Abstract
Limestone forests are an unusual habitat for primates, but little information is available for the genus Macaca in such habitats, making a comparative understanding of extant limestone primates’ behavioral adaptation incomplete. We collected data on the diet of rhesus macaques (Macaca mulatta) in a limestone habitat at Nonggang Nature Reserve, southwestern Guangxi, China, and examined the effects of forest seasonality on their diet. Our results indicated that a total of 114 species of plants are consumed by macaques. Young leaves are a preferred food, accounting for 48.9 and 56.9 % of the overall diets. One group significantly increased young leaf consumption in response to availability. Fruits contributed to only 27.3 and 28.7 % of overall diet. The macaque diet varied according to season. They fed on more fruits in the rainy season. Consumption of mature leaves increased when the availability of young leaves and fruits declined in the dry season, indicating that mature leaves are a fallback food for macaques in a limestone habitat. Similar to sympatric Assamese macaques, Bonia saxatilis, a shrubby, karst-endemic bamboo was consumed by rhesus macaques throughout the year, and was the top food species through most of the year, suggesting that bamboo consumption represents a key factor in the macaque’s dietary adaptation to limestone habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primates exhibit highly flexible diets in response to spatial and temporal variation of food resources (Yeager and Kool 2000; Hemingway and Bynum 2005; Tsuji et al. 2013). Primates living in temperate or high altitude forests generally consume more fiber-rich foods (e.g. leaves and bark), and fewer fruits than primates in tropical forests (Fan et al. 2009; Tsuji et al. 2013). Even populations within a single species show considerable variation in diet composition (Chapman et al. 2002; Hanya et al. 2003; Chaves and Bicca-Marques 2013). For example, Hanya et al. (2003) compared the diets of Japanese macaques (Macaca fuscata) living at different altitudes and found that more seeds and fruits were consumed in the lower zones, whereas fiber and fungi were eaten more often in the higher zones. This intra-specific variation in diet often reflects differences in fruit availability due to, for example, a lower diversity of fruiting plant species and shorter length of the fruiting season in temperate forests relative to tropical forests (Ting et al. 2008; Hanya et al. 2003).
In addition, primates can adopt different foraging strategies in response to seasonal changes in food availability (Hemingway and Bynum 2005; Tsuji et al. 2013). Some primates increase foraging efforts during lean times by moving farther and covering larger seasonal home ranges to search for scarce but high-quality foods such as fruits and seeds (e.g. Hylobates lar, Bartlett 1999; Macaca leonine, Albert et al. 2003). In contrast, most other species rely on lower quality fallback foods, such as mature leaves, until conditions improve (e.g. Macaca fuscata, Hanya et al. 2003; Nomascus concolor, Fan et al. 2009; Rhinopithecus roxellana, Guo et al. 2007; Trachypithecus francoisi Zhou et al. 2009a). Additionally, some species split into smaller subgroups in response to food scarcity (e.g. Ateles geoffroyi, Chapman et al. 1995). Different foraging strategies are attributable to differences in both socioecological and digestive characteristics of the species in question (Hemingway and Bynum 2005; Tsuji et al. 2013).
Limestone forests are an unusual habitat for primates. Features that differentiate them from mountain forests include steep cliffs, which represent approximately 10–20 % of total habitat area, and a shortage of surface water (Huang 2002). The vegetation is poor due to shallow soils that characterize limestone (Xu 1993). Thus, limestone-living primates may have evolved specific behavioral strategies to adapt to this unusual habitat. For example, limestone-living langurs (e.g. Trachypithecus delacouri, T. leucocephalus) are the most folivorous of the Asian langurs (Li and Rogers 2006; Workman 2010). This may be related to relatively low fruit availability; fruit abundance markedly decreases in the dry season (Huang et al. 2015; Li and Rogers 2006; Zhou et al. 2009a). Limestone-living primates have been found to consume more leaves in response to seasonal scarcity of fruits (Nomascus nasutus, Fan et al. 2009; Macaca assamensis, Huang et al. 2015; Trachypithecus francoisi, Zhou et al. 2009a).
The rhesus macaque (Macaca mulatta) has the broadest geographic distribution of any primate and inhabits a diverse range of tropical and temperate forests (Fooden 2000). Their diet varies strongly depending on environmental conditions; rhesus macaques in temperate forests of northwestern Pakistan are mostly folivorous (Goldstein and Richard 1989), whereas those living in tropical forests in northern India are frugivorous (Lindburg 1976). But very little quantitative information is available concerning the diet of limestone-living rhesus macaques, except for a preliminary study in a small park located in northern Guangxi (Zhou et al. 2009b). They found that leaves constituted 41 % of the macaques’ diet, whereas fruits and seeds accounted for only 7.6 %. Because of serious human disturbance, however, their results do not allow for a full understanding of dietary adaptations to limestone habitats. Thus, systematic studies on the feeding ecology of rhesus macaques in a natural limestone habitat will be important in understanding macaque behavioral adaptation. We also provide important comparative information for the general study of dietary adaptation in macaque species.
This study is part of a larger project comparing sympatric Assamese macaques and rhesus macaques living in the limestone forests of Nonggang Nature Reserve. In this paper, we present quantitative data on the diet and food choice of rhesus macaques. The major objective is to characterize the diet of limestone-living rhesus macaques, specifically how they allocate their feeding time between various food items throughout the seasons in relation to plant phenology. We offer an assessment of rhesus macaque feeding strategy in limestone habitats. We also put our results within a comparative context by offering a preliminary review of feeding strategies in limestone-living primates.
Methods
Study site and species
This study was conducted from August 2012 to July 2013 at Nonggang Nature Reserve (106°42′–107°4′E, 22°13′–22°33′N), southern Guangxi Province, China, which is located at the northern limit of the tropics (Huang et al. 2015). The reserve comprises three localities separated from each other by farmlands and villages: Nonggang (5426 ha), Longhu (1034 ha), and Longshan (3949 ha). The reserve consists of limestone hills with elevations ranging from 300 to 700 m above sea level (Guangxi Forestry Department 1993). The vegetation profile is limestone seasonal rain forest with significant changes at different levels due to differences of temperature, humidity and soil quality. The lower sections of hills, including the valley floor and hillsides, are wet and rich in soil. As a result they are covered by large trees rich with vines and epiphytes. Common tree species include Deutzianthus tonkinensis, Dracontomelon dao, Cinnamomum burmannii, Burretiodendron hsienmu, and Cephalomappa sinensis. Higher levels, including cliffs and hilltops, consist of bare rocks where water and soil are scarce. Drought-enduring trees, such as Sinosideroxylon pedunculatum, Tirpitzia ovoidea, Pittosporum puchrum, and Leptodermis affinis, grow in these locations (Shu et al. 1988). Climatic data, including rainfall and maximum and minimum temperatures, were collected daily from August 2012 to July 2013 (Fig. 1). Annual precipitation during the study period was 1055.8 mm, with a distinct rainy season and dry season. The rainy season lasted from April to September with greater than 80 mm of precipitation per month; during the subsequent dry season monthly precipitation declined to less than 80 mm. Monthly mean temperature ranged from a minimum of 6.1 °C in January 2013 to 31 °C in August 2012.
The main study area is located in the northwestern portion of Nonggang (Huang et al. 2015). We focused on 2 differently sized groups of rhesus macaques that ranged near our camp. The larger group consisted of 63 individuals (6 adult males, 14 adult females, 15 subadult individuals, 14 juveniles and 14 infants) throughout the study period. The smaller group contained 22 individuals initially (2 adult males, 7 adult females, 10 subadult individuals, 3 infants), and increased to 24 individuals after the birth of 2 infants on June 2013.
Data collection
We observed the behavior of the larger group for 82 full days (5–11 days per month), and of the smaller group for 91 full days (5–9 days per month), in addition to many other partial-day follows over the course of the study. For full-day follows, we began our observations when the monkeys left their sleeping sites and ended them when they reentered the sleeping sites at the end of the day. Partial-day follows began when we first encountered the focal group. The duration of contact in partial-day follows was variable, with an average of 3.9 h (range 1–9 h). During the study period, we collected a total of 720 h of behavioral data for the larger group, and 838 h for the smaller group.
We collected behavioral data using scan sampling with 15-min intervals. Scans lasted 5 min, followed by 10 min of non-sampling until the next scan began. We recorded the activity of each individual seen during each scan. We watched each individual for 5 s after detection and recorded its predominant behavior during that interval. To avoid sampling bias toward certain individuals or a particular age-sex class, we collected behavioral records on as many different individuals as possible during a scan so that all individuals in the focal group were included without sampling any individual more than once. We divided behavior into six general categories: resting, moving, feeding, grooming, playing, and other. Feeding was defined as manually or orally handling a food item, and bringing it into the mouth. When the individual was feeding, we recorded plant species and parts eaten: young leaves, mature leaves, fruits, flowers, seeds, stems, and other items (including petioles and roots). When we could not discern the food items, we scored them as unknown. We marked all food species and collected specimens for later identification.
To evaluate the influence of forest phenology on macaques’ feeding behavior, we selected 39 plant species known from previous observation to be eaten by macaques for phenology monitoring (Tang 2008; Jiang 2009). We opportunistically selected and tagged 417 trees in the main study area. We inspected them for the presence of mature leaves, young leaves, fruits and flowers at the end of each month during the study period. We scored each tree in terms of the proportion of the crown comprising plant parts of interest on a five-point scale: 0 = absent, 1 = 0.1–25 %, 2 = 25.1–50 %, 3 = 50.1–75 %, 4: 75.1–100 %. Monthly availability indices of different plant phenophases were expressed as a percentage of the maximum possible phenology score of 1668, i.e., 4 for each of the 417 target trees.
Data analysis
We excluded records for dependent infants and juveniles from analysis because these animals were not foraging independently and infant and juvenile mouthing of prospective foods can be indistinguishable from actual feeding. We first calculated the percentage of scanned individuals devoted to feeding on each food item/species out of the total number of individuals recorded in each scan, then divided this value by the percentage of all individuals engaged in feeding activity to determine the time allocated to consuming each food item/species for that scan. Each scan budget was treated as an independent data point and used in subsequent analyses. This method can reduce potential biases introduced by the scan sampling technique (Hanya and Bernard 2012). To avoid potential biases introduced by unequally distributed samples in different months or at different times of the day, we first calculated hourly budgets by averaging scan budgets in an hour, and then averaged hourly budgets within each month to determine monthly percentages of feeding time devoted to each food item/species. Seasonal and annual dietary compositions were expressed as the average proportion across the relevant months. We defined main food species as those which contributed to >1 % of the total feeding records. Of these species, staple species was defined as plants eaten in large quantities in the rainy and dry seasons; seasonal species as plant eaten in large quantities in one season but not in another; and fallback species as plants eaten in large quantities in the dry season when preferred food was scarce. We used the Shannon–Weaver diversity index to examine inter-group and seasonal variation in dietary diversity. The index is calculated as follows: H = –ΣP i × ln P i , where P i is the proportion of feeding records of the ith plant species.
We used the Mann–Whitney U test to compare seasonal variations in diet composition. A Spearman rank correlation coefficient test was used to examine the relationship between food availability and rainfall, as well as between food availability and the consumption of different plant species and parts. All tests were two-tailed, with significance levels of P < 0.05.
Results
Forest phenology
The production of young leaves, fruits, and flowers varied considerably among months (Fig. 2). The production of young leaves and flowers peaked in March, and the majority of species produced new leaves and flowers in the rainy season or during the transition between the dry season and rainy season. Fruit production peaked in June, and its abundance was markedly higher in the rainy season than in the dry season. A significant and positive relationship was documented between the abundance of fruits and rainfall (r s = 0.692, n = 12, P = 0.013).
Overall diet
During the study period, we collected 8109 individual feeding records for the larger group, and 5355 for the smaller group, of which we identified the food species for 8009 records of the larger group, and for 5293 records of the smaller group. We identified 114 species of plants consumed by macaques belonging to 92 genera from 46 families. Of the 114 identified species, there were 59 species of trees (39.7 % of feeding records for species), 20 species of shrubs (6.2 %), 28 species of lianas (25.0 %), 6 species of herbs (28.9 %), and 1 species of epiphyte (0.3 %). It is worth noting that macaques fed on herbs almost exclusively from a shrubby bamboo, Bonia saxatilis (73.3 % of 3848 herb feeding records), and from cultivated sugarcane (Saccharum officinarum, 25.1 %).
In general, young leaves were by far the most important item in the diet. The larger group fed on young leaves of 78 species, while the smaller group fed on 58 species, comprising 48.9 and 56.9 % of the overall diets (Table 1). Although mature leaves accounted for only 7.6 % of the overall diet for the larger group and 5.6 % of that of the smaller group, they fed on the mature leaves of 53 (larger group) and 44 (smaller group) species. The larger group ate fruits from 43 species (27.3 % of overall diet) while the smaller group ate 39 species (28.7 %). Both groups consumed more immature fruits than mature fruits in terms of species consumed and percentage of total fruit feeding records (the larger group: 33 vs 30 and 63 vs 37 %; the smaller group: 36 vs 21 and 75 vs 25 %). Other items, including flowers, stems, petioles, and seeds, contributed to a small proportion of the overall diet (flowers: 1.8 % for the larger group and 5.1 % for the smaller group; stems: 8.7 and 0.4 %; petioles: 4.6 and 0.2 %; seeds: 0.8 and 3.2 %).
Seasonal variation in diet
Each month, the larger group consumed between 16 species (July) and 61 species (January), with a monthly average of 32.5 (SD 11.9) (Table 1). Monthly dietary diversity varied from 2.31 (July) to 2.86 (March), with an average of 2.65 (SD 0.18). The number of species eaten by the smaller group in each month varied from 7 (August) to 37 (December) (mean 25.5, SD 9.4). The mean monthly dietary diversity of the smaller group was 2.17 (SD 0.37), ranging from 1.16 (August) to 2.70 (November).
Among the main food species, 9 species (Bonia saxatilis, Pueraria montana, Embelia scandens, Ventilago inaequilateralis, Cissus subtetragona, Ficus cyrtophylla, Boniodendron minor, Vitex kwangsiensis, Ecdysanthera rosea, and Ficus microcarpa) were consumed for 9 or more months out of the year (Table 2). However, of the top 3 food species, only Bonia saxatilis and Pueraria montana were consumed in large quantities in both the dry and rainy seasons (Table 3). Thus, these species were the staple foods for the macaques. Of the seasonal species, sugarcane, Ficus nervosa, Ficus gibbosa, Polygonum chinense, Radermachera sinica, and Broussonetia papyrifera were the main fallback foods for the macaques in the dry season.
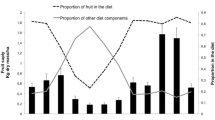
The relative representation of food items in the diets of the two groups varied widely among months (Table 1). Young leaves ranked first in the diet in most months (larger group: U = 11.000, n 1 = 6, n 2 = 6, P = 0.310; smaller group: U = 13.000, P = 0.485), except during August 2012 (smaller group), October 2012 (larger group), November 2012 (smaller group), July 2013 (both groups) when fruits dominated the diet. There was a significant and positive correlation between young leaf consumption and its abundance for the larger group (r s = 0.594, n = 12, P = 0.042), but not for the small group (r s = 0.147, P = 0.649). The two groups consumed more fruits in the rainy season than in the dry season (larger group: U = 4.000, P = 0.026; smaller group: U = 4.000, P = 0.026; Fig. 3). However, there was no significant correlation between fruit consumption and its abundance (larger group: r s = 0.329, P = 0.297; smaller group: r s = 0.378, P = 0.225). The two groups significantly increased their consumption of mature leaves in the dry season (larger group: U = 4.500, P = 0.026; smaller group: U = 0.000, P = 0.002; Fig. 3). Although flowers accounted for only a small proportion of the diet, flower consumption was correlated positively with abundance (larger group: r s = 0.707, P = 0.010; smaller group: r s = 0.832, P = 0.001). As a result of sugarcane-raiding in the dry season (Table 1), the larger group fed on more stems in the dry season than in the rainy season (U = 2.000, P = 0.009; Fig. 3).
The importance of Bonia saxatilis
Bonia saxatilis, the species the macaques consumed most frequently, contributed to 18.0 and 32.3 % of annual diet (Table 2), and their young leaves accounted for 36.8 and 56.8 % of young leaf consumption for the larger group and smaller group, respectively (Table 1). This bamboo was consumed each month, and was the top food species in 7 months for the larger group, and in 10 months for the smaller group (Table 3). There was no significant difference in the consumption of this item between the rainy and dry season for either group (larger group: U = 17.000, n 1 = 6, n 2 = 6, P = 0.937; smaller group: U = 11.000, P = 0.310). There was neither a significant correlation between the consumption of this item and abundance of young leaves (larger group: r s = 0.231, n = 12, P = 0.471; smaller group: r s = 0.210, P = 0.513) nor between the consumption of bamboo and abundance of fruits (larger group: r s = 0.154, P = 0.633; smaller group: r s = −0.007, P = 0.983).
Discussion
Dietary characteristics of limestone-living Rhesus macaques
In this study, the study groups consumed parts of 114 different species. Two other studies of rhesus macaques at Nonggang reported 71 and 93 plant species (Tang 2008; Jiang 2009). According to these studies, rhesus macaques at Nonggang fed on 174 species in total. Thus, rhesus macaques at Nonggang have a broader diet than that of congeners at other sites (92 species in the tropical forests of north India, Lindburg 1976; 35 species in the temperate forests of northwestern Pakistan, Goldstein and Richard 1989; 60 species in the limestone hills of northern Guangxi, Zhou et al. 2009a, b; 51 species in the semi-evergreen forests of Bangladesh, Feeroz 2012). However, this conclusion should be interpreted cautiously because of potential bias resulting from difference in the length of observation time.
In terms of foods selected, the diet of rhesus macaque in limestone forests at Nonggang falls within the range of that of other Asian macaques (see review in Tsuji et al. 2013). However, rhesus macaques at Nonggang were unusually folivorous. Leaves accounted for 56.5–62.5 % of the total diet, whereas fruits and flowers contributed 27.3–29.8 %. This pattern is in accordance with other research on limestone-living macaques. For example, the diet of rhesus macaques in the limestone hills of northern Guangxi province included more leaves (41 %) than fruits (6.2 %) (Zhou et al. 2009b). At our study site, sympatric Assamese macaques (Macaca assamensis) consumed even more leaves than the rhesus macaques, accounting for 77 % of the diet (Zhou et al. 2011; Huang et al. 2015). The proportion of leaves in the diets of limestone-living macaques is much higher than the mean value (25.1 %) for all Asian macaques, but is similar to or even higher than the mean value (48 %) for the Asian colobines, which are traditionally regarded as folivorous (Tsuji et al. 2013). The highly folivorous diet of limestone-living macaques may be partially explained by relatively low fruit availability as a result of low rainfall in limestone habitats (see review in Huang et al. 2015). Fan et al. (2009) documented that cao vit gibbons (Nomascus nasutus) inhabiting limestone forests are more folivorous and less frugivorous than other gibbon species living in tropical forests. In addition, the folivory of limestone-living macaques may be related to seasonal scarcity of fruits in limestone forests. Many studies have documented marked seasonal variation in fruit availability in response to rainfall, and fruit abundance decreased significantly in the dry season (this study; Li and Rogers 2006; Zhou et al. 2009a). Thus, seasonal fruit scarcity forces macaques to rely more on leaves. Similarly, Fan et al. (2009) reported that cao vit gibbons in limestone forests increased their consumption of young leaves during periods of fruit scarcity.
Dietary response to phenology change
Rhesus macaques at Nonggang increased their consumption of young leaves in response to increased availability, even though the correlation was only significant for the larger group. This is the same response to changes in leaf availability as that reported by sympatric François’ langurs (Trachypithecus francoisi) at Nonggang (Zhou et al. 2009a), as well as other limestone-living primates (e.g. Trachypitheus leucocephalus, Li and Rogers 2006; Li et al. 2015). Although we did not find a significant correlation between young leaf consumption and availability for sympatric Assamese macaques at Nonggang, young leaves formed a large proportion of their monthly diet (Zhou et al. 2011; Huang et al. 2015). Similar findings have been reported in limestone-living Delacour’s langurs (Trachypithcus delacouri) at Van Long Nature Reserve, Vietnam (Workman 2010). Thus, young leaves are a preferred food for limestone-living macaques and langurs. Although there was no significant correlation between fruit consumption and availability, rhesus macaques at Nonggang noticeably increased fruit consumption as it became more available in the rainy season. We also documented that sympatric Assamese macaques at Nonggang increased fruit consumption in response to increased fruit availability, even though fruits represented a small proportion (20.1 %) of the diet (Huang et al. 2015). Thus, similar to other macaques (Tsuji et al. 2013), fruits are still a preferred food for macaques in limestone habitats.
When preferred young leaves and fruits became scarce in the dry season, rhesus macaques at Nonggang significantly increased mature leaf consumption. A significant and negative correlation between mature leaf consumption and fruit availability (larger group: r s = −0.591, n = 12, P = 0.044; smaller group: r s = −0.609, P = 0.035) indicates that mature leaves are fallback foods for rhesus macaques at Nonggang (Marshall and Wrangham 2007). Similarly, rhesus macaques in the limestone hills of northern Guangxi consumed mature leaves in large quantities in winter (Zhou et al. 2009b). A similar foraging strategy has been reported for other Macaca species (e.g. M. cyclopis, Su and Lee 2001; M. fascicularis, Yeager 1996; M. fuscata, Hanya 2004; M. siberu, Richter et al. 2013), as well as sympatric François’ langurs at Nonggang (Zhou et al. 2009a). In contrast, sympatric Assamese macaques used young leaves as fallback foods (Huang et al. 2015). This variation may be related to two factors. First, Assamese macaques relied much more heavily on the young leaves of Bonia saxatilis than rhesus macaques did (77 vs 18–32 %). Secondly, Assamese macaques significantly increased Bonia saxatilis consumption while fruits were scarce, whereas there was no such seasonal pattern to the consumption of these bamboo leaves for rhesus macaques. In this study, we also found that one group consumed cultivated sugarcane in large quantities in the dry season. Crop-raiding has been documented as one of the many means by which primates buffer themselves against food shortages (Naughton-Treves et al. 1998).
The importance of Bonia saxatilis
Bonia saxatilis, a small shrubby bamboo, was the most important food for rhesus macaques at Nonggang. This bamboo was eaten throughout the year, and was the top food species through most of the year for rhesus macaques at Nonggang. Similarly, this species also constituted the bulk of the monthly diet (48.8–94.1 %) of sympatric Assamese macaques at Nonggang (Zhou et al. 2011; Huang et al., 2015). Bonia saxatilis plays a crucial role in the foraging ecology of macaques in limestone habitats. This small bamboo is superabundant and endemic to limestone hills (Liang et al. 1988). Thus, macaques can reduce search time by eating abundant species. Moreover, young bamboo leaves are plentiful throughout the year, and their availability invariant between seasons (Huang et al. 2015), which may provide a long-term staple food for macaques in limestone habitats. In a preliminary analysis of the nutritional content of plant species eaten by the macaques, Bonia saxatilis has the highest crude protein content and lower crude fiber content (unpublished data). These factors may be important in determining the reliance on Bonia saxatilis by limestone-living macaques. However, one problem for primates that consume large quantities of bamboo is the cyanide content (Glander et al. 1989; Tan 1999; Mekonnen et al. 2010; Nowak and Lee 2011). How do macaques cope with the potential toxin when they consume bamboo leaves? In addition to nutritional data, more information on digestive physiology is needed to improve our understanding of this dietary strategy.
Limestone-living macaques display similar feeding behavior to that of other limestone-living primates, shifting to a folivorous diet, and increasing dietary breadth when preferred foods are scarce. However, they continue to consume young bamboo leaves in large quantities year-round, which is significantly different from most other primates.
References
Albert A, Hambuckers A, Culot L, Savini T, Huynen M (2003) Frugivory and seed dispersal by northern pigtailed macaques (Macaca leonina), in Thailand. Int J Primatol 34:170–193
Bartlett TQ (1999) Feeding and ranging behavior of the white-handed gibbon (Hylobates lar) in Khao Yai National Park, Thailand. Ph.D. thesis, Washington University, St. Louis
Chapman CA, Chapman LJ, Wrangham RW (1995) Ecological constraints on group size: an analysis of spider monkey and chimpanzee subgroups. Behav Ecol Sociobiol 36:59–70
Chapman CA, Chapman LJ, Gillespie TR (2002) Scale issues in the study of primate foraging: red colobus of Kibale National Nature Park. Am J Phys Anthropol 117:349–363
Chaves OM, Bicca-Marques JB (2013) Dietary flexibility of the brown howler monkey throughout its geographic distribution. Am J Primatol 75:16–29
Fan PF, Fei HL, Scott MB, Zhang W, Ma CY (2009) Habitat and food choice of the critically endangered cao vit gibbon (Nomascus nasutus) in China: implication for conservation. Biol Conserv 144:2247–2254
Feeroz MM (2012) Niche separation between sympatric pig-tailed macaque (Macaca leonina) and rhesus macaque (M. mulatta) in Bangladesh. J Primatol 1:106
Fooden J (2000) Systematic review of the rhesus macaque, Macaca mulatta (Zimmermann, 1780). Field Zool 96:1–180
Glander KE, Wright PC, Seigler DS, Randrianasolo V, Randrianasolo B (1989) Consumption of cyanogenic bamboo by a newly discovered species of bamboo lemur. Am J Primatol 19:119–124
Goldstein SJ, Richard AF (1989) Ecology of rhesus macaques (Macaca nulatta) in northwest Pakistan. Int J Primatol 10:531–567
Guangxi Forestry Department (1993) Nature reserves in Guangxi. China Forestry Publishing House, Beijing (in Chinese)
Guo ST, Li BG, Wadanabe K (2007) Diet and activity budget of Rhinopithecus roxellana in the Qinling Mountains, China. Primates 48:268–276
Hanya G (2004) Diet of a Japanese macaque troop in the coniferous forest of Yakushima. Int J Primatol 33:322–337
Hanya G, Bernard H (2012) Fallback foods of red leaf monkeys (Presbytis rubicunda) in Danum Valley, Borneo. Int J Primatol 33:322–337
Hanya G, Noma N, Agetsuma N (2003) Altitudinal and seasonal variations in the diet of Japanese macaques in Yakushima. Primates 44:51–59
Hemingway CA, Bynum N (2005) The influence of seasonality on primate diet and ranging. In: Brockman D, Van Schaik CP (eds) Seasonality in primates. Cambridge University Press, Cambridge, pp 57–104
Huang CM (2002) The white-headed langur in China. Guangxi Normal University Press, Guilin (in Chinese)
Huang ZH, Huang CM, Tang CB, Huang LB, Tang HX, Ma GZ, Zhou QH (2015) Dietary adaptations of Assamese macaques (Macaca assamensis) in limestone forests in southwest China. Am J Primatol 77:171–185
Jiang JB (2009) Diet, activity rhythm and time budget of semi-provision rhesus macaques (Macaca mulatta) in Nonggang Nature Reserve, China. Master thesis, Guangxi Normal University, Guilin, China (in Chinese)
Li ZY, Rogers ME (2006) Food items consumed by white-headed langurs in Fusui, China. Int J Primatol 27:1551–1567
Li DY, Yuan PS, Krzton A, Huang CM, Zhou QH (2015) Dietary adaptation of white-headed langurs in a fragmented limestone habitat. Mammalia. doi:10.1515/mammalian-2014-0152
Liang ZF, Liang JY, Liu LF, Mo XL (1988) A report on vegetation in Nonggang Nature Reserve. Guihaia (Suppl) 1:83–184 (in Chinese)
Lindburg DG (1976) Dietary habits of rhesus monkeys (Macaca mulatta Zimmermann) in Indian forests. J Bombay Nat Hist Soc 73:261–269
Marshall AJ, Wrangham RW (2007) Evolutionary consequences of fallback foods. Int J Primatol 28:1219–1235
Mekonnen A, Bekele A, Fashing PJ, Hemson G (2010) Diet, activity patterns, and ranging ecology of the Bale monkey (Chlorocebus djamdjamensis) in Odobulu forest, Ethiopia. Int J Primatol 31:339–362
Naughton-Treves L, Treves A, Chapman C, Wrangham R (1998) Temporal patterns of crop-raiding by primates, linking food availability in croplands and adjacent forest. J Appl Ecol 35:596–606
Nowak K, Lee PC (2011) Consumption of cycads Encephalartos hildebrandtii by Zanzibar red colobus Procolobus kirkii. J East Africa Nat Hist 100:123–131
Richter C, Taufiq A, Hodges K, Ostner J, Schülke O (2013) Ecology of an endemic primate species (Macaca siberu) on Siberut Island, Indonesia. SpringerPlus 2:137
Shu ZM, Zhao TL, Huang QC (1988) Vegetation survey in Nonggang Nature Reserve. Guihaia (Suppl) 1:185–214 (in Chinese)
Su HH, Lee LL (2001) Food habits of Formosan rock macaques (Macaca cyclopis) in Jentse, northeastern Taiwan, assessed by fecal analysis and behavioral observation. Int J Primatol 22:359–377
Tan CL (1999) Group composition, home range size, and diet of three sympatric bamboo lemur species (Genus Hapalemur) in Ranomafana National Park, Madagascar. Int J Primatol 20:547–566
Tang HX (2008) Feeding ecology of the rhesus macaque (Macaca mulatta) at Nonggang Nature Reserve, China. Master thesis, Guangxi Normal University, Gulin, Chian (in Chinese)
Ting S, Hartley S, Burns KC (2008) Global patterns in fruiting seasons. Glob Ecol Biogeo 17:648–657
Tsuji Y, Hanya G, Grueter CC (2013) Feeding strategies of primates in temperate and alpine forest, comparison of Asian macaques and colobines. Primates 54:201–215
Workman C (2010) Diet of the Delacour’s langur (Trachypithcus delacouri) in Van Long Nature Reserve, Vietnam. Am J Primatol 72:317–324
Xu ZR (1993) The research on karst hill flora in south and southeast China. Guihaia (Suppl) 4:5–54
Yeager CP (1996) Feeding ecology of the long-tailed macaque in Ialimantan Tengah, Indonesia. Int J Primatol 17:51–62
Yeager CP, Kool K (2000) The behavioral ecology of Asian colobines. In: Whitehead PF, Jolly CJ (eds) Old World monkeys. Cambridge University Press, Cambridge, pp 497–521
Zhou QH, Huang ZH, Wei H, Wei FW, Huang CM (2009a) Factors influencing interannual and intersite variability in the diet of Trachypithecus francoisi. Int J Primatol 30:583–599
Zhou QH, Tang HX, Wei CQ, Huang CM (2009b) Diet and seasonal changes in rhesus macaques (Macaca mulatta) at Seven-Star Park, Guilin. Acta Theriol Sinica 29:419–426 (in Chinese)
Zhou QH, Wei H, Huang AH, Huang CM (2011) Diet of the Assamese macaque Macaca assamensis in limestone habitats of Nonggang, China. Curr Zool 57:18–25
Acknowledgments
This study was supported by the National Nature Science Foundation of China (No. 31360093, 31172122), Guangxi Natural Science Foundation (2014GXNSFAA118068, 2015GXNSFDA139013), Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection, Ministry of Education, China, and Guangxi Key Laboratory of Rare and Endangered Animal Ecology, Guangxi Normal University. We are very grateful to Guangxi Forestry Bureau and Nonggang National Nature Reserve for permitting us to conduct our research at the study site. We also acknowledge Dr. Xu Weibin and Dr. Huang Yusong for botanic identification. We also thank Dr. Katarzyna Nowak, Dr. John Oates, and one anonymous Associate Editor for instructive comments on this manuscript.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Tang, C., Huang, L., Huang, Z. et al. Forest seasonality shapes diet of limestone-living rhesus macaques at Nonggang, China. Primates 57, 83–92 (2016). https://doi.org/10.1007/s10329-015-0498-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-015-0498-7