability was unclear. Our results suggest that mandrill groups in the study area respond to seasonal fruit scarcity by consuming seeds and woody tissue and by ranging more widely than in fruit-rich seasons. These flexible dietary and ranging behaviors may contribute to the maintenance of extremely large groups in mandrills.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primates show a wide variety of diets and habitat use patterns between and within species. While body size and morphology largely determine primate diet and habitat use (Fleagle 2013), social organization and environmental factors, including disturbance (Johns and Skorupa 1987), seasonal inundation (Terada et al. 2015), and topographic steepness (Etiendem et al. 2013), also affect habitat use patterns. Since food resources change seasonally in most of the primate habitats (Hanya et al. 2013; van Schaik and Pfannes 2005), behavioral responses to resource seasonality, including changes in diet and habitat use, are important adaptations for most primates (Hemingway and Bynum 2005; Tsuji et al. 2013). For example, rhesus macaques (Macaca mulatta) eat more mature leaves (Tang et al. 2016) and eastern lowland gorillas (Gorilla beringei) eat more bark and leaves (Yamagiwa et al. 1994) when fruits become scarce. During fruit-scarce seasons, larger species except great apes (5–15 kg) tend to eat lower-quality foods, such as mature leaves and other vegetative matter, than smaller species, which rely on higher-quality exudate and nectar for alternative foods (Hemingway and Bynum 2005). Some primates show seasonal differences in dietary diversity. For example, Japanese macaques (Macaca fuscata: Nakagawa 1989) and black-and-white colobus (Colobus guereza: Harris et al. 2010) have more diverse diets in seasons in which their main foods are scarce, whereas blue monkeys (Cercopithecus mitis: Kaplin et al. 1998) decrease dietary diversity by eating a particular seed species frequently when fruits are scarce. Dietary responses can also vary between populations of the same species (e.g., gray-cheeked mangabeys, Lophocebus albigena: Hemingway and Bynum 2005). Changes in ranging patterns include seasonal changes in habitat (e.g., common brown lemurs, Eulemur fulvus: Sato 2013) and increased home range size (e.g., tufted capuchins, Sapajus apella: Di Bitetti 2001).
Biogeographic differences in phenology and environments affect primate behavioral responses to food seasonality (Hemingway and Bynum 2005). For example, whereas African primates often show increased dietary diversity during seasons of food scarcity but do not change in home range size seasonally, New World monkeys, particularly Atelinae and Cebinae species with relatively large home ranges, rarely increase diversity of their diets but often expand their home range or change their habitats when foods are scarce. These regional contrasts may be due to differences in phenology and forest structure: the interval between peak leaf flush and peak fruiting is shorter in American than in African forests (van Schaik and Pfannes 2005), and the Amazonian waterways cause high heterogeneity of habitat types in American tropical forests (Hemingway and Bynum 2005).
Group size may also influence primate seasonal behaviors, and behavioral responses may be particularly important for large groups because larger groups experience stronger scramble feeding competition (Janson 1988). For example, the dietary diversity of red colobus (Procolobus rufomitratus) correlates positively with their group size (Gogarten et al. 2014), and seasonal changes in habitat are confined to species with the largest group sizes among the primate community at Uruku River, Brazil (Peres 1994).
Mandrills (Mandrillus sphinx) and drills (M. leucophaeus) live in coastal tropical forests in central Africa where resource production varies seasonally (Newbery et al. 1998; White 1994). They form among the largest wild primate groups of up to 845 (mandrills: Abernethy et al. 2002) and 400 (drills: Wild et al. 2005) individuals. However, the dense vegetation of their habitats make it difficult to observe them directly without habituation, which is nearly impossible owing to their large group sizes and large home ranges. Consequently, no ecological studies of mandrills or drills based on direct behavioral observations of identified individuals have been achieved in the wild, and available data are limited to a provisioned mandrill group established by releasing captive individuals in Lékédi Park, Gabon (Brockmeyer et al. 2015; Nsi Akoue et al. 2017).
Based on indirect data from feces and food remnants, wild mandrills and drills are omnivorous, with a high preference for fruits (Astaras and Waltert 2010; Hoshino 1985; Lahm 1986; Rogers et al. 1996). Provisioned mandrills are also omnivorous, with a clear frugivorous tendency (Nsi Akoue et al. 2017). Mandrillus species, and closely related Cercocebus species, have enlarged premolars, which are adapted to processing hard nuts and seeds (Fleagle and McGraw 2002), and monkeys of both genera eat seeds frequently (Astaras and Waltert 2010; Hoshino 1985; McGrew et al. 2009). However, seasonal patterns in diets differ between the genera: whereas Mandrillus species increase the diversity of food types by eating fallen seeds and monocotyledonous herbs in fruit-scarce seasons (Astaras and Waltert 2010; Hoshino 1985; Tutin et al. 1997), Cercocebus species eat a large amount of fruits and seeds year-round, and changes in their diet do not always relate to fruiting seasonality (McGraw et al. 2014; Mitani 1989).
We know much less about ranging behaviors of wild mandrills and drills than about their diets. A study in Lopé National Park, Gabon, estimated the home range of a wild mandrill group of ca. 700 individuals at 182 km2, including 89 km2 of forested area (White et al. 2010), whereas a provisioned mandrill group of 120 individuals has a much smaller home range of 8.7 km2 (Brockmeyer et al. 2015). Surveys report that mandrills prefer primary forests and avoid savannah (Lahm 1986; Rogers et al. 1996), and drills occur from lowland to montane forests at up to a 2000 m elevation (Wild et al. 2005). Their seasonality in habitat use is barely understood. Researchers at Lopé (Rogers et al. 1996) observed groups in gallery forests more frequently during dry seasons than rainy seasons, but a subsequent report at the same site (White 2007) did not confirm this pattern because the core area of the group was in gallery forests regardless of season. Conversely, Cercocebus mangabeys mainly inhabit riverine and swamp forests with groups of 10–125 individuals and much smaller home ranges of 1–3 km2 (Swedell 2011), and red-capped mangabeys (C. torquatus) show seasonal changes in habitat (Mitani 1989).
In this study, we examined seasonal changes in the diet and habitat use of wild mandrills in Moukalaba–Doudou National Park, Gabon. A previous study at the same site (Hongo 2014) obtained three full counts of mandrill groups of 169, 350, and 442 individuals, but we do not know how many groups there are in our study population. We obtained data on diet from fecal samples, and used camera traps to collect data on differential habitat use, both for multiple unhabituated groups. Digestive efficiencies vary with food type (Litvaitis 2000), so we used fecal analysis to examine seasonal differences in the consumption of each food type, but did not compare the relative importance of food types.
We had three objectives. First, we assessed fruiting phenology in the study area to define seasons based on fruit availability. Second, we examined seasonal changes in mandrill diet by comparing the relative volumes of each food type and the number of food types in fecal remains between seasons. Third, we examined seasonal changes in mandrill habitat use based on the frequency of visits to camera traps. We explored correlations between visit frequency and the availability of important mandrill fruits, seasons, and habitat parameters, and compared the spatial distribution of visits to camera traps between seasons to explore seasonality in mandrill ranging patterns.
Methods
Study Area
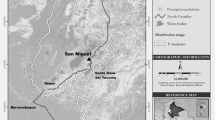
Our study area encompassed ca. 400 km2 in the eastern part of Moukalaba–Doudou National Park, Gabon. Given that a home range of the mandrill group at Lopé includes 89 km2 of forested area (White et al. 2010), the study area is likely to cover the home ranges of several groups. Our base camp was located at S2° 19′ and E 10° 34′. The study area comprises different habitat types, according to Landsat™, radar, and aerial imagery (Ministère des Eaux et Forêts et du Reboisement, Tecsult International, Quebec, Canada; provided by WWF Gamba; Fig. 1). Annual rainfall during 2002–2013 was 1176–2043 mm, and the mean monthly minimum and maximum temperatures during 2006–2013 were 18.7–25.0 °C and 26.7–34.3 °C, respectively (PROCOBHA research team, unpubl. Data). Typically, the dry season is from May to September, and the rainy season is from October to April, but there is little rain from December to February in some years (Takenoshita et al. 2008).
Map of the study area in Moukalaba–Doudou National Park, Gabon, with transects and vegetation types. Dark green (transect MD) is mountainous primary forest; olive green (NK, MB, DB, DT, FD, and G5) is lowland primary forest; lime green (A and KO) is disturbed secondary forest; light blue (G22 and BV) is seasonally inundated riverine forest; white is savannah.
Fruiting Phenology and Definition of Seasons
To monitor fruiting phenology in the study area, we conducted a monthly fallen-fruit census (Furuichi et al. 2001) from January 2012 to January 2014. We established 11 line transects separated by ≥2 km and covering all types of vegetation in the study area (Fig. 1). We set the length of transects to 2 km to prevent transects from crossing different vegetation types. We defined a fruit cluster as one or more fresh fruits that had fallen on the ground from the same tree. We noted and photographed fruit clusters of all species within 1 m of the center of the transects (total area censused = 4.4 ha). We also counted partially eaten fruits as fruit clusters if we found a fruiting tree of the same species above them. To avoid counting multiple clusters from the same fruiting tree, we did not record clusters that fell within 10 m of the previous cluster of the same species. When we found two fruit clusters of the same species >10 m apart, we counted the second cluster only if we found a different fruiting tree above them. Thus, the number of fruit clusters should match the number of fruiting trees near the transects, although we may have failed to count tree species whose fruits seldom fall to the ground or rarely remain for a long time because of consumption by animals (Furuichi et al. 2001). Our measure is a rough indicator of fruit abundance, as we did not quantify the numbers or the mass of the fruits.
We attempted to classify the fruit clusters taxonomically using photographs and plant lists for our study area (Takenoshita et al. 2007; Yumoto et al. 2015). We finished the classification of the fruits found in mandrill feces; the classification of other fruit species is ongoing.
We used fruit census data from January 2012 to November 2013 for analysis. To examine seasonal variation in fruit availability, we drew boxplots of the monthly numbers of fruit clusters of all species, based on which we defined four seasons: fruit-peak season from December to February, early fruit-scarce season from March to May, late fruit-scarce season from June to August, and fruit-increase season from September to November. We tested whether numbers of fruit clusters are statistically different among the four seasons using pairwise Welch t-tests with the Holm’s P-value adjustment (Holm 1979).
Fecal Sample Collection and Analysis
We searched for mandrill groups with research assistants over the whole study area for 25 mo between 2009 and 2013 (August–November 2009, January–June 2010, November 2011–March 2012, June–August 2012, October 2012–February 2013, and June–September 2013). When we found a group, we followed it and collected fecal samples ad libitum.
We conducted fecal analysis following a standardized protocol (McGrew et al. 2009). On the day of collection, we washed fecal samples in a 1-mm2 sieve until the waste water was clear and dried the samples in the shade. We then sorted fecal remains into nine food types using a magnifying glass: fruit fiber (fruit skin, pulp, and intact seeds); crushed seeds (including crushed seed coats); dicotyledonous leaves; monocotyledonous herbs (blades and pith); woody tissue (bark, woody liana, roots, and subterranean stems); flowers; invertebrates; vertebrates (hairs and feathers); and other (including soil, stones, and dead leaves). Unlike several previous studies of Mandrillus species, where intact seeds were discarded and/or fruit fiber and seeds were both categorized as fruits (Hoshino 1985; Owens et al. 2015), we categorized intact and crushed seeds into fruit fiber and seeds, respectively, because fruit fiber and seeds are considerably different in terms of phenology and nutrition intake. Since fruit skin and pulp rarely occurred in feces, and the occurrence of intact seeds means that mandrills receive nutrition from fruit skin and/or pulp not from seeds, excluding intact seeds would substantially underestimate the dietary contribution of fruit fiber. We estimated the relative volume of each food type in the fecal remains on a five-point scale at 25% intervals (i.e., 0%, 12.5%, 37.5%, 62.5%, and 87.5%).
We identified fruit items to the lowest possible taxonomic group based on their morphological traits. We identified “important” fruits for mandrills from the list of fruit items found in their feces. To find an objective threshold for importance, we calculated proportion of occurrence by date and fruit item whenever we collected more than five feces during a group follow, and plotted a density curve. The curve showed a bimodal distribution with a local minimum at 61.7%. We therefore defined important fruits as fruit items that occurred ≥60% at least once.
Camera Trapping and Capture Rate of Mandrill Groups
From January 2012 to February 2014, we deployed 10 camera traps (Bushnell® Trophy Cam 2010, Overland Park, MO) along each transect at 200-m intervals, as part of a comprehensive study of mammalian ecology (Nakashima 2015). We strapped each camera to a tree 10 m from the transect and adjusted it to be parallel to the ground at a height of 30 cm. We did not move cameras during the study period. We configured the cameras to start in response to the passage of animals and to record a video image of 30 s or 60 s at a minimum interval of 30 s (3 min from January to July 2012). We checked the conditions of the cameras monthly and replaced them as soon as possible when they broke.
We used camera-trap data from January 2012 to November 2013 for analysis, because the number of deployed camera traps decreased substantially in December 2013. To count the number of mandrill group visits to transects, we first counted camera visits, where a camera took videos at intervals of <30 min (O’Brien et al. 2003). We regarded visits as group visits only when two or more reproductive females, juveniles, or infants passed within 5 m of a camera. Next, we calculated time intervals between consecutive group visits recorded by cameras along the same transect and plotted a density curve. We used only intervals of <3 days (N = 157) and excluded nighttime (18:00–06:00 h) from the intervals. The curve showed an exponent function-like shape with a clear change in slope at 10 h, so we pooled camera-based group visits recorded within 10 h (excluding nighttime) by cameras along the same transect. That is, we regarded group visits filmed in the same transect at an interval of >10 h or recorded in different transects as independent. Finally, we counted independent group visits for each transect and season, and calculated capture rates as the number of independent group visits divided by the total number of days when cameras worked.
Habitat Parameters
We categorized habitat types of the transects in secondary forests as “disturbed” and those in riverine forests as “seasonally inundated” habitats. We quantified the topographic steepness of all transects by measuring the inclination of the ground in front of each camera trap using a laser range finder (Laser Technology TruPulse® 200, Centennial, CO). We used the mean of the inclination angles as an indicator of the steepness of transects.
Statistical Analysis
We performed all statistical analyses using R version 3.3.3 (R Core Team 2017). All statistical tests were performed as two-tailed tests, and we considered P < 0.05 as significant.
Diet Seasonality
We examined seasonal variation in the relative volume of each food type in fecal samples, except for flower, vertebrate, and other, which rarely occurred in fecal samples. Since the relative volumes are nonbinomial, we logit-transformed them using the following equation based on a previous study (Warton and Hui 2011): z = log([y + 0.05]/[1 – y + 0.05]), where y is a relative volume. We added 0.05 to both the numerator and denominator of the logit function because the simple logit function does not accept 0. We then constructed linear mixed models (LMMs) using the lmer function in the lme4 package (Bates et al. 2015). The full model contained the response variable logit-transformed relative volume (z), a fixed effect of season (four-level categorical variable with fruit-peak season as a control level), and a random effect of date of group follow (random intercept). We included the date of group follows as a random effect because we collected multiple fecal samples in each group follow. We did not include the number of fruit clusters as a fixed effect in the model because we did not conduct the fruit census before 2012 and we collected fecal samples both along the transects and elsewhere in the study area.
To explore the statistical differences in the relative volumes among seasons, we used the grouping model comparison (Mori et al. 2009). We generated 15 candidate models, including a full model where all four seasons were different levels, 13 possible group models where two or more seasons were grouped as identical levels, and a null model where all the seasons were regarded as a single level. We conducted model selection based on Akaike information criterion (AIC) values (Akaike 1974) and probabilities that a given model has the smallest AIC among the candidate models (model selection frequencies, Burnham and Anderson 2002) from a nonparametric bootstrap of 1000 replicates. We considered models with a model selection frequency of ≥5% as confident models (Shimodaira 1998) and used them to interpret the results. We checked the residual plots and normal Q–Q plots of both the full model and the smallest-AIC model for diagnostics and confirmed model stability.
To examine seasonality in the number of food types, we constructed a generalized linear mixed model (GLMM) with a binomial error distribution and a logit link function using the glmer function in the lme4 package. We used the number of food types (except flowers, vertebrates, and others) in a fecal sample as the response variable (integer variable of 1–6). The full model also contained a fixed effect of season and a random effect of date of group follow. We evaluated differences across seasons using the grouping model comparison, followed by model selection and diagnostics similar to those described in the foregoing.
Habitat Use Seasonality
To examine the influence of fruit availability and other environmental factors on the capture rates, we constructed a GLMM with a Poisson error distribution and a log link function, using the glmer function. We created a data set by counting the number of independent group visits for each transect and season. The full model contained a response variable of the number of group visits (integer variable) corrected by an offset of log(camera days), fixed effects of mean number of the “important” fruit clusters (continuous variable), season, the interaction between mean number of the “important” fruit clusters and season, and three habitat parameters [steepness (continuous variable), seasonally inundated habitat and disturbed habitat (binary variables of Yes or No)], and a random effect of transect (random intercept). We standardized all the continuous variables. We included an interaction between the number of fruits and season because the effect of fruit availability on habitat preference may differ between seasons. We generated 40 candidate models using all possible combinations of the fixed effects and conducted model selection and diagnostics as for the analysis of diet seasonality.
To test for seasonality in ranging patterns, we calculated variances in capture rates across transects for each season and compared them among the four seasons using a Levene’s test (Levene 1960). If the result of the Levene’s test was significant, we then tested the differences in variance for all pairwise comparisons using F tests with a Holm’s P-value adjustment. Large variances of capture rates indicated seasonally intensive use of particular transects, whereas small variances meant an even distribution of habitat use.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Note
This study complied with the IPS Code of Best Practices for Field Primatology and the laws of the Gabonese Republic and was conducted with approval from the Centre National de la Recherche Scientifique et Technologique (N° AR0031/11/MENESRSIC/CENAREST/CG/CST/CSAR) and the Agence Nationale des Parcs Nationaux (N° 000017/PR/ANPN/SE/CS/AEPN, N° 000022/PR/ANPN/SE/CS/AEPN). The authors declare that they have no conflict of interest.
Results
Fruiting Phenology
We conducted the monthly fallen fruit census in 240 transect months from January 2012 to November 2013. We could not census in the other 13 transect months (Fig. 2), because it was impossible to access to the transects owing to logistic problems. This lack of data may mean that we underestimate variance in the number of fruit clusters across transects, and underestimate the mean for February 2013. The number of fruit clusters of all species showed a seasonal pattern: numbers were largest in December–February, decreased substantially in March–May, reached their lowest numbers in June–August, and increased again in September–November (Table I, and Fig. 2). The differences among the four seasons were all statistically significant (Table I).
Seasonality in the number of fallen fruit clusters on transects in Moukalaba–Doudou National Park, Gabon (January 2012–November 2013). Circles show monthly means, horizontal lines in boxes show monthly medians, boxes show interquartile ranges, and whiskers show ranges. Characters below boxes indicate transect IDs where the census was not conducted (see also Fig. 1). White and shaded areas indicate the dry and rainy seasons, respectively.
Seasonality in Diet
We sought mandrill groups on 432 days and located them on 49 days, during which we followed groups for a mean of 4.1 h per day (range: 0.2–10.1 h) and collected a mean of 12.3 feces (range: 2–52). We analyzed 417 fecal samples and distinguished 54 fruit items: we identified 31 items to species and 12 items to genus (Table I); the remaining 11 items were unclassified. We classified 22 fruit items (17 species and 5 genera) as “important” fruits for mandrill groups (Table II).
Fruit fiber and crushed seeds both occurred in a large proportion of the fecal remains, but their seasonal patterns differed. Fruit fiber occurrence was high during the fruit-peak and fruit-increase seasons, and decreased substantially in the late fruit-scarce season (Fig. 3). The results of LMMs and AIC model selection identified two confident models which showed that relative volumes in the fruit-peak and fruit-increase seasons (mean relative volume = 45.0%) were much larger than in the early and late fruit-scarce seasons (9.3%) (Table III (i), Fig. 4a). In contrast, the occurrence of crushed seeds was high from the fruit-peak to the late fruit-scarce season (Fig. 3). Four confident models (Table III (ii)) showed that relative volumes were largest in the fruit-peak and late fruit-scarce seasons (mean relative volume = 42.6%), smallest in the fruit-increase season (9.3%), and intermediate in early the fruit-scarce season (22.8%) (Fig. 4b). Most of the seeds found in feces of fruit-scarce seasons were finely crushed and unidentifiable, but we identified nuts of Coula edulis and seeds of Sacoglottis gabonensis as “important” foods in fruit-scarce seasons, when these species do not produce many fruits (Table II).
Seasonality in relative volumes of fruit fiber, crushed seeds, dicotyledonous leaves, monocotyledonous herbs, woody tissue, and invertebrates in mandrill fecal samples from Moukalaba–Doudou National Park, Gabon (August 2009–September 2013). Areas of gray circles are proportional to the number of fecal samples. Bold horizontal lines and fine horizontal lines show estimates of the smallest-AIC models and those of the other confident models, respectively.
Nonfruit vegetable foods (dicotyledonous leaves, monocotyledonous herbs, and woody tissue) in feces showed different seasonal patterns. Relative volumes of dicotyledonous leaves showed no seasonal pattern (mean relative volume = 15.6%) (Table III (iii), Fig. 4c). Mandrills fed on the pith of Aframomum spp. (Zingiberaceae), Marantochloa spp. (Marantaceae), and on Palisota hirsuta (Commelinaceae), and blades of forest grasses (Poaceae) in various seasons. Relative volumes of monocotyledonous herbs were larger in the early fruit-scarce and fruit-increase seasons (mean relative volume = 20.0%) than in the fruit-peak and late fruit-scarce seasons (8.4%) (Table III (iv), Fig. 4d). Conversely, woody tissue showed a clear seasonal pattern. Proportions of occurrence in the early and late fruit-scarce seasons were twice as high as those in the other seasons (Fig. 3), and relative volumes in the early and late fruit-scarce seasons (mean relative volume = 17.5%) were much larger than those in the fruit-peak and fruit-increase seasons (4.4%) (Table III (v), Fig. 4e).
Invertebrates, mainly ants (Formicidae) and grasshoppers (Acrididae), occurred frequently in feces (Fig. 3) but at consistently low relative volumes (Fig. 4f). The relative volumes were stable from early fruit-scarce to fruit-increase seasons (mean relative volume = 12.5%), and decreased in the fruit-peak seasons (7.5%) (Table III (vi)).
The number of food types in feces varied seasonally. Numbers were smallest in the fruit-peak season (mean number of food types = 3.5), largest in the early fruit-scarce season (4.5), and intermediate in the late fruit-scarce and fruit-increase seasons (4.0) (Table III (vii)).
Seasonality in Habitat Use
We calculated capture rates in 87 transect seasons from January 2012 to November 2013 (total camera days = 54,541). We identified 155 independent group visits on 169 days (overall capture rate = 0.0028 visit/camera day). Groups visited two different transects on 14 days but never three or more transects on any given day. Given that the estimated mean ranging speed of groups in the study area is 0.9 km/h (Hongo 2016), all but one case of these visits to two different transects were too distant for a group to arrive within the time intervals of the visits, suggesting that they were unlikely to be two consecutive visits by the same group.
We identified seven confident models showing that the mean number of “important” fruit clusters affected the capture rates positively, and that habitat disturbance had a negative effect on capture rates (Table IV). Four of these models also included the interaction term between important fruits and season. According to the second smallest-AIC model, which had the highest model selection frequency, the mean number of important fruit clusters correlated positively with capture rates in the fruit-peak and fruit-increase seasons, but correlations in the early and late fruit-scarce seasons were not reliable, as the standard errors of the interaction term were large (Fig. 5, Table IV). Although some models included other habitat parameters, the effects were unclear because their standard errors were large (Table IV).
The influence of the number of “important” fruit clusters and season on camera-trap capture rates of mandrill groups in Moukalaba–Doudou National Park, Gabon (January 2012–November 2013). Data points represent values for individual transect seasons. Solid black line shows the regression curve of the smallest-AIC model, whereas dashed lines show those of the second smallest-AIC model, which had the highest model selection frequency.
Variance in capture rates was significantly different among seasons (Levene’s test: F = 13.9, P = 2.1 × 10−7). Post hoc pairwise comparisons showed that the variance in the fruit-peak season was significantly higher than in the other seasons (Table V). In fruit-peak seasons, cameras in more than half of the transects recorded no mandrill groups, and capture rates of transects DB and DT were particularly high (Fig. 6a, e). In contrast, in most of the other seasons, cameras in most transects recorded groups at relatively lower rates (Fig. 6).
Discussion
We found seasonal changes in diet and habitat use patterns in wild mandrill groups. In fruit-rich seasons, mandrill feces contained a high proportion of fruit fiber, and groups visited transects where fruit production was high intensively. In contrast, in fruit-scarce seasons, crushed seeds made up a large volume of the fecal samples, the proportion of woody tissue and the number of food types increased. Groups also visited larger numbers of transects, and their visit frequencies were distributed more uniformly in fruit-scarce seasons.
The relative volume of fruit fiber in feces varied with fruit availability, and the number of food types was smallest in the fruit-peak season, although feces contained multiple food types year-round. These results suggest that mandrills at Moukalaba–Doudou are omnivorous year-round but become more frugivorous when fruits are available. The relative volume of crushed seeds was high even in fruit-scarce seasons, and seeds of several species that do not produce many fruits in these seasons appeared in feces frequently, suggesting that mandrills in the study area respond to seasonal fruit scarcity by foraging on buried seeds. These dietary patterns are in common with those of mandrills at other sites (Rogers et al. 1996; Tutin et al. 1997; White 2007) and drills in lowland forests (Astaras and Waltert 2010). Increased dietary diversity during food scarcity is also a common strategy for other African primates (Hemingway and Bynum 2005).
The relative volume of woody tissue increased in fruit-scarce seasons, but that of monocotyledonous herbs did not show a seasonal pattern according to fruiting phenology. These results differ from previous findings, in which mandrills increase their herb consumption when fruits are scarce (Hoshino 1985; Tutin et al. 1997), and may imply intraspecies variation in feeding strategy, as observed in drills on Bioko Island (Owens et al. 2015). Herbs and woody tissue are both low-quality foods, which are common alternative foods for large-sized monkeys (Hemingway and Bynum 2005). Mandrills have powerful fore limb flexion (Fleagle and McGraw 2002), which may enable them to excavate roots and subterranean stems buried in leaf litter.
Camera-trap capture rates of mandrill groups showed positive correlations with availability of “important” fruits in fruit-rich seasons. Moreover, group visits in fruit-peak seasons were highly biased toward two or three transects, whereas those in the other seasons were more uniformly distributed across most of the transects. These results suggest seasonal changes in mandrill habitat use. In fruit-rich seasons, mandrill groups in our study area may show a high selectivity for habitats where fruits are highly available, to forage on fresh fruits. In contrast, during fruit-scarce seasons, when mandrills consume more uniformly distributed seeds and woody tissue, they may become less selective in fruit availability and range over a much wider area. Our findings on seasonal habitat use differ from those for a mandrill group at Lopé, which used gallery forests intensively regardless of season (White 2007). This difference may reflect differences in habitat. At Lopé, human-introduced Elaeis guineensis trees are abundant in gallery forests (Ukizintambara et al. 2007). Their fruits are available year-round, and mandrills consume them frequently (White 2007). This all-year-round available food may retain the group in gallery forests. At Moukalaba–Doudou, no fruit species was available year-round, and therefore mandrills may need change both diets and ranging patterns seasonally.
Habitat disturbance affected the capture rates negatively, suggesting that mandrill groups avoid disturbed forests. Mandrills are large-sized, frugivorous primates and this result is consistent with a general pattern in which the negative effect of disturbance on habitat suitability increases with body weight and degree of frugivory (Johns and Skorupa 1987). The effects of seasonal inundation and steepness on the capture rates were unclear. These habitat parameters may not affect mandrill habitat use: in central Gabon, mandrills are observed in forests close to streams frequently (Lahm 1986), and drills range in montane forests with steep altitudinal gradients (Owens et al. 2015; Wild et al. 2005).
There is so far no clear evidence for seasonal range expansion in African primates, but this is observed frequently in New World primates, particularly species with large home ranges (Hemingway and Bynum 2005; Terborgh 1983). The unusually large range of mandrills and heterogeneous vegetation distribution in the study area may allow them to show this flexible ranging behavior. Moreover, our findings highlight intergeneric differences between Mandrillus and Cercocebus in adaptations to food seasonality. Although these genera share morphology adapted to hard-object eating and terrestrial foraging (Fleagle and McGraw 2002), Cercocebus species do not exhibit clear dietary seasonality (McGraw et al. 2014; Mitani 1989). This gap may come from considerable difference in group size between the genera (Swedell 2011). Seasonal change in diet and seasonal range expansion may be both important for Mandrillus species to maintain their large groups year-round, whereas changing habitat may be sufficient for Cercocebus species of small group size to keep their diets stable year-round. To clarify the effect of group size on behavioral flexibility in African primates, future studies should compare both dietary and ranging responses to food seasonality between closely related sympatric species with different group sizes, as conducted in New World forests (Peres 1994). Mandrills and red-capped mangabeys may be good candidates for the comparison of this kind.
Our study has two limitations. First, we conducted the fruit census for 2 years, which covered only part of the periods when we collected fecal samples. Although fruiting phenology showed a regular pattern, it may vary between years. We need longer-term studies to confirm our findings. Second, we investigated seasonality in habitat use of mandrills at a population level, but did not examine seasonality in group ranging behaviors per se. Field studies of the ranging patterns of identified groups are needed to understand the ranging seasonality of wild mandrills at a finer level.
Conclusion and Perspectives
Mandrills in the study area changed their diets from highly frugivorous to more omnivorous when fruit availability decreased. Groups also reduced their habitat selectivity and used different habitats more evenly when fruit availability decreased. These flexible feeding behaviors may allow mandrills to maintain their extremely large groups. Using different habitats evenly in fruit-scarce seasons may also benefit mandrill reproduction. The mandrill mating season coincides with the fruit-scarce season (Hongo et al. 2016), and large groups that travel widely and contain many sexually receptive females may favor influxes by many solitary males. Receptive females may be able to mate with multiple males, including subordinate males (Setchell et al. 2005), and choose among males (Setchell 2005). Future studies should examine the relationships between the ranging patterns of groups and solitary males.
References
Abernethy, K. A., White, L. J. T., & Wickings, E. J. (2002). Hordes of mandrills (Mandrillus sphinx): Extreme group size and seasonal male presence. Journal of Zoology, 258(1), 131–137. https://doi.org/10.1017/S0952836902001267.
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6), 716–723. https://doi.org/10.1109/TAC.1974.1100705.
Astaras, C., & Waltert, M. (2010). What does seed handling by the drill tell us about the ecological services of terrestrial cercopithecines in African forests? Animal Conservation, 13(6), 568–578. https://doi.org/10.1111/j.1469-1795.2010.00378.x.
Bates, D., Mächler, M., Bolker, B. M., & Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48.
Brockmeyer, T., Kappeler, P. M., Willaume, E., Benoit, L., Mboumba, S., & Charpentier, M. J. (2015). Social organization and space use of a wild mandrill (Mandrillus sphinx) group. American Journal of Primatology, 77(10), 1036–1048. https://doi.org/10.1002/ajp.22439.
Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information-theoretic approach. New York: Springer Science+Business Media.
R Core Team (2017). R: A language and environment for statistical computing. In R foundation for statistical computing. Vienna: Austria. URL https://www.R-project.org/.
Di Bitetti, M. S. (2001). Home-range use by the tufted capuchin monkey (Cebus apella nigritus) in a subtropical rainforest of Argentina. Journal of Zoology, 253(1), 33–45. https://doi.org/10.1017/S0952836901000048.
Etiendem, D. N., Funwi-Gabga, N., Tagg, N., Hens, L., & Indah, E. K. (2013). The Cross River gorillas (Gorilla gorilla diehli) at Mawambi Hills, south-west Cameroon: Habitat suitability and vulnerability to anthropogenic disturbance. Folia Primatologica, 84(1), 18–31. https://doi.org/10.1159/000345853.
Fleagle, J. G. (2013). Primate adaptation and evolution (3rd ed.). San Diego: Academic Press.
Fleagle, J. G., & McGraw, W. S. (2002). Skeletal and dental morphology of African papionins: Unmasking a cryptic clade. Journal of Human Evolution, 42(3), 267–292. https://doi.org/10.1006/jhev.2001.0526.
Furuichi, T., Hashimoto, C., & Tashiro, Y. (2001). Fruit availability and habitat use by chimpanzees in the Kalinzu Forest, Uganda: Examination of fallback foods. International Journal of Primatology, 22(6), 929–945. https://doi.org/10.1023/A:1012009520350.
Gogarten, J. F., Bonnell, T. R., Brown, L. M., Campenni, M., Wasserman, M. D., & Chapman, C. A. (2014). Increasing group size alters behavior of a folivorous primate. International Journal of Primatology, 35(2), 590–608. https://doi.org/10.1007/s10764-014-9770-8.
Hanya, G., Tsuji, Y., & Grueter, C. C. (2013). Fruiting and flushing phenology in Asian tropical and temperate forests: Implications for primate ecology. Primates, 54(2), 101–110. https://doi.org/10.1007/s10329-012-0341-3.
Harris, T. R., Chapman, C. A., & Monfort, S. L. (2010). Small folivorous primate groups exhibit behavioral and physiological effects of food scarcity. Behavioral Ecology, 21(1), 46–56. https://doi.org/10.1093/beheco/arp150.
Hemingway, C. A., & Bynum, N. (2005). The influence of seasonality on primate diet and ranging. In D. K. Brockman & C. P. van Schaik (Eds.), Seasonality in primates: Studies of living and extinct human and non-human primates (pp. 57–104). New York: Cambridge University Press. https://doi.org/10.1017/CBO9780511542343.004.
Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6(2), 65–70.
Hongo, S. (2014). New evidence from observations of progressions of mandrills (Mandrillus sphinx): A multilevel or non-nested society? Primates, 55(4), 473–481. https://doi.org/10.1007/s10329-014-0438-y.
Hongo, S. (2016). Socioecology of mandrills (Mandrillus sphinx): Mating and feeding tactics in a primate with extremely large group. Ph. D. dissertation, Kyoto University.
Hongo, S., Nakashima, Y., Akomo-Okoue, E. F., & Mindonga-Nguelet, F. L. (2016). Female reproductive seasonality and male influxes in wild mandrills (Mandrillus sphinx). International Journal of Primatology, 37(3), 416–437. https://doi.org/10.1007/s10764-016-9909-x.
Hoshino, J. (1985). Feeding ecology of mandrills (Mandrillus sphinx) in campo animal reserve, Cameroon. Primates, 26(3), 248–273. https://doi.org/10.1007/BF02382401.
Janson, C. H. (1988). Food competition in brown capuchin monkeys (Cebus apella): Quantitative effects of group size and tree productivity. Behaviour, 105(1), 53–76. https://doi.org/10.1163/156853988X00449.
Johns, A. D., & Skorupa, J. P. (1987). Responses of rain-forest primates to habitat disturbance: A review. International Journal of Primatology, 8(2), 157–191. https://doi.org/10.1007/BF02735162.
Kaplin, B. A., Munyaligoga, V., & Moermond, T. C. (1998). The influence of temporal changes in fruit availability on diet composition and seed handling in blue monkeys (Cercopithecus mitis doggetti). Biotropica, 30(1), 56–71. https://doi.org/10.1111/j.1744-7429.1998.tb00369.x.
Lahm, S. A. (1986). Diet and habitat preference of Mandrillus sphinx in Gabon: Implications of foraging strategy. American Journal of Primatology, 11(1), 9–26. https://doi.org/10.1002/ajp.1350110103.
Levene, H. (1960). Robust tests for equality of variances. In I. Olkin, S. G. Ghurye, W. Hoeffding, W. G. Madow, & H. B. Mann (Eds.), Contributions to probability and statistics: Essays in honor of Harold Hotelling (pp. 278–292). Stanford, CA: Stanford University Press.
Litvaitis, J. A. (2000). Investigating food habits of terrestrial vertebrates. In M. C. Pearl (Ed.), Research techniques in animal ecology (pp. 165–190). New York: Columbia University Press.
McGraw, W. S., Vick, A. E., & Daegling, D. J. (2014). Dietary variation and food hardness in sooty mangabeys (Cercocebus atys): Implications for fallback foods and dental adaptation. American Journal of Physical Anthropology, 154(3), 413–423. https://doi.org/10.1002/ajpa.22525.
McGrew, W. C., Marchant, L. F., & Phillips, C. A. (2009). Standardised protocol for primate faecal analysis. Primates, 50(4), 363–366. https://doi.org/10.1007/s10329-009-0148-z.
Mitani, M. (1989). Cercocebus torquatus: Adaptive feeding and ranging behaviors related to seasonal fluctuations of food resources in the tropical rain forest of south-western Cameroon. Primates, 30(3), 307–323. https://doi.org/10.1007/BF02381257.
Mori, Y., Nagamitsu, T., & Kubo, T. (2009). Clonal growth and its effects on male and female reproductive success in Prunus ssiori (Rosaceae). Population Ecology, 51(1), 175–186. https://doi.org/10.1007/s10144-008-0099-z.
Nakagawa, N. (1989). Bioenergetics of Japanese monkeys (Macaca fuscata) on Kinkazan Island during winter. Primates, 30(4), 441–460. https://doi.org/10.1007/BF02380873.
Nakashima, Y. (2015). Inventorying medium- and large-sized mammals in the African lowland rainforest using camera trapping. Tropics, 23(4), 151–164. https://doi.org/10.3759/tropics.23.151.
Newbery, D. M., Songwe, N. C., & Chuyong, G. B. (1998). Phenology and dynamics of an African rainforest at Korup, Cameroon. In D. M. Newbery, H. H. T. Prins, & N. D. Brown (Eds.), Dynamics of tropical communities: 37th symposium of the British Ecological Society (pp. 267–308). Oxford: Blackwell Science.
Nsi Akoue, G., Mbading-Mbading, W., Willaume, E., Souza, A., Mbatchi, B., & Charpentier, M. J. E. (2017). Seasonal and individual predictors of diet in a free-ranging population of mandrills. Ethology, 123(9), 600–613. https://doi.org/10.1111/eth.12633.
O’Brien, T. G., Kinnaird, M. F., & Wibisono, H. T. (2003). Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Animal Conservation, 6(2), 131–139. https://doi.org/10.1017/S1367943003003172.
Owens, J. R., Honarvar, S., Nessel, M., & Hearn, G. W. (2015). From frugivore to folivore: Altitudinal variations in the diet and feeding ecology of the Bioko Island drill (Mandrillus leucophaeus poensis). American Journal of Primatology, 77(12), 1263–1275. https://doi.org/10.1002/ajp.22479.
Peres, C. A. (1994). Primate responses to phenological changes in an Amazonian terra firme forest. Biotropica, 26(1), 98–112. https://doi.org/10.2307/2389114.
Rogers, M. E., Abernethy, K. A., Fontaine, B., Wickings, E. J., White, L. J. T., & Tutin, C. E. G. (1996). Ten days in the life of a mandrill horde in the Lopé reserve, Gabon. American Journal of Primatology, 40(4), 297–313. https://doi.org/10.1002/(SICI)1098-2345(1996)40:4<297::AID-AJP1>3.0.CO;2-T.
Sato, H. (2013). Habitat shifting by the common brown lemur (Eulemur fulvus fulvus): A response to food scarcity. Primates, 54(3), 229–235. https://doi.org/10.1007/s10329-013-0353-7.
van Schaik, C. P., & Pfannes, K. R. (2005). Tropical climates and phenology: A primate perspective. In D. K. Brockman & C. P. van Schaik (Eds.), Seasonality in primates: Studies of living and extinct human and non-human primates (pp. 23–54). New York: Cambridge University Press. https://doi.org/10.1017/CBO9780511542343.003.
Setchell, J. M. (2005). Do female mandrills prefer brightly colored males? International Journal of Primatology, 26(4), 715–735. https://doi.org/10.1007/s10764-005-5305-7.
Setchell, J. M., Charpentier, M., & Wickings, E. J. (2005). Mate guarding and paternity in mandrills: Factors influencing alpha male monopoly. Animal Behaviour, 70(5), 1105–1120. https://doi.org/10.1016/j.anbehav.2005.02.021.
Shimodaira, H. (1998). An application of multiple comparison techniques to model selection. Annals of the Institute of Statistical Mathematics, 50(1), 1–13. https://doi.org/10.1023/A:1003483128844.
Swedell, L. (2011). African papionins: Diversity of social organization and ecological flexibility. In C. J. Campbell, A. Fuentes, K. C. MacKinnon, S. K. Bearder, & R. M. Stumpf (Eds.), Primates in perspective (2nd ed., pp. 241–277). New York: Oxford Unversity Press.
Takenoshita, Y., Ando, C., Iwata, Y., Okayasu, N., Tashiro, Y., et al (2007). Liste d’espèces de plantes vasculaire dans la partie nord du Parc National de la Moukalaba-Doudou, Gabon. In J. Projet en Primatologie de l’Université de Kyoto (Ed.), Rapport de recherches effectuées au Parc National de Moukalaba–Doudou, Gabon (pp. 41–50). Kyoto, Japan: Mars 2006–Février 2007.
Takenoshita, Y., Ando, C., Iwata, Y., & Yamagiwa, J. (2008). Fruit phenology of the great ape habitat in the Moukalaba–Doudou National Park, Gabon. African Study Monographs, Supplement, 39, 23–39.
Tang, C., Huang, L., Huang, Z., Krzton, A., Lu, C., & Zhou, Q. (2016). Forest seasonality shapes diet of limestone-living rhesus macaques at Nonggang, China. Primates, 57(1), 83–92. https://doi.org/10.1007/s10329-015-0498-7.
Terada, S., Nackoney, J., Sakamaki, T., Mulavwa, M. N., Yumoto, T., & Furuichi, T. (2015). Habitat use of bonobos (Pan paniscus) at Wamba: Selection of vegetation types for ranging, feeding, and night-sleeping. American Journal of Primatology, 77(6), 701–713. https://doi.org/10.1002/ajp.22392.
Terborgh, J. (1983). Five new world primates: A study in comparative ecology. Princeton, NJ: Princeton University Press.
Tsuji, Y., Hanya, G., & Grueter, C. C. (2013). Feeding strategies of primates in temperate and alpine forests: Comparison of Asian macaques and colobines. Primates, 54(3), 201–215. https://doi.org/10.1007/s10329-013-0359-1.
Tutin, C. E. G., Ham, R. M., White, L. J. T., & Harrison, M. J. S. (1997). The primate community of the Lopé reserve, Gabon: Diets, responses to fruit scarcity, and effects on biomass. American Journal of Primatology, 42(1), 1–24. https://doi.org/10.1002/(SICI)1098-2345(1997)42:1<1::AID-AJP1>3.0.CO;2-0.
Ukizintambara, T., White, L., Abernethy, K., & Thébaud, C. (2007). Gallery forests versus bosquets: Conservation of natural fragments at Lopé National Park in central Gabon. African Journal of Ecology, 45(4), 476–482. https://doi.org/10.1111/j.1365-2028.2007.00757.x.
Warton, D. I., & Hui, F. K. C. (2011). The arcsine is asinine: The analysis of proportions in ecology. Ecology, 92(1), 3–10. https://doi.org/10.1890/10-0340.1.
White, L. J. T. (1994). Patterns of fruit-fall phenology in the Lopé reserve, Gabon. Journal of Tropical Ecology, 10(3), 289–312. https://doi.org/10.1017/S0266467400007975.
White, E. C. (2007). Ecology of Mandrillus sphinx: Ranging, diet and social structure of a mandrill horde in Lopé National Park, Gabon. Ph. D. dissertation, University of Exeter.
White, E. C., Dikangadissi, J.-T., Dimoto, E., Karesh, W. B., Kock, M. D., Ona Abiaga, N., Starkey, R., Ukizintambara, T., White, L. J. T., & Abernethy, K. A. (2010). Home-range use by a large horde of wild Mandrillus sphinx. International Journal of Primatology, 31(4), 627–645. https://doi.org/10.1007/s10764-010-9417-3.
Wild, C., Morgan, B. J., & Dixson, A. (2005). Conservation of drill populations in Bakossiland, Cameroon: Historical trends and current status. International Journal of Primatology, 26(4), 759–773. https://doi.org/10.1007/s10764-005-5307-5.
Yamagiwa, J., Mwanza, N., Yumoto, T., & Maruhashi, T. (1994). Seasonal change in the composition of the diet of eastern lowland gorillas. Primates, 35(1), 1–14. https://doi.org/10.1007/BF02381481.
Yumoto, T., Terakawa, M., Terada, S., Boupoya, A., & Nzabi, T. (2015). Species composition of a middle altitude forest in Moukalaba–Doudou National Park, Gabon. Tropics, 23(4), 205–213. https://doi.org/10.3759/tropics.23.205.
Acknowledgments
We are grateful to CENAREST and ANPN for permission to conduct the study. PROCOBHA members including Yuji Takenoshita, Shiho Fujita, Pierre Philippe Mbehang-Nguema, Keiko Tsubokawa, and Saeko Terada helped us in the field. Field assistants including Biviga Steven and Nzamba Victor supported our fieldwork. Hiroshi Himori, Hikari Ishijima, Aya Kokubu, and Takahiro Yamagishi assisted us in the video analysis. We appreciate Naofumi Nakagawa, Juichi Yamagiwa, Michio Nakamura, Hiroshi Ihobe, Eiji Inoue, Joanna M. Setchell, and two anonymous reviewers for constructive comments on earlier versions of the manuscript. Marina Cords and two anonymous reviewers also provided comments to the manuscript submitted elsewhere. We thank Editage for the English language review. This study was funded by Japan Society for the Promotion of Science (JSPS) KAKENHI (19107007 for Juichi Yamagiwa and 12 J01884 for Shun Hongo), Kyoto University Global COE Program (A06), and Japan Science and Technology Agency/Japan International Cooperation Agency, Science and Technology Research Partnership for Sustainable Development (JST/JICA-SATREPS). Authors’ contributions: S. Hongo, Y. Nakashima, E. F. Akomo-Okoue, and F. L. Mindonga-Nguelet conducted fieldwork; S. Hongo and Y. Nakashima performed the analysis; S. Hongo wrote the first draft of the paper; and Y. Nakashima, E. F. Akomo-Okoue, and F. L. Mindonga-Nguelet improved the manuscript substantially.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Joanna M. Setchell
Rights and permissions
About this article
Cite this article
Hongo, S., Nakashima, Y., Akomo-Okoue, E.F. et al. Seasonal Change in Diet and Habitat Use in Wild Mandrills (Mandrillus sphinx). Int J Primatol 39, 27–48 (2018). https://doi.org/10.1007/s10764-017-0007-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-017-0007-5